Abstract
This retrospective review explores delayed-onset hearing loss in 85 individuals receiving cochlear implants designed to preserve acoustic hearing at the University of Iowa Hospitals and Clinics between 2001 and 2015. Repeated measures of unaided behavioral audiometric thresholds, electrode impedance, and electrically evoked compound action potential (ECAP) amplitude growth functions were used to characterize longitudinal changes in auditory status. Participants were grouped into two primary categories according to changes in unaided behavioral thresholds: (1) stable hearing or symmetrical hearing loss and (2) delayed loss of hearing in the implanted ear. Thirty-eight percent of this sample presented with delayed-onset hearing loss of various degrees and rates of change. Neither array type nor insertion approach (round window or cochleostomy) had a significant effect on prevalence. Electrode impedance increased abruptly for many individuals exhibiting precipitous hearing loss; the increase was often transient. The impedance increases were significantly larger than the impedance changes observed for individuals with stable or symmetrical hearing loss. Moreover, the impedance changes were associated with changes in behavioral thresholds for individuals with a precipitous drop in behavioral thresholds. These findings suggest a change in the electrode environment coincident with the change in auditory status. Changes in ECAP thresholds, growth function slopes, and suprathreshold amplitudes were not correlated with changes in behavioral thresholds, suggesting that neural responsiveness in the region excited by the implant is relatively stable. Further exploration into etiology of delayed-onset hearing loss post implantation is needed, with particular interest in mechanisms associated with changes in the intracochlear environment.
Keywords: cochlear implant, hearing preservation, electrode impedance, electrically evoked compound action potential
1. Introduction
Recent modifications to surgical procedures and electrode array designs facilitate the preservation of intracochlear structure and function and have thus expanded the number of patients who benefit from cochlear implantation. Although retention of acoustic hearing following cochlear implant (CI) surgery has become a common outcome, the degree ranges from minimal to complete, with some individuals experiencing total loss of acoustic hearing in the implanted ear (e.g. Helbig et al. 2016; Moteki et al. 2016; Gantz et al. 2016; Roland et al. 2016; van Abel et al. 2015; Hunter et al. 2016; Santa Maria et al. 2013). A decrease in hearing sensitivity identified at the first post-operative appointment is commonly attributed to surgical trauma, an acute inflammatory response, or change in the system mechanics due to the presence of the array, but decreases in hearing sensitivity have also been observed months to years after surgery; the cause: undetermined. It is this delayed-onset hearing loss that is the focus of the present report.
The University of Iowa has been involved in hearing-preservation research since the 1990s (see Gantz et al. 2016 for an overview of research involving the first short-electrode array). Retrospective review of data from all individuals who have undergone hearing-preservation CI surgery at this institute allowed us to characterize delayed hearing loss across a longer time span than previously reported (up to 15 years for participants implanted with earliest generation devices). We also explored whether electrode impedance (Z) or electrically evoked compound action potential (ECAP) measures might provide insight into the underlying etiology.
Delayed-onset hearing loss as observed in this population may result from the body’s reaction to the implanted materials. Histologic analysis often reveals fibrotic encapsulation of the electrode array and tissue comprised of various cell types within the cochlear labyrinth (e.g. Linthicum et al. 1991; Nadol et al. 2008; Seyyedi and Nadol, 2014; Quesnel et al. 2015). A change in the physical structure of the inner ear scalae has the potential to change the passive mechanics of the system. Modeled as an increase in damping, fibrotic tissue has the potential to reduce basilar membrane vibration. Reduced movement in apical cochlear regions, which would decrease the ear’s sensitivity to low-frequency stimuli, could occur when fibrotic tissue invades the scala tympani (Choi and Oghalai 2005). Moreover, the biological processes responsible for the fibrotic tissue may be toxic to the neurosensory structures of the inner ear (Bas et al. 2015; Eshraghi et al. 2015), which could also account for a decrease in acoustic hearing. Significant correlations between the degree of tissue reaction and the amount of acoustic hearing loss have been observed in animals (e.g. O’Leary et al. 2013), which suggests that the tissue response may also be relevant to humans experiencing delayed loss of acoustic hearing.
Changes in the physical structure of the cochlear scalae cannot be detected using current noninvasive imaging techniques; however, clues may be gleaned from impedance measures that are routinely recorded from each intracochlear electrode during clinical appointments. Electrode impedance provides information about the status of the electrode and the surrounding environment, and has been shown to be sensitive to changes in tissue growth around the electrode array (Wilk et al. 2016). Histological analysis of a temporal bone from a deceased CI recipient who experienced a complete loss of acoustic hearing in the implanted ear between one and four months after implantation revealed that although post mortem hair cell and neural counts were not significantly different for implanted and unimplanted ears, the implanted ear presented with extensive fibrous tissue in the cochlear scalae. Electrode impedance values available at the final pre mortem audiological appointment were similar to impedance values recorded shortly after implantation; however, a transient increase occurred around the time of the acoustic hearing loss (see Fig 3B in Quesnel et al. 2015). CI audiologists at this institute (and from other institutes) have also observed variations in electrode impedance at the time a drop in acoustic hearing is identified, but these anecdotal reports have yet to be systematically evaluated.
Figure 3.
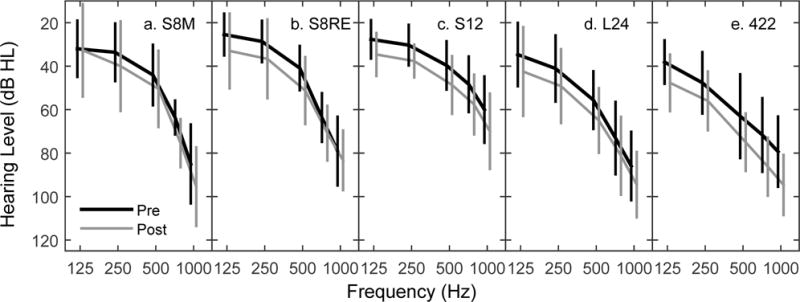
Grand mean unaided behavioral audiometric thresholds (± 1 standard deviation) at low frequencies measured prior to surgery (black) and at the first post-operative visit (gray). Pre- and post-operative data points are shifted horizontally for easier visualization of error bars. Panels separate data by device type.
Electrically evoked compound action potentials (ECAPs) can also be recorded using clinical software and are routinely measured from many individuals implanted at the University of Iowa during research appointments. These potentials are the synchronous response of electrically stimulated auditory neurons, and as such, provide information about the status of the auditory nerve. Correlations between spiral ganglion neuron loss and the degree of inflammation responses have been observed (e.g. Xu et al. 1997; Fayad et al. 2009), which is consistent with the suggestion that an inflammatory response also may be toxic to the neurosensory structures in the cochlea (Bas et al. 2015; Eshraghi et al. 2015). A hypersensitivity reaction to the electrode array may be more common than originally thought (e.g. Seyyedi and Nadol 2014), and although ECAP measures generally have been shown to be relatively stable over time (e.g. Hughes et al. 2001; Brown et al. 2010), a closer evaluation of within-subject changes, particularly for individuals who demonstrate changes in acoustic hearing, is warranted.
This retrospective review aims to characterize the prevalence and nature of delayed loss of acoustic hearing by compiling repeated measures of unaided audiometric thresholds, electrode impedance, and ECAP amplitude growth functions in a relatively large sample of hearing-preservation CI recipients. Systematic changes over time were interpreted as reflecting a change in the status of the auditory system. Because the data set contained several electrode array designs and surgical approaches, they were evaluated as factors relating to the prevalence of delayed hearing loss. Evaluation of the physical and physiological measures also allowed us to explore etiology, albeit indirectly. It was hypothesized that an increase in electrode impedance would be observed more often for individuals with delayed hearing loss than individuals with stable hearing if a change in the intracochlear environment, as would be the case with fibrous tissue growth, was in fact a contributing mechanism. Correlation analysis was used to evaluate whether changes in ECAPs (i.e. threshold, slope and suprathreshold amplitude) and changes in unaided audiometric thresholds were related. It was hypothesized that a significant correlation would be consistent with a global change in neural status as a contributing factor.
2. Methods
2.1. Participants
As of October 14, 2015, 143 adults (ages 18 years and older) had been implanted at the University of Iowa with hearing preservation electrode arrays manufactured by Cochlear Ltd (Cochlear Americas, CO, USA): S8 (24M and 24RE based receiver-stimulator), S12, L24, and 422. Characteristics of these arrays are provided in Table 1. The electrode arrays vary in physical dimensions, number of electrodes, overall length and insertion depth. Some electrode arrays were designed specifically for insertion via a cochleostomy while others were designed for insertion through either a cochleostomy or the round window (Table 1).
Table 1.
Electrode array information and study sample size.
| Array | Active Contacts | Active Array Length (mm) | Insertion Depth (mm) | Insertion Approach | Earliest Implant Date (yr) | NTot | NIncl | NECAP |
|---|---|---|---|---|---|---|---|---|
| S8M | 6 | 4.05 | 10.0 | C | 2001 | 12 | 11 | 10 |
| S8RE | 6 | 4.05 | 10.0 | C | 2005 | 12 | 12 | 12 |
| S12 | 10 | 5.40 | 10.0 | C | 2008 | 15 | 15 | 11 |
| L24 | 22 | 14.50 | 17.5–18.0 | C/RW | 2009 | 41 | 32 | 13 |
| 422 | 22 | 20.00 | 20.0–25.0 | C/RW | 2012 | 63 | 15 | 2 |
|
| ||||||||
| Total | 143 | 85 | 48 | |||||
NTot: Total number of participants identified in the query. NIncl: Number of participants included in the data set. NECAP: Subset of included participants with ECAP data in addition to audiometric threshold and impedance data. All other participants had both behavioral audiometric thresholds and impedance data available for review. C: Cochleostomy. RW: Round window.
