Abstract
Objective
To report the changing incidence, clinical presentation, microbiologic spectrum, and outcomes of pyogenic liver abscess (PLA) in Olmsted County, Minnesota, over the past 35 years.
Patients and Methods
The Rochester Epidemiology Project was used to identify residents with PLA from January 1, 1980, through December 31, 2014. The study included all patients older than 18 years, with the diagnosis of PLA confirmed through radiographic review and microbiologic cultures.
Results
In total, 72 patients received a diagnosis of PLA from 1980 through 2014. The age-adjusted incidence for men was 3.92 cases per 100,000 person-years (95% CI, 2.76-5.09 cases per 100,000 person-years) compared with 1.87 cases per 100,000 person-years (95% CI, 1.15-2.59 cases per 100,000 person-years) for women. Incidence was higher in the period from January 1, 2001, through December 31, 2014, than in the period from January 1, 1980, through December 31, 2000, for women (incidence rate ratio [IRR], 3.8; 95% CI, 1.43-10.09; P=.007) but not for men (IRR, 0.99; 95% CI, 0.55-1.76; P=.96). Fifteen additional patients had postintervention PLA (1980-2000: n=3 of 29 [10.3%] vs 2001-2015: n=12 of 58 [20.6%]). A significant association was seen between age- and sex-adjusted incidence rates of PLA and year of diagnosis (per year since 1980: IRR, 1.04; 95% CI, 1.02-1.07; P<.001) after including postintervention PLA. Streptococcus milleri was the most common organism identified (52.5%). Organisms with multidrug resistance were more common in the period from 2001 through 2014 than in the period from 1980 through 2000 (51% vs 14%; P=.005). The overall mortality rate of PLA was 16.8% (95% CI, 7.6%-25.0%) at 6 months.
Conclusion
The incidence of PLA is increasing, probably because of increase in frequency of hepatobiliary interventions and organisms with multidrug resistance.
Abbreviations and Acronyms: CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; HR, hazard ratio; IRR, incidence rate ratio; MDR, multidrug resistance; PLA, pyogenic liver abscess; PY, person-year; REP, Rochester Epidemiology Project; RFA, radiofrequency ablation; TACE, transarterial chemotherapy embolization
The epidemiology of pyogenic liver abscess (PLA) in the United States is not clearly known. Pyogenic liver abscess is more prevalent in Asian countries and is endemic in some parts of Asia, such as Taiwan, where its annual incidence is 17.6 cases per 100,000 population.1 In contrast, the incidence is lower in Europe and Canada, ranging from 1.1 to 2.3 cases per 100,000 population per year.2, 3, 4 Similarly in the United States, the estimated incidence based on a nationwide inpatient sample database was 3.6 cases per 100,000 population per year.5 That study has limitations, such as (1) lack of diagnosis confirmation because patient records were not manually reviewed and (2) inability to assess a change in PLA incidence. Inability to confirm the diagnosis of PLA through manual record review may have resulted in an overestimate of incidence rates.
Pyogenic liver abscess occurs secondary to bacterial, parasitic, or fungal infection. Microbes gain access to the liver parenchyma via a hematogenous or biliary route, through contiguous spread from adjacent organs, or from direct microbial inoculation after a hepatobiliary procedure or operation. The most common route of acquiring PLA is unclear for patients who have not undergone hepatobiliary procedures.2, 4, 6, 7, 8 Whether the epidemiologic factors of PLA have changed over time is also not known. The objectives of this study were to determine the incidence of PLA over the past 35 years in a US population–based cohort and compare the trends in underlying risk factors, clinical presentation, microbial spectrum, treatment modalities, and death due to PLA.
Patients and Methods
Study Design
Population-based epidemiologic research can be conducted in Olmsted County, Minnesota, because medical care is effectively self-contained in the community, with 2 major health care providers (Mayo Clinic and Olmsted Medical Center) serving almost the entire population.9 The system and infrastructure that collect, collate, and index patient-level data are composed in the Rochester Epidemiology Project (REP), which has been supported since 1966 by the National Institute on Aging of the National Institutes of Health. Because of this unique linkage system, Olmsted County is one of the few areas in the United States where accurate population-based studies can be conducted. In this study, we used REP to help define the epidemiologic factors of liver abscess in the Olmsted County population.
Study data were collected and managed with the Research Electronic Data Capture tool hosted at Mayo Clinic in Rochester, Minnesota.10 Research Electronic Data Capture is a secure web application designed to support data capture for research studies, providing an intuitive interface for validated data entry, audit trails for tracking data manipulation and export procedures, automated export procedures for seamless data downloads to common statistical packages, and procedures for importing data from external sources.
