SUMMARY
Despite therapeutic advances in management, the prognosis of patients with brain metastasis remains dismal. Treatment options include surgical resection, whole brain radiation therapy (WBRT), and stereotactic radiosurgery (SRS). Patients who undergo surgical resection typically receive WBRT as adjuvant therapy. However, several studies have demonstrated an association between WBRT and neurotoxicity. Thus, clinicians are increasingly delaying WBRT in favor of postoperative use of SRS. In this review, we will discuss the current literature exploring the efficacy and toxicity of postoperative SRS in the treatment of patients with resected brain metastasis.
Practice Points.
Management of brain metastasis has traditionally been palliative, in some cases combining surgery with postoperative whole brain radiation therapy (WBRT) to treat patients with solitary brain metastasis (typically >3–4 cm in diameter) and lesions with mass effect.
With improved systemic therapy including targeted agents, patients are living longer with metastatic disease. Therefore, a more aggressive approach for brain metastasis management with less long-term side effects is important to consider.
To limit neurotoxicity associated with WBRT, clinicians are now attempting to delay WBRT and instead are adopting strategies to use postoperative stereotactic radiosurgery (SRS) to treat resected brain metastasis.
The current evidence is limited primarily to retrospective case series. Several ongoing clinical trials are examining efficacy and complications of surgical resection and postoperative SRS. The results from these trials will ideally guide clinical practice in the management of brain metastasis.
Brain metastasis, an end-stage complication of cancer, remains the most common intracranial tumor in adults [1,2]. There are approximately 170,000 new cases per year in the USA and it is estimated that 20–40% of cancer patients will develop brain metastases during the course of their disease [3]. The incidence of brain metastasis is expected to increase with patients surviving longer due to better control of their systemic disease. The most common primary cancers in adults with a predilection to metastasize to the brain include lung and breast carcinomas, accounting for approximately 60% of brain metastases. Other cancers that commonly metastasize to the brain include melanoma, colon, and renal cell carcinoma. Despite advances in treatment modalities, prognosis remains poor with a median survival of 7 weeks in untreated patients [4,5].
Management of brain metastasis is typically palliative, incorporating an interdisciplinary, multimodality approach that includes medical management with corticosteroids, surgical resection, whole-brain radiation therapy (WBRT) and stereotactic radiosurgery (SRS) [6]. The main goals for management of brain metastasis are to control symptoms caused by the metastatic lesion, prevent recurrence at resected tumor beds, and prevent formation of new intracranial metastases.
Surgical resection is often reserved for the treatment of large brain metastasis, typically greater than 3 cm in diameter in surgically accessible locations of the brain [7]. Surgery is beneficial for symptomatic relief of metastatic lesions that are causing focal neurological deficits and seizures related to mass effect, cerebral edema, or obstructive hydrocephalus [4]. Surgery also allows the opportunity to biopsy the lesion for pathologic confirmation of disease. Although surgical resection alone can be sufficient in certain cases, adjuvant therapy is often indicated for patients with single brain metastasis due to the higher rates of local recurrence following surgery alone.
WBRT has been the mainstay of adjuvant treatment to prevent local recurrence following surgical resection of metastatic lesions in the brain [8,9]. Patchell et al. showed in a randomized study that the addition of WBRT after documented complete tumor mass resection decreased intracranial failure from 70 to 18% (p < 0.001) and local recurrence from 46 to 10% (p < 0.001) [8,9]. Despite success in decreasing local recurrence, WBRT has been associated with significant neurotoxicities, including neurocognitive decline [10–12]. Thus, treatment strategies are being investigated to delay WBRT in patients with brain metastases.
The strategies currently being explored are based on clinical trials that have supported the equivalency of using SRS alone at diagnosis versus SRS plus WBRT [13]. Although WBRT remains the standard of care, SRS is now increasingly being used as adjuvant therapy following surgical resection of brain metastasis to prevent local recurrence while delaying the potential toxicities associated with WBRT (Figure 1) [14]. In this review, we will present studies examining the use of postoperative SRS in the management of resected brain metastasis. We will discuss efficacy of postoperative SRS in preventing local and distant recurrence, the effect of adding a margin on local control rate, the role of postoperative SRS in multiple brain metastases, the prevalence of leptomeningeal disease, and complications following postoperative SRS. Lastly, we will review ongoing clinical trials examining postoperative SRS in brain metastasis.
