Abstract
Immune-mediated encephalitis related to immune checkpoint inhibitor therapy is a rare but increasingly described condition that can cause significant morbidity. There are several reported cases in the literature but no previously described cases of immune-mediated cerebellitis. We describe a case of acute cerebellitis that developed in a 20-year-old man with primary refractory Hodgkin lymphoma being treated with the immune checkpoint inhibitor nivolumab. After exposure to 3 cycles of nivolumab, the patient had acute onset of headache, ataxia, nausea, and vomiting, with imaging findings of cerebellar edema, early tonsillar herniation, and early hydrocephalus. Immune-mediated cerebellar encephalitis was suspected and high-dose dexamethasone therapy (8 mg every 6 hours) was initiated. Within 4 days of dexamethasone therapy, his symptoms greatly improved with near-complete resolution of symptoms after a 4-week taper. Differential diagnosis of his condition included viral cerebellitis and paraneoplastic cerebellar degeneration. In cerebellar encephalitis suspected to be due to immune checkpoint inhibitor therapy, prompt recognition and early initiation of high-dose corticosteroids is essential for symptom resolution and treatment success, including the prevention of hydrocephalus and tonsillar herniation. Currently, there are no evidence-based guidelines to guide the initial dose, type, or duration of corticosteroids. Further investigation is needed in the pathogenesis and treatment of cerebellar encephalitis related to immune checkpoint inhibitor therapy to effectively treat this rare, disabling condition.
Abbreviations and Acronyms: CSF, cerebrospinal fluid; GAD, glutamic acid decarboxylase; ICI, immune checkpoint inhibitor; irAE, immune-related adverse effect; MRI, magnetic resonance imaging; PCD, paraneoplastic cerebellar degeneration; PD-1, programmed death-1
Since the approval of ipilimumab for the treatment of melanoma in 2011, immune checkpoint inhibitors (ICIs) have been changing the landscape of oncology. They up-regulate the body's own antitumor immunity and include anti–cytotoxic T-lymphocyte antigen 4, anti–programmed death-1 (PD-1), and anti–programmed death-ligand 1 (PD-L1) monoclonal antibodies. The toxicity profile of ICIs is unique in that they often trigger the development of immune-related adverse effects (irAEs). Commonly reported irAEs include colitis, hepatitis, endocrinopathies, dermatitis, thyroiditis, and pneumonitis; however, virtually all organ systems can develop immune toxicities.1, 2 Neurologic irAEs include neuropathy, myelopathy, Guillain-Barré syndrome, meningitis, myasthenia gravis, and encephalitis and occur in less than 1% of patients.1, 3 Immune-mediated encephalitis is especially uncommon and occurs in less than 0.2% of patients.4 We describe a case of anti–PD-1 antibody-related immune-mediated cerebellar encephalitis. To our knowledge, this is the first described case of ICI-associated immune-mediated cerebellar encephalitis in the literature.
Case Study
A 20-year-old white man with stage IVB primary refractory Hodgkin lymphoma that progressed despite 3 previous therapies (doxorubicin-bleomycin-vinblastine-dacarbazine [ABVD], ifosfamide-carboplatin-etoposide [ICE], and brentuximab vedotin) was treated with nivolumab 3 mg/kg every 2 weeks. He presented 13 days after his third cycle of nivolumab to an emergency department with 1 day of bilateral stabbing headache, diplopia, confusion, nausea, and vomiting. He did not have any history of infection. On examination he was afebrile and hemodynamically stable. Neurologic examination was notable for ataxia and dysmetria. Meningeal signs were absent. Initial head computed tomography showed cerebellar edema. Brain magnetic resonance imaging (MRI) showed a diffusely edematous cerebellum with patchy enhancement, signs of early tonsillar herniation, and early hydrocephalus. Antibiotics were initiated for possible meningitis. Initial lumbar puncture was unsuccessful. After consultation with his primary oncologist, high-dose dexamethasone (8 mg every 6 hours) was initiated because of a high possibility of immune-mediated encephalitis.
