Abstract
Objective
To determine whether a pharmacist visit after hospital dismissal for patients taking at least 1 medication that places patients at high risk for emergent hospital admissions (termed high-risk medication) would decrease the risk of hospital readmission at 30 days compared with usual care.
Patients and Methods
This was a retrospective study at a tertiary care center conducted from July 26, 2013, through April 1, 2016. We reviewed outcomes among patients who did or did not have a post–hospital dismissal pharmacist visit immediately before a clinician visit. We included patients who were at least 18 years old and were taking at least 10 total medications at hospital dismissal, 1 or more of which were high-risk medications. A Cox proportional hazards model was used to compare the risk of 30-day readmission between the groups.
Results
The study cohort included 502 patients in each group (pharmacist + clinician group and clinician-only group). After adjusting for differences in background demographic characteristics, patients in the pharmacist + clinician group were significantly less likely to be readmitted to the hospital within 30 days postdismissal compared with the clinician-only group (hazard ratio, 0.49; 95% CI, 0.35-0.69; P<.001).
Conclusion
Patients seen by a pharmacist immediately before a clinician visit after hospital dismissal had a lower risk of readmission than patients who had a clinician-only visit. Patients taking high-risk medications for hospital admissions are ideal candidates for pharmacist involvement.
Abbreviations and Acronyms: EHR, electronic health record; HR, hazard ratio; PCC, pharmacist and clinician collaborative; UC, usual care
Adverse drug events are the most common preventable adverse events that occur after hospital dismissal.1 In patients older than 80 years, adverse drug events were found to be involved in nearly 25% of readmissions.2 Because of their expertise in pharmaceutical care, pharmacists have had more opportunities to be involved in transitions of care to help prevent adverse drug events. Several initiatives involving pharmacist visits in primary care during care transitions have led to decreases in hospital readmission rates.3, 4, 5, 6, 7, 8 Expanding pharmacist involvement has the potential for substantial financial impact, given the potential for decreased reimbursement for higher 30-day readmission rates, per the Affordable Care Act of 2010.
Although many drugs may cause adverse events, 3 studies have highlighted certain medications that place patients at high risk for adverse drug events specifically leading to hospital admission, termed high-risk medications.2, 9, 10 Studies have identified anticoagulants, antiplatelet agents, and diuretics as high-risk medications. Other medications, however, such as insulin and oral hypoglycemic agents, have also been listed as among the medications with the highest risk.2, 9, 10
Given that adverse drug events requiring hospitalization are more common with certain classes of medications, focusing pharmacist visits on care transitions involving these medications may decrease readmission risk. The existing literature has yet to focus on pharmacist care transition visits in patients taking only high-risk medications.
The objective of this study was to determine whether a pharmacist visit after hospital dismissal for adult patients taking at least 10 medications, with a minimum of 1 high-risk medication, would decrease the risk of hospital readmission at 30 days compared with usual care (UC).
Patients and Methods
Design, Setting, and Participants
The Mayo Clinic Institutional Review Board approved this study. This retrospective study includes patients seen at 6 different primary care practice sites (in either the Department of Family Medicine or the Division of Primary Care Internal Medicine) of Mayo Clinic in southeastern Minnesota from July 26, 2013, to April 1, 2016. Patients with any admission diagnosis were eligible for inclusion if they were 18 years or older, had been dismissed from the hospital within 30 days, had at least 10 medications on their discharge summary list, and had at least 1 high-risk medication on their discharge summary list. High-risk medication classes included oral anticoagulants, low-molecular-weight heparins, antiplatelet agents, insulin, noninsulin hypoglycemic agents, and loop diuretics (Supplemental Appendix, available online at http://www.mcpiqojournal.org). Patients were excluded if they were postpartum within 30 days, were pregnant, were prison inmates, or declined research authorization.
Patients were classified by whether they had a visit with a clinician only (the UC group) or a pharmacist plus a clinician (the pharmacist and clinician collaborative [PCC] group). If any patient had multiple qualifying visits within the time frame, the first qualifying visit was assessed. Patients in the UC group were seen by a clinician only for a 30-minute visit; patients in the PCC group were seen by a pharmacist for 30 minutes immediately before a 30-minute clinician visit. Clinicians include physicians, nurse practitioners, and physician assistants and were members of the Department of Family Medicine or the Division of Primary Care Internal Medicine.
