Short abstract
Objective
To evaluate how isoflurane affects T-cell function by assaying interleukin (IL)-2 production and the expression of two Casitas B-lineage lymphoma (Cbl) family proto-oncogenes (c-Cbl and Cbl-b) in rat peripheral blood mononuclear cells (PBMCs).
Methods
Adult male Sprague–Dawley rats were randomly allocated to those that underwent blood collection after brief isoflurane anesthesia (control group), immediately after 4 hours of isoflurane general anesthesia (4I group), and 1 day after 4 hours of isoflurane general anesthesia (1D 4I group). IL-1, IL-2, and IL-6 mRNA levels and c-Cbl and Cbl-b levels in PBMCs were determined by polymerase chain reaction. Ubiquitination of protein kinase Cθ (PKCθ) and phospholipase C-γ1 (PLC-γ1) in PBMCs was assessed by immunoprecipitation.
Results
The IL-2 mRNA level in rat PBMCs was significantly lower in the 4I and 1D 4I groups than in the control group. c-Cbl, Cbl-b, and ubiquitin expression was significantly increased and zeta-chain-associated protein kinase 70, PLC-γ1, and PKCθ protein levels were significantly decreased in the 4I group. Ubiquitination of PLC-γ1 and PKCθ was significantly increased in the 4I group.
Conclusion
Isoflurane influences ubiquitin, c-Cbl, and Cbl-b expression in rat PBMCs, indicating suppression of receptor tyrosine kinase signaling pathways. These results suggest that isoflurane suppresses T-cell function.
Keywords: Cbl family, interleukin-2, isoflurane, PKCθ, PLC-γ1, ubiquitin
Introduction
Various factors, such as surgical stress, tissue damage, and opioid use, influence the immune response in the perioperative period.1,2 In addition, inhalational anesthetic agents (e.g., isoflurane, sevoflurane, and desflurane) modulate the immune response via direct effects on immune cells.3 The effect of inhalational anesthetic agents on immune cells is complex and involves both the innate and adaptive immune systems in terms of cytokine production, expression of cytokine receptors, and the balance of adaptive cell-mediated immunity.4–8 This is important because maintenance of immune system balance in the postoperative period influences the outcomes of surgery.9,10 Furthermore, inhalational anesthetic agents are associated with recurrence of cancer after surgery.11 Therefore, the importance of the immediate postoperative immunologic response cannot be ignored, and the effect of isoflurane on the immune system should be investigated.
Interleukin (IL)-2 is produced mainly by CD4+ T cells but also by CD8+ T cells, natural killer cells, activated dendritic cells, and mast cells.12,13 Although the roles of IL-2 include facilitation of T-cell differentiation as a growth factor and promotion of the cytolytic activity of CD8+ T cells,14 it also modulates immune system homeostasis by regulating T helper 17, T follicular helper, and T regulatory cells.15 Furthermore, IL-2, in association with increased expression of the E3 ubiquitin ligases Casitas B-lineage lymphoma proto-oncogene (c-Cbl) and Cbl-b, is an indicator of functional suppression of T cells.16 Increased expression of c-Cbl and Cbl-b suppresses T-cell function by promoting ubiquitination and proteosomal degradation of phospholipase C-γ1 (PLC-γ1) and zeta-chain-associated protein kinase 70 (ZAP70), which are involved in receptor tyrosine kinase signaling pathways and IL-2 production. This results in decreased production of IL-2.16–18 Helmy and Al-Attiyah19 reported that isoflurane anesthesia significantly decreased IL-2 in patients undergoing minor elective surgery. An enhanced understanding of the mechanism underlying the effects of inhalational anesthetic agents on the immune system would thus improve surgical outcomes.
Therefore, to investigate the effects of isoflurane on T-cell function, we first evaluated its effect on IL-2 production in rat peripheral blood mononuclear cells (PBMCs). Next, we investigated whether c-Cbl and Cbl-b expression in PBMCs changed in parallel with the altered level of IL-2. c-Cbl and Cbl-b regulate intracellular signaling by ubiquitinating target proteins; therefore, we evaluated the effect of isoflurane on the ubiquitin level and the effect of isoflurane exposure on enzymes involved in receptor tyrosine kinase signaling, which affect IL-2 production in rat PBMCs.
