Short abstract
Objective
To report a case of successful allogeneic grafting of mesenchymal dental pulp stem cells (DPSCs) as preliminary findings in a patient with periodontal disease enrolled into clinical trial ISRCTN12831118.
Methods
Mesenchymal stem cells from the dental pulp of a deciduous tooth from a 7-year-old donor were separated from the pulp chamber and processed via enzymatic digestion and centrifugation. DPSCs were passaged and cultured on a 35 × 13 mm culture dish in minimum essential medium-alpha, without supplementation. After reaching 80% confluency, 5 x 106 allogeneic DPSCs in 250 µl phosphate buffered saline were seeded onto a dry scaffold of lyophilized collagen-polyvinylpyrrolidone sponge placed in the left lower premolar area of a 61-year-old patient with periodontal disease. Surgical access to the lower premolar area was achieved using the flap technique.
Results
At 3 and 6 months following allogeneic graft, the patient showed no sign of rejection and exhibited decreases in tooth mobility, periodontal pocket depth and bone defect area. Bone mineral density had increased at the graft site.
Conclusions
Regenerative periodontal therapy using DPSCs of allogeneic origin may be a promising treatment for periodontal disease-induced bone defects.
Keywords: Periodontitis, tissue regeneration, periodontal treatment
Introduction
Periodontal disease is a chronic inflammatory condition that is highly prevalent in elderly patients.1 Disease progression is marked by the destruction of underlying support structures of the teeth, which eventually leads to definitive tooth loss.1 Periodontal disease has negative effects on digestive, nutritional and glycaemic control in patients with diabetes, and is also associated with an increased incidence of Alzheimer's disease, rheumatoid arthritis and heart disease.2 Moreover, periodontal disease is common in pregnant females and may lead to premature birth and a low birth weight.3 Conventional periodontal disease treatments include changes in hygiene and dietary habits, scaling and root planing, and even flap periodontal surgery. In general, however, these treatments control only the acute phase of disease and do not restore the tissues damaged by periodontal disease.4 Thus, recent research has focused on regenerative approaches, including mesenchymal stem-cell grafting, which has promising applications. Mesenchymal stem cells are involved in growth, wound healing and replacing cells that have been lost through either daily exfoliation or pathological conditions.5
Traditionally, bone marrow has served as the main source of mesenchymal stem cells for basic research and therapeutic use; however, there are mesenchymal stem cells in almost all tissues, including teeth. Because of their accessibility and ability to differentiate into multiple lineages, mesenchymal dental pulp stem cells (DPSCs) are increasingly recognized as a viable source for the development of effective cell-based therapies.6,7
Although patient trials are limited, multiple groups view mesenchymal stem cells as a promising and safe treatment, regardless of human leukocyte antigen (HLA) compatibility.8,9 In this sense, there is evidence that allogeneic mesenchymal stem-cell infusion appears to be well tolerated. Most trials have reported only mild or transient effects, and some have even reported the absence of adverse reaction to allogeneic mesenchymal stem cells.10 Of patients with osteogenesis imperfecta treated with mesenchymal stem cells, none exhibited clinical symptoms of an autoimmune response to allogeneic cells.11 Furthermore, an intravenous infusion of allogeneic mesenchymal stem cells in patients with aplastic anaemia has been shown to be safe.12 More recently, myocardial regeneration was safely achieved using human allogeneic umbilical cord matrix-derived mesenchymal stem cells.13,14 Thus, the present authors hypothesized that an infusion of allogeneic DPSCs would similarly induce periodontal tissue regeneration in adult patients with periodontal disease. This case report presents clinical, radiographic and surgical evidence of successful periodontal regeneration following the therapeutic application of allogeneic DPSCs, as preliminary findings in one patient enrolled into trial ISRCTN12831118, which is a pilot trial aimed at standardizing techniques for periodontal regeneration.
