Abstract
Background:
A critical agenda for NIH’s Research Domain Criteria (RDoC) initiative is establishing whether domains within the RDoC matrix are truly transdiagnostic. Rates of anxiety disorders are elevated in autism spectrum disorder (ASD), but it is unclear whether the same mechanisms contribute to anxiety in those with and without ASD. As changes in selective attention are a hallmark of anxiety disorders in non-ASD samples, the identification of these changes in ASD would support the transdiagnostic nature of anxiety.
Methods:
This functional MRI study focused on the Negative Valence domain from RDoC (manifest as anxiety symptoms) in youth with ASD (N = 38) and typically developing control (TDC) participants (N = 25). The task required selective attention toward and away from social information (faces) with negative and neutral affect. Participants underwent in-depth characterization for both anxiety and ASD symptoms.
Results:
Dimensional and categorical measures of anxiety were significantly related to increased amygdala activation – evidence of enhanced attentional capture by social information.
Conclusions:
This pattern fits with decades of research among non-ASD samples using selective attention and attentional bias paradigms, suggesting that anxiety in ASD shares mechanisms with anxiety alone. Overall, results from this study support the transdiagnostic nature of the Negative Valence domain from RDoC, and increase the likelihood that anxiety in ASD should be responsive to interventions targeting maladaptive responses to negative information.
Keywords: autism spectrum disorder, anxiety disorders, amygdala, selective attention, Research Domain Criteria, negative valence
Introduction
Although anxiety disorders constitute their own diagnostic grouping within the Diagnostic and Statistical Manual (1), anxiety symptoms occur in varying levels across normal and abnormal psychology – as reflected by the Negative Valence dimension of NIH’s Research Domain Criteria (RDoC; 2). The Fear (Acute Threat) construct within the RDoC Negative Valence dimension, defined as a “defensive motivational system to promote behaviors that protect the organism from perceived danger” (https://www.nimh.nih.gov/research-priorities/rdoc/negative-valence-systems-workshop-proceedings.shtml), manifests clinically as symptoms of anxiety*. Robust dimensional models of anxiety and negative valence (or affect) actually precede RDoC by decades (in particular, see the tripartite model of anxiety and depression by Clark, Watson, and colleagues; 3–5). And yet, the recent emergence of RDoC brings renewed focus onto the strengths and weaknesses of categorical and dimensional approaches to psychopathology (6, 7).
The case of anxiety among individuals with autism spectrum disorder (ASD) illustrates the problems that can arise when dimensional models intersect categorical ones. Estimated rates of co-occurring anxiety disorders in ASD exceed 40% (well above population norms; 8–11). Many individuals with ASD present with symptoms of anxiety that fall clearly within DSM diagnostic criteria. However, individuals with ASD also present with symptoms that are best described as anxiety, but focused on themes that are rare in non-ASD populations. For example, individuals with ASD frequently present with highly unusual specific phobias (e.g., the sound of a toilet flushing, specific songs on the radio), generalized anxiety surrounding unusual themes (in particular, minor changes in the order of daily events and activities), and social anxiety without fear of negative evaluation (for review see 8, 12). These symptoms do fit within many prevailing models of anxiety (e.g., the negative affect and hyperarousal dimension of Clark and Watson’s tripartite model; 3-5), but their absence outside of ASD complicates the premise that they fall on the same continuum as anxiety symptoms generally.
This experiment tests whether individuals with ASD share cognitive profiles that are known to relate to anxiety in the typically developing population – specifically, abnormal selective attention for social and emotional information. Selective attention refers to the capacity to focus on specific information in the environment, while diminishing attention paid to irrelevant information. Selective attention is closely associated with fear, and is therefore integral to the Negative Valence domain of RDoC.
Deficits in selective attention are considered to be part of the etiology of anxiety disorders, and to predispose anxious individuals towards threats in the environment that would otherwise be disregarded – both initiating and perpetuating anxiety symptoms (13–17). However, almost all of the data we have on the selective attention/anxiety relationship come from typically developing samples. The present study focuses on visual selective attention in ASD – specifically, responses to irrelevant social and emotional information occurring outside of one’s attentional focus. Amygdala is widely considered to be a major brain structure in attentional orienting toward emotional information, and may in fact have privileged access to input from very early in the sensory information processing stream (18–20; though see (21) for alternative accounts). Until fairly recently, the prevailing view has been that ASD is associated with decreased amgydala activation, which in turn has been related to diminished social and emotional information processing (for reviews see 22–24). However, this view has generally failed to explain why anxiety – associated with increased amygdala function – occurs so frequently in ASD. The present results add to a growing number of studies suggesting that prior perspectives on diminished amygdala function and social deficits in ASD that fail to consider the role of anxiety are at best incomplete.
