Short abstract
Objective
Hyperbaric oxygen (HBO) is an emerging complementary alternative medical approach in glioma treatment. However, its mode of action is unknown, so this was investigated in the present study.
Methods
We constructed an intracranial glioma model of congenic C57BL/6J mice. Glioma growth under HBO stimulation was assessed by bioluminescent imaging and magnetic resonance imaging. Flow cytometry assessed direct effects of HBO on reactive oxygen species (ROS) signaling of transplanted glioma cells and organs, and quantified mature T cells and subgroups in tumors, the brain, and blood.
Results
HBO promoted the growth of transplanted GL261-Luc glioma in the intracranial glioma mouse model. ROS signaling of glioma cells and brain cells was significantly downregulated under HBO stimulation, but thymus ROS levels were significantly upregulated. CD3+ T cells were significantly downregulated, while both Ti/Th cells (CD3+CD4+) and Ts/Tc cells (CD3+CD8+) were inhibited in tumors of the HBO group. The percentage of regulatory T cells in Ti/Th (CD3+CD4+) cells was elevated in the tumors and thymuses of the HBO group.
Conclusion
HBO induced ROS signaling in the thymus, inhibited CD3+ T cell generation, and facilitated malignant glioma cell growth in vivo in the intracranial glioma mouse model.
Keywords: Hyperbaric oxygen, glioma, CD3+ T cells, reactive oxygen species, regulatory T cells, glioblastoma multiforme
Introduction
Glioma, a malignant intracranial tumor accounting for more than half of all brain tumors, derives from glia cells.1 Glioblastoma multiforme (GBM) is the most commonly diagnosed and most aggressive glioma in adults.1–3 However, its carcinogenesis mechanism is poorly understood compared with other types of cancer, and the prognosis for GBM patients is always poor.4,5 Surgical removal of the primary tumor is the most important approach in GBM treatment,6 but adjuvant chemoradiotherapy is thought to reduce the recurrence rate and improve prognosis.7
Hypoxia-induced angiogenesis and apoptosis are important signs used to make a diagnosis of GBM.8,9 Hypoxia-related features, such as the size of the hypoxic area, extent of aggravation, and presence of reactive oxygen species (ROS) indicate GBM prognosis and drug response.10 Hyperbaric oxygen (HBO) stimulation before chemotherapy and radiotherapy has been applied with the aim of increasing the therapeutic efficacy of GBM.8 Some pilot studies suggested that HBO stimulation elongated median survival time, and reduced the side effects in GBM patients,8,11,12 but this has not been confirmed in a formalized multicenter clinical trial.8 Additionally, HBO was found to increase the cytotoxicity of temozolomide, a novel GBM drug, in both chemotherapy-resistant and chemotherapy-sensitive cells in vitro13 and in vivo.14–17
Though many studies have focused on the clinical effects of HBO in GBM, little is known about its biological effects on malignant GBM.18 HBO was shown to inhibit the growth of subcutaneously transplanted gliomas in C57BL/6J mice.19 Furthermore, we constructed an intracranial GBM mouse model to better mimic the biological behavior of GBM cells,20 and found that HBO promoted intracranial transplanted GBM growth in vivo.20 Further investigation suggested that HBO promoted the proliferation of GBM cells, enabling them to escape cell cycle arrest and apoptosis, and increased angiogenesis.20 However, the detailed mechanisms and direct effects of HBO on GBM are unknown.
HBO stimulation not only targets tumors, but also affects every organ of the body.21 Additionally, it appears to influence the immune system.22,23 In this study, we used an intracranial transplanted congenic mouse model of GBM to investigate the direct effects of HBO on ROS signaling of transplanted glioma cells and organs to understand its immunological effects.
Materials and methods
Glioma cell culture
GL261/GL261-Luc cells were obtained from the Division of Cancer Treatment and Diagnosis Tumor Repository, National Cancer Institute (NCI; Frederick, MD, USA), and maintained in our laboratory according to NCI standard procedures. GL261/GL261-Luc cells were cultured in RPMI 1640 medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) in a 37°C incubator with 5% CO2.
Tumor xenograft assay
A total of 5 × 105 GL261-Luc glioma cells (1 × 108 cells/mL suspension, 5 µL) in an equal volume of Matrigel (BD Biosciences, San Jose, CA, USA) was injected into the caudate nucleus of the right brain of 12 inbred female congenic C57BL/6J mice (10 weeks old; NCI). Mice were then randomly separated into the experimental group (n=6) and the control group (n=6), and treated accordingly as described below. Both groups of mice were maintained under pathogen-free conditions at 24°C, with 40% humidity, 12-hour light/dark cycles (light on from 08:00 to 20:00), and with free access to food and water.
