Short abstract
Objective
Osteonectin plays a central role in various processes during the development of pancreatic adenocarcinoma. This prospective pilot study was performed to determine the feasibility of serum osteonectin as a screening tool for pancreatic cancer.
Methods
Blood samples were collected from 15 consecutive patients with newly diagnosed pancreatic cancer and 30 matched healthy controls. Serum osteonectin was measured using an osteonectin enzyme-linked immunosorbent assay kit. The primary outcomes were the diagnostic performance of serum osteonectin and the threshold value for differentiation of patients from controls.
Results
The median/quartile range of serum osteonectin in patients and controls were 306.8/288.5 ng/mL and 67.5/39.8 ng/mL, respectively. Osteonectin concentrations significantly differed among the study groups. A plasma osteonectin concentration of >100.18 ng/mL as selected by the receiver operating characteristic curves demonstrated an estimated area under the curve of 86% for prediction of pancreatic cancer. Tumour size was a significant predictor of serum osteonectin. A statistically significant difference in serum osteonectin between T1/T2 and T3/T4 tumours was found. Post-hoc comparisons revealed statistically significant differences in the serum osteonectin among the control, T1/T2, and T3/T4 groups.
Conclusion
Osteonectin may be used as a screening tool for pancreatic cancer, although this must be validated in prospective studies.
Keywords: Osteonectin, pancreatic cancer, prospective study, screening marker, biomarkers, adenocarcinoma
Introduction
More than 200,000 people annually are diagnosed with pancreatic cancer, which is the fourth most common cause of cancer-related mortality in the Western world, and increasing incidence rates have been described.1,2 Pancreatic adenocarcinoma is consistently diagnosed in its late stages because of the lack of screening biomarkers for clinical practice. Remarkably, tumours amenable to resection are found in only 10% to 15% of patients, and the overall 5-year survival rate is approximately 2% among patients with tumours of advanced stages.3 Therefore, the introduction of population-based screening markers could potentially change the natural course of this detrimental disease.
Osteonectin is also known as secreted protein acidic and rich in cysteine. This glycoprotein seems to have a critical function in pancreatic adenocarcinoma, not only in regulation of its connection to the tumour stroma but also in engagement in various cancer processes such as cell cycle progression, proliferation, angiogenesis, metastasis, migration, adhesion, and apoptosis.4,5
The tumour stroma in pancreatic cancer encompasses a large amount of the tumour volume (approximately 80%–90%). Dense fibrotic tissue comprising blood and lymphatic vessels, adipocytes, immune-inflammatory cells, pancreatic stellate cells, and extracellular matrix proteins constitute the stroma.6 Pancreatic stellate cells stimulate myofibroblasts in charge of stromal development, adding to inadequate vascularisation7 and activating signalling pathways through soluble substances that are associated with endurance and proliferation of pancreatic cancer cell lines.8 Osteonectin is momentarily secreted into the extracellular matrix but is not incorporated as a part of the extracellular matrix network.9 Osteonectin is highly expressed in cancer-associated fibroblasts and pancreatic stellate cells because the abnormal methylation pattern found in pancreatic adenocarcinoma cell lines is absent. Moreover, osteonectin expression in fibroblasts obtained from cancerous tissue is substantially different from that in cancer-free tissue from patients with pancreatic adenocarcinoma.10
We investigated whether the serum osteonectin concentration can distinguish healthy controls from patients with pancreatic cancer with clinically relevant accuracy and aimed to determine the practicability of osteonectin for population screening.
Materials and methods
This study included consecutive patients with newly diagnosed pancreatic cancer, matched patients with acute pancreatitis (only relevant data concerning acute pancreatitis are presented in this manuscript), and matched healthy controls. Blood samples for measurement of osteonectin were collected from December 2013 to January 2015.
The final histologic report and/or operating theatre notes were deemed necessary for patients with pancreatic cancer to take part in the analysis. The workup for patients with acute pancreatitis included haematological and biochemical parameters, abdominal imaging findings, and clinical evaluation results.
