Abstract
Objective
To develop clinical decision support (CDS) for familial hypercholesterolemia (FH), based on physician input obtained by a mixed methods approach.
Introduction
Awareness, detection, and control of FH—a relatively common genetic disorder—is low. Clinical decision support could address knowledge gaps and provide point-of-care guidance for the management of FH.
Methods
A 16-question survey that assessed familiarity with FH and sought input on potential content of the CDS tool was emailed to 1161 clinicians including 208 cardiologists. In addition, 4 physician focus groups were held to gather input on the structure and form of the CDS tool. This study took place between September 12, 2016, and January 16, 2017.
Results
The response rate to the survey was 18.1%. Clinicians were overwhelmingly (97.6%) in favor of a CDS tool that assists in managing patients with FH at the point of care and this was confirmed in the focus group discussions. Key themes emerged during the focus groups including providers' knowledge and understanding of FH, facilitators and barriers to implementing a CDS tool, and suggestions for its design and content.
Conclusion
Clinicians were supportive of development of a CDS tool to assist with the evaluation and treatment of FH and provided feedback related to the design and implementation of such a tool.
Abbreviations and Acronyms: CDS, clinical decision support; CHD, coronary heart disease; EHR, electronic health record; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol
Familial hypercholesterolemia (FH) is a relatively prevalent (∼1 in 250)1, 2, 3 autosomal-dominant disorder of lipid metabolism associated with elevated low-density lipoprotein cholesterol (LDL-C) levels and markedly increased risk of coronary heart disease (CHD).4 It is estimated that in the United States, up to 1.3 million patients are currently living with FH; however, less than 10% of these actually carry the diagnosis of FH,4 highlighting that this condition is frequently underdiagnosed5 and consequently undertreated.
Electronic health record (EHR)-based strategies can be useful in addressing low detection, awareness, and control of FH.6 We have previously developed an electronic phenotyping algorithm for automated detection of FH in the EHR.5 However, case detection needs to be linked to clinical decision support (CDS) to provide guidance on evaluation and management of FH at the point of care. Such a tool could significantly increase awareness and detection of FH. A previous survey revealed that even cardiologists struggle to diagnose FH correctly, with less than a third feeling confident of making the diagnosis consistently and 68% wishing to expand their knowledge of FH.7
To develop a CDS tool that provides guidance at the point of care for clinicians, we sought feedback for its design and content. We surveyed a diverse group of physicians, both primary care physicians and specialists, to gather input for the design of a CDS tool for FH, as well as its overall presentation and content. We also conducted focus groups that included physicians in primary care and cardiology to develop an approach on how such a tool would function within the EHR as well as how the tool could be designed to ensure it was useful, did not add to clinician burden, and was time efficient.8, 9
Methods
This study was considered exempt by the Mayo Clinic Institutional Review Board and took place between September 12, 2016, and January 16, 2017.
Survey
The survey was developed to (a) assess the overall level of knowledge regarding FH, including a provider’s ability to correctly identify a lipid profile consistent with FH; and (b) gather feedback on what clinicians would prefer in a CDS tool for FH. The survey was first administered to a group of clinical experts in FH (n=7) to evaluate content and content validity. Revisions were made on the basis of the feedback. The survey was then administered to a group of preventive cardiologists (n=10) to evaluate comprehension and validity. Following comments from both groups, the survey was deployed by the Mayo Survey Research Center via Qualtrics (Qualtrics LLC) to 1161 clinicians in the Mayo Clinic network comprising physicians in Rochester, Minnesota; Scottsdale, Arizona; Jacksonville, Florida; and satellite campuses in Wisconsin and Minnesota. Survey respondents included internists, cardiologists, endocrinologists, geneticists, family medicine physicians, pediatricians, cardiology fellows, internal medicine residents, and family medicine residents. Residents were included because they are often the first care provider to interact with patients and uniquely positioned to diagnose this condition. Reminders were sent 1 week after the initial invite, and a second reminder sent 2 weeks after the initial invite for participation. Over a period of 11 weeks, the final response rate was 18.1% (n=210).
