Short abstract
Background
Blood pressure variability (BPV) is a modifiable risk factor for stroke. This study was performed to determine the prognostic role of BPV in patients with acute hemorrhagic stroke.
Methods
The data of 131 hospitalized hypertensive patients with spontaneous intracerebral hemorrhage (sICH) were collected. All patients underwent examinations using several neurological scales (Glasgow Coma Scale, National Institutes of Health Stroke Scale, and modified Rankin scale [mRS]) and BP measurements at different time points.
Results
Sex, age, hematoma volume, and neurological scores were not significantly different between patients with a favorable and unfavorable prognosis for sICH. However, significant differences were found in hypertension, diabetes, metabolic syndrome, atrial fibrillation, smoking, and stroke history. The standard deviation (SD), coefficient of variation (CV), and maximum–minimum range (Max–Min) of diastolic BP and the mean, SD, CV, and Max–Min of systolic BP significantly differed between the groups. Statistical analysis also demonstrated correlations between the 90-day mRS score and BPV and between systolic BPV and the 90-day mRS score.
Conclusion
High systolic or diastolic BPV within 24 hours of hemorrhagic stroke onset is associated with the 90-day neurological prognosis. The 24-hour BPV plays a critical role in the neurological outcome of hemorrhagic stroke.
Keywords: Blood pressure, variability, stroke, spontaneous intracerebral hemorrhage, computed tomography, neurological outcome
Introduction
Stroke, also termed cerebrovascular accident, is a clinical emergency and may be caused by cerebrovascular stenosis, blocking, malformation, or rupture. There are two main types of stroke: ischemic stroke and hemorrhagic stroke. Hemorrhagic stroke, which constitutes about 13% of strokes, is caused by a weakened vessel that ruptures, bleeding into and compressing the surrounding brain tissue.1 The two main types of hemorrhagic stroke are intracerebral and subarachnoid. Increasing numbers of studies are strongly supporting the notion that hypertension is one of the most common causes of both ischemic stroke and hemorrhagic stroke and that it gradually damages blood vessel walls and accelerates the occurrence and development of atherosclerosis.2–4 Blood pressure variability (BPV), a natural characteristic of blood pressure (BP), is the result of complex interactions between extrinsic environmental and behavioral factors and intrinsic cardiovascular regulatory mechanisms (neural central, neural reflex, and humoral influences). BPV is correlated with the risk of stroke,5 and BPV is especially detrimental to the occurrence of hemorrhagic stroke. However, the clinical implications of markedly elevated BPV in the setting of acute hemorrhagic stroke are incompletely understood. Whether BPV predicts the clinical outcome of acute stroke remains controversial.6 Additionally, the significance and management of BP changes in patients with acute hemorrhagic stroke care are unclear. In the present study, we investigated the impact of 24-hour BPV on 90-day outcomes in patients with acute hemorrhagic stroke. The aim of this study was to determine the correlation between the 90-day modified Rankin scale (mRS) score and BPV in patients with acute hemorrhagic stroke and identify the role of BPV in the prognosis of patients with acute hemorrhagic stroke.
Methods
Study population and design
A prospective cohort study was performed from 10 March 2013 to 10 September 2016. All included patients had a diagnosis of hemorrhagic stroke and were from Renmin Hospital, Hubei University of Medicine. The patients were identified by a primary discharge diagnosis code of 431.xx (hemorrhagic stroke) according to the International Classification of Diseases, Ninth Revision. Patients with no primary diagnosis code but with a secondary diagnosis code of 431.xx were also included in this study. The inclusion criteria for hemorrhagic stroke in this study were in line with the 2013 American Heart Association/American Stroke Association guideline. All patients with spontaneous intracerebral hemorrhage (sICH) met the following conditions: age of >18 years, confirmation of hemorrhagic stroke by head computed tomography (CT) scan and/or magnetic resonance imaging (MRI) in combination with clinical history, presentation <6 hours after sICH onset, and systolic BP of >140 mmHg and/or diastolic BP of >90 mmHg. The exclusion criteria were no CT or MRI examination, inability to confirm sICH, subarachnoid hemorrhage, secondary hemorrhagic stroke due to traumatic head injury, anticoagulant or thrombolytic therapy, other intracranial pathologies, and surgical therapy for intracranial hematoma in patients with hypertensive cerebral hemorrhage. This study was performed in accordance with the relevant guidelines and regulations and approved by the Research Ethics Committee of Hubei University of Medicine. All patients provided written informed consent.
