Short abstract
Objective
The sedative dexmedetomidine plays a role in multi-organ protection by inhibiting toll-like receptor (TLR) 4 expression in ischemia/reperfusion injury. The present study investigated whether the neuroprotective effects of dexmedetomidine could be blocked by the TLR4 agonist lipopolysaccharide.
Methods
We established a cerebral ischemia/reperfusion model in neonatal Sprague-Dawley rats through bilateral carotid artery occlusion for 20 minutes followed by a 2-hour reperfusion. Rats were assigned to four groups: Sham operation, ischemia/reperfusion, ischemia/reperfusion preceded by dexmedetomidine treatment (10 µg/kg), and ischemia/reperfusion preceded by dexmedetomidine (10 µg/kg) and lipopolysaccharide (500 µg/kg) treatments. Cerebral tissue injury was assessed by hematoxylin and eosin staining, and cerebral TLR4 expression was evaluated by real-time PCR and western blot.
Results
Pretreatment with dexmedetomidine reduced ischemia-induced morphological changes in the hippocampal CA3 region and downregulated TLR4 expression, but these neuroprotective effects were partially blocked by co-treatment with the TLR4 agonist lipopolysaccharide.
Conclusion
Our results indicate that inhibition of cerebral TLR4 expression is related to the neuroprotective effects of dexmedetomidine in this neonatal rat cerebral ischemia/reperfusion model.
Keywords: Dexmedetomidine, cerebral ischemia/reperfusion injury, neuroprotection, TLR4, hippocampus, lipopolysaccharide
Introduction
Inflammation is known to play a major role in mediating brain injury caused by cerebral ischemia.1,2 Although the inflammatory mechanism of brain ischemia and ischemia/reperfusion injury has been elucidated over the past decade, numerous clinical studies targeting inflammatory mediators have so far failed to demonstrate the expected positive outcomes in stroke patients.3,4
One promising therapeutic strategy to alleviate cerebral ischemia is the targeted inhibition of toll-like receptors (TLRs), which are involved in initiating the inflammatory responses post-ischemic insult. TLR2 and TLR4 are considered the most important mediators in the pathological progression of cerebral injury resulting from ischemia and reperfusion.5 Post-injury, TLR2 and TLR4 bind with their endogenous ligands, which include heat-shock proteins, high-mobility group box 1 (HMGB1), hyaluronic acid, and fibronectin, and activate the signaling pathway that leads to proinflammatory cytokine production.5
Previous studies have shown that dexmedetomidine (DEX), an α2-adrenoceptor agonist, exerts anti-inflammatory effects and plays a protective role on a multi-organ level,6–10 including neuroprotection from cerebral ischemia/reperfusion injury.7,11,12 Inhibition of the TLR4 pathway has been suggested as the major underlying working mechanism responsible for the neuroprotective effects of DEX. Kim et al.13 showed that DEX is neuroprotective against transient cerebral ischemia/reperfusion injury in rats by inactivating the TLR-4/NF-κB pathway to inhibit inflammation. Resatorvid, a selective TLR4 antagonist, has been shown to mimic the neuroprotective effects of DEX.14
In the present study, we tested the hypothesis that the neuroprotective effects of DEX could be abolished by the TLR4 agonist lipopolysaccharide (LPS).
Materials and methods
Animals and study protocol
Neonatal Sprague-Dawley rats aged 7–10 days (13–20 g) were purchased from Tongji Medical College, Huazhong University (Wuhan, Hubei, China). All procedures were approved by the Ethics Committee for Animal Experimentation of Puai Hospital Affiliated to Huazhong University of Science and Technology. Animals were maintained in accordance with the guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (8th Edition, 2011). During the pilot study, we tested the effect of pentobarbital dose on respiration; the maximal dose that did not affect spontaneous respiration (40 mg/kg by intraperitoneal injection [i.p.]) was used throughout the study. Surgery was performed at room temperature (22–25°C) without additional ventilation. Under sodium pentobarbital (40 mg/kg i.p.) anesthesia and ECG monitoring, a sham operation or bilateral carotid artery occlusion (BCAO)15 was performed. The animals were randomly divided into four groups (Figure 1): The sham-operated group (S) received saline by i.p. injection 30 minutes post-anesthesia and did not undergo BCAO. The ischemia/reperfusion injury group (IR) received saline 30 minutes post-anesthesia. After 2 hours, rats underwent a 20-minute BCAO, followed by a 2-hour reperfusion. The DEX group (D) was pretreated with DEX (10 µg/kg i.p.) 1 hour before BCAO, followed by a 2-hour reperfusion. The fourth group (DL) received LPS (500 µg/kg i.p.) 1 hour before DEX pretreatment, followed by BCAO and reperfusion. Following the procedure, all animals were sacrificed under deep anesthesia (100 mg/kg chloral hydrate i.p.)