Among typically developing individuals, anxiety disorders have been consistently associated with increased attentional capture by emotional stimuli (15–17, 25), which have, in turn, been associated with increased amygdala activity (26–30). One widely used paradigm in this area, developed by Vuilleumier and colleagues (31–33), involves the simultaneous presentation of pairs of faces and non-face objects (e.g., houses; see Fig. 1). Participants are asked to make a same/different identity judgment on either the faces or the houses (varying from trial to trial), while ignoring the other stimuli on the screen. The faces present with either a neutral or negative expression (also varying from trial to trial) – either fear or anger (see 34 for a discussion of similarities and differences between these two facial emotions in selective attention tasks). The seminal finding from this paradigm was that amygdala activity was increased for negative faces regardless of whether participants were carrying out the same/different judgment for the faces or the houses on the screen. This has been considered evidence that amygdala responsiveness to emotional information is at least partially obligatory – i.e., independent of visual selective attention (19).
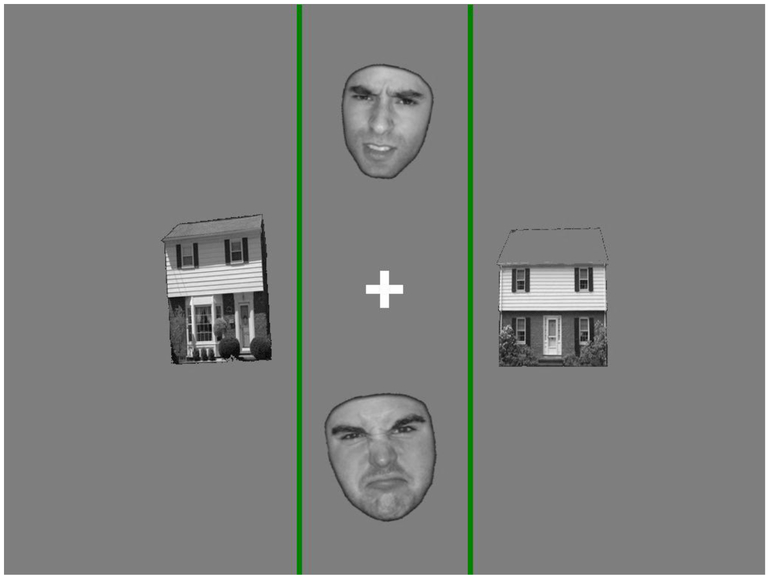
Figure 1.
Selective attention task stimuli. Participants were asked to indicate, via button press, whether the two pictures in between the green lines represented the same person (or house), or not. Stimuli alternated between faces and houses as the to-be-attended picture type (following a block design).
The present study used fMRI to test the hypothesis that anxiety in the context of ASD is associated with the enhanced processing of peripherally presented social information, as would be predicted by the Negative Valence domain of RDoC. Although evidence in favor of this hypothesis could come from several different brain areas, we focus specifically on amygdala, due to the centrality of this structure in the etiology of both anxiety and ASD (for review see (35). The analytic approach follows RDoC in taking a dimensional approach to anxiety symptoms, while also using a categorical (diagnostic) approach to examine whether anxiety disorders in the context of ASD are related to similar mechanisms as in non-ASD anxiety samples (6).
Methods and Materials
Participants
This study enrolled 76 participants. Ten youth with ASD and three typically developing control (TDC) participants were excluded from the final sample for excessive motion and/or MRI image artifact (see motion criteria below), leaving 63 participants (ASD n=38, TDC n=25; mean age=12.76 years). Exclusionary criteria for children with ASD included any known genetic or neurological disorders, current mood or psychotic disorder, extreme premature birth (gestational age<32 weeks), or other significant medical condition impacting functioning. Although ASD participants were later categorized as having or not having an anxiety disorder, all participants were enrolled regardless of anxiety level. Exclusionary criteria for TDC participants included any known genetic, neurological, language, learning, or psychiatric disorder, premature birth, or first- or second-degree relative with ASD. 18 participants in the ASD group were taking either SSRIs for mood/anxiety symptoms, stimulants for attention deficits, or atypical antipsychotics (Abilify; no other categories of psychotropic medications were reported). No TDC participant were taking psychotropic medications. Informed consent was obtained from the parent or legal guardian of all individual participants included in the study.
Participants completed a multi-day assessment battery. ASD diagnoses were informed by the Autism Diagnostic Observation Schedule (ADOS) following ADOS-2 algorithms, administered by clinicians trained to research reliability (36, 37), and parent interview guided by results from the Social Communication Questionnaire – Lifetime (SCQ; 38). Anxiety disorder diagnoses were informed by the Anxiety Disorders Interview Schedule (ADIS-IV, administered to parents only; 39). Following ADIS-IV guidelines, all individuals who received an anxiety disorder diagnosis had a Clinical Severity Rating of >= 4 for their respective diagnoses. No other psychiatric conditions were formally assessed.
General intellectual ability was established via the Differential Abilities Scale – Second Edition (DAS-II; 40). The DAS-II provides a General Conceptual Ability (GCA) scaled score that is analogous to Full Scale IQ. Following Herrington and colleagues (41), the dimensions of anxiety and ASD symptoms were measured by the parent-report version of the Screen for Child Anxiety-Related Emotional Disorders (SCARED-P; 47), and the SCQ-Lifetime, respectively. The SCARED-P provides four clinical subdomain scores (panic/somatic symptoms, generalized anxiety, social anxiety, and separation anxiety) and a total score (the sum of the four clinical subdomains). Data from these instruments are summarized in Table 1.
Table 1.