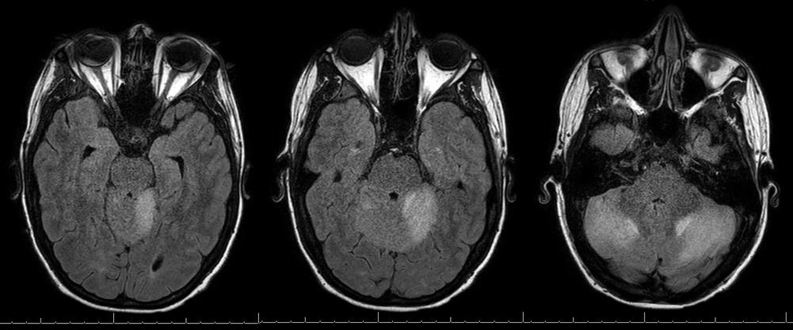
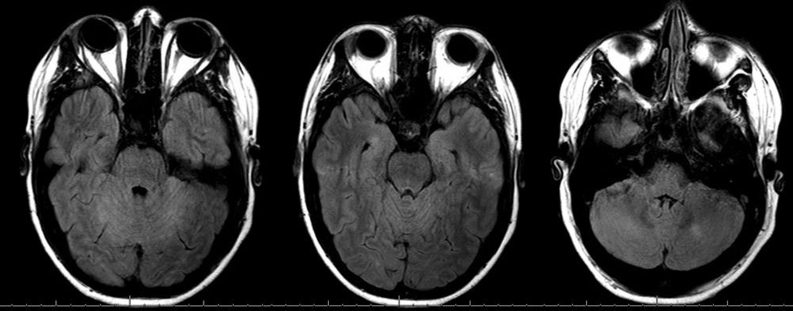
By day 6, his nausea and vomiting had resolved. He continued to have mild residual ataxia of his extremities (left greater than right), mild dysmetria, and dizziness. Repeated MRI on day 6 showed persistent but improved bilateral cerebellar hemispheric edema with mild cerebellar leptomeningeal enhancement (Figure 1). Later, cerebrospinal fluid (CSF) examination revealed normal glucose (73 mg/dL), elevated protein (161 mg/dL), and lymphocytic pleocytosis (white blood cells, 31/μL; 94% lymphocytes, 6% monocytes). Cryptococcal antigen studies, herpes simplex virus polymerase chain reaction, and routine CSF cultures yielded negative results. The CSF flow cytometry results were normal. The patient was discharged on day 8 and dexamethasone was tapered over 4 weeks. At 6-week follow-up, the patient's symptoms had essentially resolved except for mild diplopia. The brain MRI at 6-week follow-up showed near resolution of cerebellitis with subtle persistent enhancement within the cerebellar sulci (Figure 2). Positron emission tomography at 6-week follow-up showed a partial response. The cerebellitis was believed to represent an immune-mediated encephalitis related to anti–PD-1 therapy; therefore, nivolumab was discontinued with a plan to begin an alternative therapy at next follow-up.
Figure 1.
Axial T2 FLAIR MRI of patient on day 6 of hospitalization and day 4 of dexamethasone therapy. FLAIR = fluid-attenuated inversion recovery; MRI = magnetic resonance imaging.
Figure 2.
Axial T2 FLAIR MRI of patient 6 weeks after presentation, 2 weeks after completion of dexamethasone therapy. FLAIR = fluid-attenuated inversion recovery; MRI = magnetic resonance imaging.
Discussion
We describe a case of acute cerebellar encephalitis that developed after exposure to nivolumab used to treat primary refractory Hodgkin lymphoma. Although causality cannot be proven without biopsy, several clinical features suggest that his presentation was related to ICI exposure, including the timing of his symptoms, his rapid improvement with corticosteroids, and negative results of infectious work-up. Etiologies that must be considered in the differential diagnosis of ICI-related immune-mediated cerebellar encephalitis include paraneoplastic cerebellar degeneration (PCD), acute infectious cerebellitis, postinfectious cerebellitis, gliadin-associated cerebellar ataxia, glutamic acid decarboxylase (GAD)–associated cerebellar ataxia, thiamine deficiency, alcohol abuse, and cerebellar metastases.5 Thiamine deficiency and alcohol abuse were excluded on the basis of history. Brain metastases were excluded on the basis of absence of localized lesion on brain MRI and normal findings on CSF flow cytometry. Paraneoplastic cerebellar degeneration, gliadin- and GAD-associated cerebellar ataxia, and infectious and postinfectious cerebellitis were all considerations in our patient's case.
Paraneoplastic cerebellar degeneration has been well described in Hodgkin lymphoma, as well as breast, ovarian, and small cell lung cancer.6 It is associated with several antibodies, most commonly anti-Yo in ovarian and breast cancer, anti-Hu and anti-Zic4 in small cell lung cancer, and anti-Tr and anti-mGluR1 in Hodgkin lymphoma.5 Antibodies associated with autoimmune cerebellitis that can occur in the absence of malignancy include anti-GAD and antigliadin antibodies.5 Neither presence nor absence of these antibodies could definitively diagnose or exclude ICI-related immune-mediated cerebellar encephalitis in our patient's case. On initial MRI, our patient had significant cerebellar edema in contrast to most cases of PCD in which patients more commonly have an initially normal MRI result. Nonetheless, some patients with PCD do demonstrate diffuse cerebellar enlargement with cortical-meningeal enhancement early in their disease course.5 In most cases, PCD does not respond well to immunosuppressive therapy, which is also in contrast to our patient's case.7
Acute infectious cerebellitis has been associated with Epstein-Barr virus, varicella-zoster virus, influenza, enterovirus, dengue virus, JC virus, and Listeria monocytogenes.8 Acute postinfectious cerebellar ataxia presents similar to infectious cerebellitis except that infectious testing results are negative, with symptoms beginning approximately 2 weeks after a febrile illness. The most common antecedent infection is varicella-zoster virus infection in young children and Epstein-Barr virus infection in young adults. Initial MRI results can range from normal findings to significant cerebellar edema with obstructive hydrocephalus in both infectious and postinfectious cerebellitis.8 The results of CSF examination are notable for a lymphocytic pleocytosis and elevated protein. Viral polymerase chain reaction testing for the above etiologies was not done in our patient's case because he lacked a convincing history for these etiologies.