Materials and Methods
All animal experiments described in this report were performed after obtaining approval from the Institutional Animal Care and Use Committee of Samsung Biomedical Research Institute (approval number 20151118002) and followed the recommendations of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Animal experiments
Fifty-eight adult male Sprague–Dawley rats weighing 300 to 350 g (Orient Bio, Gyeonggi-do, Korea) were quarantined for at least 7 days in separate cages and acclimatized in a controlled-temperature room (24°C–25°C) with an artificial light on a 12-hour light/dark cycle. Food pellets and water were provided ad libitum.
The rats were randomly allocated to three groups: those that underwent blood collection after brief isoflurane anesthesia (control group), those that underwent blood collection immediately after 4 hours of isoflurane general anesthesia (4I group), and those that underwent blood collection 1 day after 4 hours of isoflurane general anesthesia (1D 4I group). The rats in the 4I and 1D 4I groups were briefly anesthetized in a concealed chamber, underwent tracheal intubation using a 16-gauge angiocatheter, and were mechanically ventilated. General anesthesia of rats in the 4I and 1D 4I groups was maintained by 1.5 vol% isoflurane (Forane; Ilsung Pharmaceutical, Seoul, Korea) with 100% oxygen for 4 hours. Mechanical ventilation was performed at 3.0 to 3.5 mL/breath at a respiratory rate of 70 to 75 breaths/minute (Harvard Apparatus, Holliston, MA, USA), and the end-tidal CO2 level was monitored and maintained within the range of 35 to 40 mmHg (Ohmeda Excel 210SE Anesthesia Machine; Datex Instrumentarium Corp., Helsinki, Finland). Neuromuscular blockade was maintained by continuous infusion of rocuronium (Esmeron; MSD, Kenilworth, NJ, USA) at a rate of 0.1 mg/kg/h. The rectal temperature of the rats was maintained at 37.0°C ± 0.5°C. Lactated Ringer’s solution was continuously infused into all anesthetized rats via the tail vein at a rate of 1.5 mL/kg/h for 4 hours. The rats in the control group were briefly anesthetized with isoflurane in a chamber, and their blood was collected in an ethylenediaminetetraacetic acid (EDTA) tube by cardiac puncture. The rats in the 4I group were killed at the end of the experiment, and their blood was collected in an EDTA tube by cardiac puncture. The rats in the 1D 4I group recovered from general anesthesia, and their blood was collected by cardiac puncture under anesthesia with 4 vol% isoflurane 24 hours after the experiment.
Isolation of PBMCs
Immediately after blood collection, PBMCs were isolated by density gradient centrifugation using Ficoll-Paque density gradient medium (GE Healthcare, Uppsala, Sweden) according to the manufacturer’s protocol. Briefly, 2 mL of collected anticoagulant-treated blood was gently mixed with an equal volume of balanced salt solution (0.14 M NaCl, 5.5 × 10−3 M anhydrous D-glucose, 5.0 × 10−5 M CaCl2⋅2H2O, 9.8 × 10–4 M MgCl2⋅6H2O, 5.4 × 10−3 M KCl, and 0.145 M Tris) in a 10-mL centrifuge tube. Next, 4 mL of the mixture was carefully layered on 3 mL of Ficoll-Paque medium. After centrifugation at 400 × g for 30 minutes at 18°C, PBMCs were harvested, washed twice with balanced salt solution, and centrifuged at 400 × g for 10 minutes at 18°C.
Quantitative real-time polymerase chain reaction analysis
The expression of IL-1β, IL-2, and IL-6 in seven rats in each group was assayed by quantitative real-time polymerase chain reaction (qRT-PCR). Total RNAs were extracted from PBMCs using TRIzol reagent (Sigma-Aldrich Co., St. Louis, MO, USA) according to the manufacturer’s instructions. For qRT-PCR, 2 µg of total RNA was reverse-transcribed by M-MLV reverse transcriptase (Promega, Madison, WI, USA); 2 µL of the product was then used as a template for PCR. qRT-PCR was performed using iQ SYBR Green SuperMix and the iCycleriQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). PCR conditions consisted of denaturation at 95°C for 3 minutes followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 58°C for 15 s, and extension at 72°C for 30 s. A dissociation curve was generated at the end of each cycle to verify amplification of a single product. The mRNA levels were quantified using the 2−ΔΔCT method.20 The mRNA level of the target gene was normalized to that of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers used are presented in Table 1.
Table 1.