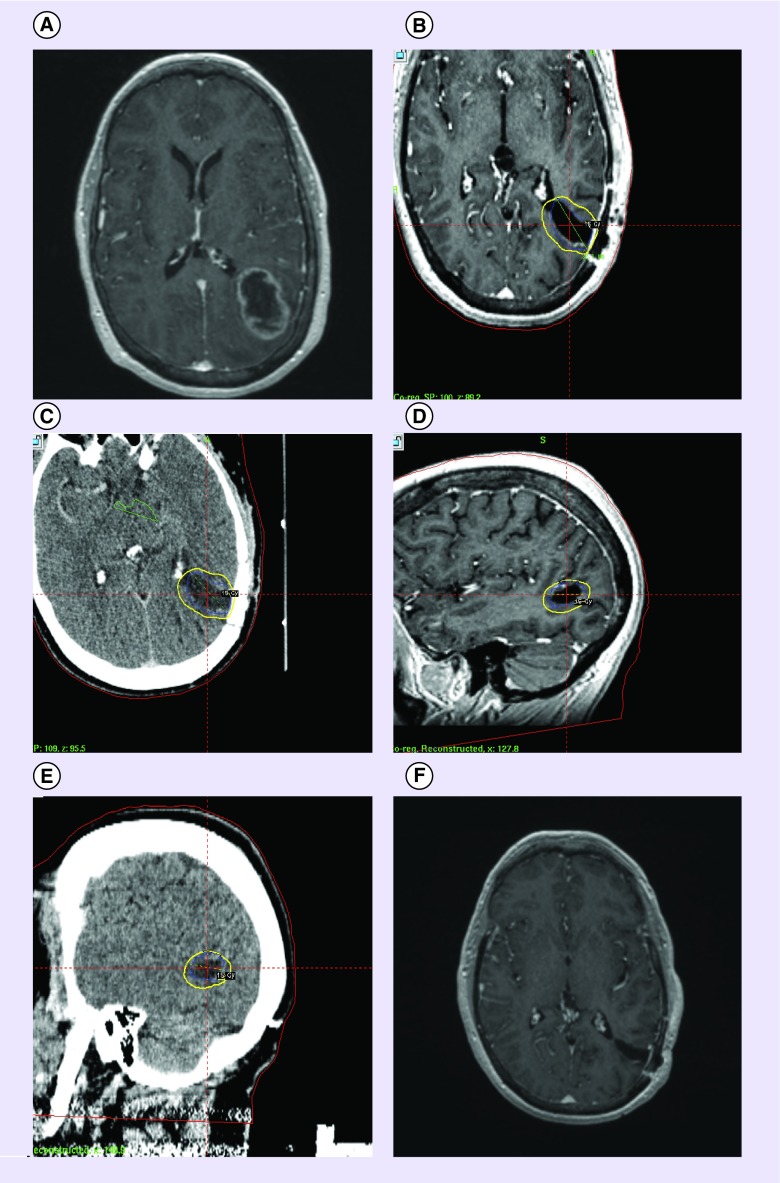
Figure 1. . Resection bed stereotactic radiosurgery.
(A) Imaging scans of a 56 year old female with a history of ovarian cancer, who presented with a 5 cm mass in the left parietal lobe consistent with brain metastasis. (B) The tumor was resected and (C–E) followed with postoperative stereotactic radiosurgery 1 month later (15 Gy, 32.1 mm). Note the dosimetric margin around the resection bed. (F) MRI scan taken following resection and postoperative stereotactic radiosurgery.
Effect of postoperative SRS on local control and local & distant recurrence in patients with brain metastasis
Several studies have examined the efficacy of postoperative SRS in preventing local recurrence and the incidence of distant recurrence (Table 1) [15–27]. A retrospective study examined 21 patients who had received surgical resection followed by radiosurgery boost [16]. Of the patient population examined, 76% had single brain metastasis, with 57% in supratentorial location. The primary cancers in this population included colon cancer (5/21), lung (5/21), breast (2/21), and gastric (2/21) cancer, with adenocarcinoma being the most prevalent (15/21). The mean time for diagnosis of primary cancer to diagnosis of brain metastasis was 2.6 years with a range of 0–15 years. Surgical resection resulted in gross total resection for 86% of lesions. Localized tumor recurrence occurred in 24% of patients, new intracranial lesions developed in 48% patients and leptomeningeal disease was seen in 24% of the patients, specifically more commonly in patients with posterior fossa lesions. The median time to local recurrence was 7 months with range of 3–30 months. Recurrence occurred in patients treated at lower doses (<18 Gy, n = 5) and none who received >18 Gy. Mean survival time was 20 months. Thus, this study suggested that higher doses of treatment are required to prevent recurrence and that leptomeningeal disease occurs more commonly in posterior fossa lesions.