Immune-mediated encephalitis related to ICI therapy is still not well described, with only 13 cases in the literature (Supplemental Table, available online at http://mcpiqojournal.org/).4, 7, 9, 10, 11, 12, 13, 14, 15 It is considered a grade 3 or 4 irAE and warrants immediate and permanent discontinuation of ICI therapy. Cases have been reported in patients with various cancer types: non–small cell lung cancer, small cell lung cancer, advanced melanoma, and prostate cancer. Nivolumab, ipilimumab, and pembrolizumab have all been associated with the syndrome. There are no reported cases with anti–PD-L1 therapy. Signs and symptoms are variable and can include headache, weakness, confusion, aphasia, ataxia, bradykinesia, somnolence, and seizures. Time from initiation of ICI therapy to symptom development varies widely from 4 days to 11 months. Time from corticosteroid initiation to symptom resolution is also widely variable from 1 day to 6 months. Initial MRI findings are usually normal but can demonstrate T2-weighted fluid-attenuated inversion recovery (FLAIR) hyperintensities in the affected areas. The results of CSF studies usually show a lymphocytic pleocytosis and elevated protein but can be normal. Most who develop immune-mediated encephalitis have a positive tumor response to ICI therapy but about a fifth of patients experience progressive disease. In a sixth of cases symptoms persisted despite high-dose glucocorticoids, and further immunosuppressive therapy (intravenous immunoglobulin and/or rituximab) was needed to achieve a durable response.4, 9 Paraneoplastic and autoimmune antibody testing results were positive in only a fourth of cases in which testing was performed.9
Conclusion
For patients receiving ICI therapy with evidence of immune-mediated cerebellitis, prompt recognition and early initiation of high-dose corticosteroids is essential for symptom resolution and treatment success, including the prevention of hydrocephalus and tonsillar herniation. Currently, no evidence-based guidelines are available to guide the initial dose, type, or duration of corticosteroids. Because dexamethasone has good penetration and efficacy in the central nervous system, we favor it in these cases. Any patient with probable ICI-induced cerebellitis should have prompt and permanent discontinuation of ICI therapy and prolonged corticosteroid treatment until resolution of symptoms and imaging abnormalities.
Footnotes
Potential Competing Interests: The authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at http://mcpiqojournal.org/. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Naidoo J., Page D.B., Li B.T., et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champiat S., Lambotte O., Barreau E., et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559–574. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]
- 3.Larkin J., Chiarion-Sileni V., Gonzalez R., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larkin J., Chmielowski B., Lao C.D., et al. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist. 2017;22(6):709–718. doi: 10.1634/theoncologist.2016-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau J., Rosenfeld M.R. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7(4):327–340. doi: 10.1016/S1474-4422(08)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sillevis Smitt P., Kinoshita A., De Leeuw B., et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21–27. doi: 10.1056/NEJM200001063420104. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld M.R., Dalmau J. Current therapies for paraneoplastic neurologic syndromes. Curr Treat Options Neurol. 2003;5(1):69–77. doi: 10.1007/s11940-003-0023-y. [DOI] [PubMed] [Google Scholar]
- 8.Pruitt A.A. Infections of the cerebellum. Neurol Clin. 2014;32(4):1117–1131. doi: 10.1016/j.ncl.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Williams T.J., Benavides D.R., Patrice K.A., et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol. 2016;73(8):928–933. doi: 10.1001/jamaneurol.2016.1399. [DOI] [PubMed] [Google Scholar]
- 10.Schneider S., Potthast S., Komminoth P., Schwegler G., Böhm S. PD-1 checkpoint inhibitor associated autoimmune encephalitis. Case Rep Oncol. 2017;10(2):473–478. doi: 10.1159/000477162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conry R.M., Sullivan J.C., Nabors L.B., III Ipilimumab-induced encephalopathy with a reversible splenial lesion. Cancer Immunol Res. 2015;3(6):598–601. doi: 10.1158/2326-6066.CIR-15-0035. [DOI] [PubMed] [Google Scholar]
- 12.Boyd K., Kalladka D., Overell J., Waterston A. Ipilimumab induced encephalitis: a case report. Immunome Res. 2015;11:092. [Google Scholar]
- 13.Carl D., Grüllich C., Hering S., Schabet M. Steroid responsive encephalopathy associated with autoimmune thyroiditis following ipilimumab therapy: a case report. BMC Res Notes. 2015;8:316. doi: 10.1186/s13104-015-1283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salam S., Lavin T., Turan A. Limbic encephalitis following immunotherapy against metastatic malignant melanoma. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-215012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng S., Coward J., McCaffrey E., Coucher J., Kalokerinos P., O'Byrne K. Pembrolizumab induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J Thorac Oncol. 2017;12(11):1626–1635. doi: 10.1016/j.jtho.2017.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.