Primer sequences used in this study
| Primer | Sequence | |
|---|---|---|
| Interleukin-1β (Gene ID: 24494) | Forward | 5’-TGTGATGAAAGACGGCACAC |
| Reverse | 5’-CTTCTTCTTTGGGTATTGTTTGG | |
| Interleukin-6 (Gene ID: 24498) | Forward | 5’-CCCTTCAGGAACAGCTATGAA |
| Reverse | 5’-ACAACATCAGTCCCAAGAAGG | |
| Interleukin-2 (Gene ID: 116562) | Forward | 5’-AAACTCCCCATGATGCTCAC |
| Reverse | 5’-GAAAATTTCCAGCGTCTTCCA | |
| GAPDH (Gene ID: 24383) | Forward | 5’-GAACATCATCCCTGCATCCA |
| Reverse | 5’-CCAGTGAGCTTCCCGTTCA | |
Western blotting
PBMCs were lysed in lysis buffer (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10 mM KCl, 0.1 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid, 1 mM dithiothreitol, 1 µg/mL aprotinin, 1 mM phenylmethane sulfonyl fluoride [PMSF], 10% NP-40, protease inhibitor cocktail [Sigma-Aldrich Co.], and phosphatase inhibitor mixture [PhosphoSTOP; Sigma-Aldrich Co.]) for 15 minutes on ice. Protein was collected by centrifugation at 14,000 rpm for 15 minutes at 4°C. The protein concentration was quantified by the Bradford method. Equal amounts of protein (30 µg) were resolved by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA, USA) in a 4% to 15% gradient gel and transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). After blocking the membrane with 5% skim milk in Tris-buffered saline (TBS), the membranes were incubated overnight (14 hours) at 4°C with the appropriate primary antibody in 5% skim milk in TBS. The primary antibodies used were polyclonal mouse anti-Cbl-b (Santa Cruz Biotechnology, Dallas, TX, USA), monoclonal mouse anti-c-Cbl (BD Biosciences, Franklin Lakes, NJ, USA), monoclonal mouse anti-c-Cbl (pY700) (BD Biosciences), polyclonal rabbit anti-ZAP70 (Santa Cruz Biotechnology), polyclonal rabbit anti-ZAP70 (pY 318) (Abcam, Cambridge, UK), polyclonal rabbit anti-phospholipase Cγ1 (Santa Cruz Biotechnology), polyclonal rabbit anti-phospholipase Cγ1 (pY775) (Abcam), polyclonal rabbit anti-protein kinase Cθ (PKCθ) (Santa Cruz Biotechnology), polyclonal rabbit anti-PKCθ (pS 676) (Santa Cruz Biotechnology), monoclonal mouse anti-mono- and polyubiquitin (Enzo Life Sciences, Farmingdale, NY, USA), polyclonal goat anti-IL-2 (R&D Systems, Minneapolis, MN, USA), and anti-GAPDH (Abcam). An anti-phosphotyrosine antibody (Merck, Darmstadt, Germany) and protein A agarose (Thermo Fisher Scientific, Waltham, MA, USA) were used for reprobing of phosphorylation of Cbl-b. The membranes were washed three times with 0.5% Tween 20 in TBS and incubated with the appropriate horseradish peroxidase-conjugated IgG secondary antibody at room temperature for 1 hour. The membranes were washed three times with 0.5% Tween 20 in TBS and once with TBS, developed using ECL solution (Promega), and exposed to medical X-ray film (Agfa Healthcare, Mortsel, Belgium) for 1 to 10 minutes.
Immunoprecipitation
To evaluate ubiquitination of PLC-γ1 and PKCθ (which is enhanced by Cbl-b21), immunoprecipitation (IP) was performed as described previously.22 PBMC lysates in IP buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 5% glycerol, 1% Triton X-100, 1 mM PMSF, and 1× protease inhibitor cocktail [Sigma-Aldrich]) were precleared to prevent nonspecific binding by incubation with 30 µL of protein A agarose (Thermo Fisher Scientific) at 4°C for 1 hour. After centrifugation at 20,000 × g for 10 minutes, the supernatant (500 µg protein) was incubated with 2 µg of the appropriate antibody (anti-PLC-γ1 or anti-PKCθ) for IP overnight at 4°C, followed by incubation with protein A agarose at 4°C for 2 hours with constant rotation. After washing the beads five times with washing buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 0.2% Triton X-100, 1 mM PMSF, and 1× protease inhibitor cocktail), immunoprecipitated proteins were eluted using a buffer containing 2% SDS and subjected to western blotting.