Participant Characteristics
| Group | N(F) | Age | IQ(GCA) | ADOSComm | ADOSSocial | ADOSStereo | SCQTotal | SCAREDTotal | TaskAccuracy | Task RT |
|---|---|---|---|---|---|---|---|---|---|---|
| ASD+Anxiety | 24(6) | 13.08(2.41) | 99.83(16.59) | 2.23(1.41) | 6.55(1.82) | 1.75(1.12) | 27.08(11.24) | 21.67(4.63) | 0.73(0.1) | 1608.95(255.08) |
| ASD_Alone | 14(6) | 13.12(2.84) | 104.5(22.26) | 3.14(1.46) | 7.18(2.36) | 2.27(1.56) | 22.43(5.2) | 9.5(7.57) | 0.74(0.11) | 1697.31(150.46) |
| TDC | 25(10) | 12.25(2.78) | 112.16(17.22) | 0.55(0.6) | 2(1.66) | 0.35(0.61) | 6.12(5.79) | 1.6(1.68) | 0.79(0.08) | 1734.35(210.43) |
Note. Except for the N column (group sample size with number of female participants in parentheses), all data are presented as M(SD). GCA: General Conceptual Ability (40). ASD + Anxiety: the ASD+Anxiety Group. ADOS: Autism Diagnostic Observation Schedule (following ADOS-2 algorithms), with data for the Communication, Social and Stereotypical Behavior subscales (36). SCARED-P: Screen for Child Anxiety Related Emotional Disorders, parent report (42). SCQ: Social Communication Questionnaire, a measure of core ASD symptoms (38). The three groups were matched on age, F(2,60)=.77, p=.47, task accuracy, F(2,56)=2.10, p=.134, and task response time, F(2,52)=1.80, p = .175.
Experimental Design
During fMRI scanning, participants completed a modified version of Vuilleumier and colleagues’ visual attention task (31–33). This task involves the simultaneous presentation of two faces and houses (Fig. 1). Participants were asked to attend either to the faces or the houses (depending on the trial), and indicate via keypad if they represented the same or different person/house. Stimuli followed a block design, where blocks varied in whether angry or neutral facial expressions were presented, and whether the faces or the houses were to be attended for the same/different judgment (see Supplementary Material for more information on the selection of angry versus fearful faces; the latter were used in the original studies by Vuilleumier et al).
Several modifications were made to the task to ensure that it could be completed across developmental levels. First, the spatial location of the to-be-attended picture pairs was held constant across the entire experiment – the top and bottom of the screen (in the original task, the attended location varied from trial to trial). Second, to make the location of the to-be-attended stimuli even clearer, two green lines were placed adjacent to them. Finally, whereas the original task involved rapid stimulus presentation (i.e., <=250ms), this duration was increased to 2000ms (see below for discussion of stimulus duration and eye gaze). See Supplementary Information for further paradigm parameters.
Most prior iterations of this task used rapid stimulus presentation time so that participants would have insufficient time to saccade to any of the four stimuli, remaining centrally fixated. However, this increases attentional demands by making the task very rapid. We chose to increase the presentation duration to 2000ms and record simultaneous eyetracking data (using the iViewX system; Sensorimotoric Instruments [SMI], Boston, MA). Within-MRI eyetracking poses many technical challenges; we were able to successfully record eyetracking data for 50 participants (29 ASD, 21 TDC). Eyetracking data were analyzed using the SMI program Begaze and in-house software. See Supplemental Information for more detail regarding eyetracking analysis procedures.
MRI Data Acquisition, Reduction, and Analysis
Scan Parameters.
MRI data were collected on a Siemens Verio 3T scanner with a 12-channel head coil. FMRI data consisted of 218 gradient-echo echo-planar images (oblique axial orientation, TR=2110ms, TE=25ms, flip angle=60, 3.5×3.5×3.5mm with .35mm gap, corrected via gradient field map). Two structural MRI images were acquired to register fMRI data into standard (MNI) space – a FLASH sequence in the same plane as the fMRI data (TR=300ms, TE=2.46ms, flip angle=60, 0.9×0.9×3.5mm, .35mm gap), and a high-resolution MPRAGE sequence (TR=1900ms, TE=2.54ms, flip angle=9, 0.8×0.8×0.9mm).
Data Preprocessing and Time Series Analysis.
FMRI data were analyzed using the FMRIB Software Library (FSL; (43). Data were low-pass filtered (removing linear trends) and spatially smoothed (5mm gaussian kernel). Head motion was estimated and corrected using MCFLIRT (44). Data were placed into 2mm isotropic MNI space by merging affine transformation matrices (calculated via FLIRT; 44) between the following volumes: fMRI to FLASH, FLASH to MPRAGE, and MPRAGE to MNI. Participants with a maximum volume-to-volume displacement (“spikes”) greater than 1 voxel (3.5mm) were excluded from analyses. The three groups used in categorical analysis (discussed below) were matched on maximum spike magnitude, F(2,60)=.08, p=.93.
Analytic Approach.
