Short abstract
Objective
To determine whether there was an association between serum total antioxidant capacity (TAC) levels prior to in liver transplantation (LT) for hepatocellular carcinoma (HCC) and 1-year LT mortality.
Methods
This observational retrospective single-centre study of patients with LT for HCC measured serum levels of TAC and malondialdehyde (as a biomarker of lipid peroxidation) before LT. The study endpoint was 1-year LT mortality.
Results
This study included 142 patients who underwent LT for HCC. Patients who survived the first year (n = 127) had significantly lower aged liver donors, significantly higher serum TAC levels, and significantly lower serum malondialdehyde levels compared with the non-survivors (n = 15). Logistic regression analysis found that serum TAC levels (odds ratio [OR] 0.275; 95% confidence interval [CI] 0.135, 0.562) and the age of the LT donor (OR 1.050; 95% CI 1.009, 1.094) were associated with 1-year LT mortality. There was an inverse association between serum levels of TAC and malondialdehyde levels (rho = –0.22).
Conclusions
There was an association between low serum TAC levels prior to LT for HCC and mortality during the first year after LT. There was an inverse association between serum TAC levels and lipid peroxidation as measured by malondialdehyde levels.
Keywords: Serum total antioxidant capacity, hepatocellular carcinoma, liver transplantation, mortality, outcome
Introduction
Hepatocellular carcinoma (HCC), the most common primary hepatic malignant tumour, is one of the most frequent causes of cancer-attributable death.1–3 Liver transplantation (LT), which treats the hepatic insufficiency and eliminates the hepatic tumour, is the treatment of choice for some patients with HCC.1–7
The peroxidation of membrane lipids results in the production of different chemical entities.8,9 Malondialdehyde is an aldehyde produced by the effects of free radicals on polyunsaturated fatty acids in the cellular membranes.8,9 Malondialdehyde is released into the extracellular space and can reach the blood stream; therefore, it has been used as a circulating biomarker of lipid peroxidation.8,9 Reactive oxygen species (ROS) are balanced by the action of antioxidant defences, and the determination of total antioxidant capacity (TAC) can provide information about the antioxidant status of patients.10,11
Lower circulating TAC levels have been found in LT patients compared with healthy controls.12 LT patients also have lower circulating antioxidant vitamin levels than healthy controls;13,14 and patients with HCC have lower circulating TAC levels than healthy controls.15–18 Higher serum malondialdehyde levels have been demonstrated in LT patients compared with healthy controls,12–14 and higher serum malondialdehyde levels have been found in HCC patients compared with healthy controls.15,18–20 Higher circulating lipid peroxide levels have been found in patients that do not survive LT (i.e. non-survivor LT patients) compared with LT survivors.21 Higher serum concentrations of reactive oxygen metabolites were found in HCC patients with a greater risk of disease recurrence after curative treatment.22 The levels of circulating malondialdehyde were higher in non-survivor LT patients than survivors.23 However, to the best of our knowledge, there are no published data describing circulating TAC levels in non-survivor and survivor patients with HCC who have undergone LT. Thus, the objective of this present study was to determine whether there was an association between serum TAC levels prior to LT and 1-year LT mortality in patients with HCC.
Patients and methods
Study design and patient population
This observational retrospective single-centre study included consecutive patients who underwent LT for HCC in the Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain between January 1996 and January 2016. Livers for LT were collected from brain dead donors in all cases. The study was carried out with the approval of the Institutional Review Board of the Hospital Universitario Nuestra Señora de Candelaria and written informed consent was obtained from all patients or their family members.
The serum levels of malondialdehyde were determined previously in some of the patients who participated in this current study.23 Serum TAC levels were analysed in the current study to determine their association with 1-year LT mortality.
Variables recorded
The following variables were recorded for each patient: degree of tumour differentiation; the Child-Pugh score;24 presence of a multinodular tumour; met the Milan criteria before and after LT;25 macrovascular invasion; microvascular invasion; model for end-stage liver disease (MELD) score26 by hepatic function; age of liver recipient; infiltration of adjacent tissues; age of liver donor; serum alpha-fetoprotein (AFP) levels; portal hypertension (determined clinically or by hepatic venous pressure gradient); LT technique; treatment prior to LT; nodule size; ABO blood type; leukocyte count; serum albumin levels; serum protein levels; and sex. The 1-year LT survival was the study endpoint.