Immunohistochemistry
PBMCs were post-fixed for 15 minutes in 4% paraformaldehyde and washed three times with 0.1 M phosphate-buffered saline (PBS). Nonspecific protein binding was then blocked by incubation in blocking buffer (10% goat serum and 0.1% Triton X-100 in 0.1 M PBS) at room temperature for 60 minutes. Next, PBMCs were incubated overnight at 4°C with the appropriate primary antibody in 0.1% Triton X-100 in 0.1 M PBS with constant rotation. After washing three times with 0.1 M PBS, the PBMCs were visualized using the corresponding Alexa Fluor-conjugated IgG secondary antibody (Invitrogen, Carlsbad, CA, USA) at 1:200 dilution for 2 hours at room temperature. The primary antibody was omitted in the negative controls. Images were acquired using a Zeiss fluorescence microscope (Zeiss, Oberkochen, Germany). We focused on CD4+ and CD28+ T cells because their function is reportedly influenced by c-Cbl and Cbl-b expression.23–25 CD4+ T cells are the T-cell subset primarily responsible for IL-2 production. IL-2 production by CD28+ T cells, a subset of CD4+ T cells, is more prominently regulated by Vav protein kinase,26 and this subset increases IL-2 production 30- to 100-fold by co-stimulation with the ligand B7.27
Statistical analysis
Data are presented as mean ± standard error of the mean. The data were analyzed with the Kruskal–Wallis test, followed by Tukey’s test using ranks. A P-value of <0.05 was considered statistically significant.
Results
Effect of isoflurane on IL-2 mRNA level in rat PBMCs
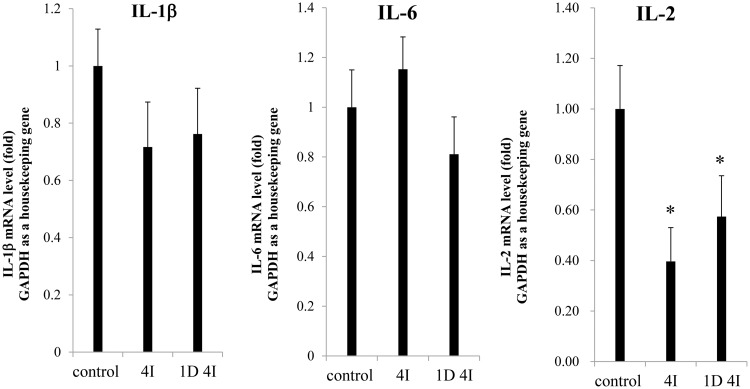
The mRNA levels of IL-1β and IL-6 were not significantly different between the 4I and 1D 4I groups and the control group. However, the IL-2 mRNA level was significantly downregulated (0.40-fold) in the 4I group compared with in the control group and was downregulated in the 1D 4I group (0.57-fold) (P < 0.05) (Figure 1).
Figure 1.
Interleukin (IL)-1β, IL-6, and IL-2 mRNA levels in peripheral blood mononuclear cells (PBMCs) of the control, 4I, and 1D 4I groups. The IL-2 mRNA level was significantly decreased (0.40-fold) in the 4I group and non-significantly decreased (0.57-fold) in the 1D 4I group compared with in the control group. ∗P < 0.05.
Effect of isoflurane on c-Cbl, Cbl-b, and ubiquitin levels in rat PBMCs
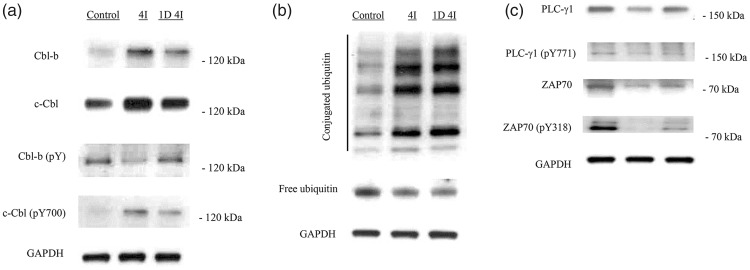
Next, we investigated the protein levels of c-Cbl and Cbl-b in PBMCs by immunoblotting. The c-Cbl and Cbl-b protein levels in rat PBMCs were increased in the 4I and 1D 4I groups. The level of phosphorylated c-Cbl was also increased in the 4I and 1D 4I groups, but that of phosphorylated Cbl-b was decreased (Figure 2(a)). The conjugated ubiquitin level was increased up to 1 day after isoflurane anesthesia. However, the mono-ubiquitin level was decreased in the 4I and 1D 4I groups (Figure 2(b)).