Table 1. . Summary of published studies assessing post-operative stereotactic radiosurgery in brain metastasis .
| Study | Sample size | Dose (range) | Local control | Distant recurrence | Survival |
|---|---|---|---|---|---|
| Minniti G et al. (2013) | 101 patients with large resection cavities (>3 cm). included 2-mm margin |
Multidose 9 Gy x 3 | 1 year: 93% 2 year: 84% |
1 year: 50% 2 year: 66% |
1-year: 69% 2-year: 34% |
| Hwang SW et al. (2010) | 25 patients underwent surgical resection + SRS. 18 patients underwent surgical resection + WBRT |
(15–20 Gy) depending on size of cavity |
GKS patients: No known local failure WBRT: 3 patients with local failures |
GKS patients: 28% WBRT: 17% |
Median survival treated with GKS: 15 months. Median survival treated with WBRT: 6.81 months |
| Iwai Y et al. (2008) | 21 patients | 17 Gy (13–20 Gy) | 76% | 48% | Median survival: 20 months |
| Jensen CA et al. (2010) | 106 patients (112 resection cavities) | Median dose: 17 Gy | 1 year: 80.3% | 1 year: 35.4% | Median overall survival: 10.9 months Overall survival at 1 year: 46.8% |
| Jagannathan J et al. (2009) | 47 patients | Mean dose: 19 Gy (6–22 Gy) | 94% | 34 patients (72%) underwent additional radiosurgery for 140 new metastases | Mean of 12 months |
| Hartford AC et al. (2013) | 47 patients (49 lesions) | 15.3 Gy (10.75–23.5 Gy) depending on size of tumor | 1 year: 85.5% 2 year: 66.9% |
63% patients developed DR 1-year actuarial rate: 56% 2-year acturial: 76% |
1 year: 52.5% 2 year: 31.7% |
| Do L et al. (2009) | 30 patients | (15–18 Gy) | 86.70% | 63% developed recurrences in new intracranial sites | 1 year overall survival: 51% |
| Quigley MR et al. (2008) | 32 patients | 14 Gy (10–18 Gy) | 93.75% | 4 patients required SRS for new lesions | Median survival: 16.4 months |
| Kelly PJ et al. (2012) | 18 patients | 18 Gy (15–18 Gy) | 89% | Distant recurrence occurred in 6 patients | 1 year actuarial overall survival rate: 93% |
| Karlovits BJ et al. (2011) | 52 patients | 15 Gy (8–18 Gy) | 92.30% | 23 patients (44%) developed distant brain recurrences at a median of 16 months postresection | Median survival: 15 months |
| Soltys SG et al. (2008) | 72 patients (76 cavities) | 18.6 Gy (15–30 Gy) | 6 month: 88%. 1 year: 79% |
49% | 6 month: 77% 12 month: 57% |
| Choi CYH et al. (2012) | 112 patients (120 cavities). added a 2-mm margin |
Median marginal dose: 20 Gy (12–30 Gy) in 1–5 fractions. | 1 year LF: 9.5% 1 year cumulative incidence rate of LF: with 2-mm margin: 3% without margin: 16% |
1 year DF: 54% | Median OS time: 17 months 12-month OS: 62% |
| Brennan C et al. (2014) (Phase II study) |
49 patients (50 lesions) | 18 Gy (15–22 Gy) | 70% | Cumulative 1 year RF rate: 44% | |
| Limbrick DD et al. (2009) | 15 patients | (16–24 Gy) | 83.30% | 6 developed remote disease | Overall median survival: 20.0 months |
| Nataf F et al. (2008) | 93 patients had single metastasis. 2-mm margin: 51/93 patients. No margin. 42/93 patients |
10–20 Gy | 2 mm margin: 1 year: 69.1% 2 year: 64% No margin: 1 year: 72.4% 2 year: 54.7% |
2 mm margin: Median: 19 months 1 year: 60.2% No margin: Median: 11.3 months 1 year: 41.6% |
|
| Atalar B et al. (2013) | 63 patients (68 cavities) | 18 Gy (13–27) in 1–3 fractions | LF: 7 of 68 cavities Cumulative incidence rate of LF: 6 month: 2.9% 12 month: 12% 24 month: 11.2% |
Median: 17 months 6 month: 83% 12 month: 60% 24 month: 42% |
|
| Jarvis LA et al. (2012) | 41 patients (43 lesions) | 5 cavities had local progression | 4 patients had progression elsewhere in brain 1 patient had both local and distant progression |
||
SRS: Stereotactic radiosurgery; WBRT: Whole brain radiation therapy.