All healthy controls had normal findings of endoscopy, abdominal computed tomography, and mammography (for female patients) previously performed for various reasons upon study entry and underwent a repeat magnetic resonance imaging examination of the pancreas in January 2016 to check for the absence of pancreatic cancer. Healthy controls were chosen from the department’s database by applying certain criteria (described below).
Information was collected concerning sex, age, body mass index (BMI), presence of comorbidities, smoking, performance status, and medications. In accordance with the above-mentioned risk factors for pancreatic cancer, controls and patients were matched for age (classified by 5-year age groups), BMI (according to BMI classes), and the presence of diabetes, smoking, and alcohol consumption (yes/no).
Sequential filters were applied to the Microsoft Excel database to create this standard population so that the frequency of the confounder was identical among the groups (e.g., for every smoker with pancreatic cancer aged 40–49 years, we enrolled three healthy smokers and three smokers with acute pancreatitis aged 40–49 years).
Inclusion/exclusion criteria
The inclusion criterion for patients was a confirmed diagnosis of pancreatic cancer. The exclusion criteria were urgent surgery, exposure to certain chemicals, a history of other primary types of cancer, appearance of liver metastases, and lack of informed consent.
The inclusion criteria for healthy controls were negative findings on endoscopy, abdominal computed tomography, and mammography and the presence of at least one confounding factor. The exclusion criteria were liver cirrhosis, acute/chronic pancreatitis, inherited genetic syndromes, a personal or family history of cancer, haemolysed serum samples, exposure to certain chemicals, cancer development during follow-up, and lack of informed consent.
Ethical approval for this study was obtained by the Institutional Review Board and the World Medical Association. Every patient and control provided written informed consent prior to study entry.
Sample collection and storage
Blood samples were collected from the umbilical vein and allowed to clot for 2 hours at room temperature. The samples were then centrifuged for 15 minutes at 1000 × g. The serum was removed and immediately assayed, and the samples were stored at −20°C or −80°C.
Human osteonectin was detected by a quantitative sandwich enzyme immunoassay technique (Human Osteonectin ELISA kit; Cusabio, College Park, MD, USA) according to the manufacturer’s instructions. The detection range was 0.78 to 50 ng/mL, and the minimum detectable concentration of osteonectin (sensitivity) was 0.195 ng/mL.
CurveExpert 1.3 professional software was used to create a standard curve for calculation of the results. We averaged the duplicate readings for each standard and sample and subtracted the average zero standard optical density. A four-parameter logistic curve-fitting analysis was performed using capable computer software to create a standard curve by reducing the data.
Primary and secondary outcomes
The primary outcomes of the study were the diagnostic performance of the serum osteonectin concentration to distinguish healthy controls from patients with pancreatic cancer and the threshold value that can indicate the presence of pancreatic cancer. The secondary outcome was the relationship of the serum osteonectin concentration with tumour size.
Statistical analyses
The chi-square test was used to correlate the two categorical variables. The Mann–Whitney U test was used to test both the differences in the shapes of the distributions of the two groups and the significance of the difference between two independent samples (groups) of an ordinal variable.
The median test and the Kruskal–Wallis analysis of variance (ANOVA) by ranks were used to correlate multiple independent samples (groups) in data containing a coding variable with codes to uniquely identify the group membership of each case. The significance levels for the individual post-hoc correlations were adjusted by one-way ANOVA and the Bonferroni test.
The performance of a two-class classifier across the range of possible thresholds was summarised by a receiver operating characteristic (ROC) curve, taking into account the pancreatic cancer prevalence of 0.0124%.3 The validation of our results was achieved using the sample size for two means, t-test, independent samples, and several-means one-way ANOVA.
Statistical significance was defined as p < 0.05. MedCalc for Windows, version 14.8.1 (MedCalc Software, Ostend, Belgium) and Statistica version 7 (StatSoft, Inc., Tulsa, OK, USA) were used for data analysis.
