Abstract
Objective
To assess the extent to which (1) clinicians, using or not using conversation aids, foster choice awareness during clinical encounters and (2) fostering choice awareness, with or without conversation aids, is associated with greater patient involvement in shared decision making (SDM).
Patients and Methods
We randomly selected 100 video-recorded encounters, stratified by topic and study arm, from a database of 10 clinical trials of SDM interventions in 7 clinical contexts: low-risk acute chest pain, stable angina, diabetes, depression, osteoporosis, and Graves disease. Reviewers, unaware of our hypothesis, coded recordings with the OPTION-12 scale to quantify the extent to which clinicians involved patients in decision making (SDM, 0-100 score). Blinded to OPTION-12 scale scores, we used a self-developed coding scale to code whether and how choice awareness was fostered.
Results
Clinicians fostered choice awareness in 53 of 100 encounters. Fostering choice awareness was associated with a higher OPTION-12 scale score (adjusted [for using vs not using a conversation aid] predicted mean difference, 20; 95% CI, 11-29). Using a conversation aid was associated with a higher, nonsignificant chance of fostering choice awareness (N=31 of 50 [62%] vs N=22 of 50 [44%]; adjusted [for trial] P=.34) and with a higher OPTION-12 scale score, although adjusting for fostering choice awareness mitigated this effect (adjusted predicted mean difference 5.8; 95% CI, −1.3-12.8).
Conclusion
Fostering choice awareness is linked to a better execution of other SDM steps, such as informing patients or discussing preferences, even when SDM tools are not available or not used.
Abbreviations and Acronyms: SDM, shared decision making
Two roads diverged in a yellow wood,
And sorry I could not travel both
—Robert Frost “The Road Not Taken”
The recognition that care should be patient-centered and that patients should be involved in their care is growing. This is considered particularly pertinent when more than one reasonable approach is available to manage the patient's situation (including doing nothing else) and when these approaches differ in ways that matter to these patients.1, 2, 3 In shared decision making (SDM), clinicians and patients work together to figure out how to best address the patient's situation and to make decisions about health and care that fit each patient and their lives.4 Most SDM models distinguish 3 key steps before reaching a decision: (1) creating choice awareness, (2) discussing the relevant options, and (3) discussing patient preferences.2, 3, 5 To date, most SDM research and implementation, including the efforts to develop, test, and implement SDM tools, have mainly focused on the second and third steps of SDM and on making the final decision. Nevertheless, the first step of creating choice awareness—that is, acknowledging that the patient's situation is mutable and that there is more than one sensible way to address or change this situation—is considered pivotal.2
Creating choice awareness may engender subsequent steps of SDM, alerting patients that decisions about their health or care are about to be made and that these decisions require their input insofar as these decisions should reflect what matters to patients. Adequate choice awareness could therefore potentially lead to better or easier execution of these subsequent SDM steps. Despite its importance, what is and how to measure the process of fostering choice awareness has received little attention. We recently showed that oncologists express the need to make a treatment decisions about (neo-)adjuvant cancer treatment in only 3% of pretreatment encounters, and instead, use the encounter to explain the one approach they recommend.6 Also, Couët et al7 reported that in only 1 in 3 SDM studies, clinicians state that “there is more than one way to deal with the identified problem.”
Tools to support the process of SDM, such as (patient) decision aids and conversation aids, may explicitly mention that there is more than one sensible option available to address the patient's situation.4, 8 Access and use of these tools during the encounter may make it easier for clinicians to act toward creating choice awareness (henceforth referred to as “fostering choice awareness”) or to skip this step, assuming the tool alone could do the work.
The aims of this study were to assess the extent to which (1) clinicians, using or not using SDM tools, foster choice awareness during clinical encounters and (2) fostering choice awareness, with or without SDM tools, is associated with greater patient involvement in decision making.
Patients and Methods
Study Design
A random sample of recorded clinical encounters from 10 clinical trials (9 randomized and 1 before-after design) was analyzed to assess communication between patients and clinicians. We first selected 20 encounters as a training set and to define behaviors likely to foster choice awareness. We then randomly selected a convenience sample of 100 additional encounters to code such behaviors. The Mayo Clinic Institutional Review Board approved each of the included trials (along with the boards of participating sites) and this secondary analysis. Patients and clinicians provided written informed consent about the use of trial data and video recordings for research before the encounter.