Tumor xenografts were evaluated by in vivo bioluminescence imaging (BLI) 10 days after the intracranial transplantation of GL261-Luc glioma cells. In accordance with the manufacturer’s protocol, individual mice were put into the chamber of an ICE Chemi & Fluo System P80 BLI system (Photometrics; Tucson, AZ, USA). The exposure time was set to 8 min. The tumor size was quantified with normalized photon flux.
Hyperbaric oxygen intervention process
Mice of the experimental group were placed into NG90-IIIB medical hyperbaric oxygen chambers (Ningbo Hyperbaric Oxygen Chamber Factory, Ningbo, China), and underwent standard HBO intervention according to the manufacturer’s protocol (2.5× atmospheres, 0.015 MPa/minute for 10 minutes; maintain for 60 minutes and decompress at the same rate). HBO treatment was started 10 days after tumor cell injection, and was performed daily for 10 days. All applicable international, national, and institutional guidelines for the care and use of animals were followed. The complete study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University.
Small animal magnetic resonance imaging
MRI of mouse brains was performed 20 days after glioma cell injection (10 days after HBO treatment). Mice were anesthetized (1% isoflurane treatment, and intraperitoneal injection of 0.8 mL/kg gadopentetate dimeglumine), then imaged with an 8.5-T Biospec vertical bore system (Bruker, Billerica, MA, USA). MRI images were generated and the tumor volume (V) was measured using a threshold method according to an evaluation of length (L), width (W), and height (H), using the formula: V = (L × W × H)/2.
Flow cytometry assays
The ROS level was evaluated by flow cytometry using the DCFDA Cellular Reactive Oxygen Species Detection Assay Kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s protocol. Briefly, cells were stained with 2′,7′-dichlorofluorescin diacetate (DCFA) at 37°C for 30 minutes, washed with 1× buffer, and the signal was read at an excitation of 485 nm and an emission of 535 nm. The expression of markers (including CD3, CD4, CD8, CD25, and FoxP3) on T cells from different organs was determined by flow cytometry as previously described.20
Statistical analysis
In comparisons of the HBO group and the control group, P values were calculated by two-tailed Student’s t-test and the Mann–Whitney U test according to data distribution. P<0.05 was considered statistically significant. IBM SPSS Statistics, version 22.0 (IBM Corp., Armonk, NY, USA) was used for all data analyses.
Results
Hyperbaric oxygen treatment facilitated transplanted glioma growth
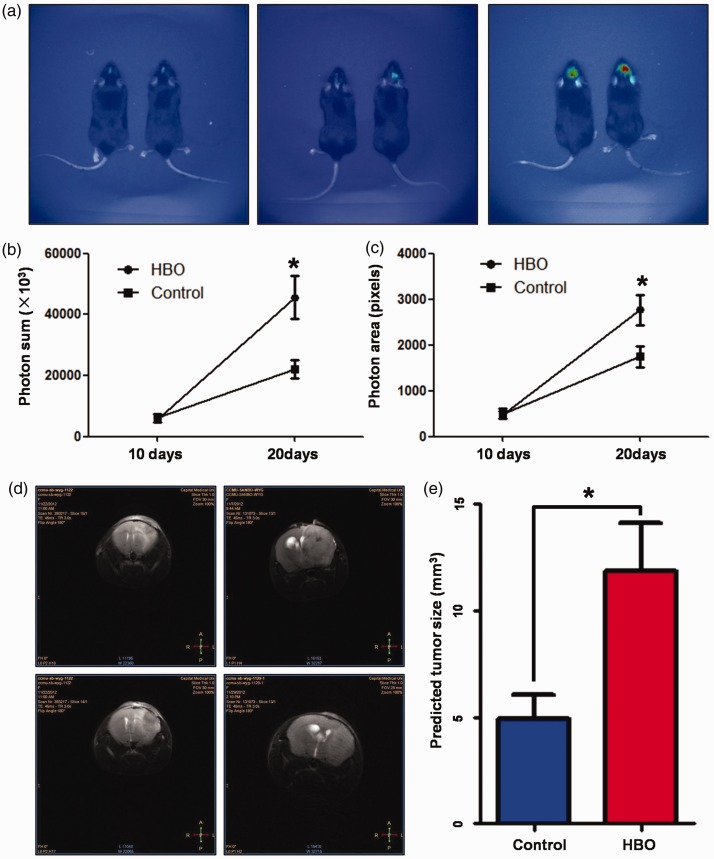
Tumor size was measured in vivo by BLI 10 days after GL261-Luc glioma cells intracranial transplantation. Tumor growth rates were similar between the HBO experimental group and the control group (Photon area pixels: 5678±957 vs. 6069±1400, respectively; Photon sum ×1000: 498.8±111.8 vs. 476.7±77.7, respectively). However, 10 days after HBO treatment, the HBO group showed significantly larger tumors than the control group (Photon area pixels: 22220±7780 vs. 45640±7191, respectively, P=0.013; Photon sum ×1000: 1745.0±233.5 vs. 2768.1±331.3, respectively, P=0.030). Representative real-time images of the tumors are shown in Figure 1(a), and the statistics of the photon sum and photon area are shown in Figure 1(b, c).
