Short abstract
Tumour necrosis factor (TNF)-α inhibitors are highly used in Romania for the treatment of autoimmune disorders, such as rheumatoid arthritis (RA), psoriasis, inflammatory bowel diseases, and ankylosing spondylitis. Biological therapy using TNF-α inhibitors is very effective but is associated with an increased risk of opportunistic infections, including active tuberculosis. Here, two cases are presented of patients with RA and psoriasis under biological therapy who developed very aggressive forms of disseminated tuberculosis, with a rapid progression to death. The authors conclude that patients undergoing biological therapy require thorough evaluation prior to initiating treatment, followed by continuous and rigorous monitoring by a multidisciplinary team during biological treatment, particularly in countries with a high incidence of tuberculosis.
Keywords: Rheumatoid arthritis, psoriasis, TNF-α inhibitors, lethal disseminated tuberculosis
Introduction
The use of tumour necrosis factor (TNF)-α inhibitor therapy in patients diagnosed with autoimmune diseases, such as rheumatoid arthritis (RA) and psoriasis, has proven highly effective in suppressing the pathologic inflammation responsible for specific symptoms and joint damage, but is associated with a decrease in host defence against opportunistic infections, including active tuberculosis (TB).1–4 All anti-TNF drugs are associated with an increased risk of TB reactivation in patients positive for latent TB infection, with a lower risk associated with etanercept,5,6 and a consistent number of cases have also been reported from countries with a low TB incidence (less than 20 per 100 000 population per year).7 Cases of tuberculosis have been described in patients with known RA even before starting biological therapy, particularly in countries with high incidence of TB.8 Only a few cases with rapid progression to death due to disseminated forms of TB have been reported to date, however.9,10 Romania has a relatively high incidence of TB (70 per 100 000 inhabitants),11 and like other countries, biological treatment is frequently used, thus, monitoring of the adverse effects of this therapy must be very rigorous, multidisciplinary, and well established in active national guidelines. The tuberculin skin test (Statens Serum Institut, Copenhagen, Denmark) and QuantiFERON-TB Gold in-tube assay (Cellestis Ltd, Carnegie, Victoria, Australia) are used in Romania to screen for latent TB infection.
Here, two cases are presented of patients with RA and psoriasis, treated using biological therapy, who developed very aggressive forms of disseminated tuberculosis. These cases are presented due to the unusual clinical features, unexpected severity, and fast progression to death.
Case reports
Approval to publish the present findings was provided by the Ethics committee of the Clinical Pneumology Hospital of Constanta (Case 1) and the Ethics committee of the Clinical Infectious Diseases Hospital of Constanta (Case 2) and each patient provided written informed consent on admission to hospital.
Case 1
A 72-year-old female patient was admitted to the First Internal Medicine Department of Constanta County Clinical Emergency Hospital in December 2015, for fever, chills, severe asthenia, productive cough, polyarthralgia, with symptomatology that had worsened over the previous 3 days. Medical history revealed arterial hypertension dating from 2010 and stage III seropositive RA since November 2004. The patient’s RA had been initially treated with 15 mg methotrexate, oral, weekly and anti-inflammatory drugs (500 mg naproxen with 20 mg esomeprazole as gastric protection, both oral, twice daily). From June 2015, because of highly active disease (Disease Activity Score 28 for Rheumatoid Arthritis using C-reactive protein [DAS28-CRP], 7.79), 40 mg adalimumab, subcutaneous, every other week, was added to the regimen. Before the initiation of adalimumab, chest X-ray was normal, tuberculin skin test (Statens Serum Institut) and QuantiFERON-TB Gold in-tube test (Cellestis) were negative, and enzyme-linked immunosorbent assay for human immunodeficiency virus (HIV) was