Data Source
We identified 838 videotaped encounters from 10 completed trials conducted by the Knowledge and Evaluation Research Unit, Mayo Clinic (Rochester, Minnesota).9 Most of these trials included patients outside the referral practice of the Mayo Clinic and involved primary and specialty care, physicians and nurses, and emergency and ambulatory settings (Table 1).10, 11, 12, 13, 14, 15, 16, 17, 18, 19 These multicenter trials compared usual care (with clinicians conducting the encounter as they saw fit) with the use of a within-encounter conversation aid, an SDM tool designed to convey evidence and promote SDM during the encounter. Participating clinicians received training on how to use the conversation aid before their first use, in the form of either a brief (<10 minutes) demonstration or a video-clip or storyboard.20
Table 1.
Trial Characteristics
| Trial | Reference | Disease state | No. of patients | Site | Clinical context | Randomization level | Date | Locale | Conversation aid type | Details of choice | Total encounters included for this secondary analysis/encounters with conversation aid |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Statin choice | Weymiller et al,10 2007 | Cardiovascular primary prevention | 93 | Single | Diabetes clinic visit for prevention of CAD | Clinician | 2005 | Suburban | Risk calculator | Dichotomous (statin or not) | 6 (6%)/3 (50%) |
| Diabetes | Mullan et al,11 2009 | Diabetes | 85 | Single | Primary care clinic visit for diabetic management | Clinician | 2007-2008 | Suburban and rural | Issue cards | Multiple drug options for diabetes: oral vs injectable medications | 7 (7%)/2 (29%) |
| Osteo I | Montori et al,12 2011 | Osteoporosis | 100 | Single | Primary care clinic visit for osteoporosis prevention | Patient | 2007-2008 | Suburban | Risk calculator | Dichotomous (bisphosphonate or not) | 10 (10%)/6 (60%) |
| Osteo II | LeBlanc et al,13 2015 | Osteoporosis | 79 | Single | Primary care clinic visit for osteoporosis prevention | Patient | 2009-2010 | Suburban | Risk calculator | Dichotomous (bisphosphonate or not) | 2 (2%)/1 (50%) |
| DAD | Branda et al,14 2013 | Diabetes/cardiovascular primary prevention | 104 | Multisite | Primary care clinic visit for diabetic management | Clinician | 2010-2011 | Suburban and rural | Issue cards/risk calculator | Multiple drug options for diabetes: oral vs injectable medications/dichotomous (statin or not) | 18 (18%)/10 (56%) |
| Chest pain | Hess et al,15 2012 | Chest pain | 204 | Single | Emergency department visit for chest pain | Patient | 2010-2011 | Suburban | Risk calculator | Dichotomous (admit for observation or discharge home with follow-up) | 14 (14%)/6 (43%) |
| iADAPT | LeBlanc et al,16 2015 | Depression | 297 | Multisite | Primary care clinic for depression management | Site | 2011-2013 | Suburban and rural | Issue cards | Multiple medication options | 13 (13%)/5 (38%) |
| TRICEP | ClinicalTrials.gov NCT0129357817 | Diabetes | 350 | Multisite | Primary care clinic visit for diabetic management | Site | 2011-2013 | Suburban and rural | Issue cards | Multiple drug options for diabetes: oral vs injectable medications | 11 (11%)/6 (55%) |
| PCI Choice | Coylewright et al,18 2016 | Stable CAD | 124 | Single | Outpatient cardiology clinic | Patient | 2012-2014 | Suburban | Issue cards | Medicine alone vs medicine plus stents | 7 (7%)/4 (57%) |
| Graves disease | Brito et al,19 2015 | Graves disease | 68 | Single | Outpatient discussion within specialty care (endocrinology) | No randomization pre vs post |
2013-2015 | Suburban | Issue cards | Treatment choice: antithyroid drugs, radioiodine ablation, surgery | 12 (12%)/7 (58%) |
CAD = coronary artery disease; DAD = Decision Aids to Enhance Shared Decision Making for Diabetes; iADAPT = Innovative Adaptation and Dissemination of AHRQ Comparative Effectiveness Research Products; PCI = percutaneous coronary intervention; TRICEP = Translating Information on Comparative Effectiveness into Practice.