Data collection
A flow chart of this study is shown in Figure 1. All clinical assessments were performed by experienced neurologists. Hospitalized patients with sICH and hypertension were recruited. All patients underwent examinations using various neurological scales and BP measurements at different time points.
Figure 1.
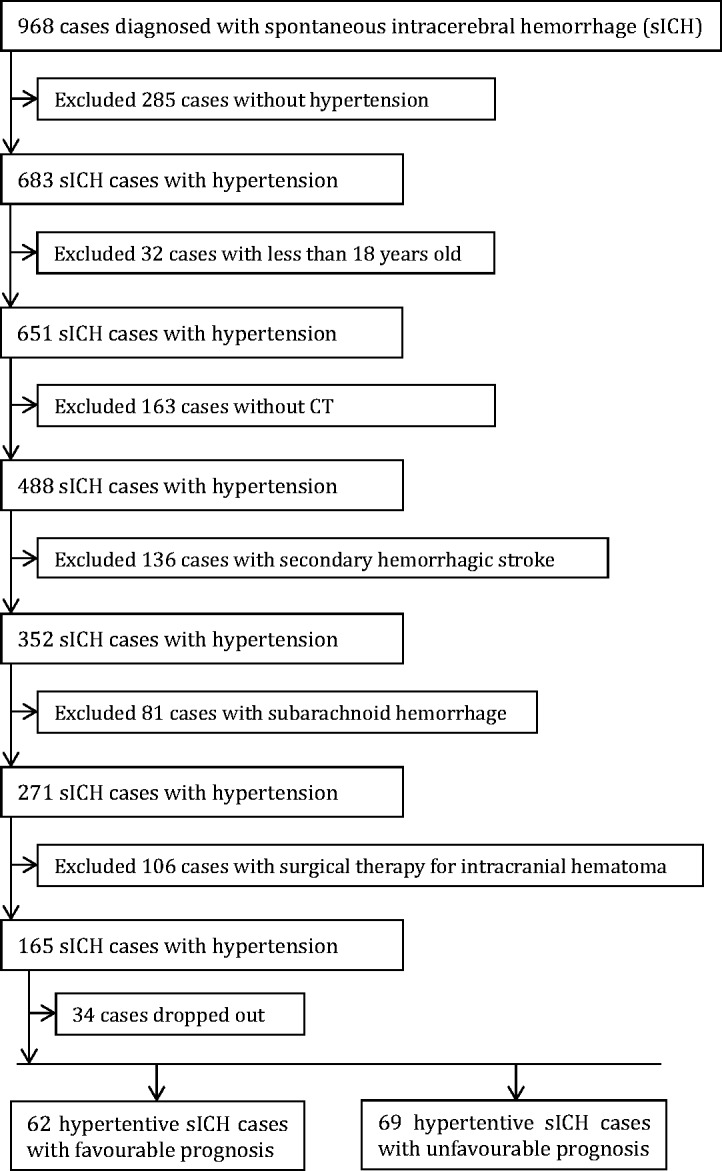
Flow chart of patient recruitment and selection.
Each patient’s BP was measured using an electronic sphygmomanometer once every 15 minutes from admission to 1 hour post-admission, once every 30 minutes from 1 to 6 hours post-admission, and once every hour from 6 to 24 hours post-admission.
The sICH-associated hematoma volumes were measured with the simple formula ABC/2, where A is the longest diameter of the hematoma on the CT slice with the largest hematoma area, B is the longest diameter perpendicular to A on the same slice, and C is the approximate number of CT slices with hemorrhage multiplied by the slice thickness.7–9
The Glasgow coma scale (GCS), a neurological scale, was used to assess each patient’s conscious level. A GCS score of <8 indicated severe impairment of conscious (coma), a GCS score of 8 to 12 indicated moderate impairment, and a GCS score of ≥13 indicated minor impairment. The National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin scale (mRS) were used to evaluate the clinical outcomes at admission, discharge, and 90 days after stroke. The mRS was used to evaluate the neurological deficits both within 6 hours of hospital admission and 90 days after hemorrhagic stroke. An mRS score of 0 to 1 indicated a favorable prognosis for patients with sICH, and an mRS score of 2 to 6 indicated an unfavorable prognosis.
