Short abstract
Objective
This study was performed to determine whether transarterial chemoembolization (TACE) plus multi-imaging–guided radiofrequency ablation (MIG-RFA) can completely eliminate 3.1- to 5.0-cm hepatocellular carcinoma (HCC) nodules and identify factors that may influence the complete elimination rate (CER) of this therapy.
Methods
Patients who underwent TACE+MIG-RFA for initial treatment of HCC from January 2008 to January 2016 were retrospectively reviewed. In total, 162 patients with 216 HCC nodules (3.1–5.0 cm) were enrolled. TACE was performed first; MIG-RFA was performed 2 to 4 weeks later. Contrast-enhanced computed tomography was performed 1, 3, 6, and 12 months after TACE+MIG-RFA. If tumor enhancement was not detected by the end of the 12-month follow-up, the lesion was considered completely eliminated. Additional TACE+MIG-RFA was performed for residual lesions. The CER was calculated 12 months after the last therapy. Factors that may influence the CER were analyzed.
Results
In total, 207 (95.8%) nodules showed no residual lesions and were completely eliminated after one or more TACE+MIG-RFA sessions. Nine (4.2%) nodules were incompletely eliminated even with repeated TACE+MIG-RFA. Tumor location was the only significant prognostic factor influencing the CER.
Conclusions
TACE+MIG-RFA can eliminate 3.1- to 5.0-cm HCC nodules; the tumor location may affect the treatment outcome.
Keywords: Hepatocellular carcinoma, multi-imaging–guided radiofrequency ablation, transarterial chemoembolization, complete elimination rate, computed tomography, tumor enhancement, residual lesions
Introduction
Elimination of local lesions is very important for the treatment of small hepatocellular carcinoma (HCC). For tumor nodules measuring ≤3 cm, radiofrequency ablation (RFA) alone can be curative and as effective as surgical resection or liver transplantation. For nodules measuring >3 cm, however, RFA alone has inferior outcomes that may result from incomplete ablation, which may lead to recurrence of the original tumor.1–3 Therefore, new techniques with which to improve the local therapeutic effects for HCC should be considered. The present study was performed to determine whether transarterial chemoembolization (TACE) plus multi-imaging–guided RFA (MIG-RFA) can successfully eliminate local HCC nodules measuring 3.1 to 5.0 cm. We also analyzed factors that may influence the complete elimination rate (CER) of the combined therapy.
Materials and methods
Patient selection
This study was approved by the Ethics Committee of Peking University First Hospital. This was a retrospective study, and we only evaluated treatment efficacy. Written informed consent for all therapeutic procedures performed was obtained from the patients before the procedures.
Records of patients who underwent TACE+MIG-RFA for HCC from January 2008 to January 2016 in our medical center were reviewed. The inclusion criteria were HCC nodules measuring 3.1 to 5.0 cm in diameter, no previous treatment for target nodules, and follow-up for at least 1 year after the last TACE+MIG-RFA session. HCC was diagnosed according to the criteria of the American Association for the Study of Liver Disease.4
Tumors in special locations
Special locations of the liver were defined in our study as areas adjacent to the diaphragm, liver surface, bile ducts, large vessels, or visceral organs with a range of ≤5 mm.
TACE procedure
A 5-French catheter was introduced into the celiac trunk from the right femoral artery, and common hepatic artery angiography was performed to evaluate the arterial blood supply to the tumor. A 2.8-French microcatheter was then superselectively introduced into the feeding artery of the tumor. TACE was performed using 20 to 40 mg of epirubicin mixed with lipiodol, and further embolization was performed with a gelatin sponge or polyvinyl alcohol granules. After embolization, angiography was performed to determine the extent of vascular occlusion and assess blood flow in other arteries.