Case report
A 61-year-old male patient was referred to a dental clinic at the National Autonomous University of Mexico to receive treatment for periodontal disease. The patient first presented at the clinic in March 2016, at which time oral diagnosis was confirmed, as were supra- and subgingival dental calculus, bleeding upon probing, grade II mobility (graded by holding one side of the tooth with a metal instrument and the other side with the index finger and moving in buccolingual and vertical directions, as previously described)15 and a periodontal pocket depth of 6.5 mm in the second left lower premolar (No. 35). Radiological analysis showed a radiolucent zone on the mesial side of the root of the same tooth. No deep caries, changes in tooth mobility, or root involvement were detected in any other teeth.
The patient reported a history of systemic arterial hypertension since the age of 35, which he controlled by taking metoprolol and nifedipine every 24 h. The mother had a history of systemic arterial hypertension and diabetes, and the father had no history of pathology. The patient provided written informed consent for treatment and publication of the case and was enrolled into trial ISRCTN12831118. The surgical protocol followed the principles of the Helsinki Declaration and was approved by the Bioethics and Biosafety Committee of the Research Committee of the Faculty of Higher Studies Zaragoza, National Autonomous University of Mexico, ref: 25/11/SO/3.4.1.
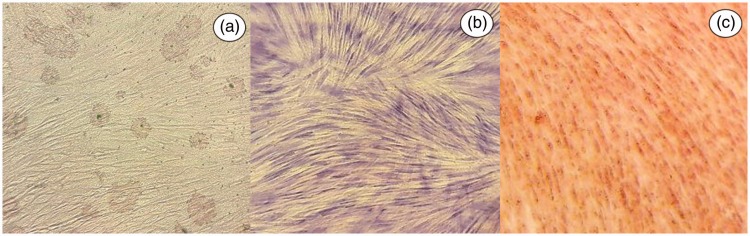
Mesenchymal stem cells were obtained from the dental pulp of a 7-year-old male donor under aseptic conditions and under the strict criteria of good manufacturing practices, using reagents that were free of products of animal origin. The dental pulp was gently removed from the teeth and was immersed in a digestive solution (3 mg/ml type I collagenase plus 4 mg/ml dispase in Minimum Essential Medium [MEM]-α [Life Technologies; Grand Island, NY, USA]) for 1 h at 37 °C. Once digested, the dental pulp was dissociated and centrifuged at 497 g for 5 min. After centrifugation, the dissociated tissue was resuspended in MEM-α and seeded at 2 × 104 cells/ml into a 35 mm × 13 mm dish, then incubated at 37 °C until 80% confluence was achieved. The cells were expanded by serial passage into 35 mm dishes, and at the third passage, cells were cultured to 80% confluence for analyses by flow cytometry and for use in treating the recipient patient. Cell phenotype was analysed using BD antibodies to human cell antigens and a BD FACSCalibur™ flow cytometer with BD Pro v.5.1.1 software (all BD Biosciences, San José, CA, USA), according to the manufacturer’s instructions. Cells were found to be positive for cluster of differentiation (CD)105 (85.81%), CD73 (99.81%), and CD90 (99.54%) and negative for CD45 (0.06%), CD34 (0.09%), CD31 (0.20%), CD14 (0.04%), CD11b (0.04%) and HLA–D related (DR) (1.06%). In addition, differentiation assays (Figure 1) were performed for osteogenic, adipogenic and chondrogenic lineages as described previously.16,17 The present findings were consistent with the International Society for Cellular Therapy criteria for mesenchymal stem cells.18
Figure 1.
Representative images showing in vitro multi-lineage differentiation of dental pulp stem cells obtained from a 7-year-old donor: (a) oil red O staining showing lipid deposits (arrows), indicative of adipogenic lineage; (b) alcian blue staining showing glycosaminoglycans deposits (arrows), indicative of chondrogenic lineage; and (c) alizarin red staining showing more densely stained areas with mineral deposits (arrows), indicative of osteogenic lineage; (all images, original magnification × 40).