The “S” arrays were all implanted under an Investigational Device Exemption (IDE) approved by the Food and Drug Administration (FDA; S8: G990155; S12: G070016). Preoperative unaided audiometric thresholds ≤ 60 dB HL at 125 and 250 Hz and ≥ 75 dB HL above 1500 Hz were required for candidacy. The L24 array was approved by the FDA for clinical use in March of 2014; arrays implanted prior to that date were approved using an IDE (G070191 and G110089). For L24 arrays implanted under IDE G070191, audiometric threshold criteria were the same as for the S8 and S12 arrays. For individuals implanted under IDE G110089, a five-frequency pure tone average (PTA; 125, 250, 500, 750 and 1000 Hz) between 60 – 90 dB HL was required. The 422 array is FDA approved for clinical use. It was designed to preserve cochlear structures rather than acoustic hearing, and has been used in individuals both with and without acoustic hearing prior to surgery. In this report, only individuals with 422s whose preoperative audiometric thresholds were ≤ 60 dB HL at 125 and 250 Hz were included for review. This criterion was based on in-house clinical guidelines for identifying individuals who are likely to benefit from the fitting of an acoustic component on the implanted ear. Candidacy criteria used to determine which electrode array is chosen for the individual CI recipient also include factors such as word recognition ability, status of the contralateral ear, age of onset of hearing loss, and/or duration of severe-profound hearing loss. These details have been reported elsewhere (e.g. Gantz et al. 2016; Roland et al. 2016; van Abel et al. 2015).
Of the 143 individuals identified as potential subjects, 85 were included for the retrospective review (Table 1). This sample included 41 males and 44 females, with age at implantation ranging from 18 to 82 years (mean: 55.7, sd: 14.2). Reasons for exclusion were (1) they were recently implanted; no data beyond 3 months post initial activation were available, (though one individual who lost all residual hearing at 2.3 months was included), (2) audiometric thresholds from the contralateral ear were not available, (3) only audiometric threshold data were available; Z and ECAP data were absent or available only for a single time point, (4) extended periods of non-use of the CI (N=1), (5) experimental programming adjustments involved deactivating or reactivating individual electrodes, potentially affecting the Z data (N=1), (6) complete loss of residual hearing at initial activation (N=5), (7) loss of residual hearing following head trauma (N=1), and (8) fluctuating hearing associated with an etiology of Meniere’s/autoimmune disease (N=2). Sample sizes for L24 and 422 arrays are affected most by the exclusion criteria, primarily because these arrays were more recently introduced into clinical practice and therefore fewer time points are available for many implanted individuals. One patient with an S12 array was explanted and reimplanted with a 422 array. Because acoustic hearing was lost following the revision surgery, only data up to the time of explantation are included. One individual retained acoustic hearing following explant of an S12 array and reimplantation with an L24 array (Dunn et al. 2015). Thus, the data associated with both devices are included. The data for individuals who eventually obtained an implant in the contralateral ear are included up to the time of the second implant, after which behavioral thresholds in the previously unimplanted ear could no longer be used as a control. The University of Iowa Institutional Review Board approved both the research registry and use of past data collected from consenting adults.
2.2. Surgical Protocol
All surgeries were performed by authors B.J.G or M.R.H. The earliest hearing preservation arrays (S8 and S12) were inserted through a cochleostomy, typically drilled antero-inferior to the round window (Gantz et al. 2005). Some L24 arrays were also initially inserted via cochleostomy. Surgeons transitioned to a round window approach with the intent to minimize both acoustic and mechanical trauma around 2013 (e.g. Adunka et al., 2004). In this data set, approximately half of the L24 arrays and most 422 arrays were inserted via the round window. Anatomy occasionally dictated cochleostomy location and general approach. Principles of soft insertion include: (1) blue lining of the otic capsule bone to expose the endosteum followed by sharp opening of the endosteum for cochleostomy, (2) copious irrigation to remove any bone dust prior to opening of the scala tympani, (3) avoidance of suctioning once the scala is opened, (4) slow, steady insertion of the electrode array, (5) use of suture at the tegmen mastoideum to stabilize the electrode array, and (6) sealing of the round window or cochleostomy with a small fascia or muscle plug. All patients received 10 mg intravenous dexamethasone during the surgery. Neither intra- nor post-operative imaging to is a routine procedure at this clinic, and radiographs were not available for this subset of patients, including those with progressive hearing loss. In 2012, surgeons began prescribing a 1-week course of oral steroids (prednisone at 1mg/kg/day) beginning immediately post operatively and a second 1-week course beginning the day prior to initial activation in an effort to reduce inflammatory and cytotoxic responses that may be associated with surgical trauma or device activation.
2.3. Data Range and Appointment Schedule
There were no attempts to control for duration of follow up, but rather, all of the time points available for each individual were used. Because this review spans more than 15 years, a longer time period of follow up is available for individuals implanted with older generation electrode arrays compared to individuals receiving newer generation electrode arrays. This is relevant when comparing results across array types, as the potential for observing changes in auditory status is greatest for individuals implanted over a longer time period. (We address the issue of different durations of follow-up in further detail in Section 3.1). Moreover, the appointment schedule changed over time, which affects the number and frequency of repeated measures. The current appointment schedule at this institute for recipients of an implant designed to preserve hearing is an audiometric assessment prior to surgery, at initial activation of the CI, at 1, 2 and 3 weeks post activation, and then at 1, 3, 6, and 12 months post activation, and annually thereafter. During each appointment, CI recipients participate in a variety of clinical and research tasks. The appointment itinerary depends upon many factors, including the clinical and research priorities, both of which changed across the period of this review. Each visit involved a combination of unaided behavioral threshold, impedance, and/or ECAP measures. Appointment dates were often adjusted or cancelled to accommodate personal schedules, illness and weather. Thus, the time intervals containing the data of interest were not identical across participants. “Missing” time intervals may not be completely random since individuals with concerns were more likely to return for follow-up appointments. Moreover, appointments were added on occasion, particularly if there were concerns about changes in hearing sensitivity. Thus, individuals with changes in hearing status may be over-represented in this sample because these subjects tended to have more data available for review.
2.4. Behavioral Audiometric Thresholds
Unaided air and bone conduction thresholds were monitored during clinical appointments using standard clinical procedures (e.g. modified Hughson Westlake ascending method; 5 dB step-size, Carhart & Jerger, 1959) and entered into a research database. For this study, air conduction thresholds at 125, 250, 500 and 1000 Hz were averaged at each time point (a four-frequency PTA). Previous studies have included 750 Hz, but this frequency was not consistently measured for this sample of various array types for the extended time period. If the majority of time points for an individual were missing threshold information at one of the four included frequencies, a three-frequency average was used (typically the thresholds at 250, 500 and 1000 Hz); otherwise, time points with missing threshold data were excluded. When a clinic note or bone conduction thresholds indicated a possible conductive component, the time point was excluded because middle ear pathology not only has the potential to affect air conduction thresholds, but accumulation of fluid in the middle ear space may also result in transitory increases in electrode impedance (Sainz et al., 2003). When no response could be obtained at the limit of the audiometer output, the threshold was coded as 125 dB HL.
Many studies define hearing preservation relative to pre-operative hearing status. In this study, because the interest was to characterize delayed hearing loss, the audiogram measured at the initial activation appointment was used as the baseline hearing level. Participants were categorized into four groups according to the pattern of PTA changes relative to initial activation: (1) stable, (2) symmetrical, (3) implanted: gradual, and (4) implanted: precipitous (see Figure 1 for examples). Because the goal was to better understand mechanisms underlying delayed hearing loss rather than to report on the success of surgical techniques/devices for preserving hearing, relatively strict criteria were used to categorize changes in hearing sensitivity over time. A drop in the implanted ear PTA ≤ 10 dB from initial activation was considered “stable” (Fig. 1a). For participants whose PTA in the implanted ear was > 10 dB at any time point relative to initial activation, the unimplanted ear thresholds and time course were used for categorization. If a change in unimplanted ear PTA was also observed and was within 10 dB of the change in the implanted ear, the hearing loss was categorized as “symmetrical” (Fig. 1b). In many of these instances, the absolute hearing sensitivity across the two ears was asymmetrical, with hearing thresholds poorer in the implanted ear. However, the symmetry categorization was determined by comparing the amount that hearing declined from baseline across the two ears. For hearing sensitivity that changed more in the implanted ear than the unimplanted ear, visual inspection of the data suggested that some subjects tended to gradually lose hearing over time while others (the majority) showed a precipitous drop in hearing thresholds. In an attempt to objectify this observation, the rate of change was calculated in dB/month across time points for which thresholds increased by > 10 dB. If the rate of change was < 5 dB/month, the hearing loss was considered “gradual” (Fig 1c). If the rate of change was ≥ 5 dB/month, the hearing loss was considered “precipitous” (Fig 1d). One subject with a precipitous loss regained auditory function over time (i.e. PTA returned to within 10 dB of initial activation).