MIG-RFA procedure
All RFA procedures were performed under multi-imaging guidance with intravenous anesthesia. MIG-RFA was defined as the performance of RFA under the guidance of more than one imaging system, including ultrasonography (US) + computed tomography (CT), in which US guidance was used during the puncture process and CT was then used to make fine adjustments of the location of the needle tip; US + fluoroscopy, in which fluoroscopy was used to adjust the needle deployment after puncture of the lesion by US guidance; and US + cone-beam CT, in which RFA was performed under US guidance and both fluoroscopy and CT were performed during the same procedure. The guiding method was chosen according to the location of the HCC nodule and the appearance of lipiodol deployment. Coordination of the patient's breathing rhythm was also an important factor in choosing the guiding method; if the patient could not breathe evenly, we chose a real-time guiding method with US or fluoroscopy.
The RFA system was a Model 1500X radiofrequency generator manufactured by RITA Medical Systems (Latham, NY, USA). The radiofrequency needle was a StarBurst open type (AngioDynamics, Latham, NY, USA), and the cluster needle contained nine electrodes. Upon completion of ablation, the needle was withdrawn and track ablation was simultaneously performed to prevent bleeding and tumor seeding. Electrocardiographic monitoring was performed for 12 hours after RFA.
Follow-up procedure
Contrast-enhanced CT was performed 1, 3, 6, and 12 months after the combined therapy. If tumor enhancement was not detected after the 12-month follow-up, the HCC nodules were considered completely eliminated. If residual lesions were detected during the follow-up, additional TACE+MIG-RFA was performed. The CER was calculated 12 months after the last combined therapy. Factors that may influence the CER were also analyzed.
Statistical analysis
The patients’ baseline characteristics were documented. The following treatment-related variables were assessed by univariate analysis of variance: age, sex, tumor size, tumor location, Karnofsky performance score, Barcelona Clinic Liver Cancer grade, and Child–Pugh classification. The differences in CER were compared by the log-rank test. A significant difference was considered to be present when P≤0.05. All statistical analyses were performed using IBM SPSS Statistics, version 20.0 (IBM Corp., Armonk, NY, USA).
Results
In total, 162 patients (127 male, 35 female) with 216 nodules measuring 3.1 to 5.0 cm were enrolled in our study. The patients’ general conditions are shown in Table 1. Technical success was achieved in all patients. No severe complications occurred.
Table 1.
Patients’ baseline demographic and clinical characteristics.
| Age, y | 60.2 ± 11.2 |
| 60 | 81 (50.0) |
| ≥60 | 81 (50.0) |
| Sex | |
| Male | 127 (78.4) |
| Female | 35 (21.6) |
| Child–Pugh classification | |
| A | 143 (88.2) |
| B | 19 (11.8) |
| BCLC grade | |
| A | 74 (45.7) |
| B | 53 (33.7) |
| C | 35 (21.6) |
| AFP, ng/L | 255.6 ± 428.5 |
| <400 | 132 (81.4) |
| ≥400 | 30 (19.6) |
| KPS | 92.2 ± 8.2 |
| <90 | 133 (82.1) |
| ≥90 | 29 (17.9) |
| Tumor size, cm | 3.81 ± 0.748 |
| 3.1–4.0 | 133 (61.6) |
| 4.1–5.0 | 83 (38.4) |
| Tumor location | |
| Normal | 123 (56.9) |
| Special | 93 (43.1) |
Data are presented as mean ± standard deviation or n (%). BCLC, Barcelona Clinic Liver Cancer; AFP, alpha fetoprotein; KPS, Karnofsky performance score
At the end of the follow-up, 207 (95.8%) nodules were completely eliminated, and 9 (4.2%) nodules remained viable. Complete elimination after just one TACE+MIG-RFA session occurred in 180 (83.3%) nodules, after two TACE+MIG-RFA sessions in 24 (11.1%) nodules, and after three TACE+MIG-RFA sessions in 3 (1.4%) nodules (Table 2).
Table 2.
Therapeutic effects after transarterial chemoembolization plus multi-imaging–guided radiofrequency ablation.
| Viable nodules | Complete elimination | Incomplete elimination | |
|---|---|---|---|
| 1 Session | 216 | 180 | 36 |
| 2 Sessions | 36 | 24 | 12 |
| 3 Sessions | 12 | 3 | 9 |
Data are presented as number of nodules.