The recipient patient’s blood chemistry, blood cytometry, prothrombin time and activated partial thromboplastin time were all within normal parameters (Table 1). Cone beam volumetric tomography was performed to calculate the size of the periodontal defect and the bone mineral density (Figure 2a; Table 2) using OnDemand3D™ Project Viewer software (Cybermed Inc., Seoul, Korea). Subsequently, phase I periodontal therapy (non-surgical) was performed, comprising the removal of supra- and subgingival calculus using a Tigon Piezo Scaler (W&H Impex, Windsor, OH, Canada), root scaling/root planing, replacement of restorations, occlusal adjustment and instructing the patient in proper oral hygiene. After 12 weeks, the amount of biofilm detected was minimal, and the periodontal defect remained unchanged, with a depth of 6.5 mm in the mesial, middle and distal areas.
Table 1.
Blood chemistry, blood cytometry, prothrombin time and activated partial thromboplastin time before and after allogeneic dental pulp stem cell grafting in a 61-year-old male patient with periodontal disease.
|
Assessment time-point |
Normal range | ||
|---|---|---|---|
| Blood parameter | Pre-surgery | 6 months post-surgery | |
| Haematocrit, % | 48.0 | 47.5 | 38–54 |
| Haemoglobin, g/dl | 15.8 | 15.6 | 12–16 |
| White cell count, × 103/mm3 | 8.5 | 8.3 | 5.0–10.0 |
| Red cell count, × 106/mm3 | 5.23 | 5.11 | 4.2–6.2 |
| Platelet count, × 103/mm3 | 172 | 162 | 150–400 |
| MCHC, % | 31 | 32 | 26–32 |
| MCH, % | 29 | 30.5 | 27–32 |
| MCV, fl | 94 | 93 | 82–98 |
| Lymphocytes, % | 22 | 21 | 20–40 |
| Neutrophils, % | 67 | 67 | 40–70 |
| Eosinophils, % | 1 | 0 | 0–1 |
| Monocytes, % | 8 | 7 | 2–8 |
| Basophils, % | 0 | 0 | 2–4 |
| Biochemistry | |||
| Glucose, mg/dl | 101 | 100 | 70–100 |
| Urea, mg/dl | 50.3 | 49 | 10–50 |
| Creatinine, mg/dl | 0.95 | 0.89 | 0.50–1.20 |
| Uric acid, mg/dl | 5.0 | 5.0 | 2.40–5.4 |
| Cholesterol, mg/dl | 160 | 150 | 150–200 |
| Triglycerides, mg/dl | 89 | 83 | 50–160 |
| Bilirubin, mg/dl | 0.25 | 0.10 | 0–0.3 |
| C-reactive protein, mg/l | 0 | 0 | 0–10 |
| Coagulation | |||
| PT, s | 12.8 | 12.0 | 10–15 |
| aPTT, s | 33.1 | 32.1 | 27–45 |
MCHC, mean corpuscular haemoglobin concentration; MCH, mean corpuscular haemoglobin; MCV, mean corpuscular volume; PT, prothrombin time; aPTT, activated partial thromboplastin time.
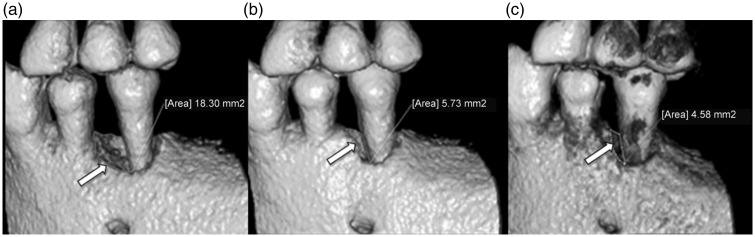
Figure 2.
Cone beam volumetric tomography images of the left lower premolar area of a 61-year-old male patient with periodontal disease, showing: (a) boundary of the initial bone defect area of 18.30 mm2 (arrow) at baseline; (b) bone defect area reduced to 5.73 mm2 (arrow) at three months following dental pulp stem cell graft; and (c) bone defect area reduced to 4.58 mm2 (arrow) at six months following dental pulp stem cell graft.