Following prior research (34, 45), we predicted increased amygdala activation when participants attended to faces – i.e., the contrast of Attended Face (AF)>Unattended Face (UF – i.e., attended house). Based on evidence from anxiety disorder groups (34, 45), we predicted that this effect would diminish among individuals with higher levels of anxiety. In other words, the higher the level of anxiety symptoms, the greater the magnitude of amygdala activation for unattended faces (i.e., UF>AF). Contrast maps were calculated via general linear modeling at each intracerebral voxel, including explanatory variables (EVs) representing each unique condition (AF-Negative Faces, AF-Neutral Faces, UF-Negative Faces, and UF-Neutral faces), and post hoc contrasts of these EVs (AF>UF and UF>AF). Boxcar waveforms for each of these EVs were convolved with a double-gamma hemodynamic response function. Temporal derivatives of each EV were also included, to account for potentional variation in hemodynamic response timing. Lastly, motion parameters were included as nuisance EVs.
Dimensional Analyses.
The key hypothesis for this study was that anxiety in the context of ASD would be associated with increased attentional capture by peripherally presented social information – manifesting as increased amygdala activity. For both dimensional and categorical analyses, we relied on per-voxel as well as region of interest (ROI) approaches. The dimensional hypothesis of increased attentional capture by social information was tested using multiple regression predicting UF>AF maps from the SCARED-P total score, for all participants. For all per-voxel statistical maps, family-wise error (FWE) was controlled via permutation testing and Threshold-Free Cluster Enhancement, using the program randomise (46). FWE correction was implemented separately within our a priori brain area of interest (amygdala), and the rest of the brain’s gray matter. Bilateral amygdalae were segmented by creating a sample-specific structural MRI template (47, 48), running this template through FSL’s program FIRST (49), and dilating the result to allow for minor individual differences in amygdala boundaries.
To test the specificity of observed amydala activity, an ROI approach was used, extracting and averaging data within a 4 mm sphere around the location of the peak correlation between anxiety symptoms (SCARED-P total) and the UF>AF contrast. ROI activation was examined with respect to age, GCA, task performance, and eyetracking data, using multiple regression.
Categorical Analyses.
Based on ADIS-IV results, the overall ASD group (N=38) was subdivided into groups with at least one anxiety disorder (henceforth called the ASD+Anxiety group; N=24), and those with no anxiety disorder diagnosis (ASD Alone; N=14). Within the ASD+Anxiety group, there were 7 participants with generalized anxiety disorder, 3 participants with separation anxiety disorder, 7 participants with specific phobia, and 8 participants with anxiety disorder not otherwise specified (many participants had more than one anxiety disorder diagnosis). Per-voxel tests among the three groups (including TDC) were conducted using FSL’s FLAME (50). Per-voxel analyses followed a 3 (Group) x 2 (Attention: UF vs. AF) x 2 (Valence) ANOVA design. ROI analyses also followed this general design, but were tailored primary to rule out the effects of nuisance variable (GCA, eyetracking, etc.) on the ANOVA effects observed in per-voxel analyses.
Results
Dimensional Analyses
Per-Voxel Analyses.
As predicted, the entire sample showed bilateral amygdala activation when attending to faces (i.e., AF>UF; see Fig 2 Panel A). Simple effects tests indicated that this effect was significant for ASD+Anxiety, ASD Alone, and TDC groups. This pattern was also observed when looking at neutral and negative expression conditions separately, showing that the effect did not vary as a function of emotional valence. Although activation was observed in several additional clusters, only one survived FWE correction – a cluster in inferior frontal gyrus (peak coordinate=50,34,8, cluster size=253, peak p(corrected)=.012; see SI Fig. 1).
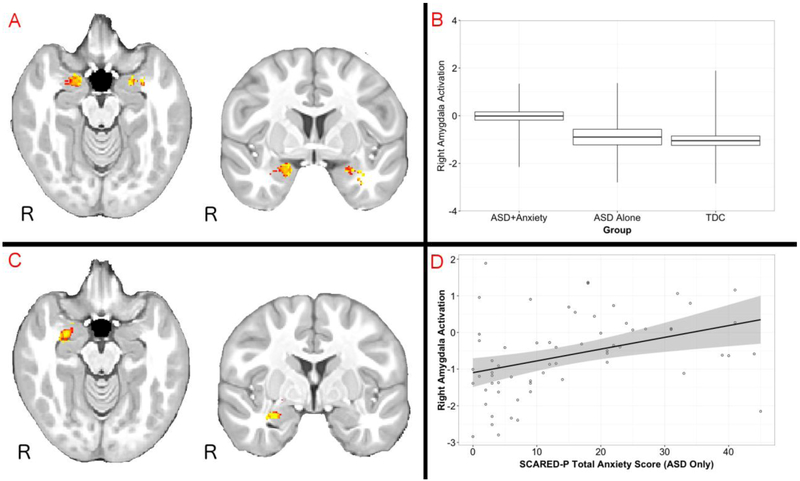
Figure 2.
Amygdala activation as a function of attention and anxiety. Panel A: Increased amygdala activation during the perception of attended versus unattended faces (AF > UF contrast) across all participants in the study. Panel B: Increased amygdala activation for UF > AF in the ASD + Anxiety group, demonstrating increased selective attention in this group for unattended faces. Panel C: per-voxel correlation map comparing the total score on the Screen for Child Anxiey-Related Emotional Disorders – Parent Version with activation for the UF > AF contrast (includes all participants). Panel D: a scatterplot of these data for the ASD group only (i.e., the ASD + Anxiety and ASD Alone groups). Panel D: Data are presented at a per-voxel threshold of p < .05 (corrected for multiple comparisons). FMRI maps are presented at a per-voxel threshold of p<.05 (corrected for multiple comparisons).