Focus Groups
The focus groups were conducted from September 23, 2016, to January 16, 2017, and included at least 4 participants per group. Clinicians at the Mayo Clinic-Rochester campus in the fields of internal medicine, family medicine, endocrinology, cardiology, and medical genetics were invited by email. Of the 417 physicians (147 cardiologists, 15 endocrinologists, 140 internists, 5 medical geneticists, and 59 family medicine physicians), a subset was selected to participate in the study.10, 11 The discussions lasted between 45 and 60 minutes.
A semi-structured interview guide was used to moderate the discussion. The sessions were audiotaped with participant consent and transcribed verbatim by a transcriptionist. The first author (A.H.) along with an experienced moderator (A.K.) led the focus groups. Data collection and analysis were guided by grounded theory methodology.9, 12, 13 Data were then coded by A.H. and A.K. using a process of open, axial, and selective coding using Nvivo software (QSR International). Both A.H. and A.K. independently read the first 2 transcripts and developed a codebook with consensus. In the open coding process, the codebook was used to independently code all the transcripts and labels were given for different themes emerging from the data. In the next step of axial coding, all the labeled ideas were grouped together on the basis of their characteristics and relationships. The final stage of analysis included selective coding. All the themes that emerged were regrouped for a descriptive presentation of key findings using a constant comparative approach. Any discrepancies between the 2 coders were resolved through discussion and consensus was reached.
Statistical Analyses
Survey data were analyzed with use of JMP Pro 10 (SAS). To facilitate analyses, some of the survey responses were recoded from free-text responses to binary data. Descriptive data were provided for relevant measures. The frequency (%) of categorical factors was compared using the χ2 test to identify statistically significant (P<.05) differences for several questions in the survey.
Results
Survey
Table 1 lists the characteristics of the survey respondents. An overwhelming majority (97.6%, n=205) were in favor of a CDS tool for FH, stating that it would be helpful for the clinician at the point of care. Only 5.3% (n=11) of clinicians reported being very familiar with FH, with the largest group (47.8%, n=99) reporting some familiarity with FH. Most (84.6%, n=176) clinicians were able to identify a lipid panel consistent with FH, but only 48.5% (n=101) correctly identified the prevalence of FH. Cardiologists were 2.89 times more likely to correctly state the prevalence of FH as compared with primary care providers (P=.01; 95% CI, 1.55-5.40) and 2.40 times more likely to correctly identify the lipid profile consistent with FH (P=.05; 95% CI, 0.97-6.79); 83.3% (n=175) of respondents indicated that a CDS tool providing an alert that a patient may have FH as well as suggestions for treatment would be the most valuable, instead of an alert focused on diagnosis or treatment only, and 4.0% (n=8) of respondents preferred no alert at all. The most frequent concern was the possibility of alert fatigue. In addition, 37.3% (n=76) of respondents believed that an order-set for FH would be very helpful and 80.3% (n=164) of respondents believed that the order-set would be somewhat to very helpful. Additional results of the survey are summarized in Table 2.
Table 1.
Characteristics of Survey Respondents (n=210)
| Characteristic | n (%) |
|---|---|
| Type of respondent | |
| Resident/Fellow | 40 (19.1) |
| Faculty | 170 (80.9) |
| Sex | |
| Male | 147 (70.0) |
| Female | 63 (30.0) |
| Specialties | |
| Internal medicine | 75 (35.7) |
| Cardiology | 65 (30.9) |
| Family medicine | 50 (23.8) |
| Medical genetics | 3 (1.42) |
| Endocrinology | 10 (4.7) |
| Pediatrics | 7 (3.3) |
| Years in practice | |
| 0-1 | 13 (6.1) |
| 2-4 | 24 (11.4) |
| 5-10 | 30 (14.2) |
| More than 10 | 103 (49.0) |
| In training (fellow/resident) | 40 (19.0) |
| Site | |
| Rochester, MN | 148 (70.4) |
| Jacksonville, FL | 13 (6.1) |
| Scottsdale, AZ | 21 (10.0) |
| Other | 28 (13.3) |
Table 2.