Figure 1.
Hyperbaric oxygen promoted the growth of intracranial transplanted glioblastoma multiforme cells in vivo. (a) Representative bioluminescence images of tumors inoculated in the HBO group and the control group in vivo (left panel: 10 days after transplantation; middle panel: 20 days after transplantation, control group; right panel: 20 days after transplantation, HBO group). (b) Photon sum of the HBO group and the control group. (c) Photon light-emitting area of the HBO group and the control group. (d) Representative magnetic resonance images of tumors inoculated in the HBO group and the control group in vivo (left panel: control group; right panel: HBO group). (e) Predicted tumor sizes of tumors inoculated in the HBO group and the control group. HBO, hyperbaric oxygen.
To further confirm the effects of HBO on intracranial glioma cell growth, MRI was performed 20 days after the glioma cell injection. The predicted sizes of tumors in the HBO group were significantly larger than those of the control group (11.83±2.28 vs. 4.925±1.13 mm3, respectively, P=0.017; Figure 1(d, e)).
Hyperbaric oxygen repressed ROS levels in glioma cells and brain cells
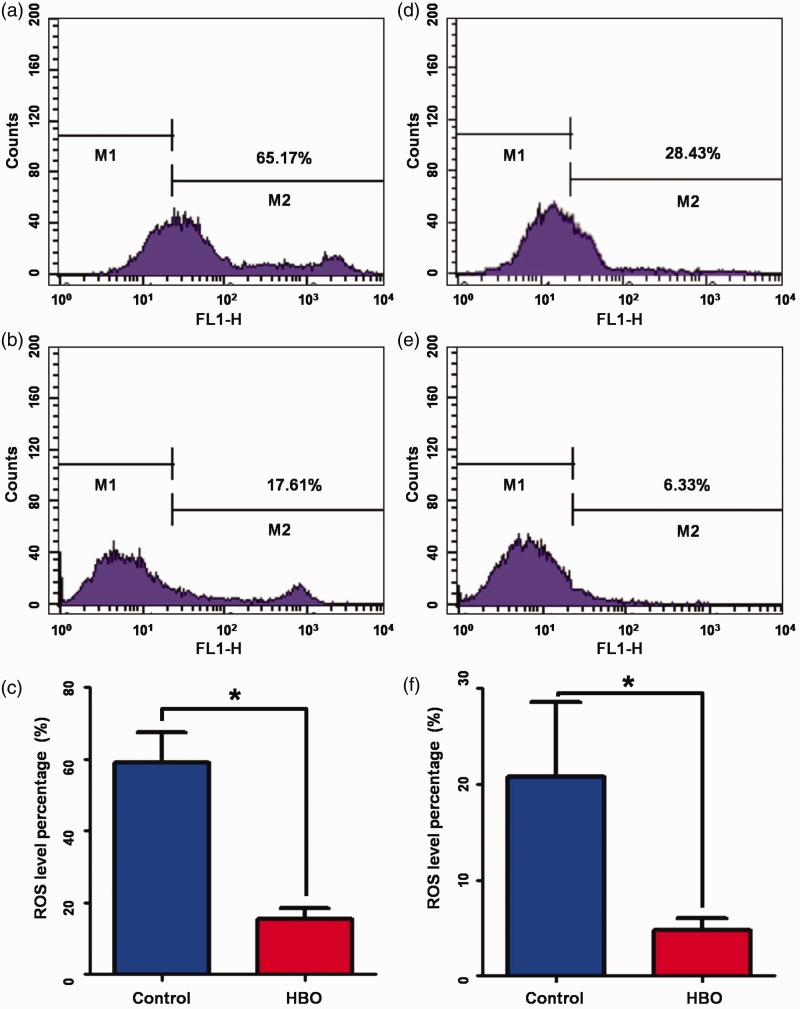
To reveal the direct effects of HBO treatment, ROS levels in glioma cells and brain cells were evaluated by flow cytometry using the cell-permeant reagent DCFDA. The ROS level of tumor cells was significantly lower in the HBO group than in the control group (15.51±2.85 vs. 59.00±8.37%, respectively, P<0.001; Figure 2(a–c)). The ROS level of brain cells was also significantly lower in the HBO group than in the control group (4.79±1.10 vs. 20.81±6.91%, respectively, P=0.038; Figure 2(d–f)). To further investigate the biological effects of HBO-induced ROS signaling, hypoxia-inducible factor (HIF-1α) protein levels were measured, and shown to be significantly increased in the HBO group compared with the control group (P=0.002). Quantitative reverse transcription PCR also showed that HIF-1α-targeted oncogenes, such as glucose transporter type-1 and ATP-binding cassette sub-family B member 1, were elevated in the HBO group compared with the control group (data not shown).