Figure 2.
Effects of isoflurane on c-Cbl, Cbl-b, ubiquitin, ZAP70, and PLC-γ1 levels in rat peripheral blood mononuclear cells. (a) Casitas B-lineage lymphoma proto-oncogene (c-Cbl) and Cbl-b protein levels were increased after exposure to isoflurane. The levels of phosphorylated c-Cbl and Cbl-b were increased and decreased, respectively, in the 4I group. (b) The level of conjugated ubiquitin was increased, and that of monoubiquitin was decreased, in the 4I group. (c) The levels of the phosphorylated and non-phosphorylated forms of zeta-chain-associated protein kinase 70 (ZAP70) and phospholipase C-γ1 (PLC-γ1) were decreased in the 4I and 1D 4I groups compared with in the control group.
Isoflurane decreased ZAP70, PLC-γ1, and PKCθ levels
Activation of ZAP70 and PLC-γ1 results in increased production of IL-2.16 Therefore, we investigated the effect of isoflurane exposure on ZAP70 and PLC-γ1 levels. The levels of the phosphorylated and non-phosphorylated forms of ZAP70 and PLC-γ1 were decreased in the 4I and 1D 4I groups (Figure 2(c)). Therefore, isoflurane reduced the activity of these components of the receptor tyrosine kinase signaling pathways. Unlike c-Cbl, which is activated by phosphorylation, phosphorylation of Cbl-b by PKCθ reportedly leads to proteosomal degradation, whereas ubiquitination of PKCθ by Cbl-b inhibits its activity.28,29 For this reason, we also investigated whether PKCθ expression decreased as Cbl-b expression increased. The level of PKCθ was decreased in the 4I and 1D 4I groups (Figure 2(c)).
Exposure to isoflurane increased ubiquitination of PLC-γ1 and PKCθ
We investigated the effect of isoflurane on ubiquitination of PLC-γ1 in rat PBMCs (Figure 3(a)). Ubiquitination of PLC-γ1 was increased in the 4I group and was increased and decreased in the 1D 4I group compared with in the control and 4I groups, respectively. Moreover, ubiquitination of PKCθ was increased in the 4I and 1D 4I groups (Figure 3(b)), possibly due to increased Cbl-b activity.
Figure 3.
Ubiquitination of PLC-γ1 and PKCθ after 4 hours of exposure to isoflurane. (a) Ubiquitination of PLC-γ1 was increased in the 4I and 1D 4I groups compared with in the control group. (b) Ubiquitination of protein kinase C (PKC)θ was increased in the 4I and 1D 4I groups.
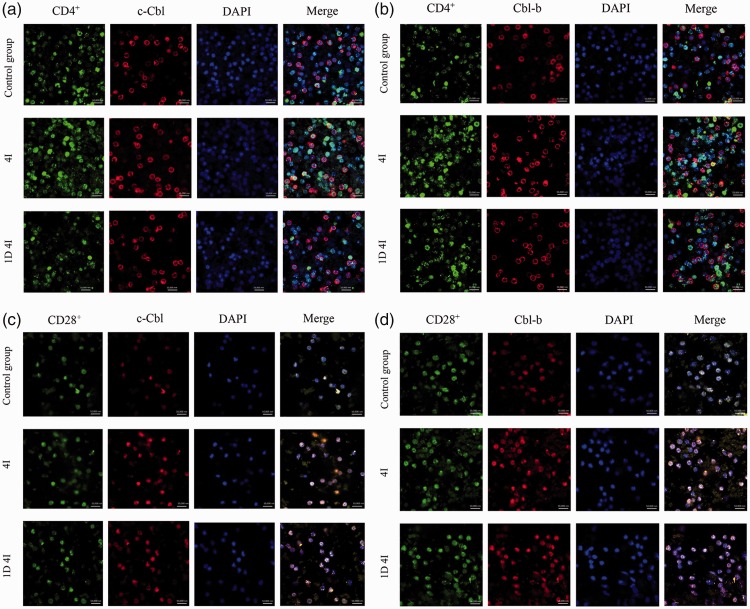
Effect of isoflurane on c-Cbl and Cbl-b expression in CD4+ and CD28+ T cells
Next, we identified by immunohistochemistry the T-cell subsets, CD4+ and CD28+, in which c-Cbl and Cbl-b expression was increased. c-Cbl and Cbl-b expression by CD4+ T cells (Figure 4(a) and (b)) and CD28+ T cells (Figure 4(c) and (d)) was increased in the 4I group and 1D 4I group compared with in the control group.