To examine the effect of anxiety across the entire study sample, multiple regression was conducted predicting UF>AF activity from the SCARED-P total score, across all participants. As predicted, these analyses revealed a portion of amygdala that was significantly correlated with anxiety symptoms (see Fig. 2 Panel C; peak coordinates=29,−13,−15, cluster size=37, peak p(corrected)=.032). In other words, within this right amygdala cluster, anxiety was associated with an increased propensity to respond to social information presented outside of the focus of visual attention.
Previous studies using this paradigm (namely, 34, 51, 52) have focused specifically on the relationship between anxiety and attentional effects for negative stimuli. Interestingly, when conducting the same analysis as above for negative faces only, no significant correlation was identified between anxiety scores and the AF>UF contrast at a corrected per-voxel threshold of p < .05.
ROI Analyses.
Post hoc correlation and multiple regression analyses were carried out to examine the specificity of amygdala activity for anxiety vis-à-vis other relevant clinical, demographic, and performance variables. Right amygdala activity was not significantly correlated with age (r=.07, p=.58), task accuracy (r=.14, p=.28), or task response time (r=.15, p=.27). However, right amygdala activity was significantly correlated with two clinical variables – GCA (r=.36, p<01), and ASD symptoms (SCQ Lifetime overall score; r=−.29, p=.02). Nevertheless, when both GCA and SCQ were entered into multiple regression predicting amygdala ROI activation, partial correlations between the SCARED-P and amygdala activity became more rather than less statistically significant. The negative correlation beween ASD symptoms (SCQ) and amygdala activationreplicates and extends findings from Herrington and colleagues using a separate sample (41).
In order of confirm that the observed per-voxel correlation was not driven entirely by differences between the ASD and TDC groups, a correlation between right amygdala activation for the UF>AF contrast was conducted for the ASD group only (including those with and without anxiety), and was significant; r=.38, p=.002 (see Fig. 2 Panel D). Because some variability was observed in amygdala activation across the range of SCARED-P values, a post hoc non-parametric correlation was run, and was also significant (Spearman’s Rho=45, p<.001).
Post hoc analyses examined which of the four SCARED-P symptom domains capured the most variance in the UF>AF contrast. Zero-order correlations including the entire sample were significant for panic/somatic (r=.34, p=.006), generalized anxiety (r=.44, p<.001), and social phobia subdomains (r=.33, p=.007), but not separation anxiety (r=.10, p=.42).
A final series of multiple regression analyses were conducted across the entire sample to examine whether the right amygdala/anxiety relationship was mediated by participant eye gaze. Eyetracking data confirmed that participants rarely fixated on unattended stimuli; from the total of 192 seconds of actual stimulus presentation time during the task, participants fixated on unattended stimuli for an average of only 5.3 seconds. For direct comparison with amygdala ROI data, fixations were summed for each participant separately for task-irrelevant stimuli for the AF and UF conditions across the entire task. Neither of these fixation scores were significantly correlated with right amygdala ROI activity (ps of .57 and .37, respectively) or with anxiety level (SCARED-P total correlation ps of .72 and .75, respectively). These results suggest that the relationship between anxiety and right amygdala activity for attended-face stimuli was unrelated to eye gaze patterns.
Categorical Analyses
Based on psychodiagnostic testing, the 63 study participants were placed into three groups – ASD+Anxiety, ASD Alone, and TDC. The three groups were matched on age, task accuracy, and task response time (see Table 1). The three groups showed a trend toward a significant difference in GCA, F(2,59)=2.79, p=.07 (GCA data were missing for 1 participant). Tukey’s Range Test indicated a trend toward greater GCA for the TDC group relative to ASD+Anxiety at p=.06 (the other two group comparisons had ps of >.4.; see below for group analyses covarying GCA).
Per-Voxel Analyses.
FMRI data were analyzed in the context of a 3 (Group) x 2 (Attention: AF vs. UF) x 2 (Valence) ANOVA. Analyses indicated a main effect of Attention, but no main effects of Group or Valence. No significant clusters of amygdala activity were observed in the three-way interaction, or the Group X Valence or Valence X Attention interactions.
However, a significant interaction was observed in right amydala for the interaction between Group and Attention. Both the ASD Alone and TDC groups showed a significant, bilateral increase in amygdala activity for AF>UF contrast. However, the ASD+Anxiety group showed comparable activation in bilateral amygdala for the AF and UF conditions, yielding a null effect. Per-voxel tests indicated that the ASD+Anxiety group had significantly increased right amygdala activation than the TDC group for the UF>AF contrast (peak coordinates=26,−13,−14, cluster size=267, peak p(corrected)=.008), Again, the pattern was that the ASD+Anxiety group had an enhanced amygdala response to unattended social information. Groups were matched on time spent gazing on peripheral faces, F(2,47)=.19, p=.826, and on peripheral houses, F(2,47)=1.59, p=.214 (all pairwise comparisons between groups had p-values of >.2).