Table 2.
Clinical evaluation before and after allogeneic dental pulp stem cell grafting in a 61-year-old male patient with periodontal disease, showing baseline and post-intervention (3- and 6-month) results.
|
Study time-point |
|||
|---|---|---|---|
| Parameter | Baseline | 3 months | 6 months |
| Depth of periodontal defect, mm | 6.5 | 3.6 | 3.5 |
| Tooth mobility, grade | II | I | I |
| Bone mineral density | |||
| Distal area, Hounsfield Units | 916 | 1111 | 1328 |
| Proximal area, Hounsfield Units | 612 | 880 | 1058 |
| Middle area, Hounsfield Units | 163 | 381 | 929 |
Tooth mobility grade: I, normal; II, slight mobility.15
Prophylactic antibiotic therapy, using 500 mg metronidazole and 300 mg clindamycin, orally, every 8 h, was initiated three days prior to the surgical phase. Before proceeding to the surgical phase, a rinse was performed with chlorhexidine 0.12% for 2 min, and the area was cleaned with benzalkonium chloride. Loco-regional anaesthesia was applied (1:50 000 lidocaine and adrenaline).
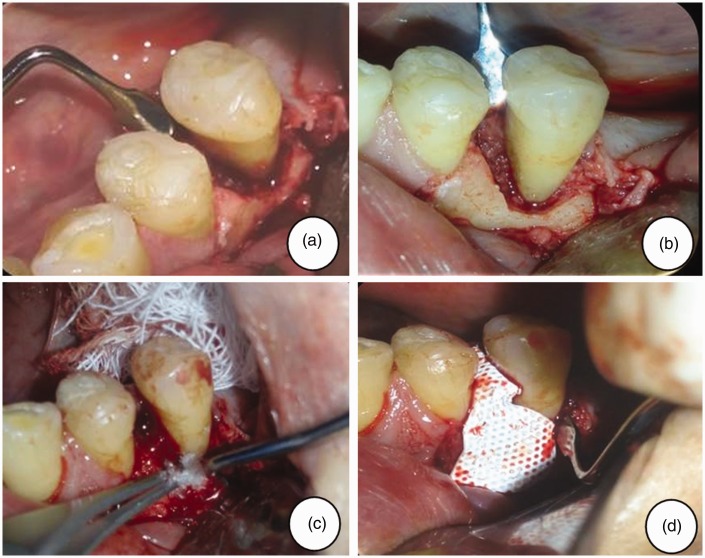
Surgical access to the left lower premolar area was achieved using the flap technique. The root surfaces were smoothed using an ultrasonic reamer (W&H Impex, Windsor, OH, Canada), the granulation tissue was removed and the area was irrigated with 0.9% NaCl in injectable water. After cleaning the surface, a dry scaffold of lyophilized collagen-polyvinylpyrrolidone sponge (Fibroquel; Aspid, Mexico City, Mexico) was placed on the bone defect in 0.5 cm2 fragments using an Adson calliper (Hu-Friedy, Chicago, IL, USA) filled with chlorhexidine gel. Following three passages, a total of 5 x 106 DPSCs in 250 μl of phosphate buffered saline were seeded onto the scaffold.19 A Teflon-coated titanium membrane (Cytoplast® Ti-250/Ti-150 non-resorbable barrier membranes; Osteogenics Biomedical, Lubbock, TX, USA) was applied, and the flap was repositioned with absorbable suture points. Surgical cement was added to the area to protect the intervention. The intervention was further protected with metronidazole and clindamycin (as above) supplemented with 100 mg nimesulide, orally, every 12 h for 5 days to control oedema and pain (Figure 3).
Figure 3.
Representative clinical views of the left lower premolar area of a 61-year-old male patient with periodontal disease, showing: (a) the mesial circumferential bone defect during periodontal surgery; (b) debridement and collocation of a dry scaffold of lyophilized collagen-polyvinylpyrrolidone sponge; (c) seeding of dental pulp stem cells; and (d) adaptation of non-resorbable membrane.