During the visit in the PCC group, a pharmacist completed medication reconciliation, identified medication discrepancies, screened for drug interactions and high-risk medications, assessed adherence, identified drug therapy problems, and documented a clinical note on a standard documentation template. All recommendations were shared with the provider via verbal, written, or electronic means before the provider appointment with the patient. During the study period, 12 different pharmacists conducted visits. All pharmacists are credentialed by the study institution to deliver medication therapy management services and were authorized via a collaborative practice agreement to initiate, modify, or discontinue medications used to treat chronic diseases on the clinician's behalf. Pharmacists intentionally limited collaborative practice agreement use during their portion of the visit so that the pharmaceutical care plan could be discussed with the clinician and agreed upon before the clinician implemented the plan with the patient.
Outcomes
Our institutional electronic health record (EHR) was reviewed for patient characteristics and clinical variables, including the LACE index (Length of stay, Acuity of admission, Comorbidities, Emergency department visits during previous 6 months), which predicts the risk of death or unplanned readmission within 30 days after dismissal from the hospital.11 The study sites have 2 affiliated hospitals within the same community. Records of hospitalizations within these hospitals and 12 affiliated hospitals in the surrounding area were available in the EHR, and these were reviewed to abstract outcomes data on readmissions. The prespecified primary outcome measure was risk of readmission at 30 days after index hospital dismissal. Secondary outcomes were risk of readmission at 60 days and 180 days after dismissal.
For the PCC group only, additional descriptive outcomes were assessed. These included the number of drug therapy problem recommendations per patient made by the pharmacist and the number of drug therapy problem recommendations per patient made by the pharmacist relating specifically to high-risk medications, as determined from the documentation. We defined a drug therapy problem as “any undesirable event experienced by a patient that involves, or is suspected to involve, drug therapy and that interferes with achieving the desired goals of therapy and requires professional judgment to resolve.”12 Drug therapy problems were classified on the basis of the approach by Cipolle et al12 and included the categories indication, efficacy, safety, and adherence. The EHR was also reviewed for the percentage of drug therapy problem recommendations that were acted on by the clinician within 7 days. We also assessed the number of medication discrepancies identified per patient through medication reconciliation for all medications and for high-risk medications. Medication discrepancies were defined as any lack of agreement between the medication list in the EHR and the patient-reported medication regimen.13 Medication discrepancies were categorized as active medications omitted, medications listed in the EHR but no longer being taken, wrong dose of medication, or schedule other than prescribed.
Statistical Analyses
Descriptive statistics are reported as mean ± SD or number (percentage) as appropriate. Readmission rates postvisit were estimated using the Kaplan-Meier method. Multivariable Cox proportional hazards models were used to compare the risk of readmission between the 2 groups after adjusting for baseline covariates. Outpatient visits with the clinician, with or without the pharmacist, occurred several days after dismissal. Because we were interested only in comparing the PCC group with the UC group for postvisit readmissions, the follow-up time for these patients was 30, 60, or 180 days from the dismissal date. Hazard ratios (HRs) and 95% CIs were estimated from the models to compare groups. P values of less than or equal to .05 were considered to be statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc) and R version 3.2.0 (R Core Team, R Foundation for Statistical Computing).
Results
Patients
Of 960 unique patients seen by a pharmacist and clinician during the study time frame, 60 declined research authorization and 398 did not meet inclusion criteria. The remaining 502 patients made up the PCC cohort. In the UC group, 2471 patients were identified, of whom 104 declined research authorization and 579 did not meet inclusion criteria. Patient lists were cross-referenced, and 322 patients were excluded for also being in the PCC group. A random sample of the remaining 1466 patients was taken, and 502 patients were selected for the UC cohort.