As a post hoc analysis, a two-group comparison of all children with ASD vs. TDCs was conducted for the UF>AF contrast. No significant effects were observed in amgydala, replicating Herrington and colleagues (41).
ROI Analyses.
In a multiple regression with Group and GCA scores predicting activity within the right amygdala ROI, the Group effect was significant, F(2,58)=8.39, p < .001, with significant differences between the ASD+Anxiety and TDC groups. This effect persisted when covarying task accuracy, F(2,51)=7.78, p < .001, and response time, F(2,51)=6.61, p=.003.
Discussion
These results replicate Herrington and colleagues (41) in associating amygdala function with negative valence and anxiety in ASD, using a separate paradigm and independent sample. Amygdala data indicated that individuals with ASD and anxiety showed increased attentional capture by peripheral, task-irrelevant social information similar to individuals with anxiety without ASD (45). This finding is consistent with decades of research on anxiety disorders, but has seldom been validated in the context of other significant psychiatric or neurodevelopmental conditions (i.e., ASD).
The present data support the transdiagnostic nature of the Negative Valence domain of RDoC, encompassing populations where fear can present in forms that do not map cleanly onto DSM diagnostic criteria. In fact, the emerging link between amygdala function in anxiety in ASD is arguably one of the first transdiagnostic neurobiological mechanisms to emerge from ASD research. Amygdala-mediated mechanisms of selective attention represent one of the most compelling endophenotypes of fear and anxiety; the measurement of this type of mechanisms is precisely what RDoC is designed to support (53).
Conversely, these data illustrate how problematic it is that the Negative Valence dimension of RDoC has been missing from many if not most models of amygdala function in ASD. Until fairly recently, the prevailing view has been that ASD is associated with decreased amgydala activation, which in turn has been related to diminished social and emotional information processing (22–24). The present results add to a growing number of studies suggesting that this perspective is likely an oversimplification of the precise relationship beween amygdala function and ASD.
Both this study and Herrington and colleagues (41) identified robust bilateral amygdalae activation in ASD during the perception of faces. Interestingly, the one published study using this paradigm in ASD (to our knowledge) did not measure anxiety symptoms, and reported selective attention differences in visual cortex, but not amygdala (54). Again, the consideration of anxiety is likely critical in identifying patterns of selective attention toward social information
Although broadly consistent with patterns observed in anxiety disorders outside of ASD, the present findings diverge from previous results in some noteworthy respects. First, the correlation between anxiety symptoms and attentional capture by peripherally presented faces did not reach significance when examining data from negative faces only – the effect emerged when combining negative and neutral faces. While our faces were normed in terms of the intensity and validity of facial expressions, it is possible that a different set of faces (or, for that matter, fearful rather than angry faces) might have yielded different results. It is also possible that valence effects with this task emerge more robustly with rapid presentation (as in prior studies), and in adults (the task has seldom been used in children). But more intriguingly, this null finding may reflect something about how individuals with ASD process emotional stimuli. Even if abnormal selective attention is a mechanism related to anxiety in ASD, it is unwise to presume that individuals with ASD respond the same way as typically developing individuals to different emotional (or neutral) expressions. In particular, it is possible that individuals with ASD and co-occurring anxiety perceive neutral facial expressions as more negative, and experience them as more anxiogenic. The best way to test this in a future study would be to include children with anxiety disorders but not ASD.
Finally, it is worth noting that, like previous imaging studies using this type of paradigm, the present study infers the presence of enhanced attentional capture primarily based on changes in brain activity, rather than changes in overt behavior (such as differences in task performance). There are a very small number of studies examining anxiety and performance-baed attentional biases in ASD, with at least two showing a null relationship (55, 56). Both of these studies point to the need to identify more precisely which mechanisms of selective attention are related to anxiety symptoms among individuals with ASD.
In summary, this study indicates that anxiety in ASD may be related to individual differences in the ability to disregard irrelevant neutral and negative social information in the environment. This result has implications for behavioral treatment. A major objective of cognitive behavioral therapy for anxiety is to help individuals improve their ability to disregard unhelpful negative information. The present results lend support to the idea that the same principle may be effective among individuals with ASD.
Supplementary Material
Acknowledgements
We are very grateful to the many families who participated in this research. The data collection, management, and analysis for the manuscript were supported by funds from Shire Pharmaceuticals. The design and conduct of the study, collection, management, and analysis were also supported by grants from the Pennsylvania Department of Health (SAP # 4100042728 to R. Schultz), the National Institute of Child Health and Development (P30 HD026979, to M. Yudkoff), and National Institute of Mental Health (RC1MH08879 and R01 MH073084-01 to R. Schultz). Portions of this manuscript were presented at the 13th Annual International Meeting for Autism Research, Atlanta, GA (2014).
Financial Disclosures
J. Herrington, J. Miller and R. Schultz reported having received lecture fees and/or research funds from Shire Pharmaceuticals. Additionally, R. Schultz reported receiving research funding from Pfizer. Ms. McVey and Drs. Maddox, Franklin, and Yerys reported no biomedical financial interests or potential conflicts of interest.