Of the 10 trials, 6 (438 encounters) took place in primary care and 4 (400 encounters) in specialty care. We randomly selected 100 encounters from the 10 trials. This sample size allowed us to have enough videos to adequately stratify them by trial arm (care as usual vs conversation aid) and conversation aid type (whether the key decisional task required either risk communication for the selection of a risk-reducing approach [“risk calculator”] or the selection of a treatment alternative based on treatment characteristics of most importance to each patient [“issue cards”]). Recordings lasted, on average, 20 minutes (range, 1-73 minutes).
The conversation of interest addressed decisions to be made about patients' health or care when more than one reasonable approach was available. Conversations recorded in the 10 trials were related to 7 clinical contexts, namely, primary prevention of coronary artery disease and the management of low-risk acute chest pain, stable angina, diabetes, depression, osteoporosis, and Graves disease.
Data Extracted
We extracted patient and clinician characteristics from each clinical trial along with arm assignment. In addition, we extracted the OPTION-12 scale scores for each encounter. This scale is the most frequently used scale to quantify the extent to which clinicians sought to involve patients in decision making (0-100 scale).7, 21, 22 Reviewers had rated each video with substantial interrater agreement (κ>.7) and, because this scoring preceded the formulation of our project, were unaware of our hypothesis. OPTION-12 scale scores of encounters coded in duplicate were averaged.
Measures
We developed a coding scheme to detect and characterize behaviors likely to foster choice awareness. Codes were drafted deductively on the basis of SDM models to capture behaviors to either foster or disrupt choice awareness.3, 6 These codes were refined on the basis of a sample of 20 encounters selected by the statistician in which varying OPTION-12 scale scores and spread across studies and trial arms was taken into account. Table 2 reports the final codes used to describe fostering choice awareness. When in doubt, coders used the higher code, that is, the one representing more fostering of choice awareness.
Table 2.
Clinician Behaviors to Foster Choice Awareness
| Fostering choice awareness behavior | Total |
Care as usual |
Conversation aid |
|||
|---|---|---|---|---|---|---|
| No. (%) | OPTION-12 scale score (mean, 95% CI) | No. (%) | OPTION-12 scale score (mean, 95% CI) | No. (%) | OPTION-12 scale score (mean, 95% CI) | |
| Choice awareness not fostered | 47 | 28 | 19 | |||
| 1. The clinician does not create choice awareness; rather, the clinician informs on the next step in management without introducing other options for consideration | 34 (72) | 19.4 (12.2-26.6) | 19 (68) | 17.4 (7.4-27.3) | 15 (79) | 21.9 (10.4-33.5) |
| 2. The clinician does not create choice awareness; rather, the clinician makes a recommendation that implies the existence of alternatives but without explicitly mentioning these | 13 (28) | 15.0 (9.3-20.7) | 9 (32) | 14.5 (6.9-22.1) | 4 (21) | 16.1 (0.5-31.8) |
| Choice awareness fostered | 53 | 22 | 31 | |||
| 3. The clinician creates choice awareness by listing relevant options followed by recommending one of these to the patient | 15 (28) | 30.9 (23.0-38.8) | 9 (41) | 27.8 (16.6-38.9) | 6 (19) | 35.6 (21.0-50.2) |
| 4. The clinician creates choice awareness by listing relevant options without recommending one of these to the patient | 38 (72) | 39.1 (32.6-45.5) | 13 (59) | 33.4 (22.6-44.2) | 25 (81) | 42.0 (33.6-50.4) |
Two investigators (M.K. and V.M.M.) coded all encounters in duplicate, blinded to the OPTION-12 scale scores. Disagreements (which occurred in 6 of the videos) were resolved by discussion and consensus.