negative. Following six months of adalimumab therapy, the patient was admitted into the hospital in a conscious state with normal body mass index (BMI, 21.1 kg/m2), fever (38.6 °C), joints with specific deformities only, normal lung examination, SpO2 of 97%, blood pressure at 120/70 mmHg, heart rate of 100 beats per min, and an enlarged abdomen with ascites. Biological tests showed normal white blood cell (WBC) count (5.910 x 103 cells/µl), mild chronic inflammatory anaemia (haemoglobin, 9.6 g/dl; haematocrit, 30.1 %) and systemic inflammatory syndrome (erythrocyte sedimentation rate [ESR], 36 mm/h; fibrinogen, 512 mg/dl). Bacteriological examination of blood culture was sterile and sputum samples were negative for pyogenic bacteria, fungi, and acid-fast bacillus. Standard chest X-ray (Figure 1) showed multiple micronodular and nodular pale opacities, disseminated in both lungs. The investigations were extended using thoracic computed tomography (CT) and revealed a bronchopneumonic aspect, mediastinal lymphadenopathy (between 12 and 20 mm), and straight lymph node calcifications (Figure 2). Antibiotic and antifungal treatment was initiated, comprising 1 g ceftazidinum twice daily and 400 mg/250 ml moxifloxacin once daily, plus 200 mg fluconazole once daily, all administered intravenously (i.v.). On day 4 of treatment, ceftazidinum was replaced with 1 g meropenem i.v., three times daily, and the treatment continued for a further 10 days, while previous treatment remained the same for the entire treatment duration. This treatment regimen was without good patient progression. Abdominal ultrasound, performed on the first day of hospitalization and repeated on day 5 due to poor patient progression, indicated splenomegaly with heterogeneous structure and multiple hypoechogenic formations, and gross ascites, confirmed by abdominal CT (Figure 3). Exploratory paracentesis revealed the presence of an exudate with protein (3.7 g/dl), lactate dehydrogenase (864 U/l), and increased adenosine deaminase (ADA; 93 U/l). At this hospitalization, the repeated QuantiFERON-TB Gold test was positive. Due to the risk of TB infection secondary to biological therapy, enforced by the patient’s lung images and ADA value, a complete range of bacteriological tests were performed. Microscopic examination of sputum was negative for acid-fast bacilli. The rapid detection of Mycobacterium tuberculosis in liquid culture from sputum specimens and ascites liquid (tested using the BD BACTEC™ MGIT™ automated mycobacterial detection system; Becton Dickinson, Sparks, MD, USA), which were both positive after 14 days of liquid culture, confirmed the diagnosis of disseminated secondary tuberculosis, with multiple organ involvement (pulmonary, peritoneal and possibly splenic). Consequently, the patient was transferred to the Pulmonology Department, Constanta County Clinical Emergency Hospital, to undergo appropriate treatment. Due to corroboration between clinical and paraclinical factors, treatment against TB had been initiated prior to having bacteriological confirmation from sputum and ascites samples in solid culture on Lowenstein-Jensen medium, which became positive after 40 days (positive culture with 1–30 colonies of M. tuberculosis, antigen MPT64 positive, fully sensitive to all TB drugs, in sputum and [++] in ascites liquid). The first standard regime of treatment, in accordance with the National Programme for Prevention, Monitoring and Control of Tuberculosis,11 was initiated with: 5 mg/kg/day isoniazid, 10 mg/kg/day rifampicin, 30 mg/kg/day pyrazinamide and 25 mg/kg/day ethambutol, all intravenous, in perfusion (approximately 30–40 min per drug), all administrated in the morning. In addition, 250 mg pyridoxine, and 20 mg esomeprazole, both oral, daily, were added. Progression was quickly and constantly unfavourable, with additional complications: upper digestive bleeding (exteriorized by haematemesis) and multiple organ failures (respiratory, kidney and liver). The patient finally died following 7 days of standard TB treatment and 21 days of hospitalization, despite all medical efforts. The family did not consent to an autopsy.