Seven variables with possible effects on the CER were analyzed. Tumor location was a significant prognostic factor for the CER (P<0.001) (Table 3). The log-rank test showed that the presence of nodules in special areas of the liver was significantly correlated with incomplete tumor elimination (P<0.001, Exp (B) (95% CI) = 63.9 (8.4–484.5)). After three therapy sessions, the remaining nine viable nodules were all located in special areas of the liver: very close to vessels (n=3), near the gallbladder or intestine (n=2), adjacent to the diaphragm (n=3), and very close to the heart (n=1).
Table 3.
Risk factors for poor treatment effects by univariate analysis of variance.
| Characteristics | n |
Incompletely eliminated tumors |
P value | ||
|---|---|---|---|---|---|
| 1st treatment | 2nd treatment | 3rd treatment | |||
| Age, y | |||||
| 60 | 104 | 15 | 4 | 3 | 0.402 |
| ≥60 | 112 | 21 | 8 | 6 | |
| Sex | 0.6 | ||||
| Male | 169 | 28 | 7 | 5 | |
| Female | 47 | 8 | 5 | 4 | |
| Child–Pugh classification | 0.134 | ||||
| A | 190 | 30 | 10 | 7 | |
| B | 26 | 6 | 2 | 2 | |
| BCLC grade | 0.484 | ||||
| A | 102 | 7 | 2 | 2 | |
| B | 63 | 13 | 4 | 3 | |
| C | 51 | 17 | 6 | 4 | |
| AFP, ng/L | 0.823 | ||||
| 400 | 177 | 25 | 8 | 5 | |
| ≥400 | 39 | 11 | 4 | 4 | |
| Tumor size, cm | 0.378 | ||||
| 3.0–4.0 | 133 | 11 | 2 | 2 | |
| 4.0–5.0 | 83 | 25 | 10 | 7 | |
| Tumor location | <0.001 | ||||
| Ordinary | 123 | 1 | 0 | 0 | |
| Special | 93 | 35 | 12 | 9 | |
| KPS score | 0.018 | ||||
| 90 | 39 | 15 | 4 | 3 | |
| ≥90 | 177 | 21 | 8 | 6 | |
Data are presented as number of tumor nodules. BCLC, Barcelona Clinic Liver Cancer; AFP, alpha fetoprotein; KPS, Karnofsky performance score
Discussion
Tumor recurrence from the residual lesion is the main problem directly affecting overall survival when RFA is compared with surgical resection or transplantation. The size of the lesion to be ablated is closely associated with the risk of local recurrence after RFA. The optimal lesion size for curative RFA is considered to be ≤3 cm. For tumors even 1 mm larger than 3 cm, a significant increase in local recurrence and lower recurrence-free and disease-free survival rates are found in association with RFA when compared with surgical resection or transplantation.5 Therefore, for HCC nodules of >3 cm, how to decrease the local recurrence rate and improve the elimination rate in RFA remains a challenging problem and may have a strong effect on the therapeutic outcome.
TACE before RFA has been confirmed to have several advantages. The decreased arterial blood flow induced by TACE may reduce the heat sink effect of large vessels adjacent to the lesion, which may result in considerable enlargement of the ablation zone by RFA.6 Moreover, the effect of chemotherapy and hypoxic injury induced by TACE on tumor cells is enhanced by the high temperature during RFA, making it possible to extend the ablation zone.7 In addition, tumor boundaries could be better delineated by deposition of lipiodol inside the tumor, providing a clear target for perfect RFA.
Kim et al.8 compared TACE+RFA with hepatectomy in patients with HCC within the Milan criteria. The 5-year overall survival and disease-free survival rates in the combination group were 75% and 27%, respectively. Kim et al.9 evaluated 37 patients who underwent TACE+RFA for treatment of a single HCC ranging from 2.0 to 5.0 cm, and the 4-year overall survival and disease-free survival rates in the combination group were 78.4% and 69.4%, respectively. In the present study, we used an innovative guidance technique during RFA and investigated whether this technique after conventional TACE can eliminate HCC nodules with confidence. The overall survival rate was not the main focus in this study.