After approval of the institutional review boards of Mayo Clinic (IRB# 16-001465) and Olmsted Medical Center (IRB# 009-OMC-16), REP was used to identify all Olmsted County residents with a diagnosis of PLA from January 1, 1980, through December 31, 2014. Among potential cases, the diagnosis was defined by meeting the following criteria: (1) clinical symptoms of the underlying infection, (2) imaging study of the abdomen exhibiting characteristic lesions in the liver that are suggestive of PLA, and (3) aspiration or drainage of pus from the PLA cavity or blood cultures positive for organism with characteristic liver abscess on imaging. Among patients without aspiration of the PLA cavity and with negative blood culture results, we considered clinical and radiologic response to antibiotic therapy on follow-up scans as diagnostic for PLA. The first documented date of the imaging study depicting the liver abscess was considered the date of diagnosis. For patients with positive culture results, multidrug resistance (MDR) was defined as organism resistance to 2 or more classes of antimicrobial agents.11, 12
To observe any change in trends of PLA over the past 35 years, we defined 2 periods: January 1, 1980, through December 31, 2000 (period 1); and January 1, 2001, through December 31, 2014 (period 2). The period before January 1, 1980, was excluded because high-quality abdominal imaging was usually not available. Because hepatobiliary interventions may have increased during the 2 decades, we analyzed the data with and without patients who had PLA after an intervention (total n=87): endoscopic retrograde cholangiopancreatography (ERCP) (n=10 [11.5%]), radiofrequency ablation (RFA) or transarterial chemotherapy embolization (TACE) for hepatocellular carcinoma (n=3 [3.4%]), and liver transplant (n=2 [2.3%]).
Study Variables
The complete inpatient and outpatient medical records were extracted to collect the following variables: date of birth, sex, race, and age at diagnosis. Clinical variables were recorded, such as fever (temperature >100.9°F), chills, abdominal pain, malaise or fatigue, weight loss, diarrhea, jaundice, hepatomegaly, and altered mental status. The diagnostic work-up included laboratory investigations—hemoglobin level, white blood cell count, aspartate transaminase level, alanine transaminase level, alkaline phosphatase level, total bilirubin level, and direct bilirubin level—as well as radiographic imaging details (eg, imaging type), location and size of the abscess, and culture growth from blood and abscess aspirate. Details of antibiotic therapies, drainage (eg, percutaneous aspiration, placement of drainage catheters, and number of drainage catheters), and surgical procedure (eg, procedure type and postoperative complications) were documented. Various outcomes on follow-up were assessed, including recurrence of liver abscess, new diagnosis of malignant tumors, and death. Death certificates were reviewed for cause of death. Through careful review of linked medical records, patients were monitored from the time of PLA diagnosis until death or date of the last clinical visit to a health care system in the community.
Statistical Analyses
Age- and sex-specific incidence rates per 100,000 person-years (PYs) in Olmsted County were calculated for each period by using the number of persons with incident PLA as the numerator and the age- and sex-specific counts of the population of Olmsted County for the 2 periods as the denominator (counts were from the Olmsted County census, with linear interpolation between census years). Rates were age- and sex-adjusted to the total white population structure of the United States in 2010. The 95% CIs were calculated for these rates by assuming a Poisson error distribution. Poisson regression models were used to evaluate the association of age, sex, and time period with incidence rates. All interactions between the 3 variables were assessed, and the likelihood ratio test was used to compare this full model with the model of main effects. Demographic characteristics, risk factors, and organisms of PLA were compared between the 2 periods using either the chi-square test or the Fisher exact test, as appropriate. Death due to PLA was estimated using the Kaplan-Meier method with a competing risk of non–PLA-related death, and Cox proportional hazards regression was used to evaluate predictors of PLA-related death. A P value less than .05 was considered statistically significant. All analyses were completed using SAS version 9.4 (SAS Institute Inc).
Results
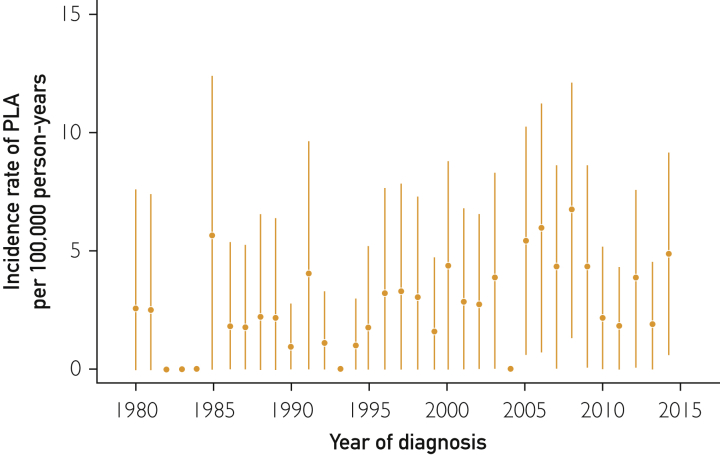
During January 1, 1980, through December 31, 2014, 72 patients received the diagnosis of PLA in Olmsted County and met the study inclusion criteria. Fifteen additional patients had PLA after a hepatobiliary intervention: 3 of 29 (10.3%) in period 1 and 12 of 58 (20.7%) in period 2, with no significant difference between the time periods (P=.37). Most patients were male (46 of 72 [63.9%]) and white (60 of 72 [83.3%]); the mean age was 63±17.5 years. The overall age- and sex-adjusted incidence of PLA was 2.9 cases per 100,000 PYs (95% CI, 2.2-3.9 cases per 100,000 PYs). Figure 1 displays the annual age- and sex-adjusted incidence rates of PLA in Olmsted County between January 1, 1980, and December 31, 2014. Table 1 summarizes the demographic and clinical characteristics of the patients.
Figure 1.
Yearly age- and sex-adjusted incidence rates of PLA per 100,000 person-years. Error bars represent 95% CIs. PLA = pyogenic liver abscess.