Statistical analysis
IBM SPSS for Windows, version 19.0 (IBM Corp., Armonk, NY, USA) and Excel (Microsoft Corp., Redmond, WA, USA) were used for statistical analysis. The BP and BPV parameters were the mean BP (Mean), maximum BP (Max), minimum BP (Min), range from Max to Min (Max–Min), standard deviation (SD), and coefficient of variation (CV, calculated as [100 × SD]/mean). A univariate analysis was first performed for each parameter to identify the independent variables. The 90-day mRS score was used as a dependent variable. Spearman’s rank correlation coefficient test was conducted. Multiple logistics regression was performed to investigate the association between the 90-day mRS score and BPV (systolic BP: Max–Min < 60, 60 ≤ Max–Min < 80, and Max–Min ≥ 80; diastolic BP: Max–Min < 20, 20 ≤ Max–Min < 40, and Max–Min ≥ 40). For parameters with normal distributions and homogeneous variances, the significance of differences between the mean values in the two groups was verified using Student’s t-test. The chi square test or Fisher’s exact test was used for numerical data to analyze the associations between two different variables. A p value of <0.05 indicated statistical significance.
Results
Demographic and baseline characteristics
In total, 131 hospitalized patients with sICH and hypertension were recruited. The demographic and baseline characteristics of the patients are shown in Table 1. According to the mRS score, 62 patients had a favorable prognosis and 69 had an unfavorable prognosis. The variables sex, age, hematoma volume, and neurological scales scores (GCS, NIHSS, and mRS scores) were not significantly different between the favorable and unfavorable prognosis groups according to the univariate analysis of each variable separately. However, significant differences were found in the following risk factors: hypertension, diabetes, metabolic syndrome, atrial fibrillation, smoking, and stroke history (p < 0.05).
Table 1.
Demographic and general characteristics of patients with sICH
| Variables | Favorable prognosis (n = 62) | Unfavorable prognosis (n = 69) | p value |
|---|---|---|---|
| Sex | 0.889 | ||
| Male | 37 (59.7) | 42 (60.9) | |
| Female | 25 (40.3) | 27 (39.1) | |
| Age, years | 58.22 ± 18.63 | 62.01 ± 19.32 | 0.675 |
| Hematoma volume, cm3 | 40.23 ± 11.93 | 43.15 ± 13.28 | 0.637 |
| Baseline GCS score | 12.68 ± 3.62 | 13.57 ± 4.09 | 0.369 |
| Baseline NIHSS score | 15.32 ± 6.73 | 16.78 ± 7.13 | 0.435 |
| Baseline mRS score | 2.02 ± 0.16 | 2.64 ± 0.28 | 0.512 |
| Hypertension | 0.021 | ||
| Yes | 46 (74.2) | 61 (88.4) | |
| No | 16 (25.8) | 8 (11.6) | |
| Diabetes | 0.018 | ||
| Yes | 24 (38.7) | 41 (59.4) | |
| No | 38 (61.3) | 28 (40.6) | |
| Hyperlipidemia | 0.334 | ||
| Yes | 28 (45.2) | 37 (53.6) | |
| No | 34 (54.8) | 32 (46.4) | |
| Metabolic syndrome | 0.008 | ||
| Yes | 23 (37.1) | 46 (66.7) | |
| No | 39 (62.9) | 23 (33.3) | |
| Atrial fibrillation | 0.105 | ||
| Yes | 21 (33.9) | 33 (47.8) | |
| No | 41 (66.1) | 36 (52.2) | |
| Carotid stenosis | 0.652 | ||
| Yes | 28 (45.2) | 30 (43.5) | |
| No | 34 (54.8) | 39 (56.5) | |
| Alcohol consumption | 0.874 | ||
| Yes | 27 (43.5) | 31 (44.9) | |
| No | 35 (56.5) | 38 (55.1) | |
| Smoking | 0.001 | ||
| Yes | 14 (22.6) | 39 (56.5) | |
| No | 48 (77.4) | 30 (43.5) | |
| HHcy, mmol/L | 12.16 ± 8.01 | 13.98 ± 10.31 | 0.314 |
| Yes (>10 mmol/L) | 36 (58.1) | 43 (62.3) | |
| No (≤10 mmol/L) | 16 (41.9) | 26 (37.7) | |
| Stroke history | 0.002 | ||
| Yes | 15 (24.2) | 35 (50.7) | |
| No | 47 (75.8) | 34 (49.3) |
Data are presented as n (%) or mean ± standard deviation. HHcy, hyperhomocysteinemia; mRS, modified rankin scale; NIHSS, National Institutes of Health Stroke Scale; sICH, spontaneous intracerebral hemorrhage.