Baseline Characteristics
Patients in the PCC group had a slightly higher mean LACE index (10.9±2.4 vs UC, 10.6±2.7; P=.02), which indicated a possible higher baseline risk of death or unplanned readmission within 30 days of dismissal (Table). The time from hospital dismissal to visit was longer in the UC group than in the PCC group (mean, 8.7±7.1 days vs 5.7±4.5 days; P=.005). In addition, a higher percentage of patients in the PCC group were taking aspirin (<325 mg/d) (58.4% vs 49.6%; P=.005). A higher percentage of patients in the PCC group were discharged from a primary care service, and a higher percentage in the UC group were discharged from a surgical service (both P<.001). Other baseline demographic characteristics were similar between groups (Table).
Table.
Baseline Characteristicsa
| Variable | Groupb |
P value | |
|---|---|---|---|
| PCC (n=502) | UC (n=502) | ||
| Age (y) | 70.9±14.2 | 70.2±13.8 | .31 |
| Men | 247 (49.2) | 265 (52.8) | .26 |
| No. of medications at dismissal | 16.3±5.3 | 15.6±4.8 | .065 |
| Time from hospital dismissal to follow-up visit (d) | 5.7±4.5 | 8.7±7.1 | .005 |
| LACE index | 10.9±2.4 | 10.6±2.7 | .02 |
| Charlson comorbidity index | 7.8±3.7 | 7.6±3.9 | .36 |
| High-risk medications | |||
| Oral anticoagulant | 310 (61.8) | 319 (63.5) | .56 |
| Injectable anticoagulant | 58 (11.6) | 40 (8.0) | .056 |
| Antiplatelet agent | 159 (31.7) | 163 (32.5) | .79 |
| Insulin | 104 (20.7) | 130 (25.9) | .052 |
| Noninsulin oral antidiabetic | 121 (24.1) | 139 (27.7) | .20 |
| Loop diuretic | 189 (37.6) | 209 (41.6) | .20 |
| 2 classes | 182 (36.3) | 176 (35.1) | .69 |
| ≥3 classes | 87 (17.3) | 63 (12.5) | .07 |
| Discharging service | |||
| Primary care | 284 (56.6) | 192 (38.2) | <.001 |
| Surgical specialty | 55 (11.0) | 132 (26.3) | <.001 |
| Nonsurgical specialty | 163 (32.5) | 178 (35.5) | .35 |
| Aspirin <325 mg | 293 (58.4) | 249 (49.6) | .005 |
LACE = Length of stay, Acuity of admission, Comorbidities, Emergency department visits during previous 6 months; PCC = pharmacist and clinician collaborative; UC = usual care.
Values are No. (percentage) of patients or mean ± SD.
Outcomes
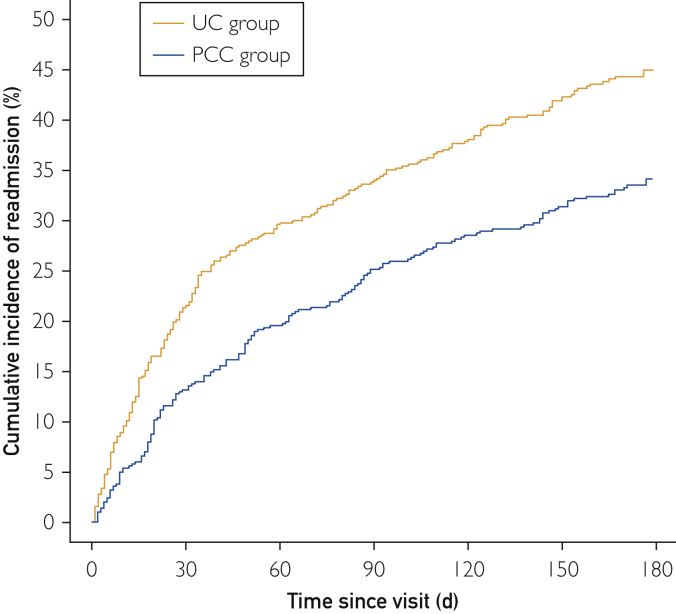
Readmission rates 30 days postvisit were 13.2% (95% CI, 10.2%-16.1%) in the PCC group and 21.6% (95% CI, 17.9%-25.1%) in the UC group. Readmission rates at 60 days and 180 days postvisit in the PCC group were 19.6% (95% CI, 16.0%-23.0%) and 34.1% (95% CI, 29.7%-38.3%), respectively; these rates were 29.8% (95% CI, 25.7%-33.7%) and 45.0% (95% CI, 40.3%-49.3%), respectively, in the UC group. The Figure shows the cumulative incidence of postvisit readmission.