Table 1.
Demographic and Clinical Characteristics of 72 Patients With Diagnosis of Pyogenic Liver Abscess in Olmsted County, Minnesota, From 1980 Through 2014
| Characteristic | Total (N=72) | Period |
P value | |
|---|---|---|---|---|
| 1980-2000 (n=26) | 2001-2014 (n=46) | |||
| Age (y) | 63±17.5 | 59.6±20.3 | 64.7±15.6 | .27 |
| Age group (y) | ||||
| 18-34 | 6 (8.3) | 4 (15.4) | 2 (4.3) | |
| 35-49 | 8 (11.1) | 3 (11.5) | 5 (10.9) | |
| 50-64 | 20 (27.8) | 9 (34.6) | 11 (23.9) | |
| ≥65 | 38 (52.8) | 10 (38.5) | 28 (60.9) | |
| Male sex | 46 (63.9) | 21 (80.8) | 25 (54.3) | .03 |
| Race | .07 | |||
| White | 60 (83.3) | 20 (76.9) | 40 (87.0) | |
| Black | 2 (2.8) | 0 (0) | 2 (4.3) | |
| Asian | 2 (2.8) | 0 (0) | 2 (4.3) | |
| Other/unknown | 8 (11.1) | 6 (23.1) | 2 (4.3) | |
| Abscess location | .14 | |||
| Right lobe | 45 (63.4) | 18 (72.0) | 27 (58.7) | |
| Left lobe | 11 (15.5) | 5 (20.0) | 6 (13.0) | |
| Both lobes | 15 (21.1) | 2 (8.0) | 13 (28.3) | |
Data are presented as mean ± SD or as No. (percentage).
Incidence of PLA
The age- and sex-adjusted incidence was 2.09 cases per 100,000 PYs (95% CI, 1.26-2.93 cases per 100,000 PYs) in period 1 and 3.63 cases per 100,000 PYs (95% CI, 2.58-4.68 cases per 100,000 PYs) in period 2 (Table 2). After adjustment for sex and year of diagnosis, older patients were at higher risk of PLA development than were younger patients (per 1-year increase in age incidence rate ratio [IRR], 1.05; 95% CI, 1.04-1.07; P ≤.001). No significant association was found between incidence rates of PLA and year of diagnosis after adjustment for age and sex (per year since 1980: IRR, 1.02; 95% CI, 0.99-1.05; P=.13). The age-adjusted incidence for women was 1.87 cases per 100,000 PYs (95% CI, 1.15-2.59 cases per 100,000 PYs); for men, it was 3.92 cases per 100,000 PYs (95% CI, 2.76-5.09 cases per 100,000 PYs), with men at higher risk of PLA than women (IRR, 2.3; 95% CI, 1.40-3.69; P<.001, with adjustment for age and year of diagnosis). The risk of PLA was significantly higher in period 2 than in period 1 for women (IRR, 3.80; 95% CI, 1.43-10.09; age-adjusted, P=.007), but no difference was seen between the 2 time periods for men (IRR, 0.99; 95% CI, 0.55-1.76; age-adjusted, P=.96).
Table 2.
Age- and Sex-Specific Incidence of Pyogenic Liver Abscess Per 100,000 Person-Years in Residents of Olmsted County, Minnesota, in 2 Periods From 1980 Through 2014
| Age group (y) | 1980-2000: Incidence (95% CI) |
2001-2014: Incidence (95% CI) |
||||
|---|---|---|---|---|---|---|
| Female sex (n=8,542,229) | Male sex (n=770,985) | Total (n=1,625,214) | Female sex (n=748,194) | Male sex (n=695,953) | Total (N=1,444,147) | |
| 18-34 | 0.0 | 1.3 (0.4-3.4) | 0.6 (0.2-1.6)a | 0.0 | 0.9 (0.1-3.3) | 0.4 (0.1-1.6)a |
| 35-49 | 0.4 (0.0-2.3) | 0.8 (0.1-3.0) | 0.6 (0.1-1.8)a | 0.5 (0.0-2.6) | 1.9 (0.5-4.9) | 1.2 (0.4-2.8)a |
| 50-64 | 0.0 | 6.5 (3.0-12.4) | 3.2 (1.5-6.1)a | 2.3 (0.6-5.8) | 4.2 (1.7-8.8) | 3.2 (1.6-5.8)a |
| ≥65 | 2.9 (0.8-7.4) | 6.8 (2.5-14.9) | 4.4 (2.1-8.1)a | 12.0 (6.8-19.4) | 11.9 (6.2-20.8) | 11.9 (7.9-17.3)a |
| Total | 0.7 (0.1-1.3)b | 3.6 (2.0-5.3)b | 2.1 (1.3-2.9)c | 3.1 (1.8-4.4)b | 4.2 (2.5-5.9)b | 3.6 (2.6-4.7)c |
Incidence is sex-adjusted to the population structure of the US white population in 2010.
Incidence is age-adjusted to the population structure of the US white population in 2010.
Incidence is age- and sex-adjusted to the population structure of the US white population in 2010.