Survey Results
| Question | n (%) |
|---|---|
| Correctly identified lipid profile | |
| • Percentage of faculty | 155 (92) |
| • Percentage of residents and fellows | 21 (53) |
| Number of patients with FH seen by the majority (>50%) of | |
| • Primary care physicians (n=74) | 0-3 (58.2) |
| • Cardiologists (n=33) | >7 (51.5) |
| • Endocrinologists (n=7) | >10 (70) |
| Respondents who believed a CDS tool would be helpful in the management of a patient with FH | 205 (98) |
| Perceived utility of FH order-set | |
| • Very helpful | 76 (37.2) |
| • Somewhat helpful | 88 (43.1) |
| • Neither helpful nor unhelpful | 19 (9.3) |
| • Somewhat unhelpful | 12 (5.8) |
| • Very unhelpful | 9 (4.4) |
| Preference for alert location | |
| • Upon accessing the patient record | 42 (20) |
| • Upon reviewing the laboratory data | 105 (50) |
| • No alert, instead highlight the patient on caregiver’s schedule | 16 (8) |
| • Inbox notification | 83 (40) |
| • No alert | 8 (4) |
| Elements related to the configuration of CDS tool | |
| • Reminder to rule out secondary causes of hyperlipidemia | 139 (66) |
| • Reminder to screen family members | 126 (60) |
| • Initiate/optimize lipid-lowering therapy | 149 (71) |
| • Cardiovascular genomics consultation for family pedigree and genetic testing | 106 (51) |
| • Link to relevant scientific statements | 76 (36) |
| • Link to AskMayoExpert | 138 (65) |
| Components of order-set | |
| • Lipid profile | 151 (72) |
| • Cardiovascular genomics consultation for possible genetic testing and family pedigree | 165 (79) |
| • Reminder to test family members | 137 (65) |
| • Other | 16 (8) |
CDS = clinical decision support; FH = familial hypercholesterolemia.
Focus Groups
Of the 19 physicians who participated in the focus groups, 6 were primary care physicians (5 internists and 1 family medicine physician) and 13 were cardiologists; 10 were men and 9 were women. Based on analysis of the transcripts of the focus group discussions, 4 major themes were identified (Table 3).
Table 3.
Major Themes of Focus Group Discussions and Key Ideas Emerging From Themes
| Major themes of focus group discussions | Key ideas emerging from themes |
|---|---|
| Theme 1: Knowledge, Perceptions, and Understanding of FH |
|
| Theme 2: Facilitators for the Successful Implementation of a CDS Tool for FH |
|
| Theme 3: Barriers to Successful Implementation of a CDS Tool for FH |
|
| Theme 4: Recommendations for the Development of a CDS Tool for FH |
|
CDS = clinical decision support; FH = familial hypercholesterolemia.
Theme 1: Knowledge, Perceptions, and Understandings of FH
A major theme that emerged in the focus group discussions was the lack of knowledge of FH and incomplete understanding of the disease among providers. Consistent with the survey results, physicians expressed relative unease dealing with FH and were forthcoming in their lack of awareness of FH and its significance. It became apparent that many clinicians do not realize the significant risk of CHD associated with FH and the implications for family members. Clinicians raised questions such as “How big of a problem is FH?” and “How will labeling a patient with FH help me or the patient any more than labeling them with hypercholesterolemia?” One physician likened hypercholesterolemia to hypertension, noting that “[FH] it is like hypertension just see it and treat it… that’s been my approach” (Focus Group [FG] 03). In addition, a physician seemed to justify the lack of knowledge as irrelevant in some cases as he described a hypothetical patient with elevated cholesterol levels, saying “So, really for me the biggest impact is, what am I going to do with this 68-year-old patient who's got high lipids, maybe on a familial basis, maybe not, but they've got high lipids. What am I going to do with them? Am I going to do anything differently?” (FG 04). This physician's perception was that knowledge of FH would likely not alter the management of this patient, and that was what the physician was most focused on, the management of that patient.
Among cardiologists, the lack of familiarity with FH may in part be due to subspecialization and contribute to underdiagnosis of FH cases. One of the physicians, a cardiologist, noted: “But for me I am focused more on valvular heart disease. I mean I do general cardiology but you focus on certain things and it gets you away from hyperlipidemia and stuff, so maybe it's a twofold thing. You know getting away from it from a stand point of specializing in your area of interest, plus maybe not being clear about that particular diagnosis, the criteria for it” (FG 04).