The data collection, management, and analysis for the manuscript were supported by funds from Shire Pharmaceuticals. The design and conduct of the study, collection, management, and analysis were also supported by grants from the Pennsylvania Department of Health (SAP # 4100042728 to R. Schultz), the National Institute of Child Health and Development (P30 HD026979, to M. Yudkoff), and National Institute of Mental Health (RC1MH08879 and R01 MH073084-01 to R. Schultz).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although the RDoC matrix defines “anxiety” as an analogue to a construct it calls “Potential Threat”, in this study we follow the tradition of referring to anxiety as a superordinate construct that subsumes fear as well as other related constructs (such as worry, anxious arousal, etc.).
References
- 1.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association. [Google Scholar]
- 2.Kozak MJ, Cuthbert BN (2016): The NIMH Research Domain Criteria Initiative: Background, Issues, and Pragmatics: NIMH Research Domain Criteria initiative. Psychophysiology. 53:286–297. [DOI] [PubMed] [Google Scholar]
- 3.Clark L, Watson D (1991): Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 100: 316–336. [DOI] [PubMed] [Google Scholar]
- 4.Watson D, Clark L, Weber K, Assenheimer J, Strauss M, McCormick R (1995): Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 104: 15–25. [DOI] [PubMed] [Google Scholar]
- 5.Watson D, Weber K, Assenheimer J, Clark L, Strauss M, McCormick R (1995): Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 104: 3–14. [DOI] [PubMed] [Google Scholar]
- 6.Kraemer HC (2015): Research Domain Criteria (RDoC) and the DSM--Two Methodological Approaches to Mental Health Diagnosis. JAMA Psychiatry. 72: 1163–1164. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger DR, Glick ID, Klein DF (2015): Whither Research Domain Criteria (RDoC)?: The Good, the Bad, and the Ugly. JAMA Psychiatry. 72: 1161–1162. [DOI] [PubMed] [Google Scholar]
- 8.Kerns CM, Kendall PC, Berry L, Souders MC, Franklin ME, Schultz RT, et al. (2014): Traditional and atypical presentations of anxiety in youth with autism spectrum disorder. J Autism Dev Disord. 44: 2851–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G (2008): Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 47: 921–929. [DOI] [PubMed] [Google Scholar]
- 10.Sukhodolsky DG, Scahill L, Gadow KD, Arnold LE, Aman MG, McDougle CJ, et al. (2008): Parent-rated anxiety symptoms in children with pervasive developmental disorders: frequency and association with core autism symptoms and cognitive functioning. J Abnorm Child Psychol. 36: 117–128. [DOI] [PubMed] [Google Scholar]
- 11.van Steensel FJA, Bögels SM, Perrin S (2011): Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev. 14: 302–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerns CM, Kendall PC, Zickgraf H, Franklin ME, Miller J, Herrington J (2015): Not to be overshadowed or overlooked: functional impairments associated with comorbid anxiety disorders in youth with ASD. Behav Ther. 46: 29–39. [DOI] [PubMed] [Google Scholar]
- 13.Watts SE, Weems CF (2006): Associations Among Selective Attention, Memory Bias, Cognitive Errors and Symptoms of Anxiety in Youth. J Abnorm Child Psychol Off Publ Int Soc Res Child Adolesc Psychopathol Vol 346 841–852. [DOI] [PubMed] [Google Scholar]
- 14.Dalgleish T, Watts F (1990): Biases of attention and memory in disorders of anxiety and depression. Clin Psychol Rev. 10Mood: 589–604. [Google Scholar]
- 15.MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L (2002): Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol. Vol 111: 107–123. [PubMed] [Google Scholar]
- 16.Mogg K, Bradley B (1998): A cognitive-motivational analysis of anxiety. Behav Res Ther. Vol 36: 809–848. [DOI] [PubMed] [Google Scholar]
- 17.Ohman A, Mineka S (2001): Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 108: 483–522. [DOI] [PubMed] [Google Scholar]
- 18.Pasley B, Mayes L, Schultz R (2004): Subcortical discrimination of unperceived objects during binocular rivalry. Neuron. 42: 163–72. [DOI] [PubMed] [Google Scholar]
- 19.Vuilleumier P, Pourtois G (2007): Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 45: 174–194. [DOI] [PubMed] [Google Scholar]
- 20.Méndez-Bértolo C, Moratti S, Toledano R, Lopez-Sosa F, Martínez-Alvarez R, Mah YH, et al. (2016): A fast pathway for fear in human amygdala. Nat Neurosci. 19: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 21.Pessoa L, Adolphs R (2010): Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci. 11: 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelphrey KA, Carter EJ (2008): Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 1145: 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrington JD, Schultz RT (2010): Neuroimaging of developmental disorders In: Shenton M, Turetsky BI, editors. Underst Neuropsychiatr Disord Insights Neuroimaging. Cambridge: Cambridge University Press. [Google Scholar]
- 24.Baron-Cohen S, Ring H, Bullmore E, Wheelwright S, Ashwin C, Williams S (2000): The amygdala theory of autism. Neurosci Biobehav Rev. 24: 355–364. [DOI] [PubMed] [Google Scholar]