Following surgical intervention, the patient was instructed to rinse with Oxoral® solution for 2 min twice daily for two weeks. In addition, the patient was instructed to use Tebodont® toothpaste and to perform dental cleaning with a Curaprox Surgical brush. Weekly observations were performed to monitor the healing process.
The surgical cement was removed at 7 days following surgery, and cone beam volumetric tomography was performed at 3 and 6 months following surgery (Figure 2b and 2c). The patient will be examined monthly up to 1 year following the surgical intervention and every 6 months for 3 years after the intervention to confirm that the defect has filled with bone tissue and that the new tissue remains stable over time.
At 3 and 6 months following surgical intervention, the patient showed no signs or symptoms of rejection. Throughout this time, the gingiva showed no signs of inflammation, and depth of the periodontal pocket and dental mobility both decreased compared with initial baseline findings (Table 2). Furthermore, the patient’s blood chemistry and blood cytometry parameters at 6 months postoperatively (white blood cell count, red blood cell count, the percentage of neutrophils, the percentage of lymphocytes, blood bilirubin and C-reactive protein) were comparable with values at 2 weeks prior to surgery (Table 1), suggesting that there was no immunological rejection, as also noted in a previously published study.20
The clinical results were in agreement with the findings of cone beam volumetric tomography and bone densitometry assays. Cone beam volumetric tomography revealed that an initial damaged area of 18.30 mm2 was reduced to 5.73 and 4.58 mm2 at 3 and 6 months, respectively, following the DPSC graft (Figure 2). Densitometry assays revealed an increase in bone mineral density in the walls of the defect at 3 and 6 months post-treatment, which is suggestive of bone tissue regeneration (Table 2).
Discussion
Tissue engineering using stem cells is an approach that might address the deficiencies of more conventional therapeutic options by regenerating living and functional periodontal structures.21 For many years, bone marrow has been the main source of stem cells but harvesting mesenchymal stem cells from bone marrow is difficult and painful. DPSCs represent an ideal stem cell source, as they are easily accessible, can be harvested using a non-invasive protocol, and are rapidly expandable in vitro. Animal studies have suggested that the application of DPSCs could promote the growth of human dental tissues in vivo. When transplanted into mice and immunodeficient rats, DPSCs isolated from human third molars differentiate into cementoblast-like cells and can form complex structures, such as pulp-dentin and a periodontal ligament-cementum root.22–25
In the present study, collagen scaffold was chosen because it is the main protein of undifferentiated mesenchymal tissues and because scaffolds with a collagen base have been reported to support stem cells during the first weeks of differentiation by facilitating initial cell attachment and cell differentiation and, consequently, the formation of calcified tissue.26 Collagen is radiolucent with high periodontal tissue compatibility and degradability.27 Furthermore, the pore structure of a collagen sponge is ideal for the colonization of seeded cells and enhances bone formation by promoting the differentiation of osteoblasts,28 thus providing a suitable scaffold for the engineering of tooth tissue.
Compared with other adult tissue sources, DPSCs are an easily accessible source of adult dental stem cells.29 The supply of pulp tissue decreases with age, however, due to narrowing root canals, physiological secondary dentin formation, and pathological tertiary dentin formation and mineralization.30 Thus, older patients’ teeth contain fewer DPSC colonies than the teeth of younger patients.31 There is some conflicting evidence, as some groups have reported no such age-related change, but the majority of studies find an age-related decrease in differentiation/regeneration capacity of cells derived from dental pulp. Human DPSCs obtained from older donors are thought to lose their proliferative activity and differentiation capability, and become senescent, after fewer passages than cells derived from younger donors.32–34 Thus, the present authors believe that minimal manipulation of DPSCs in culture will increase the possibility of achieving tissue regeneration.