Figure 2.
Hyperbaric oxygen decreased ROS levels in glioma cells and brain cells. (a) Representative flow cytometry analysis of ROS levels in tumor cells of the control group. (b) Representative flow cytometry analysis of ROS levels in tumor cells of the HBO group. (c) ROS level percentage in tumor cells of the HBO group and the control group. (d) Representative flow cytometry analysis of ROS levels in brain cells of the control group. (e) Representative flow cytometry analysis of ROS levels in brain cells of the HBO group. (f) ROS level percentages in brain cells of the HBO group and the control group. HBO, hyperbaric oxygen; ROS, reactive oxygen species.
Hyperbaric oxygen elevated ROS levels in the thymus and inhibited T cell maturation
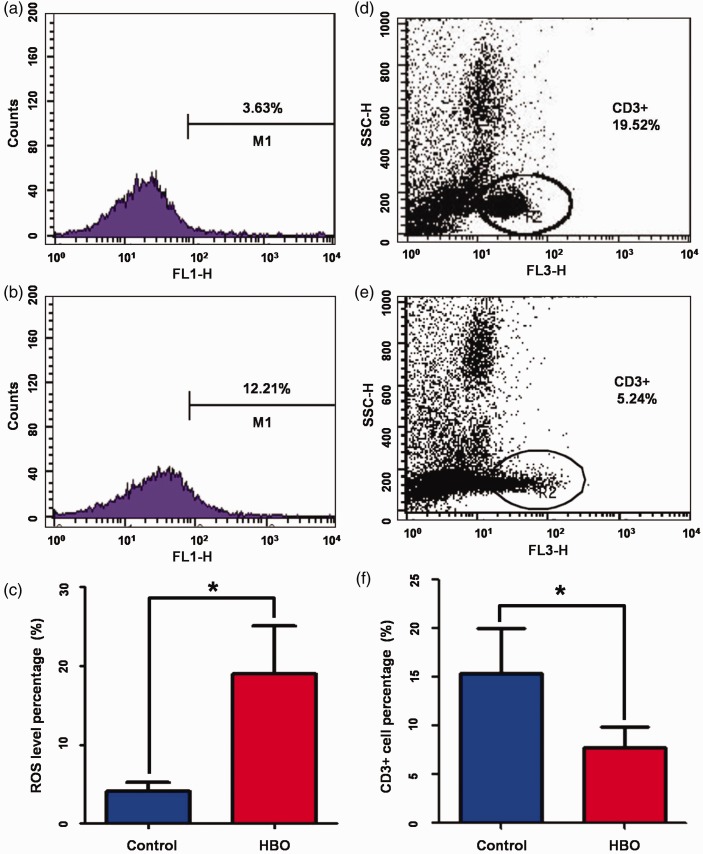
ROS levels in other organs besides the brain were also evaluated by flow cytometry. After HBO treatment, ROS levels were not significantly changed in the spleen or blood (data not shown), but were significantly increased in the thymus compared with the control group (18.98±6.14 vs. 4.05±6.91%, respectively, P=0.032; Figure 3(a–c)).
Figure 3.
Hyperbaric oxygen increased ROS levels in the thymus and inhibited T cell production. (a) Representative flow cytometry analysis of ROS levels in thymuses of the control group. (b) Representative flow cytometry analysis of ROS levels in thymuses of the HBO group. (c) ROS level percentage in thymuses of the HBO group and the control group. (d) Representative flow cytometry analysis of CD3+ cells in tumors of the control group. (e) Representative flow cytometry analysis of CD3+ cells in tumors of the HBO group. (f) CD3+ cell percentages in tumor-infiltrating lymphocytes of the HBO group and the control group. HBO, hyperbaric oxygen; ROS, reactive oxygen species.