Figure 1.
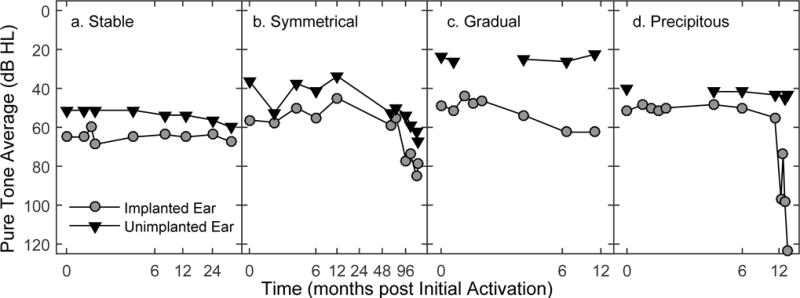
Individual examples of implanted and unimplanted ear audiometric thresholds (PTA) as a function of time for each hearing loss category. Note that the x-axis is not the same for each panel.
2.5. Electrode Impedance
Electrode impedance was measured during clinical and/or research appointments using commercial software available at the time of testing. Due to the 15-year time span of the data set, the software included various versions of NRT (Neural Response Telemetry), Custom Sound, and Custom Sound EP. “Common ground” impedance, which is calculated by relating the impedance of the electrode of interest to all other intracochlear electrodes shorted together, was used in this review. If multiple measures were available on the same day for an individual, the last measurement was used for this study.
Electrodes that were deactivated in the participant’s clinical program were excluded from the analysis. Per this institution’s clinical protocol, which was influenced by information regarding location of the basal end of the array relative to the auditory nerve, electrodes 1–4 are deactivated for L24 recipients. Note that electrode 1 is the most basal electrode for all arrays included in this review. Additional reasons for electrode deactivation included evidence of short/open circuits, nonauditory percepts associated with stimulation, adverse sound quality, and limited loudness growth. Recipients of 422 arrays often have at least 1–2 basal electrodes deactivated due to anomalous perception.
Electrode impedance is affected by the physical properties of the electrode itself, such as the surface area. There has been a trend toward the use of intracochlear electrodes with smaller surface areas, which has resulted in higher average impedance values. Moreover, electrode impedance can vary across individuals and across electrodes. Therefore, the metric of interest was not the absolute impedance value, but a change relative to a baseline value. Because electrode impedance increases from the time of surgery up to the point of activation, and then decreases once electrical stimulation is initiated (e.g. Dorman et al. 1992; Hughes et al. 2001; Busby et al. 2002), the first appointment after initial activation was used as the baseline for impedance measures. For the majority of participants (N=67), that appointment was prior to the 1-month post initial activation appointment (range: 0.2–.92 months). For ten participants, the baseline appointment was within the first month (1.02–.58 months), and for six participants it was between the second and fourth months (2.14–.55 months). For two participants with S8M devices, records of impedance measures were not available until 7 and 12 months post initial activation, though these patients had earlier appointments. One of these participants was categorized as having stable hearing thresholds and the other as symmetrical; therefore, the exact timing of impedance data was less of a concern (i.e., we were not attempting to relate impedance changes directly to changes in audiometric thresholds). Both participants were implanted in 2001, and more than 10 test dates were available for review. Across all participants, the number of time points available for reviewing impedance measurements ranged from 3 to 30.
2.6. Electrically Evoked Compound Action Potentials
Electrically evoked compound action potential amplitude growth function data were available for a subset of research appointments for 48 study participants. A variety of commercially available software packages were used to record these neural potentials for a range of stimulus levels spanning the listener’s dynamic range. These data have the potential to be less reliable than the impedance data, as a larger number of measurement parameters can be adjusted that will affect the recordings. The lack of control on these parameters due to the retrospective nature of this report was suboptimal. But, despite the limitations, it was deemed informative to review and report on the data that were available to evaluate whether changes in ECAPs over time related to changes in audiometric thresholds.
Authors V.D.T. and J.K.O. and a trained lab member analyzed ECAP growth function data collected since 2013. ECAP growth function data collected prior to 2013 were reanalyzed by V.D.T. and J.K.O. for consistency. Three metrics were calculated from each ECAP amplitude growth function: visual detection threshold (lowest current level necessary to evoke a response), slope, and amplitude for a relatively high stimulus level (Fig 2). In some cases, the ECAP growth function was nonlinear and best fit using two regression lines. When that occurred, the steeper segment was used to calculate slope. For example, the function in Figure 2 has two regression lines, but the steeper slope of 9.8 μV/CL was used. The highest current level consistently tested across time for each person/electrode was used for the response amplitude comparison. If the current level of interest wasn’t tested on a given date, but responses for current levels above and below were available, then linear interpolation was used to predict ECAP amplitude.
Figure 2.
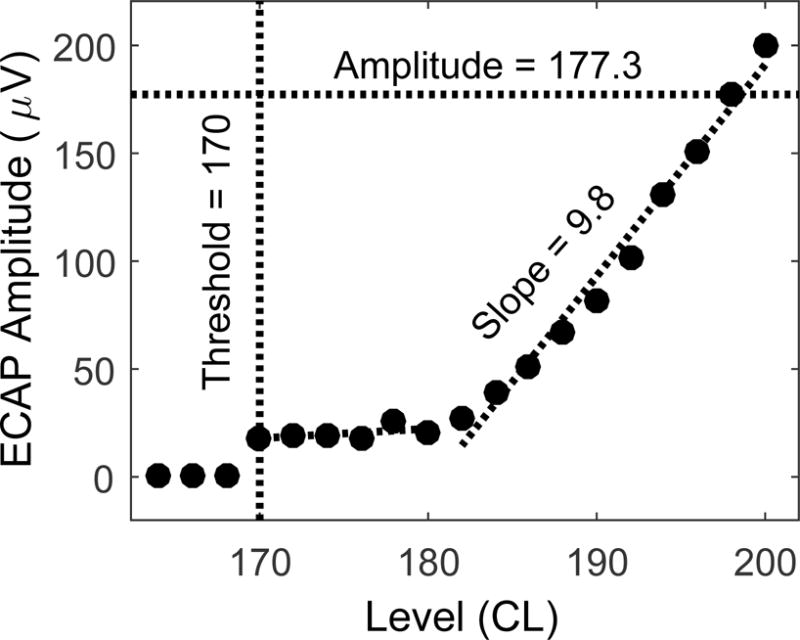
ECAP amplitude growth function for one participant and one electrode. Visual detection threshold (CL), the steeper slope (μV/CL), and the amplitude (μV) at a stimulus level of 198 CL are indicated.
The available ECAP data for each participant were evaluated to find an early and late time point with the greatest amount of consistency in stimulation and recording parameters as these variables can affect ECAP amplitude, and thus also threshold and slope measures. When measurement parameters were confirmed to be inconsistent across time, the data were omitted. If not otherwise indicated in the research note and could not be verified by looking back at the exported data, it was assumed that default parameters were used or that parameter changes necessary to optimize the recordings for an individual would have been used at follow-up appointments.
For individuals with declines in audiometric thresholds, additional attempts were made to choose time points that would straddle the drop in hearing. Because ECAP slope (and presumably response amplitude) increases significantly between initial activation and 2 months post (Hughes et al. 2001), attempts were made to avoid using ECAP data collected within this time period. It was not possible to do so for 14 of the participants due to the limited number of ECAP data and time course of hearing loss experienced by some individuals. Therefore, data were analyzed twice: once with the full data set (N=48) and once with a reduced data set which eliminated individuals whose early measurement period was < 2 months post initial activation (N=34). It was rare that all electrodes were tested on any given date or that the same electrodes were tested at each visit. However, if multiple ECAP data on multiple electrodes were available across the two time points, the threshold, slope and amplitude changes were averaged across the electrodes.
2.7. Data Analysis
Statistical analyses were performed in SPSS (analysis of variance; ANOVA), SAS (Fisher’s Exact, Pearson’s Chi-squared tests for count data, and linear mixed effects model) and MATLAB (t-tests and correlation). Two one-way ANOVAs were performed: (1) to compare amount of initial hearing loss immediately following surgery across the electrode arrays and (2) to compare the duration of data across hearing loss categories. For the latter comparison, hearing loss categories were combined into two groups and reanalyzed with a t-test. Pearson’s Chi-squared and Fisher’s Exact tests were used for analyses evaluating whether the distribution of participants across hearing loss categories was different depending upon array type or insertion approach (L24 arrays only). T-tests were used to evaluate the effect of hearing loss category on changes in electrode impedance (average and maximum). The linear mixed model was used to relate behavioral thresholds (PTA) to electrode impedance, and to quantify how that relationship varied by time and hearing loss category. A Pearson product-moment correlation was used to evaluate whether changes in the PTA across two time points were related to changes in ECAPs across the same two time points. The analysis was replicated three times (once each using ECAP threshold, slope, or amplitude). Because ECAPs are recorded using intracochlear electrodes, electrode impedance and ECAPs may be correlated. Therefore, these correlations were also evaluated.