Determination of serum levels of TAC and malondialdehyde
A 5-ml sample of venous blood was collected approximately 2 h prior to LT from a peripheral intravenous catheter inserted in the middle third of the arm. The blood sample was transferred to a serum separator tube and centrifuged within 30 min at 1000 g for 15 min at 4°C. The serum was removed and frozen at −80°C until determination of serum TAC and malondialdehyde levels. TAC concentrations were determined in the Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain using the Antioxidant Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), which is based on the ability of serum antioxidants to inhibit the oxidation of 2,2′-azino-di-(3-ethylbenzthiazoline sulphonate). The limit of detection of the assay was 0.04 nmol/ml, the inter-assay coefficient of variation was 3.0%, and the intra-assay coefficient of variation was 3.4%.
Malondialdehyde concentrations were determined in the Faculty of Medicine of La Laguna University, Santa Cruz de Tenerife, Spain using the thiobarbituric acid-reactive substance method as previously described.27 The limit of detection of the assay was 0.079 nmol/ml, the inter-assay coefficient of variation was 4.01%, and the intra-assay coefficient of variation was 1.82%.
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 17.0 (SPSS Inc., Chicago, IL, USA) for Windows® and MedCalc® version 15.2.1 (MedCalc, Ostend, Belgium). Categorical variables are presented as frequency (percentage) and were compared using χ2-test. Continuous variables are presented median (interquartile range) and were compared using Mann–Whitney U-test. A receiver operating characteristic (ROC) curve was performed to estimate the prognostic capacity of serum TAC levels for 1-year LT mortality. Kaplan–Meier 1-year LT survival analysis of patients with serum TAC ≥2.98 nmol/ml and <2.98 nmol/ml was undertaken. The Youden J index was used to select this cut-off of serum TAC levels. Logistic regression analysis was used to determine the association between serum TAC levels prior to LT and 1-year LT mortality controlled for the age of LT donor. Odds ratio (OR) and 95% confidence intervals (CI) were used to estimate the clinical impact of each predictive variable. Spearman’s rank correlation test was used to determine the association between serum levels of TAC and malondialdehyde. A P-value < 0.05 was considered statistically significant.
Results
Of the 142 patients that were included in the study, a total of 127 were alive at 1 year after LT and 15 died during the first year after LT. The demographic and clinical characteristics of the two groups of patients are presented in Table 1. Patients who survived the LT had a significantly lower age of liver donor (P = 0.03), significantly higher serum TAC levels (P = 0.003) and significantly lower serum malondialdehyde levels (P = 0.02) compared with non-survivor patients. There were no statistically significant differences between the two groups of patients (survivors versus non-survivors) in terms of sex, met the Milan criteria before and after LT, degree of tumour differentiation, presence of a multinodular tumour, portal hypertension, infiltration of adjacent tissues, macrovascular invasion, microvascular invasion, Child-Pugh score, ABO blood type, LT technique, treatment prior to LT, age of liver recipient, leukocyte count, serum albumin levels, serum protein levels, serum AFP levels, MELD score, and nodule size (Table 1).
Table 1.