Allogeneic DPSCs were obtained from a 7-year-old donor patient in the present study, following good manufacturing practices, to graft onto a periodontal defect in an elderly male patient. A previous study, published in 2009, showed that mesenchymal stem cells are only weakly immunogenic in humans and validated the clinical use of mesenchymal stem cells from HLA-mismatched donors.35 Results from the same study indicated that mesenchymal stem cells could be transplanted successfully into allogeneic recipients across HLA barriers, with little evidence that the mesenchymal stem cells would be rejected or would sensitize the recipient to other cells of the same HLA type.35 Since then, many procedures have been performed with allogeneic HLA-mismatched mesenchymal stem cells without evidence of immune rejection.36
These and other experimental data present strong evidence that DPSCs have the potential to induce the formation of human dental tissues in vivo; however, clinical data were available for only a few human cases.37 Thus, the present case report examined whether the transplantation of human DPSCs contributed to periodontal repair in a patient with a periodontal disease-induced infrabony defect.
The combination choice of metronidazole and clindamycin for pre-surgical prophylactic therapy in the present case is supported by an investigation by Sigusch et al (2001)38 that reported the use of metronidazole and clindamycin in treating periodontal disease. In addition, these antibiotics have been used in combination to treat other infectious diseases.39 Furthermore, the present authors have used the combination of metronidazole and clindamycin as a prophylactic treatment for surgical intervention and achieved good results.
The patient described here exhibited clinical improvement at 3 and 6 months following DPSC treatment, as indicated by a decreased depth of periodontal defect, normal gingiva characteristics, decreased tooth mobility, and observation of bone-like tissue in both tomographic and bone densitometry assays. To the best of the authors’ knowledge, the present case provides the first evidence that allogeneic DPSC transplantation in humans is capable of inducing bone tissue regeneration in a periodontal disease-induced infrabony defect. The findings are consistent with those of a previous study that provided the first evidence that an autologous human DPSC transplant could induce the restoration of mandibular bone tissue in patients with third molar extraction,40 and are consistent with another study demonstrating that DPSCs deposited in a collagen scaffold are able to successfully repair bone.41 In the present case, a collagen scaffold was used to generate bone tissue, however, it is possible to transplant DPSCs and obtain a remodelled and highly vascularized bone tissue without the use of scaffolding.42 Thus, the present authors would like to explore this less invasive possibility in future studies.
A possible mechanism behind the bone defect repair observed in the present study is that conditions in the oral microenvironment facilitated DPSC differentiation. Evidence suggests that local factors can modulate the physiology of mesenchymal stem cells to promote tissue regeneration in injured organs and tissues.43 Negative signals in the aged periodontium can send distress signals to the grafted allogeneic mesenchymal stem cells, thereby inducing the mesenchymal stem cells to secrete a diverse repertoire of factors, such as growth factors, cytokines, mRNA and extracellular matrix, that support cell survival. Components of the mesenchymal stem cell secretome might therefore rescue injured cells, reduce tissue damage, and accelerate repair.43,44
In addition to bone formation, the complete regeneration of periodontal tissue requires the formation of periodontal ligament and root cement.45 Further research should, therefore, include analysis of a sample of neoformed tissue in terms of histological composition, to confirm the kinds of tissue formed other than bone.
Regenerative periodontal therapy using allogeneic DPSCs did not present evidence of immunological rejection in the current case, and it was shown to help reduce the periodontal pocket and drive the formation of bone-like tissue to actively repair the periodontal disease-induced infrabony defect.
The present work represents a pilot proposal for a periodontal disease treatment protocol, and a control case is not presented for comparison. A split-mouth model could be considered in future research, in which the patient simultaneously serves as control and treatment and both sites are exposed to the same internal and external factors that could affect the outcome. In addition, a randomized clinical study is required to corroborate the present findings in a larger patient population.
A further limitation was that fact that due to the treatment protocol, it could not be determined whether the number of cells seeded onto the scaffolding was maintained on the scaffolding. Thus, it is suggested that the cells be transferred and allowed to attach to the scaffold before surgery, so that when the surgery is performed, the likelihood of a successful therapy is increased.