Results
Ninety participants were allocated to three groups: patients with pancreatic cancer (n = 15), patients with acute pancreatitis (n = 45), and healthy controls (n = 30).
The clinicopathological characteristics and confounding factors did not differ significantly between the patients with pancreatic cancer and controls. Tumour sizes were spread roughly evenly among the patients with cancer (Table 1).
Table 1.
Comparison of clinicopathological characteristics between patients and healthy controls
| Cancer (n = 15) | Controls (n = 30) | p-value | |
|---|---|---|---|
| Age, years | 68.3 ± 11.24 | 61.15 ± 9.95 | 0.095 |
| Sex, female/male | 10/5 | 16/14 | 0.393 |
| BMI, kg/m2 | 23.74 ± 4.73 | 26.26 ± 3.43 | 0.135 |
| Diabetes | 5 | 11 | 0.878 |
| Smoking | 6 | 13 | 0.891 |
| Alcohol consumption | 5 | 9 | 0.869 |
| Tumour size | |||
| T1 | 3 | ||
| T2 | 5 | ||
| T3 | 3 | ||
| T4 | 4 | ||
Data are presented as n or mean ± standard deviation. BMI, body mass index.
The duration of follow-up (median and 25th to 75th interquartile range) for healthy controls was 11 months and 5.5 to 14.5 months, respectively. None of the healthy controls developed pancreatic cancer.
The descriptive statistics of the serum osteonectin values among the patients with cancer, patients with acute pancreatitis, and healthy controls are presented in Table 2 and Figure 1.
Table 2.
Osteonectin serum concentrations in ng/mL in patients with cancer, patients with acute pancreatitis, and healthy individuals
| Median | Lower | Upper | Quartile range | |
|---|---|---|---|---|
| Cancer | 306.76 | 27.85 | 978.87 | 288.49 |
| Controls | 67.47 | 25.87 | 147.54 | 39.79 |
| Acute pancreatitis | 467.15 | 32.39 | 2412.57 | 380.00 |
Figure 1.
Box plot by group shows the median, 25th to 75th interquartile range, and minimum to maximum serum osteonectin concentrations in ng/mL.
No significant differences were found in the serum osteonectin values between the patients with acute pancreatitis and those with pancreatic cancer.
Primary outcomes
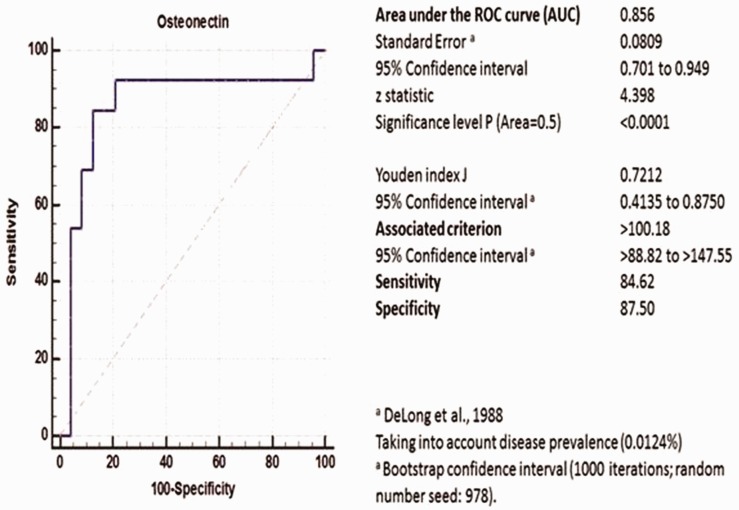
A highly statistically significant difference was found in the serum osteonectin concentration between the controls and patients with cancer (Z adjusted: −3.86, p = 0.0001). The optimum cut-off serum osteonectin concentration was 100.18 ng/mL. Serum osteonectin concentrations of >100.18 ng/mL at enrolment had a sensitivity of 84.62%, specificity of 87.50%, and area under the ROC curve of 0.856 (86% accuracy, Z-statistic = 4.398, p < 0.0001, 95% confidence interval = 0.701–0.949) for predicting pancreatic cancer (Figure 2).