3. Results
3.1. Behavioral Audiometric Categorization
Although the focus of this study is characterizing delayed hearing loss, it was useful to first quantify pre-operative hearing levels and any immediate drops in hearing observed for this sample to provide a description of baseline values that were used for determining any further declines. Figure 3 displays grand mean audiometric thresholds for low frequencies at the pre-operative (black) and first post-operative (gray) visits separated by device (panels a–e). The figure includes 750 Hz, but recall that the PTA is calculated by averaging 125, 250, 500 and 1000 Hz. On average, the immediate loss of hearing following surgery (i.e. difference between the pre- and first post-operative PTA) was 8.9 dB [S8(M): 6.4; S8(RE): 9.5; S12: 8.5; L24: 8.6; 422: 11.6]. The differences across array type were not statistically significant (one-way ANOVA, F(4,80): 0.663; P:0.62). Thresholds obtained at the first post-operative visit (typically the initial activation appointment) were the baseline measure for calculating additional changes over time.
Although not shown in Figure 3, when participants with L24 arrays were separated by IDE, those in the more severe hearing loss group (N=13) had pre- and post-operative hearing thresholds similar to recipients of the 422 arrays, and those in the standard group (N=19) had thresholds more similar to recipients of the “S” arrays. This reflects differences in candidacy criteria rather than array differences. More relevant is that the immediate drop in hearing (7.4 and 9.5 dB, respectively) was not significantly different (two-tailed T-test: T30: 0.632; P: 0.532). Because initial hearing level was not related to the amount of immediate hearing loss for the L24 recipients, it was not evaluated as a factor in subsequent analyses. When L24 users were separated by insertion approach, pre- and post-operative PTAs differed by approximately 4 dB, and the average immediate loss of hearing was 7.7 (round window; N=19) and 9.1 dB (cochleostomy; N=13). This difference was not statistically significant (two-tailed T-test using Welch adjustment for unequal variances; T13.62: 0.37; P: 0.72). Insertion approach was a variable we were interested in exploring in subsequent analyses related to delayed hearing loss (Rowe et al. 2016).
Table 2 provides the distribution of participants displaying one of four distinct patterns of long-term hearing sensitivity: stable hearing, symmetrical loss, gradual loss, precipitous loss (see methods for details). About 50% of all participants were categorized as having stable hearing, with an additional 12% showing similar loss of hearing in both ears, which likely reflects natural progression of hearing loss rather than effects of implantation. The remaining individuals (N=32; 38%) demonstrated distinctive loss of hearing in the implanted ear compared to the unimplanted ear, and in the majority of those cases (N=26; 81%) the progression occurred rapidly. It is notable that final averaged hearing thresholds were < 90 dB HL for half (N=16) of all individuals with delayed-onset hearing loss and 56% (N=18) were using an acoustic component in combination with the electrical stimulation at the conclusion of the present review.
Table 2.
Number (proportion) of participants in each category, defined using the changes in audiometric threshold (PTA) relative to initial activation compared to the unimplanted ear in addition to rate of change (see methods).
| Ipsilateral Loss ≤ 10 dB | Ipsilateral Loss >10 dB | |||
|---|---|---|---|---|
| Array | Stable | Symmetrical | Gradual | Precipitous |
| S8 (M) | 5 (0.45) | 4 (0.36) | 0 (0.00) | 2 (0.18) |
| S8 (RE) | 4 (0.33) | 4 (0.33) | 1 (0.08) | 3 (0.25) |
| S12 | 7 (0.47) | 0 (0.00) | 1 (0.07) | 7 (0.47) |
| L24 | 16 (0.50) | 2 (0.06) | 4 (0.13) | 10 (0.31) |
| 422 | 11 (0.73) | 0 (0.00) | 0 (0.00) | 4 (0.27) |
|
| ||||
| Totals | 43 (0.51) | 10 (0.12) | 6 (0.07) | 26 (0.31) |
A valid concern is that this categorization is biased according to the duration of data available for review. For example, if the duration of follow-up is shortest for individuals showing stable hearing, the proportions in Table 2 might under represent the percentage of individuals who eventually lose hearing. Figure 4 displays the duration of data available for each participant at the time of this review, ordered from least to greatest. Categorization is shown with the color coding (stable: black, symmetrical: dark gray, gradual: medium gray, precipitous: light gray). Table 3 provides the associated summary statistics. Individuals in the symmetrical group had data available across an average time period of 107 months, which is a longer time period than the other three groups. A one-way ANOVA with follow-up pairwise comparisons confirmed that these differences were significant (stable: P<0.0001; gradual: P=0.111; precipitous: P=0.002; Tukey-Kramer adjustment applied). There were no significant differences between the stable and gradual categories (P=0.8156), stable and precipitous categories (P=0.6538), or gradual and precipitous categories (P=0.9827). Because a symmetrical decline in hearing is likely unrelated to the implant, individuals characterized with stable or symmetrical hearing loss were combined into one group and individuals with gradual or precipitous loss were combined into another group. This grouping eliminates any difference in the average duration of data available (T83=0.867; P=0.3886).
Figure 4.
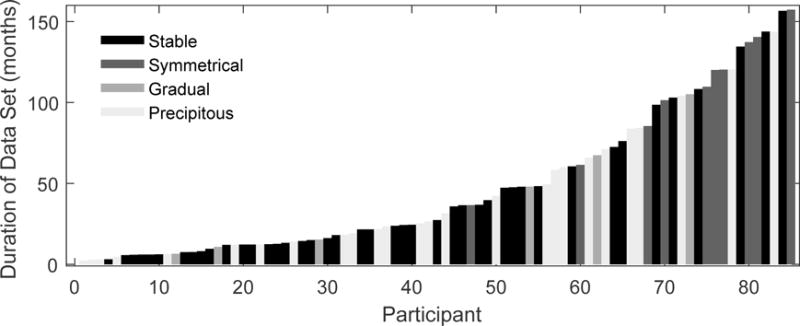
Duration of data available for each participant, ranked from least to greatest. Color coding indicates category of change observed in audiometric thresholds (see legend).
Table 3.
Summary statistics for duration of data available by hearing loss category (rounded to the nearest month).
| Ipsilateral Loss ≤ 10 dB | Ipsilateral Loss >10 dB | |||
|---|---|---|---|---|
| Stable | Symmetrical | Gradual | Precipitous | |
| Minimum | 3 | 37 | 7 | 2 |
| Maximum | 157 | 157 | 105 | 144 |
| Mean | 38 | 107 | 42 | 43 |
| Median | 22 | 115 | 32 | 26 |
| Stand Dev | 40 | 37 | 39 | 39 |
Further review of the raw data revealed that when individuals experienced delayed loss of acoustic hearing, the audiometric changes most often occurred within 6 months of device activation. Thus, having a shorter duration of data available for follow-up for some individuals does not necessarily imply that the number of individuals with delayed loss of acoustic hearing is severely underrepresented in this sample. However, in a minority of instances, audiometric changes were observed years after implantation. Figure 1d provides longitudinal audiometric results from an individual who experienced a sudden change in acoustic hearing after using the CI for nearly 1 year. Though uncommon, there were other individuals whose acoustic hearing thresholds in the implanted ear alone dropped gradually over 4–7 years of device use. Hearing loss occurring > 1-year post implantation was more common for individuals showing a symmetrical decline in both the implanted and unimplanted ears.
As expected, symmetrical declines in hearing sensitivity were observed most often for individuals with the oldest generation electrode arrays (“S8”, see Table 2), and thus, the longest range of follow-up appointments (Figure 4, Table 3). To quantitatively compare post-operative changes in hearing sensitivity across array types, individuals characterized with stable or symmetrical hearing loss were once again combined into one group while individuals with gradual or precipitous loss were combined into another group (not shown, but can be calculated from Table 2). Although it appears that a greater proportion of individuals with S12 and L24 arrays present with some loss relegated to the implanted ear compared to the other arrays, a relationship between array type and hearing loss group was not supported statistically (Fisher’s exact test, two-sided hypothesis; P = 0.3386).
We were also interested in whether the proportion of participants in each category differed by insertion approach for the L24 recipients (Table 4). As before, stable and symmetrical categories were grouped together, as were gradual and precipitous. In this sample, 47% of individuals undergoing a round window insertion experienced hearing loss specific to the implanted ear compared to 38% of individuals undergoing a cochleostomy insertion (difference of 9%). This difference was not significant (Χ2 = 0.0081; df = 1; P = 0.9285).
Table 4.
Number (proportion) of participants with L24 arrays in each hearing loss category, separated by insertion approach.
| Ipsilateral Loss ≤ 10 dB | Ipsilateral Loss >10 dB | |||
|---|---|---|---|---|
| Approach | Stable | Symmetrical | Gradual | Precipitous |
| Cochleostomy | 6 (0.43) | 2 (0.14) | 2 (0.14) | 4 (0.29) |
| Round Window | 10 (0.56) | 0 (0.00) | 2 (0.11) | 6 (0.33) |
|
| ||||
| Totals | 16 (0.50) | 2 (0.06) | 4 (0.13) | 10 (0.31) |
3.2. Impedance
For all analyses, impedance was averaged across electrodes. This might not be ideal because in addition to observations of impedance increases coincident with the time of a drop in acoustic hearing, CI audiologists at this institute often report an increase in variability of impedance across electrodes within an individual. A cursory look at the data did not reveal a consistent pattern (e.g. that basal electrodes were affected more than apical electrodes). Thus, for this initial attempt to explore the data, averaging across electrodes was sufficient for characterizing one aspect of the observed patterns of impedance changes.