Figure 4.
c-Cbl and Cbl-b expression in CD4+ and CD28+ T cells after 4 hours of exposure to isoflurane. c-Cbl and Cbl-b expression by (a and b) CD4+ T cells and (c and d) CD28+ T cells was significantly increased in the 4I group and non-significantly increased in the 1D 4I group compared with in the control group.
Discussion
Our results suggest that isoflurane increases c-Cbl and Cbl-b expression and the conjugated ubiquitin level in PBMCs, leading to a decrease in the mRNA level of IL-2. Therefore, isoflurane may lead to disturbance of immune homeostasis and suppression of T-cell function.
Isoflurane, an important inhalational anesthetic agent, can modulate the immune response.30–32 Although exposure is brief, the choice of anesthetic agent has been suggested to affect postoperative outcomes.33 Wigmore et al.11 reported that patients who underwent general anesthesia with volatile anesthetics had a lower survival rate after surgical treatment of cancer than those who underwent total intravenous anesthesia. In addition, isoflurane reportedly increases the risk of malignancy of various cancers.34–36
Clinical use of IL-2 is limited by the risk of severe complications, such as capillary leak syndrome. However, the beneficial effects of IL-2 have resulted in trials of its efficacy against various diseases, including cancer.12,37,38 Previous studies have revealed diverse IL-1β and IL-6 levels in PBMCs, likely due to different experimental conditions, durations of exposure to isoflurane, and sample types.39–41 However, our results showed that the mRNA levels of IL-1β and IL-6 did not change significantly during the experimental period, whereas 4 hours of exposure to isoflurane decreased IL-2 expression, which was associated with increased c-Cbl and Cbl-b expression. Because we controlled the effects of other factors, these findings suggest that isoflurane significantly decreased the IL-2 mRNA level in rat PBMCs, and this could have disturbed immune system homeostasis. The E3 ubiquitin ligases c-Cbl and Cbl-b suppress the production of IL-2 by promoting ubiquitination of downstream enzymes in receptor tyrosine kinase signaling pathways in T cells.17 Ubiquitination is a post-translational modification involving the attachment of ubiquitin—an evolutionarily conserved 76-amino-acid peptide—to target proteins, leading to their proteosomal degradation.42 Therefore, decreased IL-2 production together with increased c-Cbl and Cbl-b expression in T cells indicates suppression of the T-cell-mediated immune response. Maintenance of the balance of T-cell function in the perioperative period reduces the risk of disease progression after surgical treatment of cancer.43,44 Akiyoshi et al.45 reported that the IL-2 level was significantly decreased up to 8 days after major surgery, but the proportions of T-cell subsets were unchanged. We detected decreased production of IL-2 and increased expression of c-Cbl and Cbl-b in CD4+ and CD28+ T cells up to 1 day after exposure to isoflurane. This suggests a mechanism for the effect of isoflurane on IL-2 production.
Mono- or polyubiquitination of target proteins leads to their proteosomal degradation.46 The activity of c-Cbl and Cbl-b involves attachment of ubiquitin to target proteins; therefore, we evaluated the effect of increased c-Cbl and Cbl-b expression on ubiquitin expression. The conjugated ubiquitin level was significantly higher in the 4I and 1D 4I groups than in the control group. This suggests that the isoflurane-induced increased expression of ubiquitin enhanced the ubiquitination of intracellular proteins.
c-Cbl and Cbl-b belong to the Cbl family and function as adaptor proteins, but they may have different mechanisms of action. Ubiquitination of target proteins by c-Cbl is enhanced by its phosphorylation;18,47 indeed, tyrosine phosphorylation of Cbl-b results in the greatest increase in its ubiquitination activity.28 PKCθ phosphorylates Cbl-b, which suppresses the activity of the latter.29 In contrast, Cbl-b ubiquitinates PKCθ, leading to its proteosomal degradation.21 Therefore, the isoflurane-induced decrease in the PKCθ level and increase in ubiquitination of PKCθ may enhance the activity of Cbl-b, possibly leading to decreased expression of IL-2. Furthermore, the ubiquitination-mediated decrease in the levels of phosphorylated and non-phosphorylated PLC-γ1 could also suppress production of IL-2. Thus, isoflurane influences the immune response by increasing c-Cbl, Cbl-b, and ubiquitin levels in PBMCs, which modulates the activity of receptor tyrosine kinase signaling pathways.