Effects of BPV on 90-day neurological outcome of patients with sICH
As shown in Table 2, the independent-samples t test revealed significant differences in the following parameters between the favorable and unfavorable prognosis groups: SD, CV, and Max–Min of diastolic BP and Mean, SD, CV, and Max–Min of systolic BP (p < 0.05 for all).
Table 2.
Effects of BPV on 90-day neurological outcome of patients with sICH
| Variables |
Favorable prognosis (n = 62) |
Unfavorable prognosis (n = 69) |
t value | p value |
|---|---|---|---|---|
| SBP | ||||
| Mean | 151.23 ± 14.09 | 164.51 ± 16.32 | 3.562 | 0.016 |
| SD | 16.85 ± 3.36 | 19.25 ± 5.91 | 3.125 | 0.032 |
| CV | 11.82 ± 3.02 | 13.75 ± 3.87 | 2.641 | 0.006 |
| Max–Min | 71.08 ± 22.34 | 82.27 ± 23.64 | 2.712 | 0.001 |
| DBP | ||||
| Mean | 89.10 ± 15.12 | 92.16 ± 14.78 | 1.612 | 0.135 |
| SD | 11.32 ± 2.39 | 13.56 ± 2.97 | 2.160 | 0.036 |
| CV | 9.63 ± 2.52 | 12.14 ± 2.18 | 1.523 | 0.026 |
| Max–Min | 42.36 ± 12.27 | 52.38 ± 13.29 | 2.635 | 0.001 |
Data are presented as mean ± standard deviation. BPV, blood pressure variability; CV, coefficient of variation; DBP, diastolic blood pressure; Max, maximum; Min, minimum; SBP, systolic blood pressure; SD, standard deviation; sICH, spontaneous intracerebral hemorrhage
Association of greater BPV fluctuation with worse prognosis of sICH
The multiple logistics regression analysis demonstrated a correlation between the 90-day mRS score and BPV (systolic BP: Max–Min < 60, 60 ≤ Max–Min < 80, and Max–Min ≥ 80; diastolic BP: Max–Min < 20, 20 ≤ Max–Min < 40, and Max–Min ≥ 40) (p < 0.001) (Table 3). The Spearman correlation analysis revealed a positive correlation between BPV of systolic BP and the 90-day mRS score (Table 4). These results indicate greater fluctuation of BPV and a worse prognosis in patients with sICH.
Table 3.
Multiple logistic regression analysis of effects of BPV on 90-day neurological outcome of sICH
|
SD |
CV |
Max–Min |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Max–Min< 60 | 60 ≤Max–Min< 80 | Max–Min≥ 80 | Max–Min< 60 | 60 ≤Max–Min< 80 | Max–Min≥ 80 | Max–Min< 60 | 60≤ Max–Min< 80 | Max–Min≥ 80 |
| SBP | |||||||||
| OR (95% CI) | – | 2.31 (1.63–3.64) | 4.99 (3.86–6.12) | – | 4.16 (3.05–5.13) | 4.37 (2.57–6.72) | – | 1.26 (0.95–2.61) | 4.39 (4.01–6.34) |
| p value | – | 0.0012 | 0.0008 | – | 0.0056 | 0.0032 | – | 0.0001 | 0.0001 |
|
Max–Min< 20 |
20 ≤Max–Min< 40 |
Max–Min≥ 40 |
Max–Min< 20 |
20 ≤Max–Min< 40 |
Max–Min≥ 40 |
Max–Min< 20 |
20 ≤Max–Min< 40 |
Max–Min≥ 40 |
|
| DBP | |||||||||
| OR (95% CI) | – | 1.36 (1.06–1.78) | 2.02 (1.86–3.61) | – | 1.95 (1.36–3.97) | 3.87 (3.02–5.12) | – | 1.03 (1.09–2.52) | 1.41 (1.35–1.99) |
| p value | – | 0.81 | 0.009 | – | 0.23 | 0.0002 | – | 0.96 | 0.0005 |
The variables adjusted for in the multivariate model were hypertension, diabetes, metabolic syndrome, smoking, and stroke history. BPV, blood pressure variability; CI, confidence interval; CV, coefficient of variation; DBP, diastolic blood pressure; Max, maximum; Min, minimum; OR, odds ratio; SBP, systolic blood pressure; SD, standard deviation; sICH, spontaneous intracerebral hemorrhage.