Figure 2.
Receiver operating characteristic curve. Serum osteonectin concentrations of >100.18 ng/mL at enrolment had 86% accuracy in predicting pancreatic cancer.
To validate our results, we performed a two-mean t-test for independent samples with alpha = 0.05, power = 0.9, difference between mean = 239.29 ng/mL [(mean: cancer group = 306.76 ng/mL − control group = 67.47ng/mL), (standard deviation: cancer group = 265.97 ng/mL and control group = 28.76 ng/mL)], and patient per control ratio = 0.5. Fifteen patients with pancreatic cancer and 30 healthy controls constituted the estimated minimum sample size. We added an additional 10% to these numbers in case of haemolysed samples and other causes of exclusion.
Secondary outcome
Univariate analysis of one-way ANOVA (sigma-restricted parameterisation, effective hypothesis decomposition) was applied to evaluate the effect of tumour size on the serum osteonectin concentration. Tumour size was a significant factor (p = 0.021, df = 3, F-statistic = 5.329).
By applying the Bonferroni test in post-hoc comparisons (probabilities for post-hoc test error: between MS = 33972, df = 9), we found a statistically significant difference in the serum osteonectin values between T2 and T3 tumours (p = 0.045). The difference between T1 and T3 tumours was not significant (p = 0.064).
Taken together, these data revealed a statistically significant difference in the serum osteonectin concentration between T1/T2 and T3/T4 tumours (Z-statistic =2.781, p = 0.005).
Comparison of the serum osteonectin concentration among the controls and patients with T1/T2 and T3/T4 tumours also showed statistical significance (F-statistic = 40.653, df = 2, p = 0.0001).
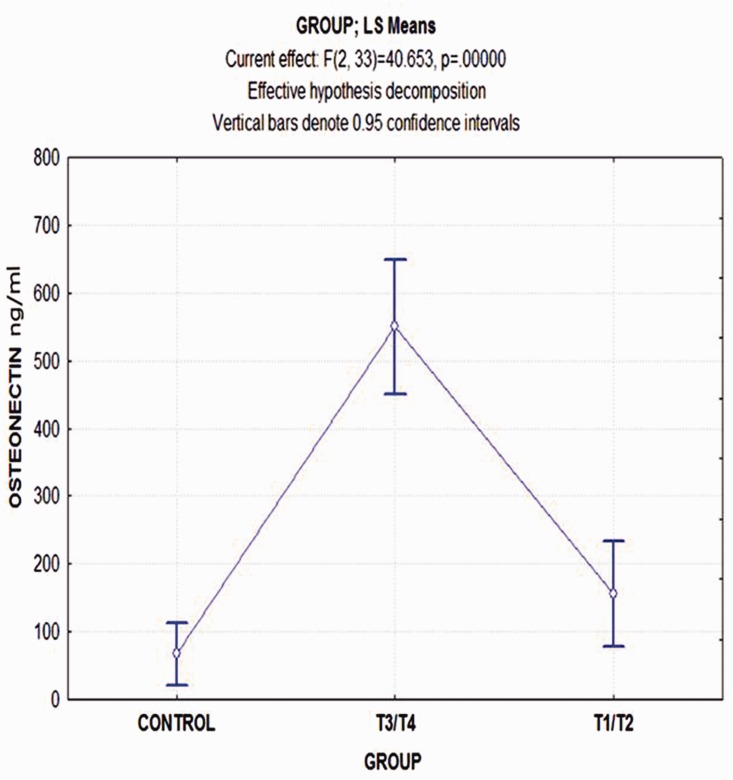
By applying the Bonferroni test in post-hoc comparisons (probabilities for post-hoc test error: between MS = 11746, df = 33), we found a significantly statistical difference in the serum osteonectin concentration between the controls and T3/T4 group, whereas the respective comparison with the T1/T2 group was not significant (p = 0.057). Likewise, the difference between the T1/T2 and T3/T4 groups was highly significant (p = 0.0001) (Figure 3).