An individual example comparing the time courses of acoustic hearing and electrode impedance changes is provided in Figure 5. For this individual, a complete loss of measurable acoustic hearing occurred between 1 and 3 months post initial activation (panel a). Electrode impedance measured around that same time period increased by more than a factor of two, and then decreased, but remained higher than the initial measured impedance (panel b). For this patient, there is a large impedance change at the onset of delayed hearing loss, consistent with the aforementioned anecdotal reports. For some individuals, average impedance returned to baseline values; for others impedance remained higher than baseline as shown in the figure. For some, impedance increased and plateaued. The time courses of hearing sensitivity and impedance changes often were similar, but not always.
Figure 5.
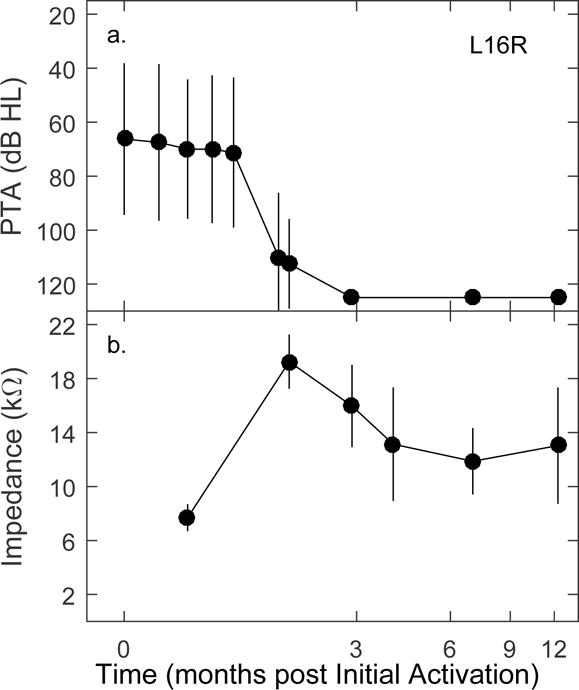
Example of one participant showing a precipitous drop in acoustic hearing (a) and coincident increase in electrode impedance (b). Symbols are the means across frequencies (a) or electrodes (b), vertical lines extend ± 1 standard deviation.
Figure 6 displays changes in impedance values (the average across electrodes) relative to the baseline value for individual study participants. Recall that the baseline impedance was taken from the first time point following initial activation that was available for each participant. Participants are grouped by hearing loss category (stable/symmetrical combined in panel a.), revealing differences in the impedance trajectories.
Figure 6.
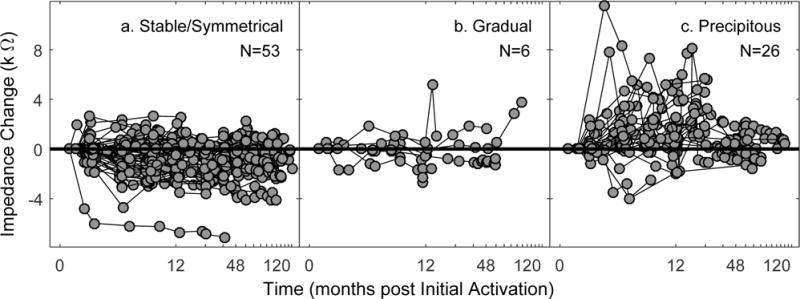
Impedance trajectories separated by hearing loss category (a. stable/symmetrical; b. gradual; c. precipitous). Each symbol is the change in average impedance across electrodes at a given time point for an individual. Individual data are connected by thin lines.
For individuals with stable hearing or symmetrical hearing loss, the majority of data points are around or below zero. For the six individuals with a gradual loss of hearing in the implanted ear, the impedance changes are near zero, with a few transient positive values. Due to the small sample size, it is not possible to make a general summary statement, and this group was not considered in the statistical analysis. For the individuals showing a precipitous loss of hearing, there are more data points above zero than below. This indicates that increasing impedance was observed more often than the slight decrease and relative stable impedance reported in the literature (e.g. Dorman et al. 1992; Hughes et al. 2001; Busby et al. 2002) and observed for the individuals in the stable/symmetrical group of the present study.
To test for group differences, the repeated impedance measures across time for an individual were reduced to a single value by calculating the (1) average and (2) maximum (in either positive or negative directions) change from baseline (horizontal line at zero; Fig 6). By averaging across time, the magnitude of impedance increases is reduced due to the transient nature, but the average retains the general decrease in impedance observed for the stable/symmetrical group. In figure 7, thick black bars are the grand mean across all individuals belonging to each group. Statistical analysis confirmed that both the average and maximum impedance changes for individuals with a precipitous drop in acoustic hearing were larger than that observed for individuals with stable/symmetrical hearing (one-tailed t-test; average: T77: 5.81; P<0.001; maximum: T77: 7.83; P<0.001).
Figure 7.
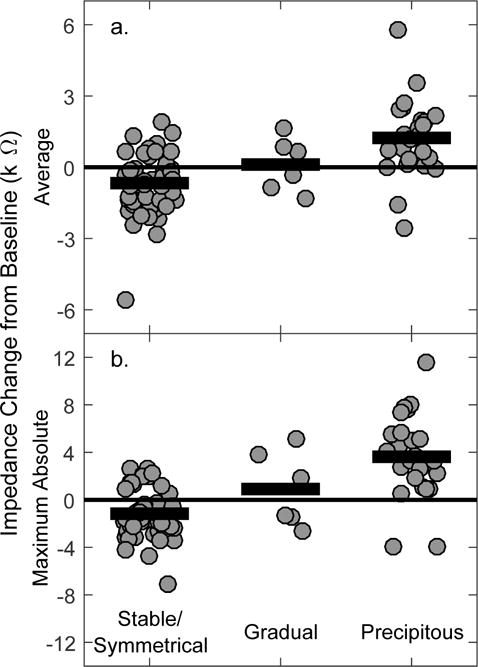
Average (a) and maximum (b) impedance changes across time separated by hearing loss category. Note the different y scales. Individual data are represented with gray circles, and group means with horizontal black bars.
To further investigate relationships among impedance changes and delayed-onset hearing loss, we implemented a linear mixed-effects model with impedance (change from baseline, averaged across electrodes) as the response variable. The mixed effects model readily allows for individuals that were not measured for the same range of time points. For this model, measurement time was split into categories of 0, 3, 6, 12, 24, and 36 months after device activation. The categorized time was then centered for each individual at the point of maximum impedance, so that we could better evaluate whether impedance changes related to changes in behavioral audiometric threshold. Due to the differences across individuals with respect to the time of maximum impedance, centering resulted in 21 time categories. Behavioral threshold, measurement time, and hearing loss category (two categories: stable/symmetrical and those with precipitous hearing loss) were the fixed explanatory variables in the model as well as an interaction between PTA and category. A random intercept for each individual was fit, along with an autoregressive model of order 1 (AR(1)) for the errors, which allows for correlation between measurements to decay at an exponential rate as time points get further apart. A random slope model could not be fit due to the non-linear way the impedance data were spread over time. We used Akaike’s Information Criterion (AIC) to compare various versions of the model; the reported model yielded the lowest AIC.
There was a significant time effect for impedance (X2=113.50; df=20; P <0.0001). The main effects for both PTA (X2=27.97; df=1; P<0.0001), and hearing loss category (X2=11.16; df=1; P=0.0008) were also significant. Most interesting in this analysis, and most relevant, is that the significant interaction between PTA and hearing loss category (X2=26.54; df=1; P<0.0001) implies that the relationship between PTA and impedance is different for the two groups. The slope between PTA and impedance for the precipitous group is 0.05. The interpretation is that at a time of 0 (maximum impedance change), a 10 dB increase in PTA is associated with a 0.5 kΩ increase in electrode impedance, on average. However, the slope is 0.001 for the stable/symmetrical group, meaning that there is essentially no relationship between change in PTA and change in impedance.
3.3. ECAP
The second objective of this study was to use the ECAP as a measure of neural responsiveness to assess whether global changes in neural health/survival might be evident for individuals demonstrating delayed hearing loss. ECAP growth functions were available for at least two time points in 48 individuals; visual detection thresholds, slope and amplitudes at a suprathreshold stimulus level were calculated. Because ECAPs are recorded using an intracochlear electrode, and ECAP measures may be affected by any change in electrode impedance, ECAP and impedance changes were evaluated for covariance. There were no correlations between impedance changes and changes in ECAP threshold (Pearson’s r: −0.13; P: 0.38), slope (r: −0.21; P: 0.15), or amplitude (r: −0.13; P: 0.38; data not shown). Scatterplots provided in Figure 8 relate changes in the three ECAP metrics to changes in PTA. There were no correlations between PTA changes and changes in ECAP threshold (Pearson’s r: −0.22; P: 0.13), slope (r: 0.10; P: 0.48), or amplitude (r: 0.12; P: 0.40). The results did not change when eliminating subjects with ECAP measures prior to 2 months (N=34; data not shown). Moreover, there is no pattern observed across hearing-loss categories with regards to ECAP changes. Individuals with and without delayed hearing loss show similar random scatter of ECAP changes.
Figure 8.