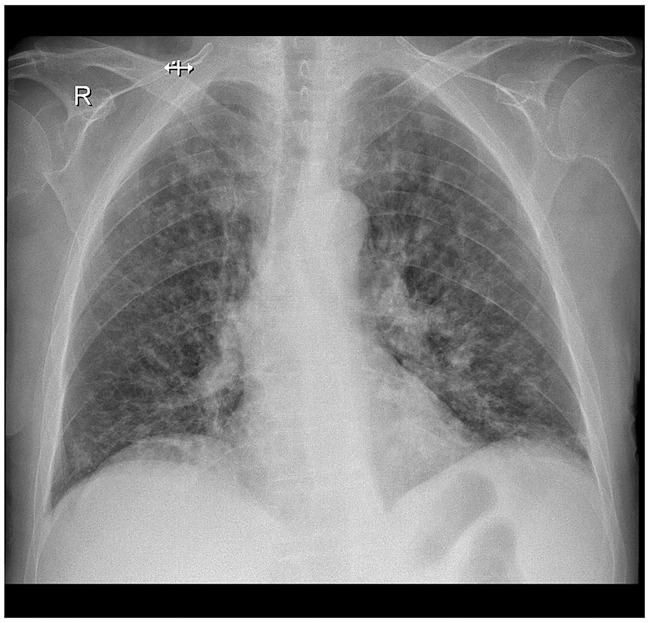
Figure 1.
Chest X-ray from a 72-year old female patient with rheumatoid arthritis, obtained following 6 months of treatment with 40 mg/day adalimumab, every 2 weeks: X-ray shows multiple pale nodular opacities disseminated bilaterally, particularly in the upper two thirds of the thorax
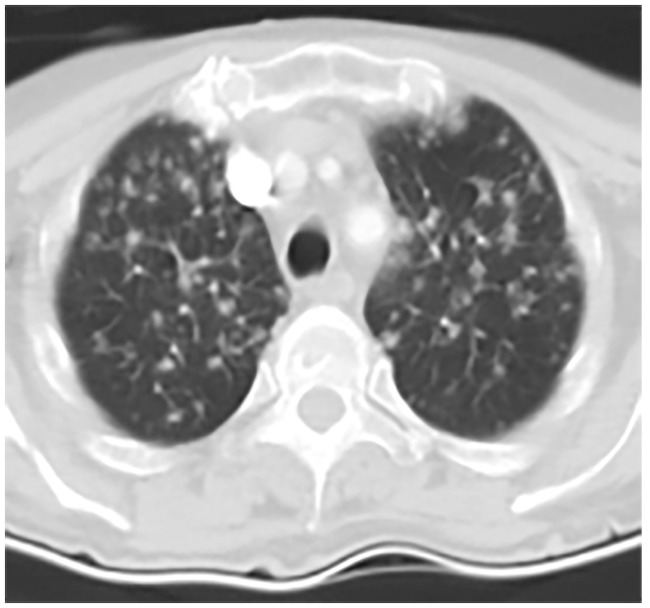
Figure 2.
Thoracic computed tomography (CT) image from a 72-year old female patient with rheumatoid arthritis, obtained following 6 months of treatment with 40 mg/day adalimumab, every 2 weeks: CT image shows bronchopneumonic aspect, mediastinal lymphadenopathies between 12 and 20 mm, and straight lymph node calcifications
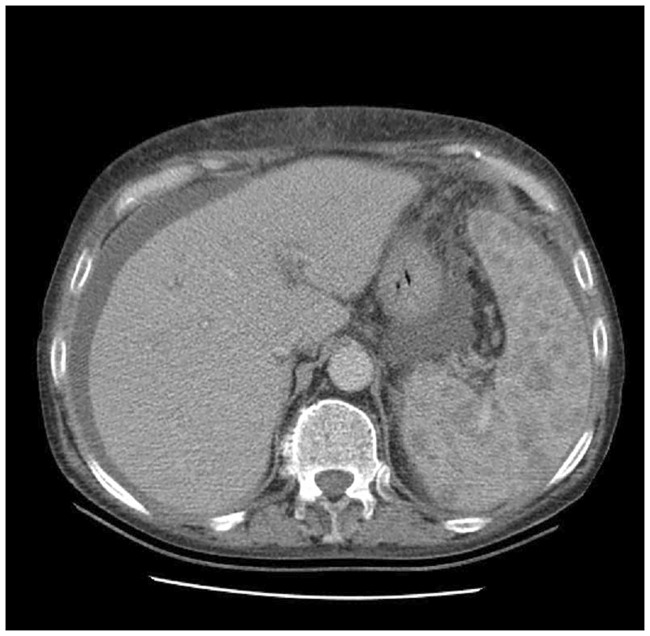
Figure 3.