Figure 3.
Least-square means and 95% confidence intervals of serum osteonectin concentrations in ng/mL among the three groups
To confirm our results, we performed one-way ANOVA for a several-means sample size test with alpha = 0.05, power = 0.9, three groups, and root mean square standardised effect = 256.90 (mean: T1/T2 group = 154.93 ng/mL, T3/T4 group = 549.68 ng/mL, and control group = 67.47 ng/mL)]. The minimum sample size was estimated as at least four patients in each group. In case of haemolysed samples and other causes of exclusion, an additional 10% was added to these samples.
Discussion
The obtainability of an effective and reliable pancreatic cancer screening tool that permits initial pancreatic cancer recognition is of vital importance to international health care systems. Better screening systems with high sensitivity and specificity for this diagnosis are needed; these systems should be technically easy to use had have high population acceptance.
We conducted this small pilot case-control study in view of the fact that matching was useful because there were many possible controls but a smaller number of cases (patients and controls were matched for confounding factors epidemiologically considered to systematically influence the occurrence of pancreatic cancer with a ratio of 2:1).
Our methodology mainly aimed to document the accuracy of serum osteonectin levels for distinguishing healthy controls from patients with pancreatic cancer, the threshold value that can indicate the presence of pancreatic cancer, and the relationship of the serum osteonectin concentration with tumour size as well as to create a knowledge base for prospective future studies.
The difference between cancer and control osteonectin serum values was highly significant. The optimal cut-off point selected for serum osteonectin was 100.18 ng/mL. Serum osteonectin concentrations of >100.18 ng/mL at enrolment had a sensitivity of 84.62%, specificity of 87.50%, and area under the ROC curve of 0.856 (accuracy) for predicting pancreatic cancer. There was a statistically significant difference in the serum osteonectin concentrations between the controls and patients with T3/T4 cancer. Likewise, the difference between the T1/T2 and T3/T4 groups was highly significant (p = 0.0001), whereas the respective comparison with the T1/T2 group was not significant (p = 0.057). However, we were expecting such a difference to be established with the enrolment of a significant number of participants.
The prospective predictive value of osteonectin is expected to be the same as that estimated by the ROC curves under the hypothesis that the osteonectin concentrations of healthy controls will remain stable and given the fact that none of the healthy controls developed pancreatic cancer after a median follow-up of 11 months.
The functions of osteonectin in cancer growth might be affected to a certain extent by the interface with several growth factors (e.g., fibroblast growth factor and transforming growth factor-β) and matrix metalloproteinases.11 Remarkably, no osteonectin receptors have been recognised, and the protein part rapidly undergoes proteolysis by various proteases.12 Interestingly, osteonectin expression in pancreatic cancer cell lines is clearly correlated with matrix metalloproteinase (MMP)-2 expression.13 Moreover, osteonectin increases the metastatic capacity of cancer cells by promoting MMP-2 expression. Additionally, osteonectin proteolysis is caused by MMPs, and derivatives of the degradations probably have diverse biological activities.14 An osteonectin peptide appears to regulate and increase apoptosis in MiaPaCa-2 cells.15
Osteonectin stimulates G1/S cell cycle detention by down-regulation of the phosphorylation pRB and up-regulation of p53 and p27Kip116; it also inhibits angiogenesis by controlling the efficiency of platelet-derived growth factor and vascular endothelial growth factor.17 Osteonectin-regulated signalling of transforming growth factor-β1 is a significant mediator in pancreatic carcinogenesis and can be either tumour-suppressive or -promoting.18
Pancreatic intraepithelial neoplasia (PanIN) is the most common type of pancreatic cancer.19 KRAS2 oncogene activation in conjunction with CDKN2A/p16 loss and TP53 and SMAD4/DPC4 inactivation appears to be distinctive in pancreatic cancer.20 Epigenetic alterations are known to affect the growth of pancreatic cancer.21 Interestingly, irregular methylation of the CpG islands on the osteonectin gene is found in 28% of PanIN tissue.22 In intraductal papillary mucinous neoplasms (IPMN), osteonectin expression is missing in 50% and 80% of tissue showing low–moderate and high-grade dysplasia, respectively.23 Methylation of CpG region 2 (CpG sites 8–12) is excessively susceptible in pancreatic carcinogenesis. Likewise, the percentage of methylation at CpG region 2 is coupled with alcohol consumption, tobacco exposure, and larger tumour size.24 Overall, it appears that the expression of osteonectin in stromal fibroblasts is mediated by pancreatic adenocarcinoma cells. Likewise, osteonectin is expressed in metastases as well as in primary tumors.25
To our knowledge, only two studies have addressed the osteonectin serum concentration in patients with pancreatic cancer. The present study is the first to assess serum osteonectin in humans with the intention of identifying a screening marker for pancreatic cancer by connecting fundamental research to clinical practice.