To further explore the effects of HBO on ROS levels in the thymus, the amount of CD3+ mature T cells in tumor-infiltrating lymphocytes was quantified by flow cytometry. The HBO group had a significantly lower CD3+ cell percentage than the control group (7.10±1.36 vs. 15.64±3.61%, respectively, P=0.044; Figure 3(d–f)).
Hyperbaric oxygen resulted in T cell immunosuppression
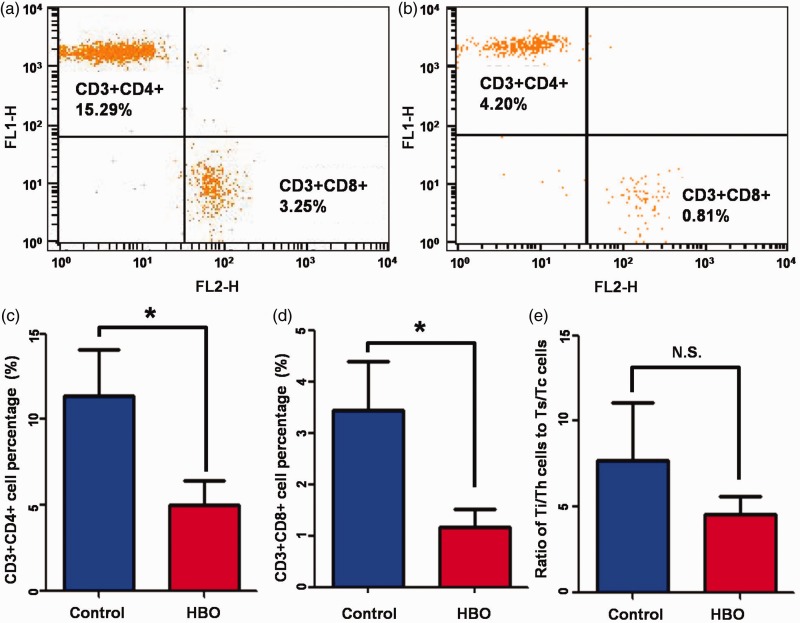
To study the immunological effects of HBO on elevated ROS signaling, T cells in tumor-infiltrating lymphocytes were further sorted by CD4 and CD8 expression. Flow cytometry indicated that the proportion of Ti/Th cells (CD3+CD4+) in tumor-infiltrating lymphocyte was significantly decreased in the HBO group compared with the control group (4.73±1.32 vs. 11.36±2.72%, respectively, P=0.046, Figure 4(a, c)). Similarly, the proportion of Ts/Tc cells (CD3+CD8+) in tumor-infiltrating lymphocytes was also significantly decreased in the HBO group compared with the control group (1.16±0.35 vs. 3.44±0.96%, respectively, P=0.043; Figure 4(b, d)).
Figure 4.
Hyperbaric oxygen resulted in T cell immunosuppression. (a) Representative flow cytometry analysis of Ti/Th cells (CD3+CD4+) and Ts/Tc cells (CD3+CD8+) in tumor-infiltrating lymphocytes of the control group. (b) Representative flow cytometry analysis of Ti/Th cells (CD3+CD4+) and Ts/Tc cells (CD3+CD8+) in tumor-infiltrating lymphocytes of the HBO group. (c) Ti/Th cells (CD3+CD4+) percentages in tumor-infiltrating lymphocytes of the HBO group and the control group. (d) Ts/Tc cells (CD3+CD8+) percentages in tumor-infiltrating lymphocytes of the HBO group and the control group. (e) Ratios of Ti/Th cells (CD3+CD4+) to Ts/Tc cells (CD3+CD8+) in tumor-infiltrating lymphocytes of the HBO group and the control group. HBO, hyperbaric oxygen.
The ratio of CD4+ to CD8+ cells reflects the type of immune disorder in clinical practice,24 so we also quantified the ratio of Ti/Th cells (CD3+CD4+) to Ts/Tc cells (CD3+CD8+). Although the ratio of Ti/Th cells (CD3+CD4+) to Ts/Tc cells (CD3+CD8+) was lower in the HBO group than in the control group, the difference was not significant (4.54±1.05 vs. 7.69±3.38%, respectively; Figure 4(e)).