Abdominal computed tomography (CT) image from a 72-year old female patient with rheumatoid arthritis, obtained following 6 months of treatment with 40 mg/day adalimumab, every 2 weeks: CT image shows ascites, and a heterogeneous spleen with multiple hypoechogenic formations
Case 2
A 65-year-old female patient was admitted to the Cardiology Department of Constanta County Clinical Emergency Hospital in February 2016, for headache, dizziness and thoracic pain with suspicion of acute myocardial infarction. This patient presented with a history of essential stage III arterial hypertension (with very high additional risk) since 2002, diabetes mellitus with nephroangiopathy and polyneuropathy treated with oral antidiabetes medications since 2006, and severe chronic psoriasis since 2009. In the previous three months the patient had received treatment for severe psoriasis with psoriatic arthritis (Psoriasis Area and Severity Index score, 23.2) comprising an anti-TNF-α agent: three doses of 5 mg/kg infliximab, i.v. (at weeks 0, 2, and 6; last dose received in December 2016), according to an approved RA protocol.12 Physical examination did not reveal pathological signs. Biological tests were without significant or specific abnormalities: normal haemogram, fibrinogen, alanine transaminase, aspartate aminotransferase, creatine kinase-muscle/brain, creatinine, and urea, but elevated ESR (68 mm/h), blood glucose (195 mg/dl) and troponin level (199.2 µg/l). Myocardial infarction was excluded, but the patient’s general condition deteriorated during hospitalization, the level of consciousness decreased, and fever with signs of meningeal irritation appeared five days following hospital admission. She was diagnosed with acute meningoencephalitis with a Glasgow coma scale score of 6 and was admitted to the Intensive Care Unit. Analyses of cerebrospinal fluid (CSF) revealed: intense positive Pandy’s reaction (++++), 235 cells/µl (85% polymorphonuclear leukocytes and 15% lymphocytes), increased albumin (132 mg/dl), hypoglycorrhachia (glucose, 0.31 g/l) and hypochlorrhachia (chloride, 9.20 µmol/l [6.35 g/dl]). Bacteriological examination showed the CSF to be positive for acid-fast bacillus (Ziehl-Neelsen staining), positive for TB nucleic acid using GeneXpert (Cepheid, Sunnyvale, CA, USA) with no detection of rifampin resistance, and positive for M. tuberculosis in liquid culture, tested using the BD BACTEC™ MGIT™ system. Chest X-ray showed a miliary TB pattern (not shown). The patient was confirmed as a new case of disseminated TB in a patient recently treated with infliximab for psoriasis. Specific anti-TB chemotherapy with four first-line drugs, as in the previous case (5 mg/kg/day isoniazid, 10 mg/kg/day rifampicin, 30 mg/kg/day pyrazinamide and 25 mg/kg/day ethambutol), oral administration, was started. Three days following initiation of treatment for TB, the patient presented upper digestive haemorrhage exteriorized by melena. Despite all aggressive treatment, the patient died with cardiorespiratory failure after 16 days of hospitalization.