Figure 1.
Experimental groups used in the study: sham operation (S); ischemia/reperfusion injury (IR); DEX and ischemia/reperfusion injury (D) and DEX, LPS, and ischemia/reperfusion injury (DL). DEX, dexmedetomidine; LPS, lipopolysaccharide; NS, normal saline.
Tissue collection
Following decapitation, the brain was removed and part of the brain tissue was successively cryopreserved at −80°C and used for western blotting and qPCR analyses. The remaining brain tissue was fixed in 4% paraformaldehyde, embedded in paraffin, sliced into sections of 4-µm thickness, and stained with hematoxylin and eosin (H&E).
H&E staining
Brain sections (4 µm thickness) were dewaxed in xylene, dehydrated using gradient alcohol, and washed in phosphate-buffered saline. The sections then underwent H&E staining, liquid differentiation, gradient alcohol and xylene dehydration, and neutral gum sealing.
Western blotting
Total protein extraction was performed by hippocampus homogenization. The mixture was incubated at 4°C for 1 hour and then centrifuged at 12,000 rpm for 5 minutes at 4°C. The supernatants were collected and stored at −80°C until protein concentration determination by bicinchoninic acid assay. For electrophoresis, 50 µg of total protein were loaded per line and separated on a 12% sodium dodecyl sulfate-polyacrylamide electrophoresis gel at 100 V for 90 minutes. Separated proteins were transferred to polyvinylidene fluoride micro porous membranes at 200 mA for 60 minutes using an electro-blotting system. The blots were stained to confirm equal protein loading, and unstained with a mixture of Tris-buffered saline and Tween (TBST, 20 mM Tris, 150 mM NaCl, 0.1% Tween 20, pH 7.5). Non-specific binding to polyvinylidene fluoride membranes was blocked by 5% Blotto non-fat dry milk in TBST for 1 hour at 24°C. Membranes were then incubated for 20 hours at 4°C with a rabbit anti-TLR4 polyclonal antibody (Abcam, Cambridge, UK) diluted 1:500 in 5% non-fat dry milk in TBST. After washing three times with TBST for 15 minutes, membranes were incubated with a secondary antibody coupled to horseradish peroxidase diluted 1:1,000 in 5% non-fat dry milk in TBST for 1 hour. We used β-actin as a loading control. Proteins were visualized in Biomax light films (Sigma-Aldrich, St. Louis, MO, USA) using the Immobilon Western Chemiluminescent Horseradish Peroxidase substrate kit. AlphaEaseFC software (Alpha Innotech, San Leandro, CA, USA) was used to measure band intensities by densitometry.
qPCR
For total RNA extraction, the dissected hippocampi were placed in sterile tubes containing 1 mL of Trizol reagent and frozen at −80°C until analysis. Total RNA was extracted using the MiniBEST Universal RNA Extraction Kit (TaKaRa Bio, Kusatsu, Japan) according to the manufacturer’s instructions. RNA samples were suspended in 20 µL of nuclease-free water. One microgram of RNA was digested with deoxyribonuclease I (DNase I, amplification grade; Gibco BRL, Gaithersburg, MD, USA). Complementary DNA was synthesized by reverse transcription using the digested RNA. Concentration and purity of the total RNA were assessed by UV spectrophotometry at 260 and 280 nm, qualifying for reverse transcription and amplification. cDNA was synthesized using PrimeScript™ One Step RT-PCR Kit Ver. 2 (TaKaRa Bio). For real-time PCR reaction, the SYBR Green PCR kit (TaKaRa Bio, RR820A) was used in a CFX96 real time-PCR system (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. Expression levels were calculated using the ΔΔCT method. Table 1 lists the primers used.
Table 1.
q-PCR primers used in the study.
| Primer | Sequence | Length (bp) | |
|---|---|---|---|
| β-actin | Forward | 5′-CGTTGACATCCGTAAAGACCTC-3′ | 110 |
| Reverse | 5′-TAGGAGCCAGGGCAGTAATCT-3′ | ||
| R-TLR4 | Forward | 5′-CCCAATTGACTCCATTCAAGC-3′ | 229 |
| Reverse | 5′-CCTGAACTCATCAATGCTCACAT-3′ | ||
Statistical analysis
Data are presented as the means ± SD. Comparisons between groups were made using ANOVA followed by Dunnett’s test. P values < 0.05 were considered statistically significant.