Demographic and clinical characteristics of patients (n = 142) with hepatocellular carcinoma who underwent liver transplantation stratified according to their 1-year survival.
| 1-year survivors n = 127 | 1-year non-survivors n = 15 | Statistical significancea | |
|---|---|---|---|
| Sex, female | 21 (16.5) | 0 (0.0) | NS |
| Met the Milan criteria prior to LT | 122 (96.1) | 14 (93.3) | NS |
| Met the Milan criteria after LT | 106 (83.5) | 11 (73.3) | NS |
| Degree of tumour differentiation | NS | ||
| Well | 95 (74.8) | 12 (80.0) | |
| Moderate | 29 (22.8) | 2 (13.3) | |
| Poor | 3 (2.4) | 1 (6.7) | |
| Multinodular tumour | 39 (30.7) | 5 (33.3) | NS |
| Portal hypertension | 87 (65.8) | 11 (73.3) | NS |
| Infiltration | 40 (31.5) | 4 (26.7) | NS |
| Macrovascular invasion | 7 (5.5) | 0 (0.0) | NS |
| Microvascular invasion | 27 (21.3) | 3 (20.0) | NS |
| Child-Pugh score | NS | ||
| A | 62 (48.8) | 10 (66.7) | |
| B | 36 (28.3) | 3 (20.0) | |
| C | 29 (22.8) | 2 (13.3) | |
| ABO blood type | NS | ||
| A | 59 (46.5) | 6 (40.0) | |
| B | 11 (8.7) | 2 (13.3) | |
| O | 51 (40.2) | 6 (40.0) | |
| AB | 6 (4.7) | 1 (6.7) | |
| Transplantation technique | NS | ||
| By-pass | 44 (34.6) | 6 (40.0) | |
| Piggy back | 83 (65.4) | 9 (60.0) | |
| Treatment prior to LT | 69 (54.3) | 10 (66.7) | NS |
| Percutaneous ethanol injection | 28 (22.0) | 7 (46.7) | NS |
| Radiofrequency ablation | 8 (6.3) | 0 (0.0) | NS |
| Transarterial chemoembolization | 27 (21.3) | 3 (20.0) | NS |
| Liver resection | 3 (2.4) | 0 (0.0) | NS |
| Mixed treatment | 3 (2.4) | 0 (0.0) | NS |
| Age of LT recipient, years | 59 (52–62) | 56 (53–62) | NS |
| Serum alpha-fetoprotein, ng/dl | 7.0 (4.0–42.0) | 12.0 (4.8–164.9) | NS |
| Leukocyte count, 103/mm3 | 4.90 (3.60–6.25) | 4.94 (3.49–7.92) | NS |
| Albumin, g/dl | 3.33 (2.90–4.10) | 3.31 (2.93–4.16) | NS |
| Protein, g/dl | 6.70 (6.10–7.10) | 6.70 (5.70–7.68) | NS |
| MELD score | 15 (12–18) | 15 (15–18) | NS |
| Nodule size, cm | 3.0 (2.0–3.5) | 3.2 (1.7–4.6) | NS |
| Age of liver donor, years | 52 (35–63) | 62 (49–72) | P = 0.03 |
| Malondialdehyde, nmol/ml | 2.96 (2.28–4.04) | 3.66 (3.39–4.62) | P = 0.02 |
| TAC, nmol/ml | 4.00 (3.40–4.80) | 2.98 (2.26–4.00) | P = 0.003 |
Data presented as n (%) or median (interquartile range).
Categorical variables were compared using χ2-test; and continuous variables were compared using Mann–Whitney U-test.
LT, liver transplantation; MELD, model for end-stage liver disease; TAC, total antioxidant capacity; NS, no significant between-group difference (P ≥ 0.05).
The logistic regression analysis found that serum TAC levels (OR 0.275; 95% CI 0.135, 0.562; P < 0.001) and the age of the LT donor (OR 1.050; 95% CI 1.009, 1.094; P = 0.02) were associated with 1-year LT mortality (Table 2).
Table 2.
Logistic regression analysis of variables associated with 1-year mortality after liver transplantation in patients (n = 142) with hepatocellular carcinoma.
| Variable | Odds ratio | 95% confidence interval | Statistical significance |
|---|---|---|---|
| Serum total antioxidant capacity levels, nmol/ml | 0.275 | 0.135, 0.562 | P < 0.001 |
| Age of liver donor, years | 1.050 | 1.009, 1.094 | P = 0.02 |
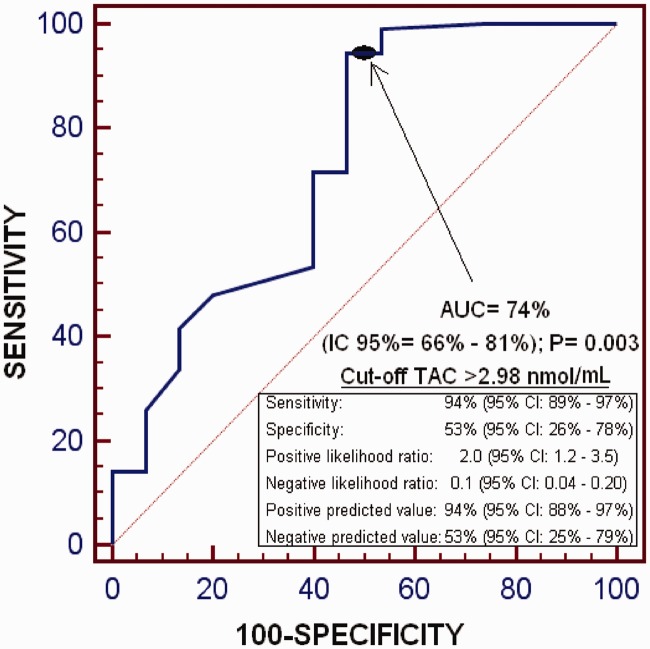
The ROC curve analysis found that the area under the curve of serum TAC levels for the prediction of 1-year LT survival was 74% (95% CI 66%, 81%; P = 0.003); and that serum TAC levels >2.98 nmol/ml had a sensitivity of 94% (95% CI 89%, 97%) and specificity of 53% (95% CI 26%, 78%) for the prediction of 1-year LT survival (Figure 1).