Figure.
Cumulative incidence of readmission. Kaplan-Meier curves estimating the incidence of readmission after follow-up visit for the PCC group and the UC group. PCC = pharmacist and clinician collaborative; UC = usual care.
After adjusting for baseline demographic characteristics (Table), patients seen in the PCC group were significantly less likely to be readmitted to the hospital within 30 days postdismissal compared with the UC group (HR, 0.49; 95% CI, 0.35-0.69; P<.001). The risk reduction was maintained at 60 days (HR, 0.50; 95% CI, 0.38-0.66; P<.001) and 180 days (HR, 0.57; 95% CI, 0.46-0.71; P<.001).
Through medication reconciliation, pharmacists identified 877 medication discrepancies (average of 1.7 per patient) for all medications and 98 medication discrepancies (average of 0.2 per patient) related only to high-risk medications. Pharmacists identified and made recommendations on 852 drug therapy problems (1.7 per patient); of these, 224 (26.3%) drug therapy problems (0.4 per patient) were related to high-risk medications. Of the drug therapy problem pharmacist recommendations, 74% of the overall medication interventions and 84% of the high-risk medication interventions were acted upon by the clinician.
Discussion
Patients taking high-risk medications, when seen by a pharmacist immediately before a clinician after hospital dismissal, had a lower risk of readmission than those who had a clinician-only visit. These patients were 50% less likely to be readmitted by 30 days compared with those seeing only a clinician. Patients taking medications that place them at high risk for hospital admissions due to adverse drug events are ideal candidates for pharmacist involvement. This study further supports the continued growth in the pharmacist's role to work collaboratively with providers during care transitions. These medications and the care plans associated with them should receive considerable attention and be addressed at every postdismissal visit with high priority. The expertise of the pharmacist and the focus on pharmaceutical care helps ensure that issues with these medications are identified and resolved. It is also possible that because the pharmacists focused on medications, the clinicians were able to spend more time on disease management.
The decreased risk of readmission seen initially was also continued through 60 and 180 days from the follow-up visits after the index hospitalization dismissals. Pharmacist interventions, particularly those related to medications with high risk of hospital admission, may have contributed to the risk reduction. On average, 2 drug therapy problems were noted for each patient during each pharmacist review. Considering that these were medically complex patients with polypharmacy who were seen within a short time after hospital dismissal, this indicates considerable potential for medication errors, even in this short transition period. It is also notable that more than one-fourth of the drug therapy problems were related to high-risk medications. The high rate of clinical acceptance of the pharmacists' interventions suggests that the patients' clinicians believed the recommendations were helpful. The decrease in readmission risk seen in our study is consistent with that seen in several studies involving pharmacist visits after hospital dismissal.3, 4, 5, 6, 7, 8 In contrast to these previous studies, we focused only on patients taking medications that confer a high risk of hospital admission.
Limitations
Our study had several limitations. It is possible that readmissions at nonaffiliated hospitals could have been missed or that patients were readmitted outside our service area. Although a standard documentation template was used by all pharmacists, there were differences in documentation between the 12 pharmacists who conducted visits. This could potentially lead to different interpretation of recommendations and discrepancies when retrospectively reviewing documentation. Patients were not case-matched on the basis of baseline variables (eg, LACE index), and some differences were noted between the 2 groups. However, all significant differences between groups were adjusted for in our analysis. Future evaluation of this intervention in other sites would be helpful. We believe that the most significant barrier to implementing a similar practice elsewhere is convincing organizations of the value of the pharmacist's medication review. In addition to the value of improved outcomes shown in our study, further exploring the financial impact of this intervention would help illustrate its benefit. Also, comparison of readmission outcomes for patients whose clinicians followed the pharmacist's recommendations with those who did not would be intriguing. However, a larger study would most likely be needed.