On comparing the periods after inclusion of 15 patients with postintervention PLA (ERCP [n=10], RFA [n=2], TACE [n=1], and liver transplant [n=2]), we found that the incidence rate changed to 2.3 cases per 100,000 PYs (95% CI, 1.4-3.2 cases per 100,000 PYs) in period 1 and to 4.49 cases per 100,000 PYs (95% CI, 3.33-5.65 cases per 100,000 PYs) in period 2. Furthermore, a significant association was found between incidence rates of PLA and year of diagnosis after adjusting for age and sex (per year since 1980: IRR, 1.04; 95% CI, 1.02-1.07; P<.001).
Presentation and Imaging
On comparing periods 1 and 2, we found that patients more commonly presented with chills (n=23, 88.5% vs n=30, 65.2%; P=.03) diarrhea (n=4, 15.4% vs n=1, 2.2%; P=.03) and hepatomegaly (n=8, 30.8% vs n=3, 6.5%; P=.006) in period 1. Presenting clinical features are summarized in Supplemental Table 1 (available online at http://www.mcpiqojournal.org). The potential risk factors associated with the development of PLA are elaborated in Table 3. The identified probable primary sources of PLA were biliary (n=24 [33%]), hematogenous (n=21 [29%]), portal (n=15 [21%]), iatrogenic (n=9 [12.5%]), and idiopathic or no source found (n=12 [16%]). We found no difference between the 2 periods in the suspected primary source of infection.
Table 3.
Underlying Risk Factors of Patients With Pyogenic Liver Abscess in Olmsted County, Minnesota, From 1980 Through 2014a,b
| Risk factor | Totalc (N=72) | Period |
P value | |
|---|---|---|---|---|
| 1980-2000c (n=26) | 2001-2014c (n=46) | |||
| Biliary disease (contiguous) | ||||
| Ascending cholangitis | 7 (9.7) | 5 (19.2) | 2 (4.3) | .09 |
| Choledocholithiasis | 10 (13.9) | 3 (11.5) | 7 (15.2) | .74 |
| Cholangiocarcinoma | 5 (6.9) | 1 (3.8) | 4 (8.7) | .65 |
| Primary sclerosing cholangitis | 2 (2.8) | 0 (0) | 2 (4.3) | .53 |
| Cholecystitis | 5 (6.9) | 1 (3.8) | 4 (8.7) | .65 |
| Biliary stricture | 1 (1.4) | 1 (3.8) | 0 (0) | .36 |
| Otherd | 5 (6.9) | 2 (7.7) | 3 (6.5) | >.99 |
| Systemic disease (hematogenous) | ||||
| Pneumonia | 10 (13.9) | 4 (15.4) | 6 (13.0) | >.99 |
| Dental work/dental caries | 8 (11.1) | 5 (19.2) | 3 (6.5) | .13 |
| Endocarditis | 2 (2.8) | 1 (3.8) | 1 (2.2) | >.99 |
| Pyelonephritis/UTI | 1 (1.4) | 0 (0) | 1 (2.2) | .53 |
| Intra-abdominal disease (portal) | ||||
| Diverticulitis | 5 (6.9) | 2 (7.7) | 3 (6.5) | >.99 |
| Inflammatory bowel disease | 2 (2.8) | 0 (0) | 2 (4.3) | .53 |
| Bowel perforation | 3 (4.2) | 0 (0) | 3 (6.5) | .55 |
| Appendicitis | 1 (1.4) | 0 (0) | 1 (2.2) | >.99 |
| Other (SBO/pancreatitis/foreign body) | 6 (8.3) | 1 (3.8) | 5 (10.9) | .41 |
| Liver disease | ||||
| Metastatic lesions | 10 (13.9) | 4 (15.4) | 6 (13.0) | >.99 |
| Liver malignancy | 1 (1.4) | 0 (0) | 1 (2.2) | >.99 |
| Hepatitis | 1 (1.4) | 0 (0) | 1 (2.2) | >.99 |
| Cirrhosis | 1 (1.4) | 0 (0) | 1 (2.2) | >.99 |
| Systemic malignancy | 17 (23.6) | 6 (24.0) | 11 (23.9) | .62 |
| Iatrogenic (operation) | ||||
| Abdominal operation | 8 (11.1) | 2 (7.7) | 6 (13.0) | .70 |
| Cholecystectomy | 1 (1.4) | 1 (3.8) | 0 (0) | .36 |
| Other | ||||
| Immunosuppression | 12 (16.7) | 4 (15.4) | 8 (17.4) | >.99 |
| Hypertension | 24 (33.3) | 5 (19.2) | 19 (41.3) | .06 |
| Excessive alcohol consumption | 15 (20.8) | 6 (23.1) | 9 (19.6) | .72 |
| Diabetes mellitus | 23 (31.9) | 6 (23.1) | 17 (37.0) | .23 |
| Cardiovascular disease | 10 (13.9) | 6 (23.1) | 4 (8.7) | .15 |
| Chronic kidney failure | 1 (1.4) | 1 (3.8) | 0 (0) | .36 |
| Recent travel | 7 (9.7) | 3 (11.5) | 4 (8.7) | .70 |
ERCP = endoscopic retrograde cholangiopancreatography; RFA = radiofrequency ablation; SBO = small-bowel obstruction; TACE = transarterial chemotherapy embolization; UTI = urinary tract infection.
Data are presented as No. (percentage).