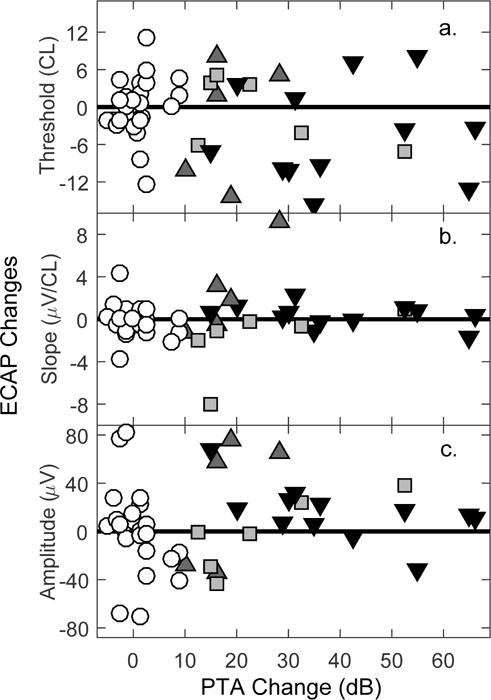
Scatterplots relating changes in ECAP measures (a. threshold, b. slope, c. amplitude) to changes in PTA. Symbols denote hearing loss category (white circles: stable; light gray squares: symmetrical; dark gray triangles: gradual; black triangles: precipitous).
4. Discussion
The data set comprising this report is unique in that the sample size is relatively large for this specialized population, in addition to the expansive time period over which repeated measures were made. Several key findings were identified, which will be discussed in more detail below. However, due to the retrospective nature and inherent limitations of design, these findings should be considered preliminary in nature and will require follow up with well-controlled, prospective studies.
4.1. Characteristics of delayed acoustic hearing loss
The majority of all patients undergoing hearing-preservation CI surgery maintain functional low-frequency acoustic hearing (e.g. Gstoettner et al. 2006; Santa Maria et al. 2013; Lenarz et al. 2013; Adunka et al. 2014; Helbig et al. 2016; van Abel et al 2015; Roland et al. 2016; Gantz et al. 2016), and that is true for the sample included in the present study as well. Approximately 80% of individuals in the present study had hearing thresholds better than 90 dB HL and were using acoustic amplification in combination with electrical stimulation at the conclusion of the study. Despite the generally positive outcomes, in this sample, delayed-onset acoustic hearing loss was relatively common (38%), and the progression was rapid in most cases (81%). This may reflect, in part, the relatively strict criteria used to define the hearing-loss categories, but also reveals that worsening hearing thresholds not apparent immediately following surgery is currently a relatively common post-operative risk that needs to be shared with patients in terms of realistic expectations.
In an attempt to present the data in a manner that reflected qualitative observations, a distinction was made between “gradual” and “precipitous” time courses of the delayed hearing loss. Although rules were used to objectify this distinction, they were chosen to result in categorization that was in agreement with our qualitative observations specific to this data set and not necessarily suggested for more general applications. Moreover, this distinction was partially dependent upon the spacing of the follow-up appointments when hearing loss was noted. It is possible that some of the changes in hearing would have been categorized as “precipitous” had follow-up appointments occurred closer together. For instance, one subject demonstrated a 10 dB loss of acoustic hearing within the first 6 months, during which 7 measurement times were available. Between 6 and 12 months, hearing dropped by another 20 dB in the implanted ear, but only two measurement times were available (at 6 and 12 months). Thus, this pattern of hearing loss was categorized as “gradual”; however, it is unknown when the 20 dB change actually occurred between those two measured time points. One might speculate that a sudden loss of hearing would be noticed by the individual, and we would hope that they would have requested an earlier follow-up appointment for possible intervention. Per experience and clinical observations, however, recipients of hearing preservation CIs are not always aware of changes in residual hearing. Based on our subjective opinion from viewing hundreds of audiograms, we felt that, despite the imperfections of categorization and inability to perform formal statistical analyses on the “gradual” group due to the small sample, keeping the distinction better reflected differences observed across individuals than grouping them with individuals demonstrating a “precipitous loss”. Although equal spacing of follow-up appointments would be beneficial, impracticality may preclude doing so for an extended period of time. An alternative method of describing changes in hearing status, such as focusing on the time of onset of delayed hearing loss may be another way to group individuals. Further exploration into the progression of delayed hearing loss, especially with respect to underlying etiology is warranted.
Although a proportionately greater number of recipients of S12 and L24 demonstrated delayed-onset hearing loss in the implanted ear compared to the S8 and 422 arrays, there was no evidence of a relationship between array type and hearing loss category in the statistical analysis. It is possible that delayed hearing loss has not yet been identified for a number of individuals receiving the 422 as a subset of recipients had used the device < 1 year at the time of creating this report. It will be important to continue to follow these recipients and newly implanted individuals to determine whether this preliminary finding holds.
4.2. Round window versus cochleostomy insertion
When hearing preservation is the goal, “soft” surgical techniques are advocated, and the trend has been to move away from cochleostomy insertions to round window insertions as one way to minimize trauma (e.g. Adunka et al. 2004 but see Briggs et al. 2006). However, a recent animal study found that progressive low-frequency hearing loss was more common in guinea pig recipients of electrode arrays that were inserted through the round window compared to a cochleostomy. One possible explanation given was that the material used to plug the incision at the round window resulted in greater damping of the scala tympani, changing the mechanics of the system (Rowe et al. 2016). Although not a primary goal of this study, the data lent itself to exploring the prevalence of delayed-onset hearing loss in humans with different insertion approaches to compare with the animal data. For recipients of L24 arrays, the proportion of individuals experiencing delayed hearing loss versus stable hearing was not significantly different according to insertion approach, which is consistent with a one-year follow-up study by Adunka and colleagues (2014). In a more recent report, Helbig and colleagues (2016) compared insertion approaches for long-term hearing preservation rates (up to 5 years). A higher proportion of individuals undergoing round window insertions tended to show partial hearing loss immediately follow surgery. Although some individuals with round window insertions also presented with delayed-onset hearing loss, a higher proportion of individuals undergoing cochleostomy insertions presented with delayed-onset complete hearing loss. The insertion approach in human subjects is not likely to be chosen at random as there are other factors, such as individual anatomy and array design, that are considered when making a decision about which approach will maximize successful insertion. Still, the issue is worth exploring further as any differences in technique could be useful for counseling patients post surgery regarding long-term expectations. An ideal insertion would minimize both immediate and progressive trauma.
4.3. Electrode Impedance
Though spontaneous increases in electrode impedance have been noted during instances of middle ear effusion, labyrinthitis and stimulation levels at or exceeding voltage compliance (Sainz et al. 2003; Neuburger et al. 2009), for adults, impedance is relatively stable for current carrying electrodes shortly after the device is activated (Dorman et al. 1992; Hughes et al. 2001; Busby et al. 2002). An exception is in the case of electrode migration (Dietz et al. 2016). A primary finding of this study was that many of the individuals who presented with a delayed loss of acoustic hearing in the implanted ear also exhibited transient increases in electrode impedance that are (1) coincident with the drop in hearing and (2) clearly deviant from the mean changes reported in the literature. Moreover, these fluctuations were not common for individuals with stable hearing or a symmetrical loss. These data provide quantitative evidence to support the anecdotal reports.
Even though our data were sufficient to provide statistical evidence of group differences in impedance changes, there may be better methods of using electrode impedance data to explore changes in the auditory system. For instance, in this study we averaged the impedance across all activated electrodes within a subject. Fibrous tissue growth, which was an underlying etiology we suspected might influence electrode impedance, is often more extensive in the base of the cochlea compared to more apical regions (e.g. Wilk et al. 2016). To the extent that measured electrode impedance reflects the extent of tissue growth, impedance measures of electrodes located in the base might be more informative than more apically located electrodes. Electrode migration, which is more common for straight arrays than curved, also affects the impedance of basally located electrodes more than apical electrodes (Dietz et al., 2016). At this center, the array is sutured to the tegmen for stabilization, which makes electrode migration unlikely, but without imaging data, electrode extrusion cannot be ruled out definitively (except for one subject whose temporal bones were available for post mortem analysis; Quesnel et al. 2015).
Electrode location was not systematically evaluated in this review partly due to the different number of electrodes, array lengths and insertion depths across devices. Moreover, a cursory look at the data suggested that even for a number of individuals with longer electrode arrays, changes in the impedance of apical electrodes were observed, and a number of clinicians have reported increased variability in impedance measures, resulting in a zigzag pattern across electrodes (reminiscent of the pattern observed with partial short circuits). Averaging across electrodes was sufficient to demonstrate that impedance changes for many individuals experiencing delayed hearing loss were atypical, but examination of individual electrodes could provide additional detail about the pattern of observed changes in this population and insight to underlying mechanisms.
A second limitation of this study is the use of total impedance. Total impedance, as measured with commercially available software, is a complex measure that can be broken down into access resistance and polarization impedance (resistive and reactive components). Access resistance is determined by the environment surrounding the electrode; whereas, polarization impedance is determined by the electrode-electrolyte interface, and reflects changes near the surface of the electrode (Dymond 1976; Tykocinski et al. 2005). The typical time course is not the same for both components, with polarization impedance more sensitive to changes in electrical stimulation than access resistance (Tykocinski et al. 2005; Newbold et al. 2013). With the data available for review in the present study, it was not possible to separate total impedance into these two components, so it is unclear whether the observed transient increases in impedance were reflecting changes in access resistance or polarization impedance, or both. We plan to continue to assess changes in impedance over time using software that will allow separation of total impedance into these two components for a more detailed description of the patterns that might provide insight into changes in auditory status.