In conclusion, isoflurane exerts an immunosuppressive effect by increasing the expression of ubiquitin, c-Cbl, and Cbl-b, leading to decreased production of IL-2 by T cells. These effects of isoflurane may result in poorer surgical outcomes.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was funded by departmental sources.
References
- 1.Walton B. Effects of anaesthesia and surgery on immune status. Br J Anaesth 1979; 51: 37–43. [DOI] [PubMed] [Google Scholar]
- 2.Plein LM, Rittner HL. Opioids and the immune system - friend or foe. Br J Pharmacol 2017. Feb 18. DOI: 10.1111/bph.13750. [DOI] [PMC free article] [PubMed]
- 3.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth 2008; 22: 263–277. [DOI] [PubMed] [Google Scholar]
- 4.Mitsuhata H, Shimizu R, Yokoyama MM. Suppressive effects of volatile anesthetics on cytokine release in human peripheral blood mononuclear cells. Int J Immunopharmacol 1995; 17: 529–534. [DOI] [PubMed] [Google Scholar]
- 5.Brand JM, Kirchner H, Poppe C, et al. [Cytokine release and changes in mononuclear cells in peripheral blood under the influence of general anesthesia]. Anaesthesist 1998; 47: 379–386. [DOI] [PubMed] [Google Scholar]
- 6.Matsuoka H, Kurosawa S, Horinouchi T, et al. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology 2001; 95: 1467–1472. [DOI] [PubMed] [Google Scholar]
- 7.Pirttikangas CO, Perttila J, Salo M. Propofol emulsion reduces proliferative responses of lymphocytes from intensive care patients. Intensive Care Med 1993; 19: 299–302. [DOI] [PubMed] [Google Scholar]
- 8.Stollings LM, Jia LJ, Tang P, et al. Immune modulation by volatile anesthetics. Anesthesiology 2016; 125: 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Homburger JA, Meiler SE. Anesthesia drugs, immunity, and long-term outcome. Curr Opin Anaesthesiol 2006; 19: 423–428. [DOI] [PubMed] [Google Scholar]
- 10.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth 2010; 105: 106–115. [DOI] [PubMed] [Google Scholar]
- 11.Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology 2016; 124: 69–79. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014; 192: 5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosalia RA, Arenas-Ramirez N, Bouchaud G, et al. Use of enhanced interleukin-2 formulations for improved immunotherapy against cancer. Curr Opin Chem Biol 2014; 23: 39–46. [DOI] [PubMed] [Google Scholar]
- 14.Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev 2014; 25: 377–390. [DOI] [PubMed] [Google Scholar]
- 15.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013; 38: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol 2004; 5: 883–890. [DOI] [PubMed] [Google Scholar]
- 17.Thien CB, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J 2005; 391: 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol 2006; 209: 21–43. [DOI] [PubMed] [Google Scholar]
- 19.Helmy SA, Al-Attiyah RJ. The effect of halothane and isoflurane on plasma cytokine levels. Anaesthesia 2000; 55: 904–910. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 21.Hsu TS, Hsiao HW, Wu PJ, et al. Deltex1 promotes protein kinase Cθ degradation and sustains Casitas B-lineage lymphoma expression. J Immunol 2014; 193: 1672–1680. [DOI] [PubMed] [Google Scholar]
- 22.Kim TW, Guan S, Sun Y, et al. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat Cell Biol 2009; 11: 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brembilla NC, Weber J, Rimoldi D, et al. c-Cbl expression levels regulate the functional responses of human central and effector memory CD4 T cells. Blood 2008; 112: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teh CE, Daley SR, Enders A, et al. T-cell regulation by casitas B-lineage lymphoma (Cblb) is a critical failsafe against autoimmune disease due to autoimmune regulator (Aire) deficiency. Proc Natl Acad Sci U S A 2010; 107: 14709–14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Bardos T, Li D, et al. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J Immunol 2002; 169: 2236–2240. [DOI] [PubMed] [Google Scholar]