Table 4.
Spearman's rank correlation test between BPV and 90-day mRS score
| Variables | Coefficient r | p value |
|---|---|---|
| SBP | ||
| SD | 0.225 | 0.009 |
| CV | 0.641 | 0.002 |
| Max–Min | 0.312 | 0.007 |
| DBP | ||
| SD | 2.186 | 0.162 |
| CV | 3.501 | 0.231 |
| Max–Min | 4.635 | 0.138 |
BPV, blood pressure variability; CV, coefficient of variation; DBP, diastolic blood pressure; Max, maximum; Min, minimum; SBP, systolic blood pressure; SD, standard deviation.
Discussion
Changes in BP are well known to occur in patients with acute stroke. Both short- and long-term BPV can act as a reliable predictor for stroke prognosis and stroke recurrence. Abundant studies have reported that 24-hour BPV plays a critical role in stroke-induced neurological impairment. The present study has shown that high systolic or diastolic BPV within 24 hours of hemorrhagic stroke onset is associated with the 90-day neurological prognosis.
BPV is considered a distinctive novel risk factor for both cardiovascular and cerebrovascular diseases. BPV is primarily divided into short-term BPV (minutes to hours) and long-term BPV (days to months). Short-term BPV is usually defined as the oscillation of BP within a 24-hour period (minute-to-minute, hour-to-hour, and day-to-night oscillation) in addition to beat-to-beat oscillation (very-short-term BPV). Long-term BPV includes day-to-day, visit-to-visit, and season-to-season variability.10 Previous studies have clearly shown that enhanced fluctuation of BP induces vascular stiffness and lesion formation, artery remodeling, and the onset of arteriosclerotic disease.11 Many clinical trials have demonstrated that short-term and long-term BPV not only independently contribute to cerebrovascular events but also implicate prognostic significance.12–15 The present study, designed as a clinical investigation of short-term BPV, confirmed that 24-hour BPV after the onset of hemorrhagic stroke is detrimental to the 90-day neurological prognosis. We also found that both systolic and diastolic BPV within 24 hours of hemorrhagic stroke onset determines the 90-day neurological prognosis; this finding differs from preliminary evidence indicating that systolic BPV is independently associated with poor functional outcomes.16
During the past decade, increasing numbers of clinical investigations have noted a relationship between BPV and the risk of stroke.17,18 A significant association has also been established between diastolic BPV and the risk of hemorrhagic but not ischemic stroke. A recent study showed that higher visit-to-visit diastolic BPV contributes to the risk of any stroke and specifically to hemorrhagic stroke, but not ischemic stroke.19 Several studies have provided evidence that exaggerated BP fluctuations may be harmful and detrimental to stroke recovery.20,21 The present study showed that short-term (24-hour) diastolic BPV was also associated with the risk of hemorrhagic stroke and determined the neurological outcome 90 days after the onset of hemorrhagic stroke. Overall, both short-term (24-hour) and long-term (visit-to-visit) diastolic BPV has a detrimental role in the risk of hemorrhagic stroke and the neurological outcome. Therefore, amelioration of BPV has been suggested as an additional clinically relevant treatment of cerebrovascular disease.
Increasing evidence implicates that the stroke outcome is strongly influenced by a multitude of variables related either to metabolic homeostasis, the inflammatory response, or cerebral perfusion and hemodynamics.22–24 All these factors may act either at the site of brain damage or at the systemic level; influence neurovascular recovery, secondary brain damage, and systemic complications; and consequently impact the clinical outcome.23,25 Accordingly, the ever-growing insight into the stroke pathophysiology and process could aid the identification of the clinical, biochemical, or brain imaging parameters that may improve prognostic algorithms.26,27 Preclinical and clinical studies have indicated the detrimental role of BPV in the stroke process,24,27,28 and because many of these variables in the stroke process can be controlled, regulation of BPV could represent a potential therapeutic target.
Most research data mainly focuses on the association between BPV and ischemic stroke, and BPV is associated with poorer functional outcomes of ischemic stroke than other types of stroke.29,30 However, one prospective study of 608 consecutive patients admitted with acute ischemic stroke demonstrated no association between BPV during the acute phase of ischemic stroke and in-hospital outcomes.31 Data on the association between BPV in patients with hemorrhagic stroke and functional outcomes are limited. Moreover, the present study had a small sample and no long-term follow-up. Hence, larger multicenter clinical observation trials with larger sample sizes are needed to clarify the role of BPV in the neurological prognosis of patients with hemorrhagic stroke.