Results
Survival rate and impact of DEX on ischemia/reperfusion-induced cerebral injury
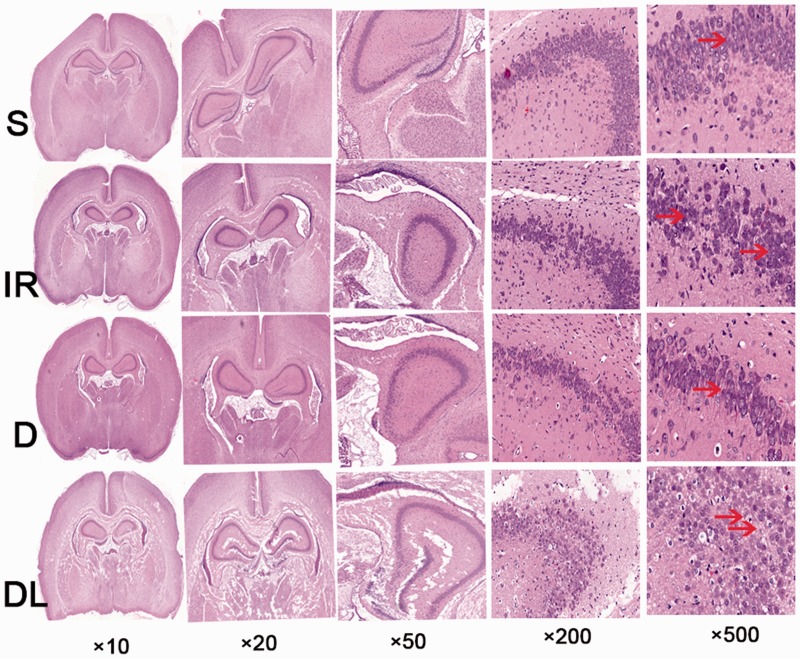
All six rats in the S (control) group survived to the end of the study. Six out of nine rats survived in the IR group and six out of eight rats survived in the D and DL groups. Figure 2 shows that the white matter was the main target of damage in this rat model of cerebral ischemia/reperfusion injury. Cells in the hippocampal CA3 region of the S group maintained a normal structure, clear nucleus, and abundant cytoplasm; no interstitial edema was observed. The pyramidal layer II and layer III cells were arranged closely and the nucleus appeared clear, large, and round. In contrast, the number of hippocampal neurons decreased in the IR group and structural degeneration was observed. As expected, the ischemia/reperfusion-induced pathological alterations were attenuated by pretreatment with DEX, while co-administration of LPS partially abolished the protective effects of DEX; smaller hippocampal neurons, loosely arranged, and pyknotic nuclei were observed in the DL group.
Figure 2.
Histopathology of the hippocampal CA3 region in rats treated with a sham operation (S), ischemia/reperfusion injury (IR), dexmedetomidine and ischemia/reperfusion injury (D), and lipopolysaccharide, dexmedetomidine, and ischemia/reperfusion injury (DL). Arrows point to structural damage.
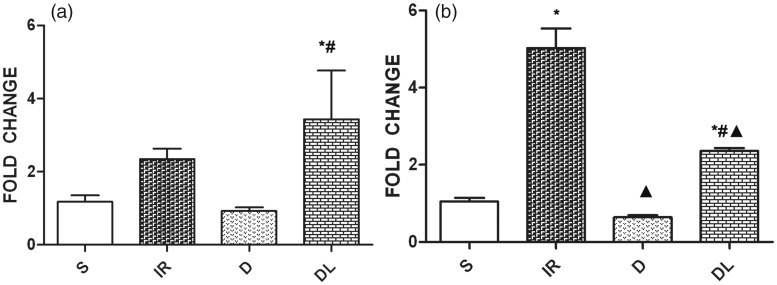
mRNA expression of TLR4 in the cortex and hippocampus
Figure 3a shows that TLR4 mRNA expression in the cortex of the IR group was higher than that of the S group. DEX pretreatment inhibited the expression of TLR4, but co-treatment with LPS abolished that effect and increased TLR4 expression significantly in the DL group. Figure 3b compares the levels of TLR4 mRNA in the hippocampus between groups. TLR4 was significantly upregulated in the IR group compared with levels in the S group. DEX pretreatment inhibited TLR4 expression, but this effect was abolished by co-treatment with LPS.
Figure 3.
Toll-like receptor 4 mRNA expression levels in the cortex (a) and hippocampus (b) of rats treated with a sham operation (S), ischemia/reperfusion injury (IR), dexmedetomidine and ischemia/reperfusion injury (D), and lipopolysaccharide, dexmedetomidine, and ischemia/reperfusion injury (DL). *P<0.05 vs. S group; #P<0.05 vs. D group; ▲P<0.05 vs. IR group.