Finally, one clinician echoed a thought shared by most of our discussants, noting the idea that even if a patient had FH, it would be beyond the scope of their practice, and thus they would just refer the patient to a lipid specialist for management. This idea that the management of this disorder is out of an individual's scope of practice likely also contributes to the relative lack of knowledge of FH and subsequently underdiagnosis of FH.
The discussion then centered on how best to address this knowledge gap, with one physician noting that to truly increase the knowledge of this disorder, one almost has to begin a campaign. Every opportunity must be taken to increase awareness of FH; otherwise, the disorder could be relegated to the background by other comorbidities that need attention. A major theme that emerged was that a CDS tool could address this knowledge gap by assisting in the diagnosis of this disorder and at the same time providing resources for managing FH, thereby increasing both awareness and knowledge of FH at every relevant encounter.
Theme 2: Facilitators for the Implementation of a CDS Tool for FH
To successfully integrate a CDS tool for FH into the EHR, clinicians noted 2 main requirements. First, the tool should not require manual input of data or laboratory values but automatically pull data necessary for guidance at the point of care. Second, the patient should be informed in some way before the encounter, thereby allowing the patient to review some of the details of this disorder and reduce the burden on the physician. More than one provider suggested relaying an alert to the patient within the Patient Portal (an online platform for patients to access medical documentation and laboratory reports) so that the patients themselves seek out information and treatment for the disorder. One physician describing genetic testing for FH stated, “I think it [the CDS tool for FH] should work in 2 ways. One is communicate it to the patient, and tell the patient we think you may have this disease, you have the phenotype and genotype …And then secondly, I think it will be helpful to flag it in the chart that the patient had the genetic sequencing; so the physician who’s seeing the patient who might be interested can go and look for it” (FG 02). Informing both the provider and the patient could enhance and create a shared decision-making environment instead of the traditional paternalistic model in which physicians inform patients of what changes to make and what medications to take. Generally, however, because of the sensitive nature of genetic test results, the information is revealed in person, usually by a certified genetic counselor.
Theme 3: Barriers to the Implementation of a CDS Tool for FH
Physicians noted several potential barriers to the adoption of the tool, some more easily addressable than others. Physicians often have limited time with their patients, and there was concern among our focus group participants at adding yet another issue to the problem list. As one physician noted, “We want to help these patients but you have to be realistic and you have to have time to help them. It’s a big conversation. And so don't just develop a machine that then puts the burden on the doctor and says ‘ok you take care of it’ and you [the doctor] say ‘I have no expertise, I have no time’ what do I do?” (FG 01) The other concern was the use of pop-up alerts, with many clinicians echoing the viewpoint that such alerts, though designed to be helpful, ultimately distract and slow down the use of the EHR and because of that, can be clicked off or ignored despite what may be a valid warning or alert.
Patient factors also can potentially limit the effectiveness and utility of a CDS tool for FH. One clinician noted that patients often are aware of their elevated lipid levels but because of lack of symptoms, they choose no action. An alert for the provider would be unlikely to change such a patient's behavior. The other concern was the utility of labeling a patient with FH with multiple comorbidities. As one physician noted, “So looking at what’s going on in their life or [if] they have end-stage cancer they don't care. I mean from [a] realistic stand point” (FG 01). Another physician echoed similar sentiments, “There are a lot of patients I care for who are end of life and [FH] is the last thing I want to address” (FG 01).
In addition, the lack of knowledge of FH may limit the impact of an alert for FH. One clinician noting that “I think a key problem is not as much how to flag the patients, but what are we going to do with the patient once they're flagged” (FG 02) suggested that the tool may not be useful unless there is guidance on the next step to pursue if the patient is flagged having FH. As another clinician suggested, “[place] a link next to that particular problem item that would take me to A and B and help me problem solve what else needs to get done. Has this person been seen by cardiology? Have they been put on medications? … [help] me to navigate what else has to be done from that stand point”(FG 01). No amount of guidance could replace core knowledge of FH, but links to such resources might allow clinicians to feel more comfortable taking the initial steps for the management of these patients.