Discussion
Incidence of RA in Romania is approximately 1% of the general population, and incidence of psoriasis is approximately 2%.12 In these population groups, the prevalence of latent TB infection remains unknown. In patients diagnosed with RA and psoriasis, TNF-α blockers are initiated only in patients who are unresponsive to conventional treatment, and after careful exclusion of latent TB infection.2,3,12,13 Romanian guidelines stipulate that prior to administration of biological treatment, the patient should follow an algorithm to identify latent TB infection, and chemoprophylactic treatment should be administered for 9 months in patients who have a positive tuberculin skin test, (tuberculin skin test, ≥ 10 mm, and without signs of active disease).11 Despite pretreatment evaluation, it is well known that the use of TNF-α inhibitor therapy is associated with an increased risk of active TB.12–15 The current report presents the cases of two female patients, both over 65 years old, with a long history of RA and psoriatic arthritis, who were diagnosed with TB after starting biological treatment, despite both being confirmed negative for latent TB infection prior to initiation of treatment. Neither of the cases had been recently exposed to TB, making it difficult to support an exogenous reinfection mechanism for TB. The possibility of a false-negative tuberculin skin test and negative QuantiFERON-TB Gold assay need to be taken into consideration, due to immunosuppression caused by disease or chronic anterior immunosuppressive therapy,16,17 making the reactivation of post-primary TB lesions more feasible. Moreover, use of the tuberculin skin test is more controversial for a patient with psoriasis due to the possibility of over-diagnosis or ambiguous results.18 Despite the fact that in many national guidelines the tuberculin skin test remains the first-line tool to screen for latent TB infection in patients with psoriasis, it is recommended that the QuantiFERON-TB Gold test is also performed, either before or on the same day as the Mantoux test, to increase the accuracy of diagnosis.18 Unusual clinical features in adults with disseminated pulmonary and extrapulmonary TB (peritoneal and meningitis), may delay diagnosis for 5 to 15 days, leading to an increased risk of mortality (authors’ personal experience). In Romania, meningitis is more frequently observed as a complication of primary TB in infants and is a very rare complication in adults.19 Knowing the paucibacillary character of serositis, the present authors consider that the positive acid-fast bacillus smear for CSF (case 2) and positive GeneXpert and liquid culture for M. tuberculosis for ascites (case 1) reflect the severity of the disease. Published studies report a positive rate for acid-fast bacillus smear in TB meningitis of between 37 and 58%.20,21 For both cases reported in the current study, death was considered to be related to disseminated TB, and it is unclear whether the occurrence of superior digestive haemorrhage aggravated the patients’ prognosis. The anti-TB treatment cannot be directly associated with this haemorrhage complication, as administration was intravenous in case 1, and the complication preceded the start of treatment in case 2.
Medical professionals should be aware of the unusual pattern of TB in patients undergoing biological treatment.22 For example, extrapulmonary or disseminated tuberculosis are more frequently observed in patients treated with TNF-α inhibitors.7,23 A review of 70 cases of TB that developed after treatment with infliximab showed an increased proportion of extrapulmonary TB (56% versus 18%) and disseminated diseases (18% versus 2%), when associated with a severe immunosuppression status (including HIV positivity).22 The four deaths out of 70 patients in this study were related to TB,22 however, in patients receiving biological treatment, very few deaths related to TB have been reported in the literature.9,10