Statistical Analyses
Patient and clinician characteristics were compared across choice awareness groups using the χ2 test or the Fisher exact test for categorical comparisons and Wilcoxon rank sum for continuous comparisons. We used cluster (trial)-adjusted χ2 tests to evaluate the hypothesis of association between arm (usual care vs conversation aid), conversation aid type (risk calculation vs issue cards), and fostering choice awareness (no/yes). We formed a generalized linear model, with trial as a random effect and adjusted by arm, to assess the association between fostering choice awareness (no/yes) or choice awareness behavior (codes 1-4 in Table 2) and clinician behavior to engage patients (OPTION-12 scale score). Because OPTION-12 scale scores did not follow a normal distribution, we transformed the mean using a log-link function. The recycled predictions23 (marginal effect) are presented for the adjusted mean effect of fostering choice awareness on OPTION-12 scale scores. The marginal effect of fostering choice awareness on OPTION-12 scale scores takes the average predicted score on OPTION-12 scale scores after fixing the value of fostering choice awareness to either being present or not. To assess whether the relationship with fostering choice awareness varied among items of the OPTION-12 scale score, we reported the unadjusted means and SDs. A cluster-adjusted t test for each OPTION-12 scale item was conducted, accounting for clustering by study. We report the adjusted mean difference and 95% CIs. Statistical analyses were performed using StataSE, version 14 (StataCorp).
Results
Participant Characteristics
Patients (N=100) had a mean age of 58 years, 50 (50%) were women, and 35 (35%) had at least a 4-year college degree. Clinicians (N=79) were mostly men (52; 66%) and 30 (38%) were postgraduate medical trainees. Most clinicians participated in 1 recorded encounter and at most in 4 (Table 3).
Table 3.
Patient and Clinician Characteristics
| Patient characteristicsa | Total (N=100) |
|---|---|
| Age (y), mean ± SD | 58±13.2 |
| Women, No. (%) | 50 (50) |
| Education | |
| High school graduation or less, No. (%) | 26 (27) |
| Clinician characteristicsa | Total (N=79) |
|---|---|
| Women, No. (%) | 27 (34) |
| Type of clinician, No. (%) | |
| Consultant | 49 (62) |
| Fellow/resident | 11 (14) |
| Registered nurse | 16 (20) |
| Physician assistant | 3 (4) |
| Practice type, No. (%) | |
| Primary care | 48 (61) |
| Specialty care | 17 (22) |
| Emergency medicine | 13 (17) |
| Encounters per clinician, mean (range) | 1 (1-4) |
| Count of clinicians with encounter that had a conversation aid, No. (%) | 47 (60) |
No significant difference across fostering choice awareness (no/yes).
Fostering Choice Awareness
In 47 of the 100 encounters, clinicians did not foster choice awareness. Instead, in most of these encounters, clinicians stated the next step in management without mention of options (N=34 [72%]). In the other 53 encounters, clinicians fostered choice awareness by listing relevant options either without (N=38 of 53 encounters [72%]) or with a recommendation (see Table 2). We found no associations between patient or clinician demographic characteristics and fostering choice awareness.
Association Between Fostering Choice Awareness and Subsequent Steps of SDM
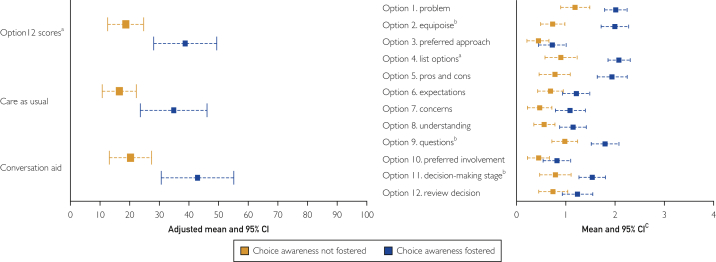
OPTION-12 scale scores were higher in encounters in which clinicians fostered choice awareness (adjusted predicted mean difference, 20; 95% CI, 11-29) (Figure). Compared with not fostering choice awareness (codes 1 and 2) or fostering choice awareness by listing the options combined with a recommendation (code 3), OPTION-12 scale scores were highest when clinicians fostered choice awareness by listing the available options without making a recommendation (code 4, see Table 2), although this association was not statistically significant. Average scores on all individual items of the OPTION-12 scale scores were higher in encounters in which choice awareness was fostered and significantly higher for items 2 (equipoise), 4 (listing of options), and 9 (opportunities to ask questions) (Figure).