In 2003, Sato et al.10 estimated the osteonectin levels in serum samples by enzyme-linked immunosorbent assay and found no significant difference in the mean osteonectin levels among patients with pancreatic adenocarcinoma, patients with benign pancreatic disorders, and healthy individuals. In contrast, Guweidhi et al.26 subsequently measured the osteonectin serum levels in patients with pancreatic cancer, patients with chronic pancreatitis, and healthy controls. They found significantly lower serum osteonectin levels in patients with pancreatic cancer but no significant correlation between osteonectin mRNA levels in tissues and serum osteonectin levels in individual patients with pancreatic cancer. In contrast to the findings of this former study, we documented significant differences (data not shown for acute pancreatitis). There were differences with the latter study as well. We found higher rather than lower serum osteonectin values in patients with cancer and a significant correlation between tumour size and serum osteonectin values. A potential explanation for the differences from the aforementioned studies may be the following: our comparison groups were matched according to confounding factors, the correlation of serum osteonectin values to pancreatic tumours was based on categorisation of tumour size instead of measurement of osteonectin mRNA levels in tissues, and different methods were used for measurement of serum osteonectin.
Likewise, none of the proposed markers in the literature is specific for pancreatic cancer; e.g., thrombospondin 2, cancer antigen 19-9, tissue inhibitor of metalloproteinase 1, and leucine-rich alpha-2-glycoprotein 1.27,28
The limitations of this study are as follows. First, the sample size was small. However, because the project is ongoing, we expect that the results will overcome this limitation (because of the small sample size, no analysis was done for confounder-adjusted estimates). Second, patients with PanIN and IPMN were not included. Third, our results require prospective validation. Fourth, a second measurement is being planned in a future study because only a single measurement per patient was calculated. Fifth, cancers originating in other sites were not examined. Finally, considering the correlation of tumour size to the serum osteonectin concentration, although our study was sufficiently powered, the results must be interpreted cautiously given the small numbers of patients with each tumour stage.
A larger-scale prospective study including healthy controls, patients with pancreatic cancer, and patients with IPMN and PanIN is needed to verify our preliminary results and determine the feasibility of the serum osteonectin concentration for screening. A combination of the serum osteonectin concentration with methylation panels including osteonectin and other usual hypermethylated genes (such as Reprimo) may be acceptable for pancreatic cancer screening and detection of PanIN and IPMN.
In summary, the serum osteonectin concentration clearly differentiates patients with pancreatic cancer from healthy individuals. The serum osteonectin concentration is positively associated with increasing tumour stage. As a promising marker for pancreatic cancer, the serum osteonectin level shows potential for screening, but further research is needed to verify this potential.
Acknowledgments
This work was presented in abstract form at the ASGBI International Surgical Congress Belfast on 11–13 May 2016. The abstract with preliminary results of the present study was awarded first place in the Short Papers of Distinction category. Following presentation at the Congress, the abstract was published in the British Journal of Surgery [2016; 103 (S6): 8–10].