Another retrospective study examined local control in 15 patients with one or two cerebral metastasis [28]. Primary tumor breakdown was 40% lung, 26.7% breast, 20% renal cell carcinoma, 6.7% ovarian and 6.7% esophageal. 80% of the cases had gross total resection while the remaining 20% had subtotal resection. All patients received SRS following surgery. Local recurrence occurred in 16.7% of those patients with GTR. Local recurrence was managed with surgical resection or additional SRS. Distant CNS progression occurred in nine patients (60.0%), with a median time to failure of 8 months; six of whom were treated with WBRT. Median survival for all 15 patients was 20 months with a range of 5–68 months. RPA class one patient had a median survival of 22 months (n = 8), class 2 patients had 13 months (n = 6), and class 3 patients survived 15 months after surgery. The authors concluded that surgical resection and postoperative SRS resulted in survival that was equivalent or greater than other case series that used surgery plus WBRT or SRS plus WBRT.
In a larger study of 112 resection cavities in 106 patients, the local control at 1 year was 80.3% with a distant brain control rate of 35.4%, with a tumor diameter >3 cm being predictive of local failure [17]. Median survival as 10.9 months, with overall 1 year survival of 46.8%. The primary tumor for the patients were non-small cell lung carcinoma (47.2%), breast cancer (14.2%), gastrointestinal cancer (13.2%), melanoma (10.4%), and renal cell carcinoma (5.7%). Preoperative tumor diameter was a median of 3.4 cm (range 0.8–7 cm). The majority of the patients (96.4%) had undergone GTR. The median dose was 17 Gy (range 11–23 Gy), with a maximum dose of 34 Gy (range 18–46 Gy). The study also reported complications and treatment failures. Leptomeningeal disease occurred in eight patients, all female, with 50% of these patients having cerebellar location in four of these eight patients. Seven patients required reoperation: two for local recurrence at the resection cavity, three for radiation necrosis (17.2, 18.8, and 41.1 months post-SRS), one for hydrocephalus, and one for a CSF cutaneous fistula (15.9 months post-SRS). Due to the high distant failure, the authors advocated for serial imaging to provide timely salvage treatment.
Multiple retrospective studies have examined the role of the size of brain metastasis on local control. Minitti G et al. reported excellent local control (1 year rate 93% and 2 year rate 84%), achieving similar rates for both radiosensitive and radioresistant brain metastasis in 101 patients with a single brain metastasis that had undergone resection and received multidose postoperative SRS (9 Gy × 3) for tumor bed cavities >3 cm along with a 2 mm margin [19]. Occurrence of distant brain metastases was seen in 50% of the patients in 1 year and 66% by the second year. Nine patients (9%) had complications, specifically brain radionecrosis with five of these patients (5%) being symptomatic. Hartford AC et al. also examined preoperative tumor size in patients (49 lesions in 47 patients) who were treated with SRS following resection [20]. They reported a 1-year local control rate of 85.5% and 2-year local control rate of 66.9%. However, tumors >3.0 cm, treated with postoperative SRS alone, were associated with a shorter time to local recurrence while tumors >2.0 cm were also associated with a shorter time to distant recurrence, intracranial recurrence, and salvage WBRT [20].
A recent Phase II study of 49 patients (50 brain metastasis) assessed local control of brain metastasis after surgical resection and postoperative SRS for brain metastasis [27]. The histology of the brain metastatic lesions found in this patient population included: NSCLC (57%) and breast cancer (18%), with gastrointestinal malignancy, melanoma and other primary cancers being equally divided at 8%. 45% of the patients had extracranial metastasis. Approximately 92% of patients had gross total resection while the remaining 8% had subtotal resection. Time from surgery to SRS was approximately 31 days, with the range being 7 days to 56 days post-resection. The median dose was 1800 cGy (range, 1500–2200 cGy). Almost all patients had a single brain metastasis, except one patient who had two lesions, with majority occurring in the supratentorial region (82%). Results demonstrated that 15 lesions (30%) had local failure post-resection, but patients who had post-operative SRS had a lower rate of local failure (p = 0.008). The cumulative 1-year local failure rate was 22% and a 1-year regional failure rate was 44%. The authors reported that non-small-cell lung cancer histology (p = 0.48), deep parenchymal tumors (p = 0.036), and tumor diameter <3 cm (p = 0.010) were all associated with higher local control. The highest risk for local failure was seen in tumors ≥3 cm with superficial involvement of the dura and pia. This Phase II study demonstrates that postoperative SRS is associated with higher rates of local control, specifically in patients with deep and smaller (<3 cm) brain metastasis.