- 25.Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS (2011): Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety. 28: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. (2005): Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 8: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson AK, Phelps EA (2001): Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 411: 305–309. [DOI] [PubMed] [Google Scholar]
- 28.Craske MG, Rauch SL, Ursano R, Prenoveau J, Pine DS, Zinbarg RE (2009): What is an anxiety disorder? Depress Anxiety. 26: 1066–1085. [DOI] [PubMed] [Google Scholar]
- 29.Davis M, Whalen P (2001): The amygdala: vigilance and emotion. Mol Psychiatry. 6: 13–34. [DOI] [PubMed] [Google Scholar]
- 30.Dolcos F, McCarthy G (2006): Brain systems mediating cognitive interference by emotional distraction. J Neurosci Off J Soc Neurosci. 26: 2072–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuilleumier P (2002): Facial expression and selective attention. Curr Opin Psychiatry. 15: 291–300. [Google Scholar]
- 32.Vuilleumier P, Armony J, Driver J, Dolan R (2001): Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 30: 829–41. [DOI] [PubMed] [Google Scholar]
- 33.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ (2004): Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 7: 1271–1278. [DOI] [PubMed] [Google Scholar]
- 34.Ewbank MP, Lawrence AD, Passamonti L, Keane J, Peers PV, Calder AJ (2009): Anxiety predicts a differential neural response to attended and unattended facial signals of anger and fear. Neuroimage. 44: 1144–1151. [DOI] [PubMed] [Google Scholar]
- 35.Mazefsky CA, Herrington JD (2014): Autism and Anxiety: Etiologic Factors and Transdiagnostic Processes In: Davis III TE, White SW, Ollendick TH, editors. Handb Autism Anxiety. New York, NY, USA: Springer, pp 91–106. [Google Scholar]
- 36.Lord C, Rutter M, DiLavore PC, Risi S (2002): Autism Diagnostic Observation Schedule. Los Angeles, CA, USA: Western Psychological Services. [Google Scholar]
- 37.Gotham K, Pickles A, Lord C (2009): Standardizing ADOS Scores for a Measure of Severity in Autism Spectrum Disorders. J Autism Dev Disord. 39: 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandler S, Charman T, Baird G, Simonoff E, Loucas T, Meldrum D, et al. (2007): Validation of the Social Communication Questionnaire in a Population Cohort of Children With Autism Spectrum Disorders. J Am Acad Child Adolesc Psychiatry. 46: 1324–1332. [DOI] [PubMed] [Google Scholar]
- 39.Silverman WK, Saavedra LM, Pina AA (2001): Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: child and parent versions. J Am Acad Child Adolesc Psychiatry. 40: 937–944. [DOI] [PubMed] [Google Scholar]
- 40.Elliot C (2007): The Differential Abilities Scale, Second Edition, 2nd ed. San Antonio, TX: Harcourt Assessments, Inc. [Google Scholar]
- 41.Herrington JD, Miller JS, Pandey J, Schultz RT (2016): Anxiety and social deficits have distinct relationships with amygdala function in autism spectrum disorder. Soc Cogn Affect Neurosci. 11: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM (1997): The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 36: 545–553. [DOI] [PubMed] [Google Scholar]
- 43.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23: S208–S219. [DOI] [PubMed] [Google Scholar]
- 44.Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17: 825–841. [DOI] [PubMed] [Google Scholar]
- 45.Bishop SJ, Duncan J, Lawrence AD (2004): State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci Off J Soc Neurosci. 24: 10364–10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. Neuroimage. 92: 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, Gee JC (2010): The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 49: 2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avants BB, Tustison NJ, Stauffer M, Song G, Wu B, Gee JC (2014): The Insight ToolKit image registration framework. Front Neuroinformatics. 8. doi: 10.3389/fninf.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011): A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 56: 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM (2004): Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 21: 1732–1747. [DOI] [PubMed] [Google Scholar]
- 51.Bishop SJ, Duncan J, Lawrence AD (2004): State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci Off J Soc Neurosci. 24: 10364–10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams MA, McGlone F, Abbott DF, Mattingley JB (2005): Differential amygdala responses to happy and fearful facial expressions depend on selective attention. Neuroimage. 24: 417–425. [DOI] [PubMed] [Google Scholar]
- 53.Yee CM, Javitt DC, Miller GA (2015): Replacing DSM Categorical Analyses With Dimensional Analyses in Psychiatry Research: The Research Domain Criteria Initiative. JAMA Psychiatry. 72: 1159. [DOI] [PubMed] [Google Scholar]
- 54.Bird G, Catmur C, Silani G, Frith C, Frith U (2006): Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage. 31: 1614–1624. [DOI] [PubMed] [Google Scholar]
- 55.Hollocks MJ, Ozsivadjian A, Matthews CE, Howlin P, Simonoff E (2013): The Relationship Between Attentional Bias and Anxiety in Children and Adolescents With Autism Spectrum Disorders: Attentional bias and anxiety in ASD. Autism Res. 6: 237–247. [DOI] [PubMed] [Google Scholar]
- 56.May T, Cornish K, Rinehart NJ (2015): Mechanisms of Anxiety Related Attentional Biases in Children with Autism Spectrum Disorder. J Autism Dev Disord. 45: 3339–3350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.