In conclusion, the findings of the present case report suggest that DPSC treatment promotes periodontal regeneration. Further research should include a randomized clinical trial to verify the findings.
Acknowledgements
The authors appreciate the support of Norma Angélica Sosa-Hernández and Itzen Aguiñiga-Sánchez, who provided biological materials and valuable technical advice regarding the management of cells; and acknowledge Rosa Diana Hernández-Palacios for her support in the clinical diagnosis and supervision of treatment.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the General Directorate of Academic Personnel Affairs (Project PAPIIT IN221815), Postgraduate in Biological Sciences of the National Autonomous University of Mexico, and DentCell.
References
- 1.Manji F, Dahlen G, Fejerskov O. Caries and periodontitis: contesting the conventional wisdom on their aetiology. Caries Res 2018; 52: 548–564. [DOI] [PubMed] [Google Scholar]
- 2.Carter CJ, France J, Crean S, et al. The Porphyromonas gingivalis/host interactome shows enrichment in GWASdb genes related to alzheimer's disease, diabetes and cardiovascular diseases. Front Aging Neurosci 2017; 9: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy BV, Tanneeru S, Chava VK. The effect of phase-I periodontal therapy on pregnancy outcome in chronic periodontitis patients. J Obstet Gynaecol 2014; 34: 29–32. [DOI] [PubMed] [Google Scholar]
- 4.Deas DE, Moritz AJ, Sagun RS, Jr, et al. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol 2000 2016; 71: 128–139. [DOI] [PubMed] [Google Scholar]
- 5.Casagrande L, Cordeiro MM, Nör SA, et al. Dental pulp stem cells in regenerative dentistry. Odontology 2011; 99: 1–7. [DOI] [PubMed] [Google Scholar]
- 6.Alraies A, Alaidaroos NY, Waddington RJ, et al. Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC Cell Biol 2017; 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledesma-Martínez E, Mendoza-Núñez VM, Santiago-Osorio E. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells Int 2016; 2016: 4709572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371: 1579–1586. [DOI] [PubMed] [Google Scholar]
- 9.Resnick IB, Barkats C, Shapira MY, et al. Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC). Am J Blood Res 2013; 3: 225–238. [PMC free article] [PubMed] [Google Scholar]
- 10.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One 2012; 7: e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA 2002; 99: 8932–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clé DV, Santana-Lemos B, Tellechea MF, et al. Intravenous infusion of allogeneic mesenchymal stromal cells in refractory or relapsed aplastic anemia. Cytotherapy 2015; 17: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 13.Musialek P, Mazurek A, Jarocha D, et al. Myocardial regeneration strategy using Wharton's jelly mesenchymal stem cells as an off-the-shelf ‘unlimited' therapeutic agent: results from the Acute Myocardial Infarction First-in-Man Study. Postepy Kardiol Interwencyjnej 2015; 11: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao LR, Chen Y, Zhang NK, et al. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med 2015; 13: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasswerman BH, Geiger AM, Turgeon LR. Relationship of occlusion and periodontal disease. VII. Mobility. J Periodontol 1973; 44: 572–578. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Yang R, Roth M, et al. Genetically transforming human osteoblasts to sarcoma: development of an osteosarcoma model. Genes Cancer 2017; 8: 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghadam FH, Tayebi T, Dehghan M, et al. Differentiation of bone marrow mesenchymal stem cells into chondrocytes after short term culture in alkaline medium. Int J Hematol Oncol Stem Cell Res 2014; 8: 12–19. [PMC free article] [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller Y, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 19.Pérez Borrego A, Domínguez Rodríguez L, Ilisástigui Ortueta ZT, et al. Stem-cells used in treatment of periodontal bone defects. Rev Cubana Estomatol 2009; 46: 122–128 [In Spanish, English abstract]. [Google Scholar]
- 20.Chen FM, Gao LN, Tian BM, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther 2016; 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, An Y, Gao LN, et al. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 2012; 33: 6974–6986. [DOI] [PubMed] [Google Scholar]