An example of this is the ordering of genetic testing of FH, with one clinician noting that “I'd have zero comfort ordering genetic tests for that. I would have no idea who would need genetic testing and what to do with it when I got it” (FG 03). Such statements suggest that it is important to have an order-set that provides the initial steps in management, but an all-inclusive order-set may not be practical.
Theme 4: Recommendations for the Development and Implementation of a CDS Tool for FH
There was significant discussion of how an alert should be embedded within the EHR. Providers wanted to have a flag or alert notifying the possibility of FH in their patient, realizing that it may not be relevant in every clinical encounter that occurs during a hospital setting (such as during a preoperative evaluation). However, a concern raised was the risk of multiplicity of flags or the recurrence of the alert. A suggestion to prevent this by one clinician was to “…have it [the alert] only once. Because you're not going to be checking it more…It would be very annoying that every year you see the patient and you have the flag…I think there should be a way that it comes up as I don't know the first time that you have lipid profile it raises…the question and then you have a way to answer no. I already thought about it” (FG 01). Another clinician echoed similar sentiments noting that in certain EHR systems, being able to click on a yellow or red symbol will trigger the appearance of an order-set as well as potential resources for additional information. Because FH is not an immediately life-threatening condition, one provider noted that even providing a “snooze” button would be helpful. “One thing that I would say would probably be useful from, from my advantage point is not to have a pop-up that doesn't allow me to do anything else in the chart until I click on it. Because those things get clicked on without being read every single time. They're not helpful; they're blocking me from doing whatever it was I went into the chart to do in the first place…At the very least add like a snooze button to it or something like that. So that you know next time I come to the chart maybe I'd like to have that alert, maybe not depending on, on the individual patient” (FG 01).
Providers felt an alert should be succinct and clear, without jargon and emphasize the diagnosis of FH and provide assistance in the management of FH. At the same time, clinicians realized they would not become experts with the provided links but rather have enough information to perform the next steps and advise the patient of this as well. As one clinician put it, “Even as an internist I don't have to be an expert on this, I don't have to be the final authority on it but I can say to them ‘this is what I think is happening with you, these are the tests we're going to do, these are the consults we're going to get, these are the consequences if we don't pay attention to what's going on here’ ” (FG 01).
Because FH is an autosomal-dominant disorder, the topic of genetic testing and family screening was discussed during the focus groups. Clinicians felt that counseling for genetic testing was outside their comfort level and the scope of their practice, and that likely those conversations would be deferred to a genetic counselor. An order-set item containing an option to order genetic testing may not be as helpful as having an order-set item to be able to refer to a genetic counselor. In addition, providers wanted to be able to provide some form of documentation or a letter to the patient that the patient could then send on to family members and alert them of their risk of this disorder as well.
Discussion
The purpose of this study was 2-fold. First, to gather input to guide the design of a CDS tool for FH using a survey; second, to conduct focus group discussions on how the tool could be designed to ensure it was useful, could be integrated within the EHR, not add to the clinician's burden, and was time efficient. We used a mixed methods approach (survey with the use of focus groups) to obtain physician perspectives related to the CDS tool, incorporating provider input from multiple medical specialties with varying experience and knowledge of both FH and the EHR workflow.
Familial hypercholesterolemia is one of the few genetic disorders that meets the World Health Organization criteria for population-based screening programs aimed at early disease detection and treatment.4 Familial hypercholesterolemia is also classified as a Tier 1 Genomic Application because it poses a significant public health burden and can be screened for and treated.4 For this reason, the diagnosis of this condition is an important public health priority.