Figure.
OPTION-12 scale scores and scores on individual OPTION-12 scale items in encounters in which choice awareness was vs was not fostered and in which a conversation aid was vs was not used. aSignificance <.001. bSignificance <.05. cSignificance reflects cluster adjusted analysis.
In encounters in which clinicians fostered choice awareness but OPTION-12 scale scores were low, we observed that right after fostering choice awareness, either the patient or the clinician quickly made a final decision, with the other party agreeing. There was no further discussion of what the options entailed or of what the patient's preferences were. In encounters in which the opposite occurred, that is, clinicians did not foster choice awareness but OPTION-12 scale scores were high, the clinician and patient spent the encounter discussing in detail the available evidence about only one option, leading to high scores on the OPTION-12 scale items assessing information-giving behavior.
Conversation Aids and Choice Awareness
The use of SDM tools, regardless of type (risk communication or issue comparison) was associated with a nonsignificant higher likelihood that clinicians would foster choice awareness (N=31 of 50 [62%] vs N=22 of 50 [44%]; adjusted P=.34). OPTION-12 scale scores were only modestly better with the conversation aid after adjusting for choice awareness (adjusted predicted mean difference, 5.8; 95% CI, −1.3 to 12.8).
Discussion
Restatements of Main Findings
Since the emergence of SDM, the literature has focused on providing patients with information about the available options, eliciting their preferences, and making a final decision. In this study, we took a first step in assessing the possible value of creating choice awareness as a prerequisite for successful SDM. We showed that, when using a generous definition, efforts to foster choice awareness are evident in more than half of the clinical encounters studied. More importantly, our results suggest that fostering choice awareness is linked to a better execution of subsequent SDM steps, both with and without the use of an effective SDM conversation aid.
Limitations and Strengths
Our study has some limitations. First, we analyzed US encounters from different clinical contexts (mostly outside the referral practice of the Mayo Clinic) involving primary and specialty care, physicians and nurses, and emergency and ambulatory settings, all obtained in the context of clinical trials of SDM interventions. We do not know the extent to which our observations apply to other settings. Second, in this study, we took a first exploratory step in assessing the fostering of choice awareness in clinical encounters. Because we expected choice awareness conversations to be rare, we used a generous approach to coding clinician behavior. It is quite likely that as we learn more about choice awareness, its occurrence will be defined more strictly and that fostering choice awareness will prove to be less prevalent in routine care than suggested by our results. In other words, our prevalence results likely represent a best-case scenario. Third, we used the OPTION-12 scale as a measure of patient involvement in decision making. Although our choice awareness codes are distinct from the OPTION-12 scale items, OPTION scale item 2 covers “equipoise” and OPTION scale item 4 covers “listing of options,” which are highly related to our definition of fostering choice awareness. Therefore, the association between fostering choice awareness and the overall OPTION-12 scale score may be partly explained by the close conceptual relationship between OPTION scale items 2 and 4 and fostering choice awareness (ie, by lack of independence between the 2 assessments). However, the effects we found were larger than could have been caused by these items alone. Furthermore, removing items 2 and 4 from the analyses did not change the results or the magnitude of the differences (data not shown). With these limitations, we see our study as an initial attempt at uncovering the prevalence and role that fostering choice awareness may have in SDM and in patient-centered care. We believe our results justify setting forth additional research into the value of this heretofore relatively neglected step in advancing SDM.