- 22.Sun HH, Chen B, Zhu QL, et al. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials 2014; 35: 9459–9472. [DOI] [PubMed] [Google Scholar]
- 23.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 2000; 97: 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Ahluwalia IP, Literman R, et al. Human dental pulp progenitor cell behavior on aqueous and hexafluoroisopropanol based silk scaffolds. J Biomed Mater Res A 2011; 97: 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 2006; 1: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumita Y, Honda MJ, Ohara T, et al. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials 2006; 27: 3238–3248. [DOI] [PubMed] [Google Scholar]
- 27.Kosen Y, Miyaji H, Kato A, et al. Application of collagen hydrogel/sponge scaffold facilitates periodontal wound healing in class II furcation defects in beagle dogs. J Periodontal Res 2012; 47: 626–634. [DOI] [PubMed] [Google Scholar]
- 28.Aimetti M, Manavella V, Cricenti L, et al. A novel procedure for the immediate reconstruction of severely resorbed alveolar sockets for advanced periodontal disease. Case Rep Dent 2017; 2017: 9370693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bansal R, Jain A. Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med 2015; 6: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray PE, Stanley HR, Matthews JB, et al. Age-related odontometric changes of human teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 93: 474–482. [DOI] [PubMed] [Google Scholar]
- 31.Iida K, Takeda-Kawaguchi T, Tezuka Y, et al. Hypoxia enhances colony formation and proliferation but inhibits differentiation of human dental pulp cells. Arch Oral Biol 2010; 55: 648–654. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Zhong Q, Yang T, et al. Comparative characterization of SHED and DPSCs during extended cultivation in vitro. Mol Med Rep 2018; 17: 6551–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda T, Tezuka Y, Horiuchi M, et al. Characterization of dental pulp stem cells of human tooth germs. J Dent Res 2008; 87: 676–681. [DOI] [PubMed] [Google Scholar]
- 34.Bressan E, Ferroni L, Gardin C, et al. Donor age-related biological properties of human dental pulp stem cells change in nanostructured scaffolds. PLoS One 2012; 7: e49146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundin M, Barrett AJ, Ringdén O, et al. HSCT recipients have specific tolerance to MSC but not to the MSC donor. J Immunother 2009; 32: 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golpanian S, DiFede DL, Khan A, et al. Allogeneic human mesenchymal stem cell infusions for aging frailty. J Gerontol A Biol Sci Med Sci 2017; 72: 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernández-Monjaraz B, Ledesma-Martínez E, Hernández-Palacios RD, et al. Mesenchymal stem cells as a treatment for periodontal disease in older adults. Odontología actual 2016; 3: 4–7 [In Spanish]. [Google Scholar]
- 38.Sigusch B, Beier M, Klinger G, et al. A 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis. J Periodontol 2001; 72: 275–283. [DOI] [PubMed] [Google Scholar]
- 39.Tchernev G. Folliculitis et perifolliculitis capitis abscedens et suffodiens controlled with a combination therapy: systemic antibiosis (metronidazole plus clindamycin), dermatosurgical approach, and high-dose isotretinoin. Indian J Dermatol 2011; 56: 318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Aquino R, Graziano A, Sampaolesi M, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 2007; 14: 1162–1171. [DOI] [PubMed] [Google Scholar]
- 41.Giuliani A, Manescu A, Langer M, et al. Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: biological and clinical implications. Stem Cells Transl Med 2013; 2: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paino F, La Noce M, Giuliani A, et al. Human DPSCs fabricate vascularized woven bone tissue: a new tool in bone tissue engineering. Clin Sci (Lond) 2017; 131: 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther 2016; 7: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang B, Yin Y, Lai RC, et al. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev 2014; 23: 1233–1244. [DOI] [PubMed] [Google Scholar]
- 45.Schüpbach P, Gaberthüel T, Lutz F, et al. Periodontal repair or regeneration: structures of different types of new attachment. J Periodontal Res 1993; 28: 281–293. [DOI] [PubMed] [Google Scholar]