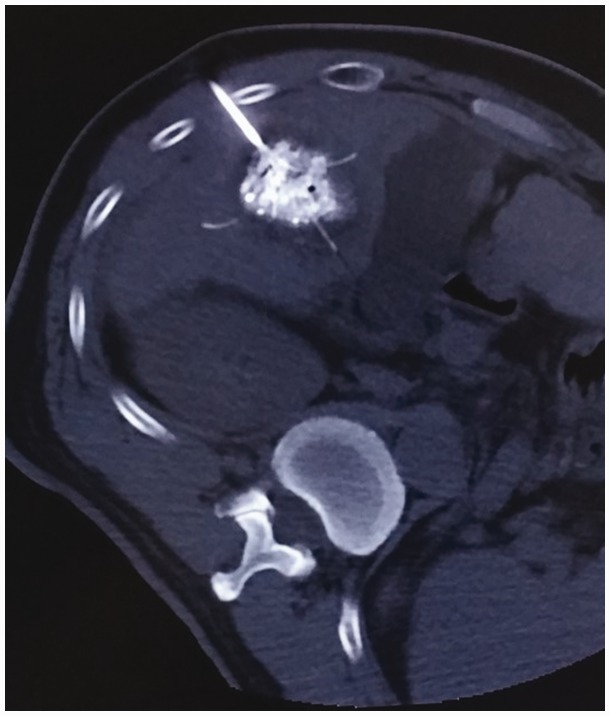
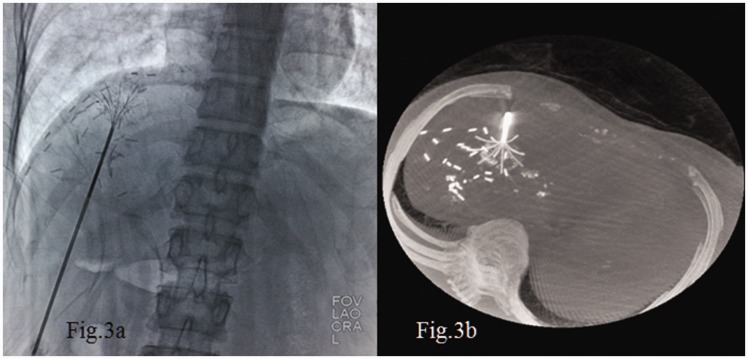
The guidance system used during RFA is a major factor for a successful procedure. New guidance systems consisting of optical, electromagnetic, or fusion imaging have been developed,10 but their current clinical application is still limited. US is the most widely used modality to guide percutaneous procedures in the liver.11 When US guidance is not suitable,12 CT is a valid alternative.13 Cone-beam CT guidance is a new emerging technique incorporating both real-time fluoroscopy and CT-like imaging in the same device.14,15 In our center, we puncture the lesion under US guidance. This provides real-time imaging to avoid breathing disturbances, and US can allow for safer treatment of lesions in some special areas such as those near the diaphragm or heart (Figure 1). CT or cone-beam CT is routinely used for further adjustment of the branch of the needle tip, which makes it easier to cover the whole lesion with the RFA needle (Figures 2 and 3).
Figure 1.
(a) Transarterial chemoembolization plus multi-imaging–guided radiofrequency ablation for a hepatocellular carcinoma nodule in an area adjacent to the diaphragm and heart. (b) Ultrasonography allowed for safe puncture, and the use of computed tomography allowed for fine adjustment of the needle tip.
Figure 2.
Computed tomography provided a very clear image of the needle tip, ensuring the safety and quality of the ablation process.
Figure 3.
Cone-beam computed tomography provided (a) real-time fluoroscopic imaging and (b) a computed tomography-like reconstructional image that clearly shows the needle tip.
In the present study, 180 (86.1%) HCC nodules achieved complete ablation after just one TACE+MIG-RFA session. For the remaining 36 incompletely ablated nodules, 24 (75.0%) achieved complete elimination after the second cycle of the combined therapy. Finally, after the third combined treatment of the remaining 12 nodules, 3 (25.0%) achieved complete elimination. Therefore, among the whole group, 207 (95.8%) nodules achieved complete elimination by the end of 3 TACE+MIG-RFA sessions. Nine (4.2%) nodules were incompletely eliminated.