The number of TB cases has been reported in the literature to be higher for infliximab than adalimumab,22,24 however, two studies (one French and one English) reported that the incidence of TB was higher for adalimumab.5,6 The interval from starting anti-TNF-α treatment until TB onset was 6 months in the present first case (treated with adalimumab), which was shorter than in a published study reporting median time to TB onset of 18.5 months for 11 patients treated with adalimumab,6 and was more comparable with timeframes reported for patients treated with infliximab.3,22,25 Clinical deterioration despite specific anti-TB treatment has been reported in cases of RA treated with adalimumab, due to a paradoxical reaction involving the lungs, with acute respiratory failure attributed to the recurrence of disease in the absence of biological treatment.23,26,27 The onset of paradoxical reaction is considered one month after TB diagnosis and treatment.23,27 In the present cases, it is difficult to maintain that death was attributed to a paradoxical reaction, because it occurred soon after starting the anti-TB treatment (7 days for case 1, and 3 days for case 2), but after 4 weeks from the last dose of adalimumab and 8 weeks from the last dose of infliximab. Unfortunately, procalcitonin level, a possible diagnostic and prognosis marker for paradoxical reaction,23 was not tested in either case. The moment of restarting therapy with TNF-α inhibitors during or following the end of anti-TB treatment differs between guidelines. It is possible to restart after 2 months,28 but it is mandatory to pay close attention to TB evolution during follow-up.29
In conclusion, because of the high endemic value of TB in Romania,30 and given the severe forms presented by these cases, there is ongoing discussion as to whether or not chemoprophylaxis with isoniazid should be provided in all cases before anti TNF-α treatment, even if the first screening excludes latent TB infection. In addition, old age, female sex, long history of autoimmune disease, and association of other comorbidities, such as diabetes, digestive haemorrhage, and HIV infection, could be risk factors for developing disseminated and lethal forms of TB during biological treatment with infliximab and adalimumab. Etanercept may be a more beneficial anti-TNF-α treatment given its relatively low incidence rate of TB, for patients in which this therapy is mandatory.31
In cases at high risk of TB reactivation, the choice of a non-anti-TNF targeted biologic is advisable.31
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Ehlers S. Role of tumor necrosis factor (TNF) in host defence against tuberculosis: implications for immunotherapies targeting TNF. Ann Rheum Dis 2003; 62 (Suppl 2): ii37–ii42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuna AA, Megeff C. New drugs for the treatment of rheumatoid arthritis. Am J Health Syst Pharm 2000; 57: 225–234. [DOI] [PubMed] [Google Scholar]
- 3.Galloway JB, Hyrich KL, Mercer LK, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011; 50: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X, Li F, Chen JW, et al. Risk of tuberculosis infection in anti-TNF-a biological therapy: from bench to bedside. J Microbiol Immunol Infect 2014; 47: 268–274. [DOI] [PubMed] [Google Scholar]
- 5.Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum 2009; 60: 1884–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis 2010; 69: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantini F.Nannini C.Niccoli L, et al. Risk of tuberculosis reactivation in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis receiving non-anti-TNF-targeted biologics. Mediators Inflamm 2017; 8909834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arghir OC, Niţu M, Trenchea M, et al. Progressive intraparenchymal lung nodules dissemination in a heavy smoker and seropositive rheumatoid arthritis suspected of tuberculosis relapse. Rom J Morphol Embryol 2013; 54: 659–663. [PubMed] [Google Scholar]
- 9.Hama M, Yamazaki Y, Kosaka M, et al. Fulminant pulmonary tuberculosis by infliximab in patient with rheumatoid arthritis. Mycobact Dis 2016; 6: 206. doi:10.4172/2161-1068.1000206. [Google Scholar]
- 10.Moyer TM, Groh B. Disseminated tuberculosis after treatment with infliximab. Hospital Physician 2006; 42: 47–51. [Google Scholar]
- 11.Arghir OC, Chiotan DI, Cioran NV ,; . Methodological guide to implementing the national program for prevention, monitoring and control of tuberculosis, 21 September 2015. Issued by the Ministry of Health. Official Monitor no. 748, 7 October 2015 [In Romanian].
- 12.Order MS - CNAS no. 1463/1036/2016 amending and supplementing Annex no. 1 to MS-CNAS Order no. 1301/500/2008 for the approval of the therapeutic protocols on the prescription of drugs corresponding to the frequent international names listed in the list of frequent international names corresponding to the drugs that insured persons have access to, with or without personal contribution, based on their prescription. http://amfms.ro/ordinul-mscnas-146310362016-privind-modificarea-si-completarea-anexei-nr-i-la-ordinul-13015002008-pentru-aprobarea-protocoalelor-terapeutice/ [In Romanian].