There is opportunity for quality improvement within the visits. Although all pharmacists were trained and credentialed as general medication therapy management providers, there was no formal training on how to conduct transition-of-care visits. Developing education targeted specifically to these visits and tracking improvements in outcomes is an important future opportunity.
Conclusion
Medically complex patients who were prescribed high-risk medications had significantly lower 30-day hospital readmission risk when their posthospital visit was paired with a pharmacist review and evaluation of pharmaceutical therapies. This effect continued for up to 180 days from the index hospitalization. Although the expertise of the pharmacist and the ability to focus considerable time on the pharmaceutical care was assumed to influence these outcomes, further evaluation of this intervention would be helpful. Furthermore, validation with a prospective study would be intriguing.
Footnotes
For editorial comment, see page 1
Potential Competing Interests: The authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Forster A.J., Murff H.J., Peterson J.F., Gandhi T.K., Bates D.W. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 2.Teymoorian S.S., Dutcher D., Woods M. Association between postdischarge adverse drug reactions and 30-day hospital readmission in patients aged 80 and older. J Am Geriatr Soc. 2011;59(5):948–949. doi: 10.1111/j.1532-5415.2011.03376.x. [DOI] [PubMed] [Google Scholar]
- 3.Pellegrin K.L., Krenk L., Oakes S.J., et al. Reductions in medication-related hospitalizations in older adults with medication management by hospital and community pharmacists: a quasi-experimental study. J Am Geriatr Soc. 2017;65(1):212–219. doi: 10.1111/jgs.14518. [DOI] [PubMed] [Google Scholar]
- 4.Cavanaugh J.J., Lindsey K.N., Shilliday B.B., Ratner S.P. Pharmacist-coordinated multidisciplinary hospital follow-up visits improve patient outcomes. J Manag Care Spec Pharm. 2015;21(3):256–260. doi: 10.18553/jmcp.2015.21.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawes E.M., Maxwell W.D., White S.F., Mangun J., Lin F.C. Impact of an outpatient pharmacist intervention on medication discrepancies and health care resource utilization in posthospitalization care transitions. J Prim Care Community Health. 2014;5(1):14–18. doi: 10.1177/2150131913502489. [DOI] [PubMed] [Google Scholar]
- 6.Ni W., Colayco D., Hashimoto J., et al. Impact of a pharmacy-based transitional care program on hospital readmissions. Am J Manag Care. 2017;23(3):170–176. [PubMed] [Google Scholar]
- 7.Bellone J.M., Barner J.C., Lopez D.A. Postdischarge interventions by pharmacists and impact on hospital readmission rates. J Am Pharm Assoc (2003) 2012;52(3):358–362. doi: 10.1331/JAPhA.2012.10172. [DOI] [PubMed] [Google Scholar]
- 8.Arnold M.E., Buys L., Fullas F. Impact of pharmacist intervention in conjunction with outpatient physician follow-up visits after hospital discharge on readmission rate. Am J Health Syst Pharm. 2015;72(11, suppl 1):S36–S42. doi: 10.2146/sp150011. [DOI] [PubMed] [Google Scholar]
- 9.Budnitz D.S., Lovegrove M.C., Shehab N., Richards C.L. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 10.Howard R.L., Avery A.J., Slavenburg S., et al. Which drugs cause preventable admissions to hospital? a systematic review. Br J Clin Pharmacol. 2007;63(2):136–147. doi: 10.1111/j.1365-2125.2006.02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Walraven C., Dhalla I.A., Bell C., et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557. doi: 10.1503/cmaj.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipolle R.J., Strand L.M., Morley P.C. 3rd ed. McGraw-Hill; New York, NY: 2012. Pharmaceutical Care Practice: The Patient-Centered Approach to Medication Management. [Google Scholar]
- 13.Orrico K.B. Sources and types of discrepancies between electronic medical records and actual outpatient medication use. J Manag Care Pharm. 2008;14(7):626–631. doi: 10.18553/jmcp.2008.14.7.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.