Comparison With Extant Literature
The research on choice awareness is scant. We previously found that the need to make a treatment decision is rarely discussed during pretreatment clinical encounters and that the clinician-patient conversation centers around the one treatment that the clinician recommends.6, 24 Toerien et al25 have also shown, by using conversation analysis, that clinicians often seem to steer decision making, both when presenting choice as accepting or rejecting a proposed course of action, and when presenting a list of available options. Such steering behavior, by framing options as an opportunity instead of a choice, or by providing a recommendation, can bias patients' preferences, their perceived involvement in decision making, and their decisions,26, 27, 28 possibly leading them to consent with approaches that go against what they would have otherwise preferred.29 Our findings are consistent with this literature. We indeed found, although with insufficient precision, that patients are more involved in the SDM process when choice awareness is fostered without providing a recommendation. When fostering choice awareness seems to open the SDM conversation, providing an (early) recommendation may sabotage the consideration of options and close the SDM conversation.30 Our coding scheme isolated the behavior to create choice awareness from other associated behaviors that may simultaneously sabotage and disrupt its effect, for example, the premature formulation of a recommendation. However, the interaction between behaviors to create choice awareness and make recommendations deserves further research. Perhaps this research may show that increasing choice awareness behaviors is insufficient, and clinicians would have to also focus on refraining from making recommendations until the patient's informed preferences have had a chance to form during deliberation.
In some cases, we found that clinicians fostered choice awareness, but patient involvement in decision making was low. In these cases, either the patient or the clinician made a final decision early in the encounter. Especially when the patient and clinician have an established relationship and the options and patients' views regarding these options have been discussed during a previous conversation, we should be cautious in concluding that an early decision reflects a poor decision-making process or poor care in general. However, when the patient's situation changes, for example after reviewing new test results, their views and preferences may change and how to proceed merits reconsideration.31
Similarly, in using measurement instruments such as the OPTION-12 scale, we must keep in mind that low scores do not necessarily mean that the patient or their views were poorly involved, nor do high scores necessarily mean that SDM took place in a high-quality, respectful, and humanistic way.32 The end goal of SDM should never be a high score on a measurement tool (a case of “measurement with a wink”32), but a way of care that reflects the patient's true and informed preferences and that makes intellectual, emotional, and practical sense.
Implications for Research, Practice, and Policy
Our research should be reproduced in other contexts and settings. Furthermore, future research should assess the association between fostering choice awareness and actual patient awareness of choice and of the role their participation has in shaping their own care. In addition, clinicians' awareness of choice should be assessed. We noticed that when choice awareness is fostered, this is usually done right after reviewing the patient's situation, thus at the start of the SDM conversation (data not shown). However, more research is needed on the optimal timing of fostering choice awareness and how this timing may affect the structure of the conversation.
We have recently characterized SDM as the development and testing of hypotheses in conversation to better understand the patient situation being addressed and the treatment required.4, 33 Patient participation is necessary to justly “try these hypotheses on for size.” In this approach, choice awareness may refer to the fostering of the open-mindedness necessary in patients and clinicians to form and work with hypotheses in pursuit of care that makes practical, intellectual, and emotional sense. We see potential to investigate this possible form of choice awareness and other questions in choice awareness through methods such as video reflexivity that develop accounts of SDM from encounter participants as a complement to observer-based evaluation tools.
A large part of the work to implement SDM has focused on developing and deploying patient decision aids and conversation tools.34, 35 Although their use can improve knowledge and reduce decisional conflict,35 their effects on the decision-making process have been smaller than anticipated.35, 36, 37, 38 Our study suggests that fostering choice awareness may do more to promote SDM than the use of effective conversation aids. The full potential of disease- and context-specific decision or conversation aids may not be reached unless their use fosters awareness that there is more than one way forward, that patient involvement matters, and that the best solution depends on what patients value. Research should be prioritized to determine the extent to which fostering choice awareness—an otherwise simple, inexpensive, and generic approach—can be an effective way to facilitate SDM and patient-centered care even when SDM tools are not available.
Conclusion
Our results suggest that clinicians foster choice awareness in about half of the clinical encounters studied and that doing so is linked to better execution of other SDM steps, both with and without the use of effective SDM conversation aids. Fostering choice awareness may behave as a prerequisite for SDM and a key preliminary step in the formulation of patient-centered conversations.