Most respondents were able to recognize a lipid profile consistent with FH, but a fewer number were able to correctly state the prevalence of FH. These results suggested that providers may not be actively considering the diagnosis of FH. Nearly half of physicians were able to state the prevalence correctly compared to a prior survey of cardiologists in which only 19% were able to do so.7 Despite what appears to be an increase in knowledge (from 2013 to 2017), a sizeable gap remains that CDS could potentially address. Most respondents favored an alert when reviewing the laboratory data. In the focus groups, participants stated that if an alert is the method of notification, it should not interrupt the workflow. Most wanted guidance related to management to accompany the alert, with 71% (n=149) favoring guidance on how to initiate/modify lipid-lowering therapy and 65% (n=138) favoring a link to AskMayoExpert (an internal database similar to UpToDate that provides recommendations for diagnosis and treatment). Clinicians wanted clear instructions on how best to manage a patient meeting criteria for FH, and this was apparent from both the survey results and the focus group discussions.
Clinical decision support can link information present in the EHR with relevant clinical guidelines and thereby reduce clinical errors and improve patient safety.14 Clinical decision support has been used for a number of purposes including diagnosis of a wide range of disorders from depression to cardiac ischemia,15 for preventive measures, including mammography reminders, influenza vaccination, and colon cancer screening,15 and for management of disorders including hypertension.16 The outcomes subsequent to the use of these CDS tools have varied, with some improving practitioner performance, some improving patient outcomes, and some achieving neither.15 A recent review noted that CDS designed to assist the provider in diagnosis was beneficial in only 4 of 10 reviewed studies, whereas reminder systems for prevention were beneficial in 76% of studies reviewed.15 To date, a CDS tool for the diagnosis and management of FH has not been developed.
Of the cardiologists in our survey 51.5% (n=33) had interacted with 7 or more patients with FH, whereas most (58.2%, n=74) primary care physicians interacted with far less (0-3 patients with FH). In one of the focus groups, there was a suggestion to consider relaying the FH alert to only specific physicians. However, in subsequent discussions, it became apparent that the maximum benefit from a CDS system would result from alerting any appropriate provider about this often-undiagnosed condition. Even seasoned cardiologists were hesitant to make the diagnosis of FH. Rather, they felt the tool would allow them to refer the patient with possible FH to an FH specialist to confirm the diagnosis.
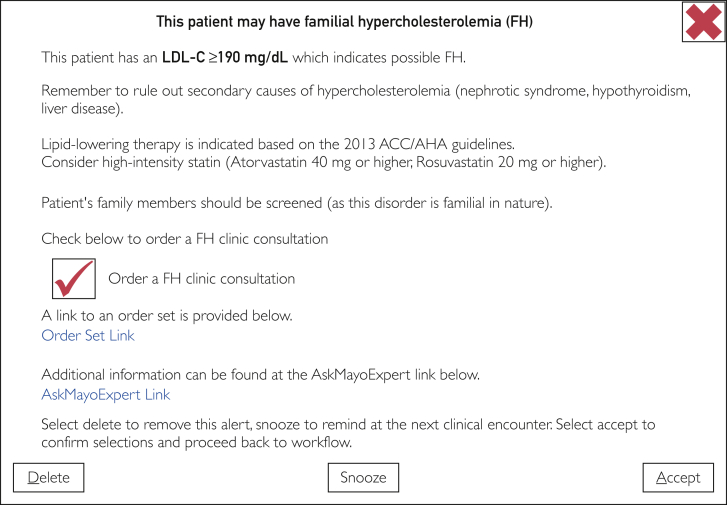
Using data from both mediums (survey and focus groups), a prototype CDS and order-set were crafted for eventual implementation into the EHR (Figures 1 and 2). The prototypes will be further modified on the basis of input (from patients and providers) before implementation in the EHR. Following this, use, feedback, and outcomes will be monitored and modifications will be made where necessary, for more widespread deployment.
Figure 1.
Prototype Clinical Decision Support (CDS) Alert. ACC = American College of Cardiology; AHA = American Heart Association; LDL-C = low-density lipoprotein cholesterol.
Figure 2.
A prototype order-set for FH.
Study Limitations
Our survey had a modest response rate. The focus group discussions included a majority of cardiologists and internists and ideally, we would have had a greater mix of clinicians particularly family medicine physicians who are often involved in caring for multiple family members and thus have a greater opportunity to alert those at risk. In addition, opinion of endocrinologists could be obtained only via the survey. An additional potential limitation was the inability to recruit our goal numbers of clinicians from each specialty from each focus group.