Figure 1.
Receiver operating characteristic curve analysis to estimate the prognostic capacity of serum total antioxidant capacity (TAC) levels for 1-year liver transplantation survival. AUC, area under the curve; IC 95%, 95% confidence interval.
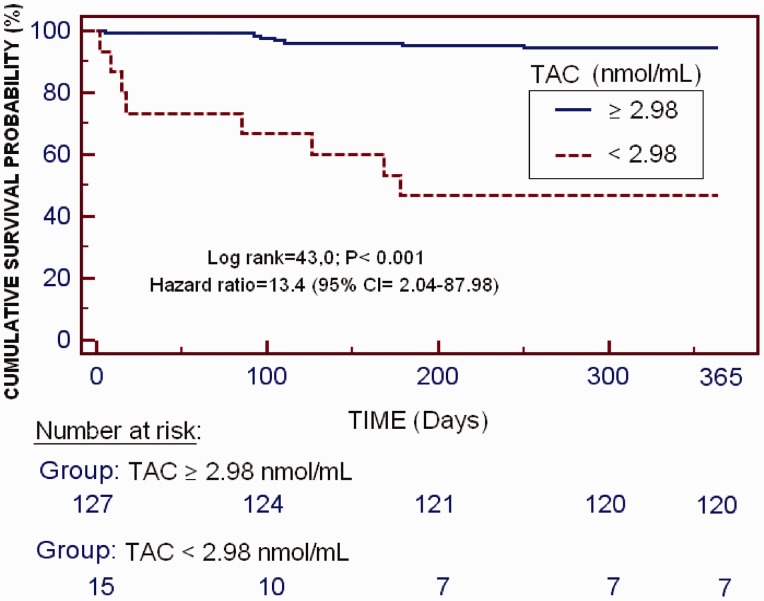
The Kaplan–Meier survival analysis found that patients with serum TAC levels ≥2.98 nmol/ml had a higher 1-year LT survival probability than patients with serum TAC levels <2.98 nmol/ml (94.5% [120/127] versus 46.7% [7/15]; hazard Ratio 13.4; 95% CI 2.04, 87.98; P < 0.001; Figure 2).
Figure 2.
Kaplan–Meier 1-year survival analysis of patients with serum total antioxidant capacity (TAC) ≥ or < 2.98 nmol/ml. 95% CI, 95% confidence interval.
There was an inverse association between serum levels of TAC and malondialdehyde levels (as a biomarker of lipid peroxidation) (rho = –0.22; P = 0.01).
Discussion
The novel findings of this current study were: (i) that there was an association between low serum TAC levels (<2.98 nmol/ml) prior to LT for HCC and mortality during the first year after LT; and (ii) that there was an inverse association between serum TAC levels and lipid peroxidation as measured by malondialdehyde levels.
Previous studies have demonstrated higher serum malondialdehyde levels in LT patients12–14 and in HCC patients15,18–20 compared with healthy controls. In addition, lower circulating TAC levels in LT patients12 and in HCC patients compared with healthy controls have been reported.15–18 Thus, it was not unexpected to get similar data in patients with LT for HCC. However, to the best of our knowledge, this current study is the first to report an association between serum TAC levels ≥2.98 nmol/ml prior to LT for HCC and survival during the first year after LT, and an inverse association between serum levels of TAC and malondialdehyde.
These current findings suggest that an imbalance in the oxidative state might be of pathophysiological importance in HCC patients undergoing to LT. It is possible that LT patients who do not survive the first year after LT continue to have lower serum TAC levels and higher serum malondialdehyde levels (representing higher lipid peroxidation due to ROS overproduction that is not balanced by sufficient antioxidant compound production) than patients that survive during the first year after LT.
The use of different antioxidant agents reduced malondialdehyde levels and increased survival in animal models of sepsis28–31 and of trauma-related brain injury.32–34 In addition, lower circulating malondialdehyde levels and higher survival rates with the administration of different antioxidant agents were observed in clinical studies of asphyxiated newborn infants,35 septic newborns,36 adult burn patients,37 and in patients with traumatic brain injury.38 Thus, the administration of antioxidant agents could be a new class of treatment for patients with HCC undergoing LT.