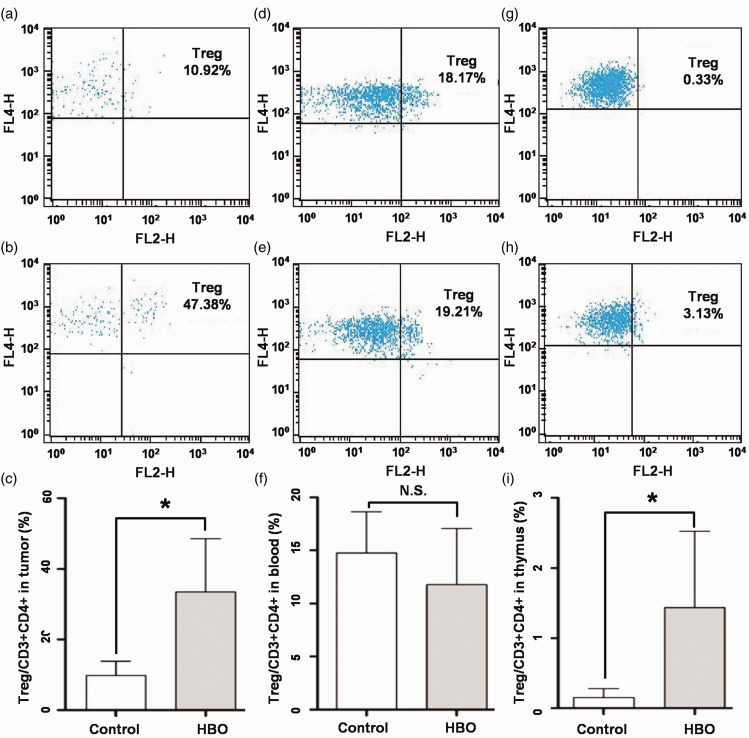
Hyperbaric oxygen promoted regulatory T cell production in the thymus
Regulatory T cells (Treg cells) are an important indicator of the cell-mediated immune response of glioma.25 We sorted Treg cells (CD3+CD4+CD25+FoxP3+) from tumor-infiltrating CD3+CD4+ cells, and found a significant elevation of the tumor-infiltrating Treg cell percentage in the HBO group compared with the control group (33.43±15.05 vs. 9.80±4.01%, respectively, P<0.001; Figure 5(a–c)).
Figure 5.
Hyperbaric oxygen promoted Treg cell production in the thymus. (a) Representative flow cytometry analysis of Treg cells (CD3+CD4+CD25+FoxP3+) in tumor-infiltrating lymphocytes of the control group. (b) Representative flow cytometry analysis of Treg cells (CD3+CD4+CD25+FoxP3+) in tumor-infiltrating lymphocytes of the HBO group. (c) Treg cells percentages of Ti/Th cells (CD3+CD4+) in tumor-infiltrating lymphocytes of the control group and the HBO group. (d) Representative flow cytometry analysis of Treg cells (CD3+CD4+CD25+FoxP3+) in blood lymphocytes of the control group. (e) Representative flow cytometry analysis of Treg cells (CD3+CD4+CD25+FoxP3+) in blood lymphocytes of the HBO group. (f) Treg cell percentages of Ti/Th cells (CD3+CD4+) in blood lymphocytes of the control group and the HBO group. (g) Representative flow cytometry analysis of Treg cells (CD3+CD4+CD25+FoxP3+) in thymus lymphocytes of the control group. (h) Representative flow cytometry analysis of Treg cells (CD3+CD4+CD25+FoxP3+) in thymus lymphocytes of the HBO group. (i) Treg cell percentages of Ti/Th cells (CD3+CD4+) in thymus lymphocytes of the control group and the HBO group. HBO, hyperbaric oxygen; Treg cells, regulatory T cells.
The percentage of Treg cells in blood CD3+CD4+ immunocytes was very similar between the HBO group and the control group (11.77±5.27 vs. 14.74±3.89, respectively; Figure 5(d–f)). However, the percentage of Treg cells in thymus CD3+CD4+ immunocytes was much higher in the HBO group than in the control group (1.44±1.09 vs. 0.15±0.13, respectively, P=0.001; Figure 5(g–i)).
Discussion
Adjuvant HBO treatment has been widely reported to improve the prognosis of glioma patients undergoing chemoradiotherapy.8,26,27 However, we previously reported that HBO alone promoted malignant glioma cell growth and inhibited its apoptosis in an intracranial transplanted glioma mouse model.20 This warned against the clinical use of HBO, and indicated that its potential pro-oncogenic effects should be managed with caution.
The molecular changes induced by HBO treatment have been investigated in several studies.13,16,17,20 However, the effects of HBO on non-cancerous cells, especially lymphocytes, might differ from those on cancer cells. Therefore, the investigation of HBO-induced immunological changes would be beneficial to understand the mechanisms underlying the pro-oncogenic effects of HBO and to improve its clinical usage in GBM treatment.