Excludes 15 patients (ERCP [n=10]; post-RFA or TACE [n=3]; liver transplant [n=2]); 1980-2000 period, 3 of 29 patients; 2001-2014 period, 12 of 58 patients.
Indicates obstruction of biliary drain (n=4), biliary enteric an anastomotic stricture (n=1), biliary obstruction postoperative of biliary bypass (n=1), and pancreatic mass obstructing biliary duct (n=1).
Characteristic findings on computed tomography (CT) of peripherally enhancing, centrally hypoattenuating lesions suggestive of PLA were found in 59 patients (81.9%), and 13 patients (18.1%) had poorly demarcated hypoechoic lesions on ultrasonography, suggestive of abscesses. Pyogenic liver abscess was found more often in the right lobe (n=45 [62.5%]) than in the left lobe (n=11 [15.3%]) or both (n=15 [20.8%]). In 1 patient, imaging was not available and PLA was diagnosed post mortem. Thirty-seven patients (51.4%) with PLA had a single abscess; 35 (48.6%) had multiple abscesses. On chest imaging scans, 9 patients (12.5%) had right lower-lobe pulmonary infiltrates.
Microbiologic Findings
Microorganisms were isolated from 61 patients (84.7%), whereas 11 patients had negative culture results (Supplemental Figure, available online at http://www.mcpiqojournal.org). One patient had a liver abscess diagnosed post mortem. Of these 61 patients, 31 had positive culture results from liver abscess and blood, 19 had positive results from liver abscess but negative blood culture results, and 11 had positive results of blood cultures but liver abscess culture results were negative or not available. Of interest, blood culture results were positive for 42 patients (58%). The most common organisms isolated were Streptococcus (52.5%) (subgroup: milleri [97%] and group D [3%]), Klebsiella (24.6%), and Escherichia coli (16.4%) (Supplemental Table 2, available online at http://www.mayoclinicproceedings.org). A comparison of the 2 periods found no significant difference in causative organisms, with Streptococcus milleri being the most common organism in both periods, followed by Klebsiella and E coli. However, MDR organisms were more frequently isolated in period 2 than in period 1 (51% vs 14%; P=.005).
After inclusion of 15 patients with postintervention PLA, the most common organisms isolated were S milleri (n=38 [52.8 - (52.8% if based on 72 pts)%]), Klebsiella (n=16 [22.2%]), and E coli (n=12 [16.7%]). No significant difference was found in the organisms isolated in the 2 periods, even after inclusion of patients who had interventions.
Of the 11 patients with no organism identified, 5 had negative results from liver abscess and blood cultures, 5 had negative blood culture results alone, and 1 had negative liver abscess culture results alone. Among the culture-negative patients, 10 had received preceding antibiotic therapy as empirical treatment. Only 1 patient without any preceding antibiotic therapy had a true culture-negative infection in accordance with liver abscess and blood cultures.
Treatment
Fourteen patients (19.4%) were treated with antibiotics alone and 1 (1.4%) received no treatment because of terminal illness. The most common route of antibiotic administration was intravenous (86%). Along with antibiotic therapy, 58 patients (80.6%) had abscesses drained with percutaneous aspiration (n=20 [27.8%]), percutaneous catheter drainage (n=31 [43.1%]), and surgical drainage (n=11 [15.5%]). Four patients required surgical drainage after percutaneous catheter drainage because of persistent abscess. There was no difference in the mean duration of antibiotic treatment in the setting of surgical vs nonsurgical drainage (4.5±2.5 weeks vs 6.2±3.3 weeks; P=.07). Recurrence of abscess was seen in 4 patients (5-year incidence, 4.4%) and was more often seen in period 2 than in period 1 (5-year incidence, 6.9% [95% CI, 0%-14.1%] vs 0%).
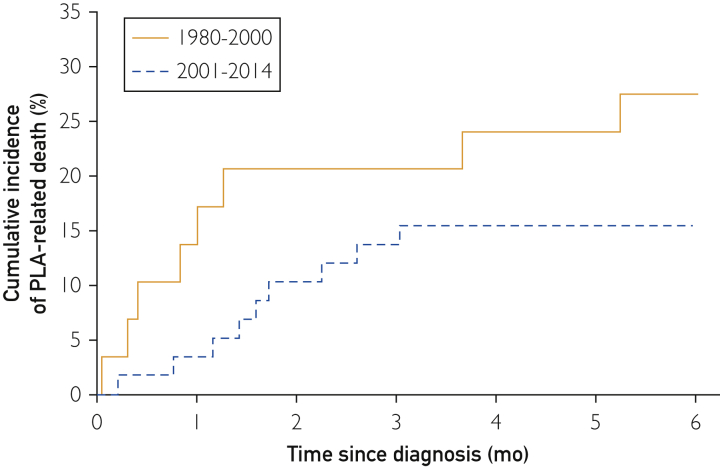
Temporal Trends in PLA-Related Death
The median follow-up duration was 8.8 years (range, 78 days to 30.8 years); 25% had follow-up for less than 4.5 years, and 75% were monitored for up to 15.8 years. The overall mortality rate of PLA was 16.8% (95% CI, 7.6%-25.0%) at 6 months. The 6-month mortality rate of PLA was 23.1% (95% CI, 5.1%-37.7%) in period 1 vs 13.1% (95% CI, 2.8%-22.3%) in period 2 (Figure 2). No significant difference was found in PLA-related death between the 2 time periods (hazard ratio [HR], 1.94; 95% CI, 0.62-6.0; P=.25). On comparing the periods after including 15 patients with postintervention PLA, we found that the 6-month mortality rate changed to 27.6% (95% CI, 9.3%-42.2%) in period 1 and to 15.5% (95% CI, 5.7%-24.3%) in period 2, with no significant difference between them (HR, 1.97; 95% CI, 0.76-5.10; P=.16).