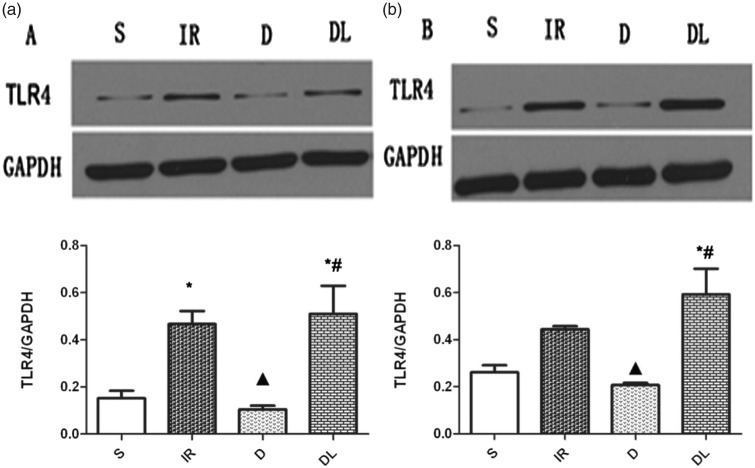
Protein expression of TLR4 in the left and right hippocampus
Figure 4a shows that TLR4 protein levels in the left hippocampus were significantly higher in the IR group than in the S group. DEX pretreatment inhibited protein expression, but this effect was abolished by co-treatment with LPS. Figure 4b shows similar results in the right hippocampus, although the difference between TLR4 protein levels in the IR and S groups did not reach statistical significance.
Figure 4.
Toll-like receptor 4 (TLR4) protein expression levels in the left (a) and right (b) hippocampus of rats treated with a sham operation (S), ischemia/reperfusion injury (IR), dexmedetomidine and ischemia/reperfusion injury (D), and lipopolysaccharide, dexmedetomidine, and ischemia/reperfusion injury (DL). GAPDH expression levels were used as the normalizing control. *P<0.05 vs. S group; #P<0.05 vs. D group; ▲P<0.05 vs. IR group.
Discussion
The present study shows that the protective effects of DEX can be abolished by the TLR4 agonist LPS, indicating that DEX downregulates TLR4 in this cerebral ischemia/reperfusion injury model in neonatal rats. To the best of our knowledge, this is the first report describing the effects of DEX in the absence or presence of a TLR4 agonist in this model.
TLR4 signaling is critical during ischemia/reperfusion injury
Exposure to multiple anesthetics and sedatives can cause neurodegeneration in the developing brain.16 Neuroprotection during anesthesia is therefore of importance, especially in children. TLR4 release is known to contribute to the initiation and development of ischemia/reperfusion injury by promoting mediators that amplify cellular damage.17 We found that cerebral ischemia/reperfusion injury could be attenuated by DEX, and that this effect was partially attributable to downregulated protein and mRNA expression in the ischemic brain area. Co-treatment with the TLR4 agonist LPS abolished the effects of DEX, indicating that TLR4 plays a pivotal role in ischemia/reperfusion injury and that downregulation of TLR4 expression may be the mechanism by which DEX alleviates ischemia/reperfusion injury in this model. Previous studies found that blocking TLR4 signal transduction attenuated cardiac injury and dysfunction in a mouse ischemia/reperfusion model,18 and that knocking out TLR4 in mice preserves renal function in renal ischemia/reperfusion injury.19 Our study provides further evidence supporting the role of TLR4 in the pathogenesis of ischemia/reperfusion injury, and inhibition of TLR4 signaling may be a promising strategy in protecting organs against injury.
TLR2 and TLR4 are both members of the innate immune system in the brain, and participate in the non-infectious immune response. In the pilot study, we found that the expression of TLR4, but not TLR2, increased significantly 2 hours after ischemia/reperfusion injury in brain tissue (data not shown), suggesting that TLR4, but not TLR2, is actively involved in the early stage of injury. Infarct volume in TLR2-deficient mice has been shown to be smaller than infarct volume in wild-type mice, indicating that TLR2 may also be involved in prolonged ischemia/reperfusion injury.20 Hyakkoku et al.21 reported that infarcts were smaller in TLR4 knockout mice, highlighting the importance of TLR4 during the early stages of ischemia/reperfusion injury.