Using two different approaches of tumor size measurement, we observed that HBO treatment promoted tumor growth, which is consistent with our previous report.20
Under HBO stimulation, the ROS signaling of glioma and brain cells was significantly downregulated, which likely reflects a decline in the oxygen partial pressure caused by cyclical HBO stimulation. However, ROS levels in the thymus were elevated. Because ROS signaling is known to regulate T cell activation in the thymus,28,29 we further investigated the quantity and composition of T cells in the HBO and control groups.
T cells play a central role in cell-mediated immunity, and different T cell subsets are characterized by cell surface biomarkers.30 For example, nearly all mature T cells express CD3+ on the cell surface. Ti/Th cells (characterized by CD3+CD4+ expression) assist other lymphocytes in immunologic processes, including the maturation of B cells and the activation of cytotoxic T cells and macrophages.30 Treg cells (characterized by CD3+CD4+CD25+FoxP3+ expression) are a subset of Ti/Th cells, which mainly display suppression activity in T cell-mediated immunity.30 Treg cells are also crucial for the maintenance of immunological tolerance. Ts/Tc cells (CD3+CD8+) recognize their targets by binding to major histocompatibility complex class I-associated antigens, and destroy virus-infected cells and tumor cells.30
The present study revealed a significantly lower percentage of mature T cells (CD3+) in tumor-infiltrating lymphocytes of the HBO group than in the control group. Further sorting of these lymphocytes by CD4 and CD8 expression revealed that the proportion of Ti/Th cells (CD3+CD4+) was largely decreased in the HBO group than in the control group, as was the proportion of Ts/Tc cells (CD3+CD8+). Additionally, the proportion of Treg cells (CD3+CD4+CD25+FoxP3+) was significantly elevated in the HBO group than in the control group, suggesting a comprehensive immunosuppression effect of HBO through inhibiting T cell maturation in the thymus. To validate whether the change of Treg cells originated from the effect of HBO on the thymus, we evaluated the percentage of Treg cells in the blood and the thymus. Though no significant difference was observed, there was a tendency for an increased percentage of Treg cells in the HBO group compared with the control group.
In summary, we confirmed that repeated exposure to HBO promoted GBM tumor growth, and revealed the influence of HBO on the immune system for the first time. The pro-oncogenic effects of HBO were not only a result of its direct influence on tumor cells, but also its strong immunosuppression effect through inhibiting T cell maturation in the thymus.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Morgan LL. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 2015; 17: 623–624. doi:10.1093/neuonc/nou358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deb P, Boruah D, Dutta V. Morphometric study of microvessels in primary CNS tumors and its correlation with tumor types and grade. Microvasc Res 2012; 84: 34–43. [DOI] [PubMed] [Google Scholar]
- 3.Sabel M, Giese A. Safety profile of carmustine wafers in malignant glioma: a review of controlled trials and a decade of clinical experience. Curr Med Res Opin 2008; 24: 3239–3257. doi:10.1185/03007990802508180. [DOI] [PubMed] [Google Scholar]
- 4.Kingwell K. Neuro-oncology: Glioblastoma prognosis linked to neuronal PD-L1 expression in tumour-adjacent tissue. Nat Rev Neurol 2013; 9: 602–603. doi:10.1038/nrneurol.2013.197. [DOI] [PubMed] [Google Scholar]
- 5.Myung JK, Cho HJ, Kim H, et al. Prognosis of glioblastoma with oligodendroglioma component is associated with the IDH1 mutation and MGMT methylation status. Transl Oncol 2014; 7: 712–719. doi:10.1016/j.tranon.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonetti G, Gaviani P, Innocenti A, et al. Update on treatment strategies for anaplastic glioma: a review of literature. Neurol Sci 2014; 35: 977–981. doi:10.1007/s10072-014-1829-y. [DOI] [PubMed] [Google Scholar]
- 7.Siu A, Wind JJ, Iorgulescu JB, et al. Radiation necrosis following treatment of high grade glioma–a review of the literature and current understanding. Acta Neurochir (Wien) 2012; 154: 191–201; discussion doi:10.1007/s00701-011-1228-6. [DOI] [PubMed] [Google Scholar]
- 8.Beppu T, Kamada K, Nakamura R, et al. A phase II study of radiotherapy after hyperbaric oxygenation combined with interferon-beta and nimustine hydrochloride to treat supratentorial malignant gliomas. J Neurooncol 2003; 61: 161–170. [DOI] [PubMed] [Google Scholar]