This current study had a number of limitations. First, serum samples to determine serum levels of TAC and malondialdehyde levels during follow-up were not obtained. Secondly, data regarding other compounds associated with oxidant and antioxidant states were not available for analysis.
In conclusion, these current findings suggest that serum TAC levels could help in predicting mortality in patients with LT for HCC. In particular, patients with serum TAC levels <2.98 nmol/ml have a higher risk of death. However, serum TAC levels should not be used as the only prognostic biomarker, but should be used in combination with other factors such as age of LT donor, outside the Milan criteria, serum AFP levels, tumour size, tumour number, hepatic microinvasion, degree of differentiation, infiltration, and vascular invasion.6,39 These current findings also suggest that further research should be undertaken to investigate the possible administration of antioxidant agents to patients with HCC undergoing LT.
Author contributions
Concept and study design: LL; acquisition of data: LL, STR, PS, APC, PAG, JP, DD, AG, MMM, PC, MAB; determination of serum total antioxidant capacity levels: APC; determination of serum malondialdehyde levels: PAG; analysis of data: LL, AJ; drafted the paper: LL. All authors revised the manuscript critically for important intellectual content and provided final approval of the version to be published.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This study was supported by a grant from Instituto de Salud Carlos III, Madrid, Spain (INT16/00165) and co-financed by Fondo Europeo de Desarrollo Regional. The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–943. [DOI] [PubMed] [Google Scholar]
- 2.Bodzin AS, Busuttil RW. Hepatocellular carcinoma: Advances in diagnosis, management, and long term outcome. World J Hepatol 2015; 7: 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerrero-Misas M, Rodríguez-Perálvarez M, De la Mata M. Strategies to improve outcome of patients with hepatocellular carcinoma receiving a liver transplantation. World J Hepatol 2015; 7: 649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol 2012; 13: e11–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verslype C, Rosmorduc O, Rougier P. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23 (Suppl. 7): vii41–vii48. [DOI] [PubMed] [Google Scholar]
- 6.Cescon M, Bertuzzo VR, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: role of inflammatory and immunological state on recurrence and prognosis. World J Gastroenterol 2013; 19: 9174–9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyoda H, Kumada T, Tada T, et al. Tumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCC. Liver Cancer 2015; 4: 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990; 186: 421–431. [DOI] [PubMed] [Google Scholar]
- 9.Dalle-Donne I, Rossi R, Colombo R, et al. Biomarkers of oxidative damage in human disease. Clin Chem 2006; 52: 601–623. [DOI] [PubMed] [Google Scholar]
- 10.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol 2001; 54: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghiselli A, Serafini M, Natella F, et al. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med 2000; 29: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 12.Thorat VN, Suryakar AN, Naik P, et al. Total antioxidant capacity and lipid peroxidation in liver transplantation. Indian J Clin Biochem 2009; 24: 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trevisani F, Caraceni P, Simoncini M, et al. Evidence of oxidative imbalance in long-term liver transplant patients. Dig Liver Dis 2002; 34: 279–284. [DOI] [PubMed] [Google Scholar]
- 14.Goode HF, Webster NR, Howdle PD, et al. Reperfusion injury, antioxidants and hemodynamics during orthotopic liver transplantation. Hepatology 1994; 19: 354–359. [PubMed] [Google Scholar]
- 15.Zhao J, Zhao Y, Wang H, et al. Association between metabolic abnormalities and HBV related hepatocellular carcinoma in Chinese: a cross-sectional study. Nutr J 2011; 10: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Y, Nie HJ, Yang D, et al. Changes of the mitochondrial DNA copy number and the antioxidant system in the PBMC of hepatocellular carcinoma. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2016; 32: 1–5 [In Chinese, English abstract]. [PubMed] [Google Scholar]