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas 2010; 39: 458–462. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Stat Facts: Pancreas Cancer. National Cancer Institute. Available from: http://seer.cancer.gov/statfacts/html/pancreas.html (Accessed 06 July 2016).
- 4.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagaraju GP, Dontula R, El-Rayes BF, et al. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis 2014; 35: 967–973. [DOI] [PubMed] [Google Scholar]
- 6.Erkan M, Hausmann S, Michalski CW, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol 2012; 9: 454–467. [DOI] [PubMed] [Google Scholar]
- 7.Erkan M. The role of pancreatic stellate cells in pancreatic cancer. Pancreatology 2013; 13: 106–109. [DOI] [PubMed] [Google Scholar]
- 8.Hwang RF, Moore T, Arumugam T. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 2008; 68: 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 2002; 14: 608–616. [DOI] [PubMed] [Google Scholar]
- 10.Sato N, Fukushima N, Maehara N, et al. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene 2003; 22: 5021–5030. [DOI] [PubMed] [Google Scholar]
- 11.Brekken RA, Sage EH. SPARC a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol 2001; 19: 816–827. [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw A, Sage EH. SPARC a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 2001; 107: 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhivkova-Galunska M, Adwan H, Eyol E, et al. Osteopontin but not osteonectin favors the metastatic growth of pancreatic cancer cell lines. Cancer Biol Ther 2010; 10: 54–64. [DOI] [PubMed] [Google Scholar]
- 14.Tai IT, Tang MJ. SPARC in cancer biology: its role in cancer progression and potential for therapy. Drug Resist Updat 2008; 11: 231–246. [DOI] [PubMed] [Google Scholar]
- 15.Rahman M, Chan AP, Tang M, et al. A peptide of SPARC interferes with the interaction between caspase8 and Bcl2 to resensitize chemoresistant tumors and enhance their regression in vivo. PLoS One 2011; 6: e26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao Z, Ma X, Fan X, et al. Secreted protein acidic and rich in cysteine inhibits the growth of human pancreatic cancer cells with G1 arrest induction. Tumour Biol 2014; 35: 10185–10193. [DOI] [PubMed] [Google Scholar]
- 17.Jendraschak E, Sage EH. Regulation of angiogenesis by SPARC and angiostatin: implications for tumor cell biology. Semin Cancer Biol 1996; 7: 139–146. [DOI] [PubMed] [Google Scholar]
- 18.Truty MJ, Urrutia R. Basics of TGF-beta and pancreatic cancer. Pancreatology 2007; 7: 423–435 [DOI] [PubMed] [Google Scholar]
- 19.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol 2008; 1: 306–316. [PMC free article] [PubMed] [Google Scholar]
- 20.Hidalgo M. New insights into pancreatic cancer biology. Ann Oncol 2012; 23: 135–138. [DOI] [PubMed] [Google Scholar]
- 21.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002; 3: 415–428. [DOI] [PubMed] [Google Scholar]
- 22.Sato N, Fukushima N, Hruban RH, et al. . CpG island methylation profile of pancreatic intraepithelial neoplasia. Mod Pathol 2008; 21: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong SM, Kelly D, Griffith M, et al. Multiple genes are hypermethylated in intraductal papillary mucinous neoplasms of the pancreas. Mod Pathol 2008; 21: 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J, Song J, Huang H, et al. Methylation of the SPARC gene promoter and its clinical implication in pancreatic cancer. J Exp Clin Cancer Res 2010; 29: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaz J, Ansari D, Sasor A, et al. SPARC: A Potential Prognostic and Therapeutic Target in Pancreatic Cancer. Pancreas 2015; 44: 1024–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guweidhi A, Kleeff J, Adwan H, et al. Osteonectin influences growth and invasion of pancreatic cancer cells. Ann Surg 2005; 242: 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capello M, Bantis LE, Scelo G, et al. Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer. J Natl Cancer Inst 2017; 1: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Bamlet WR, Oberg AL, et al. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci Transl Med 2017; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]