- 9.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol 2008; 26: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Zhou Y, Shingu T, et al. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem 2011; 286: 32843–32853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buhler H, Strohm GL, Nguemgo-Kouam P, et al. The therapeutic effect of photon irradiation on viable glioblastoma cells is reinforced by hyperbaric oxygen. Anticancer Res 2015; 35: 1977–1983. [PubMed] [Google Scholar]
- 12.Kohshi K, Yamamoto H, Nakahara A, et al. Fractionated stereotactic radiotherapy using gamma unit after hyperbaric oxygenation on recurrent high-grade gliomas. J Neurooncol 2007; 82: 297–303. [DOI] [PubMed] [Google Scholar]
- 13.Lu XY, Cao K, Li QY, et al. The synergistic therapeutic effect of temozolomide and hyperbaric oxygen on glioma U251 cell lines is accompanied by alterations in vascular endothelial growth factor and multidrug resistance-associated protein-1 levels. J Int Med Res 2012; 40: 995–1004. [DOI] [PubMed] [Google Scholar]
- 14.Stupp R, Mason WP, van den Bent MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–996. [DOI] [PubMed] [Google Scholar]
- 15.Sun S, Lee D, Lee NP, et al. Hyperoxia resensitizes chemoresistant human glioblastoma cells to temozolomide. J Neurooncol 2012; 109: 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dagistan Y, Karaca I, Bozkurt ER, et al. Combination hyperbaric oxygen and temozolomide therapy in C6 rat glioma model. Acta Cir Bras 2012; 27: 383–387. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z, Ma J, Liu B, et al. Hyperbaric oxygen therapy sensitizes nimustine treatment for glioma in mice. Cancer Med 2016; 5: 3147–3155. doi:10.1002/cam4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007; 11: 69–82. [DOI] [PubMed] [Google Scholar]
- 19.Stuhr LE, Raa A, Oyan AM, et al. Hyperoxia retards growth and induces apoptosis, changes in vascular density and gene expression in transplanted gliomas in nude rats. J Neurooncol 2007; 85: 191–202. [DOI] [PubMed] [Google Scholar]
- 20.Wang YG, Zhan YP, Pan SY, et al. Hyperbaric oxygen promotes malignant glioma cell growth and inhibits cell apoptosis. Oncol Lett 2015; 10: 189–195. doi:10.3892/ol.2015.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schonmeyr BH, Wong AK, Reid VJ, et al. The effect of hyperbaric oxygen treatment on squamous cell cancer growth and tumor hypoxia. Ann Plast Surg 2008; 60: 81–88. doi:10.1097/SAP.0b013e31804a806a. [DOI] [PubMed] [Google Scholar]
- 22.Biollaz G, Bernasconi L, Cretton C, et al. Site-specific anti-tumor immunity: differences in DC function, TGF-beta production and numbers of intratumoral Foxp3+ Treg. Eur J Immunol 2009; 39: 1323–1333. [DOI] [PubMed] [Google Scholar]
- 23.Conconi MT, Baiguera S, Guidolin D, et al. Effects of hyperbaric oxygen on proliferative and apoptotic activities and reactive oxygen species generation in mouse fibroblast 3T3/J2 cell line. J Investig Med 2003; 51: 227–232. [DOI] [PubMed] [Google Scholar]
- 24.Bruno G, Saracino A, Monno L, et al. The revival of an “old” marker: CD4/CD8 ratio. Aids Rev 2017; 19: 81–88. [PubMed] [Google Scholar]
- 25.See AP Parker JJ and Waziri A.. The role of regulatory T cells and microglia in glioblastoma-associated immunosuppression. J Neurooncol 2015; 123: 405–412. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa K, Ishiuchi S, Inoue O, et al. Phase II trial of radiotherapy after hyperbaric oxygenation with multiagent chemotherapy (procarbazine, nimustine, and vincristine) for high-grade gliomas: long-term results. Int J Radiat Oncol Biol Phys 2012; 82: 732–738. [DOI] [PubMed] [Google Scholar]
- 27.Fairchild A, Weber DC, Bar-Deroma R, et al. Quality assurance in the EORTC 22033-26033/CE5 phase III randomized trial for low grade glioma: the digital individual case review. Radiother Oncol 2012; 103: 287–292. doi:10.1016/j.radonc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Murphy MP, Siegel RM. Mitochondrial ROS fire up T cell activation. Immunity 2013; 38: 201–202. doi:10.1016/j.immuni.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Choi HJ, Jang SY, Hwang ES. High-dose nicotinamide suppresses ROS generation and augments population expansion during CD8(+) T cell activation. Mol Cells 2015; 38: 918–924. doi:10.14348/molcells.2015.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haina S, Akiko I. Tissue-resident memory T cells. Immunol Rev 2013; 255: 165–181. doi:10.1111/imr.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]