Study limitations
Our results add evidence suggestive of the link between TLR4 signaling and the neuroprotective effects of DEX, but the causal relationship should be validated with TLR4 knockout and knock-in models. We monitored the animals during the study using ECG, but did not monitor blood pressure and oxygenation; these parameters may further inform our understanding of the interaction between DEX and LPS in this model. Inflammatory mechanisms were not followed in this study, and we only focused on the pathological evidence.
In conclusion, our study confirms the involvement of TLR4 during the early stages of cerebral ischemia/reperfusion injury, and supports the hypothesis that downregulation of TLR4 in ischemic brain tissue is a key mechanism of the neuroprotective effects of DEX. Inhibition of TLR4 may thus offer a promising therapeutic strategy in treating ischemia/reperfusion injury.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Marquardt L, Ruf A, Mansmann U, et al. Inflammatory response after acute ischemic stroke. J Neurol Sci 2005; 236: 65–71. [DOI] [PubMed] [Google Scholar]
- 2.Miao YF, Wu H, Yang SF, et al. 5′-adenosine monophosphate-induced hypothermia attenuates brain ischemia/reperfusion injury in a rat model by inhibiting the inflammatory response. Mediators Inflamm 2015; 2015: 520745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuma A, Yenari MA. Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Front Neurol 2017; 8: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J Transl Med 2009; 7: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Ge P, Zhu Y. Tlr2 and tlr4 in the brain injury caused by cerebral ischemia and reperfusion. Mediators Inflamm 2013. ; 2013: 124614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu J, Sun P, Zhao H, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care 2011; 15: R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y, Xu H, Yan J, et al. Molecular targets and mechanism of action of dexmedetomidine in treatment of ischemia/reperfusion injury. Mol Med Rep 2014; 9: 1542–1550. [DOI] [PubMed] [Google Scholar]
- 8.Erer D, Ozer A, Arslan M, et al. The protective effects of dexmedetomidine on liver injury-induced myocardial ischemia reperfusion. Bratisl Lek Listy 2014; 115: 422–426. [DOI] [PubMed] [Google Scholar]
- 9.Sahin T, Begec Z, Toprak HI, et al. The effects of dexmedetomidine on liver ischemia-reperfusion injury in rats. J Surg Res 2013; 183: 385–390. [DOI] [PubMed] [Google Scholar]
- 10.Hanci V, Yurdakan G, Yurtlu S, et al. Protective effect of dexmedetomidine in a rat model of α-naphthylthiourea-induced acute lung injury. J Surg Res 2012; 178: 424–430. [DOI] [PubMed] [Google Scholar]
- 11.Luo C, Ouyang MW, Fang YY, et al. Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating hif-1α. Front Cell Neurosci 2017; 11: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu YM, Wang CC, Chen L, et al. Both pi3k/akt and erk1/2 pathways participate in the protection by dexmedetomidine against transient focal cerebral ischemia/reperfusion injury in rats. Brain Res 2013; 1494: 1–8. [DOI] [PubMed] [Google Scholar]
- 13.Kim E, Kim HC, Lee S, et al. Dexmedetomidine confers neuroprotection against transient global cerebral ischemia/reperfusion injury in rats by inhibiting inflammation through inactivation of the tlr-4/nf-kappab pathway. Neurosci Lett 2017; 649: 20–27. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Gao J, Cui Y, et al. Neuroprotective effects of resatorvid against traumatic brain injury in rat: Involvement of neuronal autophagy and tlr4 signaling pathway. Cell Mol Neurobiol 2017; 37: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uehara H, Yoshioka H, Nagai H, et al. Doxapram accentuates white matter injury in neonatal rats following bilateral carotid artery occlusion. Neurosci Lett 2000; 281: 191–194. [DOI] [PubMed] [Google Scholar]
- 16.Durga P, Yalamanchili V. Basic cellular and molecular mechanisms of anesthetic-induced developmental neurotoxicity: Potential strategies for alleviation. J Neuroanaesth Crit Care 2016; 3: 15–24. [Google Scholar]
- 17.Arslan F, Keogh B, McGuirk P, et al. TLR2 and TLR4 in ischemia reperfusion injury. Mediators Inflamm 2010; 2010: 704202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao P, Wang J, He L, et al. Deficiency in TLR4 signal transduction ameliorates cardiac injury and cardiomyocyte contractile dysfunction during ischemia. J Cell Mol Med 2009; 13: 1513–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulskens WP, Teske GJ, Butter LM, et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One 2008; 3: e3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler G, Harhausen D, Schepers C, et al. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun 2007; 359: 574–579. [DOI] [PubMed] [Google Scholar]
- 21.Hyakkoku K, Hamanaka J, Tsuruma K, et al. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience 2010; 171: 258–267. [DOI] [PubMed] [Google Scholar]