Accruing evidence is showing that a BP-lowering strategy used in the acute setting of sICH may limit the hematoma growth and be potentially beneficial to the stroke outcome.32–34 Lowering BP in the acute phase of sICH may influence BPV itself, and different classes of anti-hypertensive agents have different effects on BPV.35–37 Nevertheless, the present study provides no data about the anti-hypertensive drug classes used by the patients, or whether or how BP was lowered in the acute setting. This represents another study limitation. Further experimental research of anti-hypertensive drugs is required to shed new light on the role of BPV in patients with hemorrhagic stroke.
Acknowledgments
The authors are grateful to Professor Jia-Ning Wang for contributing to helpful discussions and to Wenbo He for expertly contributing to the clinical analysis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Albertson M, Sharma J. Stroke: current concepts. S D Med 2014; 67: 455, 457–461, 463–455. [PubMed] [Google Scholar]
- 2.Suemoto CK, Grinberg LT, Leite REP, et al. Morphometric measurements of extracranial and intracranial atherosclerotic disease: a population-based autopsy study. Atherosclerosis 2018; 270: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olatunji RB, Adekanmi AJ, Ogunseyinde AO. Intracranial arterial calcification in black Africans with acute ischaemic stroke. Cerebrovasc Dis Extra 2018; 8: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray SP, Jandeleit-Dahm KA. The role of NADPH oxidase in vascular disease –hypertension, atherosclerosis & stroke. Curr Pharm Des 2015; 21: 5933–5944. [DOI] [PubMed] [Google Scholar]
- 5.Tully PJ, Debette S, Dartigues JF, et al. Antihypertensive drug use, blood pressure variability, and incident stroke risk in older adults: three-city cohort study. Stroke 2016; 47: 1194–1200. [DOI] [PubMed] [Google Scholar]
- 6.Milonas D, Tziomalos K. Blood pressure variability: does it predict the outcome of acute ischemic stroke? Am J Hypertens 2017; 30: 476–477. [DOI] [PubMed] [Google Scholar]
- 7.Maeda AK, Aguiar LR, Martins C, et al. Hematoma volumes of spontaneous intracerebral hemorrhage: the ellipse (ABC/2) method yielded volumes smaller than those measured using the planimetric method. Arq Neuropsiquiatr 2013; 71: 540–544. [DOI] [PubMed] [Google Scholar]
- 8.Hu TT, Yan L, Yan PF, et al. Assessment of the ABC/2 method of epidural hematoma volume measurement as compared to computer-assisted planimetric analysis. Biol Res Nurs 2016; 18: 5–11. [DOI] [PubMed] [Google Scholar]
- 9.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996; 27: 1304–1305. [DOI] [PubMed] [Google Scholar]
- 10.Herpin D, Ragot S. [Variability of blood pressure. Clinical and therapeutic implications]. Rev Med Interne 1995; 16: 131–136. [DOI] [PubMed] [Google Scholar]
- 11.Nagai M, Dote K, Kato M, et al. Visit-to-visit blood pressure variability and classes of antihypertensive agents; associations with artery remodeling and the risk of stroke. Curr Pharm Des 2016; 22: 383–389. [DOI] [PubMed] [Google Scholar]
- 12.de Havenon A, Bennett A, Stoddard GJ, et al. Increased blood pressure variability is associated with worse neurologic outcome in acute anterior circulation ischemic stroke. Stroke Res Treat 2016; 2016: 7670161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muntner P, Whittle J, Lynch AI, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med 2015; 163: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning LS, Rothwell PM, Potter JF, et al. Prognostic significance of short-term blood pressure variability in acute stroke: systematic review. Stroke 2015; 46: 2482–2490. [DOI] [PubMed] [Google Scholar]
- 15.Manning LS, Mistri AK, Potter J, et al. Short-term blood pressure variability in acute stroke: post hoc analysis of the controlling hypertension and hypotension immediately post stroke and continue or stop post-stroke antihypertensives collaborative study trials. Stroke 2015; 46: 1518–1524. [DOI] [PubMed] [Google Scholar]