Univariate analysis of variance showed that the tumor location was the major factor influencing the treatment effect, and location of tumors in special areas was the major factor associated with incomplete elimination. Complications are a great concern among most practitioners when treating lesions in special areas of the liver, especially those near large vessels, the heart, or surrounding organs. In such cases, most doctors would rather use a more conservative RFA technique; however, this may lead to incomplete ablation of the target lesion. In the present study, 93 (43.1%) nodules were located in special areas of the liver, and 84 of them were eliminated by TACE+MIG-RFA; only 9 remained viable. No severe complications occurred. Thus, TACE+MIG-RFA may be very helpful in ablating lesions in special areas of the liver. The two main points that will help to manage lesions in special areas of the liver are clear delineation of the target lesion by deposition of lipiodol post-TACE and accurate RFA through the entire ablation procedure under multi-imaging monitoring.
In conclusion, the present study has confirmed that TACE followed by MIG-RFA can ablate HCC nodules ranging from 3.1 to 5.0 cm with a fairly high success rate. The tumor location is associated with the treatment effect. TACE+MIG-RFA is helpful for lesions in special areas of the liver, but a more effective technique still needs to be developed. The long-term effects of TACE+MIG-RFA on overall survival is the next subject of our research.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Kim JH, Won HJ, Shin YM, et al. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol 2011; 18: 1624–1629. [DOI] [PubMed] [Google Scholar]
- 2.Kim JW, Kim JH, Won HJ, et al. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol 2012; 81: e189–e193. [DOI] [PubMed] [Google Scholar]
- 3.Peng ZW, Chen MS, Liang HH, et al. A case-control study comparing percutaneous radiofrequency ablation alone or combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. Eur J Surg Oncol 2010; 36: 257–263. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutlu OC, Chan JA, Aloia TA, et al. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer 2017; 123: 1817–1827. [DOI] [PubMed] [Google Scholar]
- 6.Morimoto M, Numata K, Kondou M, et al. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010; 116: 5452–5460. [DOI] [PubMed] [Google Scholar]
- 7.Veltri A, Moretto P, Doriguzzi A, et al. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol 2006; 16: 661–669. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Won HJ, Shin YM, et al. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol 2011; 18: 1624–1629. [DOI] [PubMed] [Google Scholar]
- 9.Kim JW, Shin SS, Kim JK, et al. Radiofrequency ablation combined with transcatheter arterial chemoembolization for the treatment of single hepatocellular carcinoma of 2 to 5 cm in diameter: comparison with surgical resection. Korean J Radiol 2013; 14: 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JY, Choi BI, Chung YE, et al. Clinical value of CT/MR-US fusion imaging for radiofrequency ablation of hepatic nodules. Eur J Radiol 2012; 81: 2281–2289. [DOI] [PubMed] [Google Scholar]
- 11.Kim YJ, Lee MW, Park HS. Small hepatocellular carcinomas: ultrasonography guided percutaneous radiofrequency ablation. Abdom Imaging 2013; 38: 98–111 [DOI] [PubMed] [Google Scholar]
- 12.Kim JE, Kim YS, Rhim H, et al. Outcomes of patients with hepatocellular carcinoma referred for percutaneous radiofrequency ablation at a tertiary center: analysis focused on the feasibility with the use of ultrasonography guidance. Eur J Radiol 2011; 79: e80–e84. [DOI] [PubMed] [Google Scholar]
- 13.Filippousis P, Sotiropoulou E, Manataki A, et al. Radiofrequency ablation of subcapsular hepatocellular carcinoma: single center experience. Eur J Radiol 2011; 77: 299–304. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto M, Numata K, Kondo M, et al. C-arm cone beam CT for hepatic tumor ablation under real-time 3D imaging. AJR Am J Roentgenol 2010; 194: W452–W454. [DOI] [PubMed] [Google Scholar]
- 15.Braak SJ, Herder GJ, van Heesewijk JP, et al. Pulmonary masses: initial results of cone-beam CT guidance with needle planning software for percutaneous lung biopsy. Cardiovasc Intervent Radiol 2012; 35: 1414–1421. [DOI] [PubMed] [Google Scholar]