- 17.Poungpairoj P, Whongsiri P, Suwannasin S, et al. Increased oxidative stress and RUNX3 hypermethylation in patients with hepatitis B virus-associated hepatocellular carcinoma (HCC) and induction of RUNX3 hypermethylation by reactive oxygen species in HCC cells. Asian Pac J Cancer Prev 2015; 16: 5343–5348. [DOI] [PubMed] [Google Scholar]
- 18.Yahya RS, Ghanem OH, Foyouh AA, et al. Role of interleukin-8 and oxidative stress in patients with hepatocellular carcinoma. Clin Lab 2013; 59: 969–976. [DOI] [PubMed] [Google Scholar]
- 19.Galley HF, Richardson N, Howdle PD, et al. Total antioxidant capacity and lipid peroxidation during liver transplantation. Clin Sci (Lond) 1995; 89: 329–332. [DOI] [PubMed] [Google Scholar]
- 20.Tsai SM, Lin SK, Lee KT, et al. Evaluation of redox statuses in patients with hepatitis B virus-associated hepatocellular carcinoma. Ann Clin Biochem 2009; 46: 394–400. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Zhou XS, Geng QM. Evaluation oxygen free radicals related index before liver transplantation to forejudge prognosis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2003; 15: 560–562 [In Chinese, English abstract]. [PubMed] [Google Scholar]
- 22.Suzuki Y, Imai K, Takai K, et al. Hepatocellular carcinoma patients with increased oxidative stress levels are prone to recurrence after curative treatment: a prospective case series study using the d-ROM test. J Cancer Res Clin Oncol 2013; 139: 845–852. [DOI] [PubMed] [Google Scholar]
- 23.Lorente L, Rodriguez ST, Sanz P, et al. Association between pre-transplant serum malondialdehyde levels and survival one year after liver transplantation for hepatocellular carcinoma. Int J Mol Sci 2016; 17: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60: 646–649. [DOI] [PubMed] [Google Scholar]
- 25.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693–699. [DOI] [PubMed] [Google Scholar]
- 26.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001; 33: 464–470. [DOI] [PubMed] [Google Scholar]
- 27.Kikugawa K, Kojima T, Yamaki S, et al. Interpretation of the thiobarbituric acid reactivity of rat liver and brain homogenates in the presence of ferric ion and ethylediaminotetraacetic acid. Anal Biochem 1992; 202: 249–255. [DOI] [PubMed] [Google Scholar]
- 28.Sener G, Toklu H, Kapucu C, et al. Melatonin protects against oxidative organ injury in a rat model of sepsis. Surg Today 2005; 35: 52–59. [DOI] [PubMed] [Google Scholar]
- 29.Carrillo-Vico A, Lardone PJ, Naji L, et al. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J Pineal Res 2005; 39: 400–408. [DOI] [PubMed] [Google Scholar]
- 30.Lowes DA, Webster NR, Murphy MP, et al. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth 2013; 110: 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paskaloğlu K, Sener G, Kapucu C, et al. Melatonin treatment protects against sepsis-induced functional and biochemical changes in rat ileum and urinary bladder. Life Sci 2004; 74: 1093–1104. [DOI] [PubMed] [Google Scholar]
- 32.Kerman M, Cirak B, Ozguner MF, et al. Does melatonin protect or treat brain damage from traumatic oxidative stress? Exp Brain Res 2005; 163: 406–410. [DOI] [PubMed] [Google Scholar]
- 33.Horáková L, Ondrejicková O, Bachratá K, et al. Preventive effect of several antioxidants after oxidative stress on rat brain homogenates. Gen Physiol Biophys 2000; 19: 195–205. [PubMed] [Google Scholar]
- 34.Ozsüer H, Görgülü A, Kiriş T, et al. The effects of memantine on lipid peroxidation following closed-head trauma in rats. Neurosurg Rev 2005; 28: 143–147. [DOI] [PubMed] [Google Scholar]
- 35.Fulia F, Gitto E, Cuzzocrea S, et al. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: reduction by melatonin. J Pineal Res 2001; 31: 343–349. [DOI] [PubMed] [Google Scholar]
- 36.Gitto E, Karbownik M, Reiter RJ, et al. Effects of melatonin treatment in septic newborns. Pediatr Res 2001; 50: 756–60. [DOI] [PubMed] [Google Scholar]
- 37.Sahib AS, Al-Jawad FH, Alkaisy AA. Effect of antioxidants on the incidence of wound infection in burn patients. Ann Burns Fire Disasters 2010; 23: 199–205. [PMC free article] [PubMed] [Google Scholar]
- 38.Saniova B, Drobny M, Lehotsky J, et al. Biochemical and clinical improvement of cytotoxic state by amantadine sulphate. Cell Mol Neurobiol 2006; 26: 1475–1482. [DOI] [PubMed] [Google Scholar]
- 39.Varona MA, Soriano A, Aguirre-Jaime A, et al. Risk factors of hepatocellular carcinoma recurrence after liver transplantation: accuracy of the α-fetoprotein model in a single-center experience. Transplant Proc 2015; 47: 84–89. [DOI] [PubMed] [Google Scholar]