- 13.Ledingham J, Deighton C. Update on the British Society for Rheumatology guidelines for prescribing TNFα blockers in adults with rheumatoid arthritis (update of previous guidelines of April 2001). Rheumatology (Oxford) 2005; 44: 157–163. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC). Tuberculosis associated with blocking agents against tumor necrosis factor-alpha, California, 2002–2003. MMWR Morb Mortal Wkly Rep 2004; 53; 683–686. [PubMed] [Google Scholar]
- 15.Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect Dis 2003; 3: 148–155. [DOI] [PubMed] [Google Scholar]
- 16.Keystone EC, Papp KA, Wobeser W. Challenges in diagnosing latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. J Rheumatol 2011; 38: 1234–1243. [DOI] [PubMed] [Google Scholar]
- 17.Coaccioli S, Di Cato L, Marioli D, et al. Impaired cutaneous cell-mediated immunity in newly diagnosed rheumatoid arthritis. Panminerva Med 2000; 42: 263–266. [PubMed] [Google Scholar]
- 18.Balato N, Di Constanzo L, Ayala F, et al. Psoriatic disease and tuberculosis nowadays. Clin Dev Immunol 2012; 2012: 747204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard JM. Central nervous system tuberculosis. UpToDate https://www.uptodate.com/contents/central-nervous-system-tuberculosis#! (2018, accessed 29 April 2017)
- 20.Kennedy DH, Fallon RJ. Tuberculous meningitis. JAMA 1979; 241: 264–268. [PubMed] [Google Scholar]
- 21.Thwaites GE, Chau TT, Farrar JJ. Improving the bacteriological diagnosis of tuberculous meningitis. J Clin Microbiol 2004; 42: 378–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha–neutralizing agent N Engl J Med 2001; 345: 1098–1104. [DOI] [PubMed] [Google Scholar]
- 23.Wallis RS, van Vuuren C, Potgieter S. Adalimumab treatment of life-threatening tuberculosis. Clin Infect Dis 2009; 48: 1429–1432. [DOI] [PubMed] [Google Scholar]
- 24.Seong SS, Choi CB, Woo JH, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol 2007; 34: 706–711. [PubMed] [Google Scholar]
- 25.Brassard P, Kezouh A, Suissa S. Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis 2006; 43: 717–722. [DOI] [PubMed] [Google Scholar]
- 26.Salgado E, Gomez-Reino JJ. The risk of tuberculosis in patient treated with TNF antagonists. Expert Rev Clin Immunol 2011; 7: 329–340. [DOI] [PubMed] [Google Scholar]
- 27.Garcia Vidal C, Rodriguez Fernández S, Martinez Lacasa J, et al. Paradoxical response to antituberculous therapy in infliximab-treated patients with disseminated tuberculosis. Clin Infect Dis 2005; 40: 756–759. [DOI] [PubMed] [Google Scholar]
- 28.Aslanidis S, Pyrpasopoulou A, Douma S, et al. Is it safe to readminister tumor necrosis factor alpha antagonists following tuberculosis flare? Arthritis Rheum 2008; 58: 327–328. [DOI] [PubMed] [Google Scholar]
- 29.Perlmutter A, Mittal A, Menter A. Tuberculosis and tumour necrosis factor-alpha inhibitor therapy: a report of three cases in patients with psoriasis. Comprehensive screening and therapeutic guidelines for clinicians. Br J Dermatol 2009; 160: 8–15. [DOI] [PubMed] [Google Scholar]
- 30.Nitu FM, Olteanu M, Streba CT, et al. Tuberculosis and its particularities in Romania and worldwide. Rom J Morphol Embryol 2017; 58: 385.–392. [PubMed] [Google Scholar]
- 31.Cantini F, Nannini C, Niccoli L, et al. Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun Rev 2015; 14: 503–509. [DOI] [PubMed] [Google Scholar]