Figure 2.
Kaplan-Meier estimates of PLA-related death in Olmsted County, Minnesota. PLA = pyogenic liver abscess.
In univariate Cox model analysis, multiple risk factors were associated with PLA-related death (Table 4), including presence of biliary disease (HR, 3.19; 95% CI, 1.01-10.07; P=.048), systemic malignancy (eg, metastatic pancreatic cancer [n=8], leukemia/lymphoma [n=4], metastatic colon cancer [n=4]) (HR, 5.17; 95% CI, 1.64-16.32; P=.005); immunosuppression (HR, 3.90; 95% CI, 1.23-12.29; P=.02), and history of cardiovascular disease (HR, 4.17; 95% CI, 1.25-13.91; P=.01). Patients with 3 or fewer risk factors (of the comorbidities in Table 4) had a higher risk of death than did those with fewer than 3 risk factors (HR, 10.37; 95% CI, 1.33-80.40; P=.02).
Table 4.
Predictors of Pyogenic Liver Abscess–Related Death (Univariate Cox Model Analysis)
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| Age group (y) (per decade) | 1.18 (0.82-1.70) | .37 |
| 18-34 (reference group) | 1 | |
| 35-49 | 0.76 (0.05-12.13) | .85 |
| 50-64 | 0.63 (0.06-6.93) | .70 |
| ≥65 | 1.45 (0.18-11.61) | .73 |
| Sex (male vs female) | 1.10 (0.33-3.65) | .88 |
| Race (white vs other) | 1.02 (0.22-4.63) | .99 |
| Drainage (yes vs no) | 0.63 (0.17-2.32) | .49 |
| Percutaneous aspiration (yes vs no) | 1.27 (0.38-4.22) | .70 |
| Percutaneous drain (yes vs no) | 0.41 (0.11-1.50) | .18 |
| Surgery (yes vs no) | 1.20 (0.26-5.56) | .82 |
| Biliary disease (yes vs no) | 3.19 (1.01-10.07) | .048 |
| Systemic disease (yes vs no) | 2.64 (0.79-8.76) | .11 |
| Intra-abdominal disease (yes vs no) | 0.77 (0.17-3.52) | .74 |
| Liver disease (yes vs no) | 3.72 (1.176-11.80) | .03 |
| Systemic malignancy (yes vs no) | 5.17 (1.64-16.32) | .005 |
| Immunosuppression (yes vs no) | 3.90 (1.23-12.29) | .02 |
| History of cardiovascular disease (yes vs no) | 4.17 (1.25-13.91) | .01 |
Discussion
The present study reports that the incidence of PLA has increased more than 2-fold during the past 35 years in this US population of Olmsted County. The incidence of PLA increased steadily across all age groups and in women. The incidence rate was 2.9 cases per 100,000 PYs between January 1, 1980, and December 31, 2014. This rate was higher than that of some of the earlier population-based studies from Western countries,2, 3, 4, 13 but it was lower than that of East Asian countries (eg, Taiwan).1, 14, 15 Biliary disease was the most common underlying risk factor for PLA. The most common organism isolated from abscess cultures was S milleri. With the increasing prevalence of liver abscess, we also noted a marked increase in infection by organisms with MDR in the past 2 decades. We observed high 6-month mortality rates associated with PLA. Various risk factors—biliary disease, systemic malignancy, and immunosuppression—were associated with an increased risk of death.
Several population-based studies from different countries have shown increasing incidence of PLA in the past few decades.1, 2, 5, 14 In a population-based study from Denmark, Jepsen et al3 found that PLA incidence increased from 0.6 to 1.8 cases per 100,000 PYs from 1977 to 2002. Similarly, Meddings et al5 reported an incidence rate increase from 2.7 to 4.1 cases per 100,000 PYs from 1994 to 2005. However, this study by Meddings et al5 was conducted with a national inpatient sample database and had several shortcomings, including misclassification of diagnosis codes, cultures, or procedures and therefore may not reflect the true incidence. Moreover, causes for increased incidence could not be ascertained. In Taiwan, the estimated incidence increased from 10.8 to 15.4 cases per 100,000 PYs between 2000 and 2011.14 The present study reports similar trends, with increasing PLA incidence over 35 years.
This global increase in PLA incidence might be explained by changes in such factors as introduction of interventions (eg, ERCP, RFA, and TACE), increased prevalence of liver transplant, organisms with MDR, and predisposing medical conditions. Previous studies have found an association between ERCP, RFA, TACE, and liver transplant and development of PLA.2, 5, 16 In the present study, after including patients who underwent some sort of intervention or liver transplant, the incidence rate significantly increased in the recent period (P<.001), suggesting that increased frequency of these procedures or liver transplant may have caused the incidence rates to increase. Interestingly, we did not find a statistically significant difference in the frequency of interventions between period 1 and period 2 (10% vs 20%), probably because of the small sample size. In addition, the expansion of antibiotic-resistant organisms that cause PLA may contribute to the increasing incidence.