4.4. ECAP
It should be noted that the lack of prospective control has the potential to impact the validity of the ECAP data more than the impedance data, and the inferences and speculations from the results should be considered accordingly. In the present study, we hypothesized that if the underlying mechanism of delayed hearing loss involved global neural changes, we would have seen a correlation between changes in ECAPs and changes in acoustic hearing thresholds. We did not find such correlations using any of the ECAP metrics: thresholds, slopes, or suprathreshold amplitudes. It may be that for this population, neural involvement was not responsible for the changes in acoustic hearing; however, the limitations of the data set may have precluded observing a difference. For example, some individuals had repeated measures of a single electrode; others had repeated measures of all available electrodes, which provided a more complete picture of neural responsiveness across a broader region of the cochlea. Additionally, ECAP measures were not always available for the times at which changes in acoustic hearing were identified. For some individuals, this meant being recategorized as having “stable” hearing and focusing on a truncated time period. Alternatively, it may be that neural involvement occurred, but was localized to a more apical region than can be assessed with the ECAP measures that use stimulation from more basally located electrodes. This is especially true for the short (“S”) arrays, but remains an issue even for the more deeply inserted electrode arrays (L24 and 422). We are currently assessing this hypothesis using a technique we call acoustic NRT. Low-frequency acoustic stimuli are used to excite the region of residual hearing, and cochlear/neural responses are recorded using the intracochlear CI electrodes (Abbas et al. 2017; Campbell et al. 2014; Koka et al. 2017). Research in this area is ongoing. One caveat is then whole nerve data may not necessarily capture subtle changes to single nerve fibers.
The changes in ECAP threshold, slope and suprathreshold amplitude are, on average, close to zero, which is consistent with previous studies demonstrating relatively stable ECAP measures over time (Hughes et al. 2001; Brown et al. 2010). This outcome, along with the lack of correlation with changes in behavioral thresholds, is reassuring given that individuals who lose residual acoustic hearing become more reliant on the effectiveness of electrical stimulation from the CI.
4.5. Mechanisms of delayed hearing loss
The impedance data within this report are suggestive of fibrous tissue growth as a potential contributor to delayed hearing loss for at least some individuals. This conclusion is inferred based on previous studies showing that electrode impedance is sensitive to the presence of tissue cells on or surrounding the electrode (Newbold et al. 2004; Wilk et al. 2016) and that the extent of tissue growth in the cochlea is related to progressive hearing loss (O’Leary et al. 2013; but see also Wilk et al. 2016). To the extent that this inference is correct, whether the tissue is changing the passive mechanics or whether the inflammation response is resulting in sensorineural damage is not clear. Our measures of neural responsiveness did not demonstrate changes coincident with a loss of acoustic hearing, suggesting that for at the least regions stimulated electrically, neural responses did not change enough to affect the composite response. However, it is possible that changes to neurosensory structures are either more subtle than can be assessed with the ECAP or localized to a more apical region responsible for processing low frequency acoustic signals. To the extent that fibrous tissue growth contributes to delayed hearing loss following cochlear implantation, future efforts to ameliorate this growth are warranted. Such strategies could include use of pharmacological agents (e.g. corticosteroids), modification of implant materials, and/or modification of surgical approaches, among others (e.g. Bas et al. 2016; Wilk et al., 2016).
The transient nature of the impedance increase tempers the speculation regarding fibrous tissue to some extent, and other mechanisms involving changes in the intracochlear environment should also be considered. Moreover, a number of individuals with delayed hearing loss did not show impedance changes. It may be that due to the retrospective nature of this report, the impedance data available were insufficient to capture changes that occurred. Alternatively, it is unlikely that all individuals with delayed hearing loss have the same underlying mechanism. Other potential causes of acoustic hearing loss following cochlear implantation include loss of outer and/or inner hair cells, excitotoxicity affecting the hair cell-neuron synapse, and/or damage to the lateral wall/stria vascularis affecting blood supply (Tanaka et al. 2014; Reiss et al. 2015; Kopelovich et al. 2015). Moreover, the potential role of electrode migration deserves further attention. Although group data are helpful for identifying trends and patterns, it is important that we continue to find ways to understand changes in auditory status for individuals, for whom different mechanisms might be at work.
5. Conclusions
Although hearing preservation following cochlear implantation can be achieved in most cases, more than one third of individuals in this sample who had acoustic hearing immediately following surgery exhibited various degrees of delayed-onset hearing loss relegated to the implanted ear. For the majority, the loss occurred within the first year; for others, the changes in auditory status occurred after several years. Abrupt increases in electrode impedance are one characteristic of this group, which suggests a change in the physical environment surrounding the electrode (and possibly of the cochlear labyrinth). As a noninvasive, objective measure that can be easily obtained clinically for any individual with a CI, repeated measures of electrode impedance are important to consider in the evaluation of underlying mechanisms of hearing loss. Noninvasive measures sensitive to neurosensory and endocochlear potential changes should continue to be evaluated as well.
Highlights.
Delayed-onset hearing loss was observed in 38% of this sample.
A drop in hearing was associated with increasing electrode impedance.
The impedance increase was often noted to be abrupt and transient.
Behavioral threshold changes were not correlated with ECAP changes.
Acknowledgments
We gratefully acknowledge the audiologists and cochlear implant team at the University of Iowa for providing clinical care to the study participants, for their thorough evaluations which contributed to the amount of data available for review, and for sharing their observations regarding impedance fluctuations. Moreover, we thank past and current research assistants who were involved with data collection, analysis, and entry over the past 15 years. Specifically, we thank Krista Ostwinkle, Megan Rossio, Kylee McFarlin, Tyler Ellis, and Kelsey Klein for their efforts to compile and organize the data sets used for this paper. Finally, we thank the CI participants who received investigational devices and committed to an intense schedule of follow-up appointments. This work was supported in part by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders [P50-DC000242].
Abbreviations
- AIC
Akaike’s Information Criterion
- ANOVA
Analysis of Variance
- CI(s)
cochlear implant(s)
- ECAP(s)
electrically evoked compound action potential(s)
- dB
decibels
- FDA
Food and Drug Administration
- Hz
Hertz
- IDE
Investigational Device Exemption
- N
number
- NRT
Neural Response Telemetry
- PTA
pure tone average
- sd
standard deviation
- Z
impedance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
B.J.G. is a consultant for Cochlear Corporation and Advanced Bionics companies. He holds a patent on the hybrid CI, but receives no royalties. The authors declare that they have no other conflicts of interest.