- 26.Chiang YJ, Kole HK, Brown K, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature 2000; 403: 216–220. [DOI] [PubMed] [Google Scholar]
- 27.Umlauf SW, Beverly B, Lantz O, et al. Regulation of interleukin 2 gene expression by CD28 costimulation in mouse T-cell clones: both nuclear and cytoplasmic RNAs are regulated with complex kinetics. Mol Cell Biol 1995; 15: 3197–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heissmeyer V, Macian F, Im SH, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol 2004; 5: 255–265. [DOI] [PubMed] [Google Scholar]
- 29.Gruber T, Hermann-Kleiter N, Hinterleitner R, et al. PKC-theta modulates the strength of T cell responses by targeting Cbl-b for ubiquitination and degradation. Sci Signal 2009; 2: ra30. [DOI] [PubMed] [Google Scholar]
- 30.Inada T, Yamanouchi Y, Jomura S, et al. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia 2004; 59: 954–959. [DOI] [PubMed] [Google Scholar]
- 31.Markovic SN, Knight PR, Murasko DM. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology 1993; 78: 700–706. [DOI] [PubMed] [Google Scholar]
- 32.Wagner F, Radermacher P, Stahl W. Anesthesia and the immune response: evidence for an “isoflurane paradox”? Shock 2010; 34: 437–438. [DOI] [PubMed] [Google Scholar]
- 33.Bharati SJ, Chowdhury T, Bergese SD, et al. Anesthetics impact on cancer recurrence: What do we know? J Cancer Res Ther 2016; 12: 464–468. [DOI] [PubMed] [Google Scholar]
- 34.Benzonana LL, Perry NJ, Watts HR, et al. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology 2013; 119: 593–605. [DOI] [PubMed] [Google Scholar]
- 35.Jun R, Gui-he Z, Xing-xing S, et al. Isoflurane enhances malignancy of head and neck squamous cell carcinoma cell lines: a preliminary study in vitro. Oral Oncol 2011; 47: 329–333. [DOI] [PubMed] [Google Scholar]
- 36.Luo X, Zhao H, Hennah L, et al. Impact of isoflurane on malignant capability of ovarian cancer in vitro. Br J Anaesth 2015; 114: 831–839. [DOI] [PubMed] [Google Scholar]
- 37.Antony GK, Dudek AZ. Interleukin 2 in cancer therapy. Curr Med Chem 2010; 17: 3297–3302. [DOI] [PubMed] [Google Scholar]
- 38.Shablak A, Sikand K, Shanks JH, et al. High-dose interleukin-2 can produce a high rate of response and durable remissions in appropriately selected patients with metastatic renal cancer. J Immunother 2011; 34: 107–112. [DOI] [PubMed] [Google Scholar]
- 39.Callaway JK, Wood C, Jenkins TA, et al. Isoflurane in the presence or absence of surgery increases hippocampal cytokines associated with memory deficits and responses to brain injury in rats. Behav Brain Res 2016; 303: 44–52. [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Lu Y, Dong Y, et al. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol Aging 2012; 33: 1364–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Zhang J, Yang L, et al. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br J Anaesth 2013; 110 (Suppl 1): i82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fulda S, Rajalingam K, Dikic I. Ubiquitylation in immune disorders and cancer: from molecular mechanisms to therapeutic implications. EMBO Mol Med 2012; 4: 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meiler SE. Long-term outcome after anesthesia and surgery: remarks on the biology of a newly emerging principle in perioperative care. Anesthesiol Clin 2006; 24: 255–278. [DOI] [PubMed] [Google Scholar]
- 44.Kurosawa S. Anesthesia in patients with cancer disorders. Curr Opin Anaesthesiol 2012; 25: 376–384. [DOI] [PubMed] [Google Scholar]
- 45.Akiyoshi T, Koba F, Arinaga S, et al. Impaired production of interleukin-2 after surgery. Clin Exp Immunol 1985; 59: 45–49. [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt MH, Dikic I. The Cbl interactome and its functions. Nat Rev Mol Cell Biol 2005; 6: 907–918. [DOI] [PubMed] [Google Scholar]
- 47.Noble M, Mayer-Proschel M, Li Z, et al. Redox biology in normal cells and cancer: restoring function of the redox/Fyn/c-Cbl pathway in cancer cells offers new approaches to cancer treatment. Free Radic Biol Med 2015; 79: 300–323. [DOI] [PMC free article] [PubMed] [Google Scholar]