Acknowledgments
We thank all patients and clinicians who participated in our trials. We also thank our colleagues from the Knowledge and Evaluation Research Unit who designed and conducted the original studies. This study is part of the Fostering Fit by Recognizing Opportunity STudy (FROST) program, named after Robert Frost, in honor of his poem “The Road Not Taken”.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Weil A.R. The patient engagement imperative. Health Aff (Millwood) 2016;35(4):563. doi: 10.1377/hlthaff.2016.0337. [DOI] [PubMed] [Google Scholar]
- 2.Stiggelbout A.M., van der Weijden T., De Wit M.P., et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256. doi: 10.1136/bmj.e256. [DOI] [PubMed] [Google Scholar]
- 3.Stiggelbout A.M., Pieterse A.H., De Haes J.C. Shared decision making: concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172–1179. doi: 10.1016/j.pec.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Kunneman M., Montori V.M., Castaneda-Guarderas A., Hess E. What is shared decision making? (and what it is not) Acad Emerg Med. 2016;23(12):1320–1334. doi: 10.1111/acem.13065. [DOI] [PubMed] [Google Scholar]
- 5.Elwyn G., Frosch D., Thomson R., et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunneman M., Engelhardt E.G., ten Hove F.L., et al. Deciding about (neo-)adjuvant rectal and breast cancer treatment: missed opportunities for shared decision making. Acta Oncol. 2016;55(2):134–139. doi: 10.3109/0284186X.2015.1068447. [DOI] [PubMed] [Google Scholar]
- 7.Couët N., Desroches S., Robitaille H., et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect. 2015;18(4):542–561. doi: 10.1111/hex.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wieringa T.H., Kunneman M., Rodriguez-Gutierrez R., et al. A systematic review of decision aids that facilitate elements of shared decision-making in chronic illnesses: a review protocol. Syst Rev. 2017;6(1):155. doi: 10.1186/s13643-017-0557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo Clinic Shared Decision Making National Resource Center. https://shareddecisions.mayoclinic.org/. Accessed April 1, 2017.
- 10.Weymiller A.J., Montori V.M., Jones L.A., et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med. 2007;167(10):1076–1082. doi: 10.1001/archinte.167.10.1076. [DOI] [PubMed] [Google Scholar]
- 11.Mullan R.J., Montori V.M., Shah N.D., et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560–1568. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]
- 12.Montori V.M., Shah N.D., Pencille L.J., et al. Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial. Am J Med. 2011;124(6):549–556. doi: 10.1016/j.amjmed.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 13.LeBlanc A., Wang A.T., Wyatt K., et al. Encounter decision aid vs. clinical decision support or usual care to support patient-centered treatment decisions in osteoporosis: the Osteoporosis Choice randomized trial II. PLoS One. 2015;10(5):e0128063. doi: 10.1371/journal.pone.0128063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branda M.E., LeBlanc A., Shah N.D., et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301. doi: 10.1186/1472-6963-13-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess E.P., Knoedler M.A., Shah N.D., et al. The chest pain choice decision aid: a randomized trial. Circ Cardiovasc Qual Outcomes. 2012;5(3):251–259. doi: 10.1161/CIRCOUTCOMES.111.964791. [DOI] [PubMed] [Google Scholar]
- 16.LeBlanc A., Herrin J., Williams M.D., et al. Shared decision making for antidepressants in primary care: a cluster randomized trial. JAMA Intern Med. 2015;175(11):1761–1770. doi: 10.1001/jamainternmed.2015.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Study to test use of a decision aid in a clinical visit to help patients choose a diabetes medication (TRICEP) https://clinicaltrials.gov/ct2/show/NCT01293578 Accessed April 1, 2017.