- 16.Kellert L, Hametner C, Ahmed N, et al. Reciprocal interaction of 24-hour blood pressure variability and systolic blood pressure on outcome in stroke thrombolysis. Stroke 2017; 48: 1827–1834. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda K, Kai H, Kamouchi M, et al. Day-by-day blood pressure variability and functional outcome after acute ischemic stroke: Fukuoka Stroke Registry. Stroke 2015; 46: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 18.Chung JW, Kim N, Kang J, et al. Blood pressure variability and the development of early neurological deterioration following acute ischemic stroke. J Hypertens 2015; 33: 2099–2106. [DOI] [PubMed] [Google Scholar]
- 19.Dai H, Lu Y, Song L, et al. Visit-to-visit variability of blood pressure and risk of stroke: results of the Kailuan Cohort Study. Sci Rep 2017; 7: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning L, Hirakawa Y, Arima H, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: a post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol 2014; 13: 364–373. [DOI] [PubMed] [Google Scholar]
- 21.Buratti L, Cagnetti C, Balucani C, et al. Blood pressure variability and stroke outcome in patients with internal carotid artery occlusion. J Neurol Sci 2014; 339: 164–168. [DOI] [PubMed] [Google Scholar]
- 22.Lattanzi S, Bartolini M, Provinciali L, et al. Glycosylated hemoglobin and functional outcome after acute ischemic stroke. J Stroke Cerebrovasc Dis 2016; 25: 1786–1791. [DOI] [PubMed] [Google Scholar]
- 23.Zangari R, Zanier ER, Torgano G, et al. Early ficolin-1 is a sensitive prognostic marker for functional outcome in ischemic stroke. J Neuroinflammation 2016; 13: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundholm MD, Rooney M, Maas MB, et al. Wake-up stroke is associated with greater nocturnal mean arterial pressure variability. Stroke 2017; 48: 1668–1670. [DOI] [PubMed] [Google Scholar]
- 25.Lattanzi S, Cagnetti C, Provinciali L, et al. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke 2016; 47: 1654–1657. [DOI] [PubMed] [Google Scholar]
- 26.Sporns PB, Schwake M, Schmidt R, et al. Computed tomographic blend sign is associated with computed tomographic angiography spot sign and predicts secondary neurological deterioration after intracerebral hemorrhage. Stroke 2017; 48: 131–135. [DOI] [PubMed] [Google Scholar]
- 27.Shi Z, Li ES, Zhong JS, et al. Predictive significance of day-to-day blood pressure variability in acute ischemic stroke for 12-month functional outcomes. Am J Hypertens 2017; 30: 524–531. [DOI] [PubMed] [Google Scholar]
- 28.Kang J, Hong JH, Jang MU, et al. Change in blood pressure variability in patients with acute ischemic stroke and its effect on early neurologic outcome. PLoS One 2017; 12: e0189216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Men X, Sun W, Fan F, et al. China Stroke Primary Prevention Trial: visit-to-visit systolic blood pressure variability is an independent predictor of primary stroke in hypertensive patients. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian G, Xiong L, Lin W, et al. External counterpulsation reduces beat-to-beat blood pressure variability when augmenting blood pressure and cerebral blood flow in ischemic stroke. J Clin Neurol 2016; 12: 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tziomalos K, Giampatzis V, Bouziana SD, et al. No association observed between blood pressure variability during the acute phase of ischemic stroke and in-hospital outcomes. Am J Hypertens 2016; 29: 841–846. [DOI] [PubMed] [Google Scholar]
- 32.Lattanzi S, Cagnetti C, Provinciali L, et al. How should we lower blood pressure after cerebral hemorrhage? A systematic review and meta-analysis. Cerebrovasc Dis 2017; 43: 207–213. [DOI] [PubMed] [Google Scholar]
- 33.Lattanzi S, Silvestrini M. Optimal achieved blood pressure in acute intracerebral hemorrhage: INTERACT2. Neurology 2015; 85: 557–558. [DOI] [PubMed] [Google Scholar]
- 34.Arima H, Heeley E, Delcourt C, et al. Optimal achieved blood pressure in acute intracerebral hemorrhage: INTERACT2. Neurology 2015; 84: 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375: 906–915. [DOI] [PubMed] [Google Scholar]
- 36.Lattanzi S, Silvestrini M, Provinciali L. Elevated blood pressure in the acute phase of stroke and the role of Angiotensin receptor blockers. Int J Hypertens 2013. ; 2013: 941783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lattanzi S, Silvestrini M. Blood pressure in acute intra-cerebral hemorrhage. Ann Transl Med 2016; 4: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]