Conclusion
Clinicians were overwhelmingly in favor of a CDS tool that could assist in the management of patients with FH at the point of care and this was confirmed in the focus group discussions. Key themes that emerged during the focus groups included lack of knowledge about FH among discussants, facilitators and barriers to implementation, and suggestions for design of the CDS tool. Most clinicians favored an alert that appeared in a relevant location (eg, the laboratory results section of the EHR), and that also provided guidance for both the next diagnostic steps and initial options for therapy. Our study highlights the relative lack of knowledge about a common genetic disorder associated with increased risk of CHD. Physician input obtained by a survey and focus groups was informative for the development of CDS for FH.
Acknowledgments
We thank the clinicians who responded to the survey as well as those who attended the focus group discussions.
Footnotes
Grant Support: The work was supported by grant R01-HL138709 from the National Heart, Lung and Blood Institute, and an unrestricted grant (I.J.K) from Pfizer Independent Grants for Learning and Change. Dr Kullo is additionally supported by grants U01-HG006379 and K24-HL137010.
Potential Competing Interests: The authors report no competing interests.
References
- 1.Abul-Husn N.S., Manickam K., Jones L.K., et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science. 2016;354(6319) doi: 10.1126/science.aaf7000. pii: aaf7000. [DOI] [PubMed] [Google Scholar]
- 2.Austin M.A., Hutter C.M., Zimmern R.L., Humphries S.E. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol. 2004;160(5):407–420. doi: 10.1093/aje/kwh236. [DOI] [PubMed] [Google Scholar]
- 3.de Ferranti S.D., Rodday A.M., Mendelson M.M., Wong J.B., Leslie L.K., Sheldrick R.C. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES) Circulation. 2016;133(11):1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791. [DOI] [PubMed] [Google Scholar]
- 4.Safarova M.S., Kullo I.J. My approach to the patient with familial hypercholesterolemia. Mayo Clin Proc. 2016;91(6):770–786. doi: 10.1016/j.mayocp.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safarova M.S., Liu H., Kullo I.J. Rapid identification of familial hypercholesterolemia from electronic health records: the SEARCH study. J Clin Lipidol. 2016;10(5):1230–1239. doi: 10.1016/j.jacl.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safarova M.S., Kullo I.J. Lessening the burden of familial hypercholesterolemia using health information technology. Circ Res. 2018;122(1):26–27. doi: 10.1161/CIRCRESAHA.117.312319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foody J.M. Familial hypercholesterolemia: an under-recognized but significant concern in cardiology practice. Clin Cardiol. 2014;37(2):119–125. doi: 10.1002/clc.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patton M.Q. Sage; Thousand Oaks, CA: 2002. Qualitative Research Design and Evaluation Methods; p. 3. [Google Scholar]
- 9.Strauss A., Corbin J. Sage; Newbury Park, CA: 1990. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. [Google Scholar]
- 10.Krueger R.A., Casey M.A. Sage; Thousand Oaks, CA: 2015. Focus Groups: A Practical Guide for Applied Research; p. 2. [Google Scholar]
- 11.Krueger R.A., Casey M.A. Sage; Thousand Oaks, CA: 2000. Focus Groups: A Practical Guide for Applied Research; p. 3. [Google Scholar]
- 12.Charmaz K. 2006. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. Thousand Oaks, CA: Sage. [Google Scholar]
- 13.Kushniruk A. Evaluation in the design of health information systems: application of approaches emerging from usability engineering. Comput Biol Med. 2002;32(3):141–149. doi: 10.1016/s0010-4825(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 14.Bates D.W., Cohen M., Leape L.L., Overhage J.M., Shabot M.M., Sheridan T. Reducing the frequency of errors in medicine using information technology. J Am Med Inform Assoc. 2001;8(4):299–308. doi: 10.1136/jamia.2001.0080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg A.X., Adhikari N.K., McDonald H., et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. J Am Med Assoc. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery A.A., Fahey T., Peters T.J., MacIntosh C., Sharp D.J. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial. BMJ. 2000;320(7236):686–690. doi: 10.1136/bmj.320.7236.686. [DOI] [PMC free article] [PubMed] [Google Scholar]