Similar to a previous study by Huang et al,16 we observed an increased resistance of PLA-causing organisms to antibiotics across time. Although the increasing prevalence of MDR organisms may not lead to a higher incidence of PLA by itself, increased exposure of patients to such organisms could be an important factor leading to development of PLA. For instance, increasing hepatobiliary interventions with routine prophylactic antibiotics may not protect against resistant organisms and lead to PLA. This increase in resistance becomes an important factor for PLA development in light of increasing hepatobiliary interventions in a more recent era, as routine prophylactic antibiotics may not protect against resistant organisms. Another explanation may be the change in epidemiologic factors of the underlying risks. For instance, previous studies have reported an association between biliary diseases, malignancy, immunosuppression, and cardiovascular disease and increased risk of PLA.2, 17 We could not confirm these risk factors in the present study.
In this study, most patients with PLA presented with abdominal pain, fevers, and chills. On examination, hepatomegaly was the most common finding. However, this classic presentation was more common in period 1 than in period 2. The difference in presentation could be explained by more frequent use of CT in recent years, leading to earlier diagnosis of PLAs. Another explanation could be more widespread antibiotic use, which may mask symptoms in patients with PLAs.
Multiple previous studies from North America have described Streptococcus as the most common organism causing PLA.2, 5 Klebsiella and E coli are the other commonly identified microorganisms. In a study by Meddings et al,5 Streptococcus was isolated from 30% of patients with PLA and Klebsiella from 9%. Another study from Canada by Kaplan et al2 found a slightly higher incidence of Streptococcus (44%), Klebsiella (27%), and E coli (16%) infections. Our results are similar to those of Kaplan et al,2 with 52.5% Streptococcus (subspecies milleri), 25% Klebsiella, and 16% E coli isolated from PLAs. This difference in organisms across studies is likely secondary to the difference in study designs. In contrast, Klebsiella is a more common cause of PLA in East Asian countries, such as Taiwan and Hong Kong.1, 14, 15 These differences in microbiologic spectrum are unclear, but are postulated because of the high prevalence of virulent Klebsiella strains in patients living in Asia and a high prevalence of diabetes mellitus in Taiwan.18
Although PLA incidence has increased over decades, our study finds that the type of causative organism detected in PLA has not changed in the past 3 decades. However, a shift has occurred in the trend in antibiotic-resistant organisms, with increasing prevalence in more recent years. In their previous study, Huang et al16 reported that the percentage of antibiotic-resistant organisms cultured from PLAs was 25% in 1993. As observed in the present study, antibiotic-resistant organisms increased from 14% to 51% as the cause of PLA in the past 3 decades. This trend reflects frequent and prolonged use of broad-spectrum antibiotics for patients with infections.
The introduction of percutaneous aspiration of abscess, along with antibiotic use, has changed the management of PLA.19 Percutaneous drainage under ultrasonography or CT guidance has been established as the therapy of choice for PLA. Various randomized controlled trials suggest the superiority of percutaneous aspiration over surgical drainage.20, 21, 22 Similarly, we observed higher rates of percutaneous aspiration than surgical drainage. In addition, we noticed an increased rate of percutaneous aspiration, from 19% in period 1 to 33% in period 2, whereas surgical drainage decreased from 23% to 11%. In contrast to previous US studies that reported rates ranging from 4% to 9% for surgical resection and from 50% to 70% for percutaneous aspiration, we observed increased rates for both therapies (16% and 80%).2, 5 In PLA management, antibiotic therapy without drainage has a high mortality rate.23 In our study, of the 14 patients (20%) who did not undergo aspiration, 5 (36%) died within 3 months of PLA diagnosis. Better outcome after percutaneous aspiration of the abscess may be related to better source control as well as to more accurate identification of the causative organism and determination of antibiotic susceptibility.
Interestingly, the present study found that the 6-month mortality rate of PLA was 17%, which is slightly higher than that in previous studies (2%-15%).1, 2, 4, 5, 14 This discrepancy could be due to method of ascertainment of death. We reviewed and confirmed the cause of death for each patient through death certificate review in comparison to other studies that used administrative databases in which medical record review was usually not possible. Our observations of mortality rates are similar to those of the population-based study from Denmark that reported a 30-day PLA-related mortality rate of 19%.3 In comparison across the 2 periods in our study, we found that mortality rates associated with PLA were numerically smaller in 2001 to 2014 than in 1980 to 2000, suggesting a better diagnosis and management of PLA in recent years.
The main strength of this study is its population-based design. The well-established collaboration between the major health care providers in the Olmsted County area ensures that we have identified essentially all patients with PLA. Moreover, not only clinical but also microbiologic data were available for almost all patients. The study included a large number of diagnosed cases with positive culture results obtained from aspiration of PLA. Furthermore, our study extends the current medical knowledge by exhibiting increasing trends over 35 years. Finally, follow-up covered a long period (median, 8.8 years) to observe outcomes. However, this study has limitations that need to be considered when interpreting the results. The predominant population of Olmsted County is white, which may limit the generalizability of our findings.