- 18.Coylewright M., Dick S., Zmolek B., et al. PCI choice decision aid for stable coronary artery disease: a randomized trial. Circ Cardiovasc Qual Outcomes. 2016;9(6):767–776. doi: 10.1161/CIRCOUTCOMES.116.002641. [DOI] [PubMed] [Google Scholar]
- 19.Brito J.P., Castaneda-Guarderas A., Gionfriddo M.R., et al. Development and pilot testing of an encounter tool for shared decision making about the treatment of Graves' disease. Thyroid. 2015;25(11):1191–1198. doi: 10.1089/thy.2015.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyatt K.D., Branda M.E., Anderson R.T., et al. Peering into the black box: a meta-analysis of how clinicians use decision aids during clinical encounters. Implement Sci. 2014;9:26. doi: 10.1186/1748-5908-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elwyn G., Hutchings H., Edwards A., et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect. 2005;8(1):34–42. doi: 10.1111/j.1369-7625.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholl I., Koelewijn-van Loon M., Sepucha K., et al. Measurement of shared decision making—a review of instruments. Z Evid Fortbild Qual Gesundhwes. 2011;105(4):313–324. doi: 10.1016/j.zefq.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Basu A., Rathouz P.J. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93–109. doi: 10.1093/biostatistics/kxh020. [DOI] [PubMed] [Google Scholar]
- 24.Snijders H.S., Kunneman M., Bonsing B.A., et al. Preoperative risk information and patient involvement in surgical treatment for rectal and sigmoid cancer. Colorectal Dis. 2014;16(2):O43–O49. doi: 10.1111/codi.12481. [DOI] [PubMed] [Google Scholar]
- 25.Toerien M., Shaw R., Duncan R., Reuber M. Offering patients choices: a pilot study of interactions in the seizure clinic. Epilepsy Behav. 2011;20(2):312–320. doi: 10.1016/j.yebeh.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Abhyankar P., Summers B.A., Velikova G., Bekker H.L. Framing options as choice or opportunity: does the frame influence decisions? Med Decis Making. 2014;34(5):567–582. doi: 10.1177/0272989X14529624. [DOI] [PubMed] [Google Scholar]
- 27.Frongillo M., Feibelmann S., Belkora J., Lee C., Sepucha K. Is there shared decision making when the provider makes a recommendation? Patient Educ Couns. 2013;90(1):69–73. doi: 10.1016/j.pec.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelhardt E.G., Pieterse A.H., van der Hout A., et al. Use of implicit persuasion in decision making about adjuvant cancer treatment: a potential barrier to shared decision making. Eur J Cancer. 2016;66:55–66. doi: 10.1016/j.ejca.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Gurmankin A.D., Baron J., Hershey J.C., Ubel P.A. The role of physicians' recommendations in medical treatment decisions. Med Decis Making. 2002;22(3):262–271. doi: 10.1177/0272989X0202200314. [DOI] [PubMed] [Google Scholar]
- 30.Kunneman M. Shared decision making in adjuvant cancer treatment. [doctoral thesis]. Leiden, Netherlands: Leiden University Medical Center; 2016.
- 31.Montori V.M., Gafni A., Charles C. A shared treatment decision-making approach between patients with chronic conditions and their clinicians: the case of diabetes. Health Expect. 2006;9(1):25–36. doi: 10.1111/j.1369-7625.2006.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunneman M., Montori V.M., Shah N.D. Measurement with a wink. BMJ Qual Saf. 2017;26(10):849–851. doi: 10.1136/bmjqs-2017-006814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hargraves I., Kunneman M., Brito J.P., Montori V.M. Caring with evidence based medicine. BMJ. 2016;353:i3530. doi: 10.1136/bmj.i3530. [DOI] [PubMed] [Google Scholar]
- 34.Trenaman L., Bryan S., Bansback N. The cost-effectiveness of patient decision aids: a systematic review. Healthc (Amst) 2014;2(4):251–257. doi: 10.1016/j.hjdsi.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Stacey D., Legare F., Lewis K., et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silvia K.A., Sepucha K.R. Decision aids in routine practice: lessons from the breast cancer initiative. Health Expect. 2006;9(3):255–264. doi: 10.1111/j.1369-7625.2006.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stacey D., Samant R., Bennett C. Decision making in oncology: a review of patient decision aids to support patient participation. CA Cancer J Clin. 2008;58(5):293–304. doi: 10.3322/CA.2008.0006. [DOI] [PubMed] [Google Scholar]
- 38.Montori V.M., Kunneman M., Brito J.P. Shared decision making and improving health care: the answer is not in. JAMA. 2017;318(7):617–618. doi: 10.1001/jama.2017.10168. [DOI] [PubMed] [Google Scholar]