References
- Abbas PJ, Tejani VD, Scheperle RA, Brown CJ. Using neural response telemetry to monitor physiological responses to acoustic stimulation in hybrid cochlear implant users. Ear Hear. 2017 doi: 10.1097/AUD.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adunka OF, Dillon MT, Adunka MC, King ER, Pillsbury HC, Buchman CA. Cochleostomy versus round window insertions: influence on functional outcomes in electric-acoustic stimulation of the auditory system. Otol Neurotol. 2014;35:613–618. doi: 10.1097/MAO.0000000000000269. [DOI] [PubMed] [Google Scholar]
- Adunka O, Gstoettner W, Hambek M, Unkelbach MH, Radeloff A, Kiefer J. Preservation of basal inner ear structures in cochlear implantation. Orl. 2004;66:306–312. doi: 10.1159/000081887. [DOI] [PubMed] [Google Scholar]
- Bas E, Bohorquez J, Goncalves S, Perez E, Dinh CT, Garnham C, Hessler R, Eshraghi AA, Van De Water TR. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: A dose response study. Hear Res. 2016 doi: 10.1016/j.heares.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Bas E, Goncalves S, Adams M, Dinh CT, Bas JM, Van De Water TR, Eshraghi AA. Spiral ganglion cells and macrophages initiate neuro-inflammation and scarring following cochlear implantation. Front Cell Neurosci. 2015;9:1–16. doi: 10.3389/fncel.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs RJS, Tykocinski M, Xu J, Risi F, Svehla M, Cowan R, Stover T, Erfurt P, Lenarz T. Comparison of round window and cochleostomy approaches with a prototype hearing preservation electrode. Audiol Neurotol. 2006;11:42–48. doi: 10.1159/000095613. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Etler CP, O’Brien S, Oleson JJ. Effects of long-term use of a cochlear implant on the electrically evoked compound action potential. J Am Acad Audiol. 2010;21:5–15. doi: 10.3766/jaaa.21.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby PA, Plant KL, Whitford LA. Electrode impedance in adults and children using the Nucleus 24 cochlear implant system. Cochlear Implants Int. 2002;3:87–103. doi: 10.1002/cii.55. [DOI] [PubMed] [Google Scholar]
- Campbell L, Kaicer A, Briggs R, et al. Cochlear response telemetry: Intracochlear electrocochleography via cochlear implant neural response telemetry pilot study results. Otol Neurotol. 2014;36:399–405. doi: 10.1097/MAO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- Carhart R, Jerger JF. Preferred method for clinical determination of pure-tone thresholds. Journal of Speech & Hearing Disorders. 1959;24:330–345. doi: 10.1044/jshd.2404.330. [DOI] [Google Scholar]
- Choi C, Oghalai JS. Predicting the effect of post-implant cochlear fibrosis on residual hearing. Hear Res. 2005;205:193–200. doi: 10.1016/j.heares.2005.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz A, Wennström M, Lehtimäki A, Löppönen H, Valtonen H. Electrode migration after cochlear implant surgery: more common than expected? European Archives of Oto-Rhino-Laryngology. 2016;273:1411–1418. doi: 10.1007/s00405-015-3716-4. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Smith LM, Dankowski K, McCandless G, Parkin JL. Long-term measures of electrode impedance and auditory thresholds for the Ineraid cochlear implant. J Speech Hear Res. 1992;35:1126–1130. doi: 10.1044/jshr.3505.1126. [DOI] [PubMed] [Google Scholar]
- Dunn CC, Etler C, Hansen M, Gantz BJ. Successful Hearing Preservation After Reimplantation of a Failed Hybrid Cochlear Implant. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc [and] Eur Acad Otol Neurotol. 2015;36:1628–1632. doi: 10.1097/MAO.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond AM. Characteristics of the metal-tissue interface of stimulation electrodes. IEEE Trans Biomed Eng. 1976;23:274–280. doi: 10.1109/TBME.1976.324585. [DOI] [PubMed] [Google Scholar]
- Eshraghi AA, Lang DM, Roell J, Van De Water TR, Garnham C, Rodrigues H, Guardiola M, Gupta C, Mittal J. Acta Oto-Laryngologica Mechanisms of programmed cell death signaling in hair cells and support cells post-electrode insertion trauma Mechanisms of programmed cell death signaling in hair cells and support cells post-electrode insertion trauma. Acta Otolaryngol. 2015;135:328–334. doi: 10.3109/00016489.2015.1012276. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Makarem AO, Linthicum FH. Histopathologic assessment of fibrosis and new bone formation in implanted human temporal bones using 3D reconstruction. Otolaryngol - Head Neck Surg. 2009;141:247–252. doi: 10.1016/j.otohns.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: Final outcomes. Laryngoscope. 2016;126:962–973. doi: 10.1002/lary.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of Hearing in Cochlear Implant Surgery: Advantages of Combined Electrical and Acoustical Speech Processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- Gstoettner WK, Helbig S, Maier N, Kiefer J, Radeloff A, Adunka OF. Ipsilateral electric acoustic stimulation of the auditory system: results of long-term hearing preservation. Audiol Neurotol. 2006;11(suppl 1):49–56. doi: 10.1159/000095614. [DOI] [PubMed] [Google Scholar]
- Helbig S, Adel Y, Rader T, Stover T, Baumann U. Long-term hearing preservation outcomes after cochlear implantation for electric-acoustic stimulation. Otol Neurotol. 2016;37:e353–e359. doi: 10.1097/MAO.0000000000001066. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Vander Werff KR, Brown CJ, Abbas PJ, Kelsay DM, Teagle HF, Lowder MW. A longitudinal study of electrode impedance, the electrically evoked compound action potential, and behavioral measures in nucleus 24 cochlear implant users. Ear Hear. 2001;22:471–486. doi: 10.1097/00003446-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Hunter JB, Gifford RH, Wanna GB, Labadie RF, Bennett ML, Haynes DS, Rivas A. Hearing preservation outcomes with a mid-scala electrode in cochlear implantation. Otol Neurotol. 2016;37:235–240. doi: 10.1097/MAO.0000000000000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka K, Saoji AM, Litvak LM. Electrocochleography in cochlear implant recipients with residual hearing: Comparison with audiometric thresholds. Ear Hear. 2017 doi: 10.1097/AUD.0000000000000385. [DOI] [PubMed] [Google Scholar]
- Kopelovich JC, Reiss LAJ, Etler CP, Xu L, Bertroche JT, Gantz BJ, Hansen MR. Hearing loss after activation of hearing preservation cochlear implants might be related to afferent cochlear innervation injury. Otol Neurotol. 2015;36:1035–1044. doi: 10.1097/MAO.0000000000000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz T, James C, Cuda D, Fitzgerald O’Connor A, Frachet B, Frijns JH, Klenzner T, Laszig R, Manrique M, Marx M, Merkus P. European multi-centre study of the Nucleus Hybrid L24 cochlear implant. Int J Audiol. 2013;52:838–848. doi: 10.3109/14992027.2013.802032. [DOI] [PubMed] [Google Scholar]
- Linthicum FH, Fayad J, Otto SR, Galey FR, House WF. Cochlear implant histopathology. Am J Otol. 1991 doi: 10.2144/05384CI01. [DOI] [PubMed] [Google Scholar]
- Moteki H, Nishio SY, Miyagawa M, Tsukada K, Iwasaki S, Usami SI. Long-term results of hearing preservation cochlear implant surgery in patients with residual low frequency hearing. Acta Oto-Laryngologica. 2016:1–6. doi: 10.1080/00016489.2016.1252061. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Eddington DK, Burgess BJ. Foreign body hypersensitivity granuloma of the inner ear following cochlear implantation. One possible cause of a soft failure? Otol Neurotol. 2008;29:1076–1084. doi: 10.1097/MAO.0b013e31818c33cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger J, Lenarz T, Lesinski-Schiedat A, Büchner A. Spontaneous increases in impedance following cochlear implantation: suspected causes and management. Int J Audiol. 2009;48:233–239. doi: 10.1080/14992020802600808. [DOI] [PubMed] [Google Scholar]
- Newbold C, Mergen S, Richardson R, Seligman P, Millard R, Cowan R, Shepherd R. Impedance changes in chronically implanted and stimulated cochlear implant electrodes. Cochlear Implants International. 2013 doi: 10.1179/1754762813Y.0000000050. [DOI] [PubMed] [Google Scholar]
- Newbold C, Richardson R, Huang CQ, Milojevic D, Cowan R, Shepherd R. An in vitro model for investigating impedance changes with cell growth and electrical stimulation: implications for cochlear implants. J Neural Eng. 2004;1:218–27. doi: 10.1088/1741-2560/1/4/005. [DOI] [PubMed] [Google Scholar]
- O’Leary SJ, Monksfield P, Kel G, Connolly T, Souter MA, Chang A, Marovic P, O’Leary JS, Richardson R, Eastwood H. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Quesnel AM, Nakajima HH, Rosowski JJ, Hansen MR, Gantz BJ, Nadol JB. Delayed loss of hearing after hearing preservation cochlear implantation: Human temporal bone pathology and implications for etiology. Hear Res. 2015;333:225–234. doi: 10.1016/j.heares.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LAJ, Stark G, Nguyen-Huynh AT, Spear KA, Zhang H, Tanaka C, Li H. Morphological correlates of hearing loss after cochlear implantation and electro-acoustic stimulation in a hearing-impaired Guinea pig model. Hear Res. 2015;327:163–174. doi: 10.1016/j.heares.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JT, Gantz BJ, Waltzman SB, Parkinson AJ. United States multicenter clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope. 2016;126:175–181. doi: 10.1002/lary.25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D, Chambers S, Hampson A, Eastwood H, Campbell L, O’Leary S. Delayed low frequency hearing loss caused by cochlear implantation interventions via the round window but not cochleostomy. Hear Res. 2016;333:49–57. doi: 10.1016/j.heares.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Sainz M, Roldan C, de la Torre A, Gonzalez M, Ruiz J. Transitory alterations of the electrode impedances in cochlear implants associated to middle and inner ear diseases. Int Congr Ser. 2003;1240:407–410. doi: 10.1016/S0531-5131(03)00774-X. [DOI] [Google Scholar]
- Santa Maria PL, Domville-Lewis C, Sucher CM, Chester-Browne R, Atlas MD. Hearing preservation surgery for cochlear implantation–hearing and quality of life after 2 years. Otol Neurotol. 2013;34:526–31. doi: 10.1097/MAO.0b013e318281e0c9. [DOI] [PubMed] [Google Scholar]
- Seyyedi M, Nadol JB. Intracochlear inflammatory response to cochlear implant electrodes in the human. Otol Neurotol. 2014;35:1545–1551. doi: 10.1097/MAO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka C, Nguyen-Huynh A, Loera K, Stark G, Reiss L. Factors associated with hearing loss in a normal-hearing guinea pig model of hybrid cochlear implants. Hear Res. 2014;316:82–93. doi: 10.1016/j.heares.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tykocinski M, Cohen LT, Cowan RS. Measurement and analysis of access resistance and polarization impedance in cochlear implant recipients. Otol Neurotol. 2005;26:948–56. doi: 10.1097/01.mao.0000185056.99888.f3. doi:00129492-200509000-00021 [pii] [DOI] [PubMed] [Google Scholar]
- Van Abel KM, Dunn CC, Sladen DP, Oleson JJ, Beatty CW, Neff BA, Hansen M, Gantz BJ, Driscoll CLW. Hearing preservation among patients undergoing cochlear implantation. Otol Neurotol. 2015;36:416–421. doi: 10.1097/MAO.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk M, Hessler R, Mugridge K, Jolly C, Fehr M, Lenarz T, Scheper V. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can be reduced using a dexamethasone eluting electrode. PLoS One. 2016;11:1–19. doi: 10.1371/journal.pone.0147552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: A physiological and histopathological study. Hear Res. 1997;105:1–29. doi: 10.1016/S0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]