Conclusion
Our population-based study reported that PLA is more common in men than in women. The incidence of PLA has increased over the past 35 years in Olmsted County, likely because of increasing frequency of hepatobiliary interventions in the setting of a higher prevalence of MDR organisms. However, PLA-related mortality rates have numerically decreased over this period.
Footnotes
Grant Support: This study was made possible by the Rochester Epidemiology Project (grant no. R01-AG034676; Principal Investigators: Walter A. Rocca, MD, MPH, and Jennifer L. St Sauver, PhD).
Potential Competing Interests: The authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at: http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Tsai F.C., Huang Y.T., Chang L.Y., Wang J.T. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14(10):1592–1600. doi: 10.3201/eid1410.071254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan G.G., Gregson D.B., Laupland K.B. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2(11):1032–1038. doi: 10.1016/s1542-3565(04)00459-8. [DOI] [PubMed] [Google Scholar]
- 3.Jepsen P., Vilstrup H., Schønheyder H.C., Sørensen H.T. A nationwide study of the incidence and 30-day mortality rate of pyogenic liver abscess in Denmark, 1977-2002. Aliment Pharmacol Ther. 2005;21(10):1185–1188. doi: 10.1111/j.1365-2036.2005.02487.x. [DOI] [PubMed] [Google Scholar]
- 4.Hansen P.S., Schønheyder H.C. Pyogenic hepatic abscess: a 10-year population-based retrospective study. APMIS. 1998;106(3):396–402. doi: 10.1111/j.1699-0463.1998.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 5.Meddings L., Myers R.P., Hubbard J., et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105(1):117–124. doi: 10.1038/ajg.2009.614. [DOI] [PubMed] [Google Scholar]
- 6.Mohsen A.H., Green S.T., Read R.C., McKendrick M.W. Liver abscess in adults: ten years experience in a UK centre. QJM. 2002;95(12):797–802. doi: 10.1093/qjmed/95.12.797. [DOI] [PubMed] [Google Scholar]
- 7.Rahimian J., Wilson T., Oram V., Holzman R.S. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;39(11):1654–1659. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 8.Foo N.P., Chen K.T., Lin H.J., Guo H.R. Characteristics of pyogenic liver abscess patients with and without diabetes mellitus. Am J Gastroenterol. 2010;105(2):328–335. doi: 10.1038/ajg.2009.586. [DOI] [PubMed] [Google Scholar]
- 9.Melton L.J., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 10.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel J.D., Rhinehart E., Jackson M., Chiarello L., Healthcare Infection Control Practices Advisory Committee Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35(10, suppl 2):S165–S193. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Hidron A.I., Edwards J.R., Patel J., et al. National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007 [published correction appears in Infect Control Hosp Epidemiol. 2009;30(1):107] Infect Control Hosp Epidemiol. 2008;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 13.Barakate M.S., Stephen M.S., Waugh R.C., et al. Pyogenic liver abscess: a review of 10 years’ experience in management. Aust N Z J Surg. 1999;69(3):205–209. doi: 10.1046/j.1440-1622.1999.01523.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y.C., Lin C.H., Chang S.N., Shi Z.Y. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan, 2000-2011. J Microbiol Immunol Infect. 2016;49(5):646–653. doi: 10.1016/j.jmii.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Wong W.M., Wong B.C., Hui C.K., et al. Pyogenic liver abscess: retrospective analysis of 80 cases over a 10-year period. J Gastroenterol Hepatol. 2002;17(9):1001–1007. doi: 10.1046/j.1440-1746.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- 16.Huang C.J., Pitt H.A., Lipsett P.A., et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg. 1996;223(5):600–607. doi: 10.1097/00000658-199605000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez Pérez J.A., González J.J., Baldonedo R.F., et al. Clinical course, treatment, and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg. 2001;181(2):177–186. doi: 10.1016/s0002-9610(00)00564-x. [DOI] [PubMed] [Google Scholar]
- 18.Siu L.K., Yeh K.M., Lin J.C., Fung C.P., Chang F.Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 19.Lardière-Deguelte S., Ragot E., Amroun K., et al. Hepatic abscess: diagnosis and management. J Visc Surg. 2015;152(4):231–243. doi: 10.1016/j.jviscsurg.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 20.vanSonnenberg E., Wittich G.R., Goodacre B.W., Casola G., D’Agostino H.B. Percutaneous abscess drainage: update. World J Surg. 2001;25(3):362–369. doi: 10.1007/s002680020386. [DOI] [PubMed] [Google Scholar]
- 21.Giorgio A., de Stefano G., Di Sarno A., Liorre G., Ferraioli G. Percutaneous needle aspiration of multiple pyogenic abscesses of the liver: 13-year single-center experience. AJR Am J Roentgenol. 2006;187(6):1585–1590. doi: 10.2214/AJR.05.1104. [DOI] [PubMed] [Google Scholar]
- 22.Ferraioli G., Garlaschelli A., Zanaboni D., et al. Percutaneous and surgical treatment of pyogenic liver abscesses: observation over a 21-year period in 148 patients. Dig Liver Dis. 2008;40(8):690–696. doi: 10.1016/j.dld.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Branum G.D., Tyson G.S., Branum M.A., Meyers W.C. Hepatic abscess: changes in etiology, diagnosis, and management. Ann Surg. 1990;212(6):655–662. doi: 10.1097/00000658-199012000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.