Effect of adding a margin on local control rate
Given the difficulties in delineating the resection bed, studies have examined the impact of adding a margin on local control of brain metastasis. These studies, however, have yielded conflicting results [26,29]. Nataf et al. performed a retrospective analysis of 93 brain metastases with or without a 2 mm margin, but saw no differences in local control between 51 patients with brain metastasis with a 2 mm margin (69.1%) vs 42 patients without margin (72.4%). However, the authors reported higher rates of parenchymal complications in patients with a 2 mm margin (19.6% of patients with a 2 mm margin vs 7.1% patients without a 2 mm margin, p = 0.02). More recently, Choi et al. examined the effect of SRS to the resection cavity with a 2 mm margin around the resection cavity on local failure and toxicity in patients with brain metastasis (120 cavities in 112 patients) [26]. The study found that post-SRS using a 2-mm margin around the resection cavity improved local control at 12 months (3% vs 16%, p = 0.042) without increasing toxicity when compared to postoperative SRS without a margin. Given the concerns of being able to completely delineate the resection bed, in general, the addition of margins has been favored and used in the NCCTG N107C study, described later in this review.
Tumor bed dynamics post-resection: timing of postoperative SRS
SRS is typically delivered 2–4 weeks after surgery but the optimal timing for postoperative therapy remains unclear. The current hypothesis is that delaying SRS allows healing and ultimately shrinking of the resected tumor bed, thereby decreasing the treatment volumes which may in turn spare normal brain tissue. A recent retrospective study examined the dynamics of cavity volume changes after surgical resection in 68 cavities (63 patients) treated with both resection and post-surgical SRS [30]. The study found no significant association between volume change and days post-resection. The authors reported that the largest volume change occurred immediately after surgery within the first three postoperative days, but there were no significant volume changes up to 33 days post-resection. Based on these findings, the authors reported no benefit in delaying postoperative SRS to decrease the volume of the surgical cavity.
A separate retrospective analysis examined postoperative cavity dynamics and tumor progression during the period of time between resection and SRS therapy in 41 patients (43 resected cavities) [31]. The mean time between surgery and SRS was 29.8 days post-resection (range of 8–111 days) and the time between the postoperative MRI scan and the MRI scan used for SRS planning was 23.9 days (range of 2–104 days). The results demonstrated that five patients had local progression at the surgical cavity between resection to delivery of radiosurgery, four patients had progression of metastasis in other parts of the brain and one patient had both local and regional progression while 33 patients did not have local progression. Approximately 8.6% of those patients that underwent GTR had local progression as compared to 37.5% of patients that underwent STR. Thus, the study suggests that there may be significant risks in terms of local control associated with delaying SRS following surgery, especially in those patients that underwent STR. Limiting the delay may limit the extent of regrowth.
Multiple brain metastases: a role for surgery & SRS?
Surgery in patients with multiple brain metastases remains controversial. A number of retrospective studies have demonstrated a role for surgery in the management of multiple brain metastases but prospective studies are lacking [32–35]. Pollock et al. examined the efficacy of management of 52 patients with multiple brain metastases (median, 3 tumors) with surgical resection, SRS or both [36]. The median survival was 15.5 months. 35 patients (67%) had progression of intracranial disease at a median of 8 months: 12% had local intracranial progression, 44% had distant progression, and another 12% had both local and distant progression. Additional treatment of brain metastasis was performed in 74% (26 patients) with intracranial progression: two had surgical resection alone, 16 had SRS only, two had SRS twice, one patient had SRS and three craniotomies but only four patients had both resection and postoperative SRS. Of note, 20 patients in this study had received prior WBRT. Due to the small sample size and the fact that almost half the patients had received prior WBRT, it is difficult to draw conclusions regarding the efficacy of postoperative SRS in patients with multiple brain metastases.
Leptomeningeal disease
Leptomeningeal disease (LMD) occurs when cancer disseminates to cerebrospinal fluid and leptomeninges (pia mater and arachnoid) and occurs in approximately 5–8% of cancer patients [37]. Previous studies have examined the occurrence of LMD as a treatment failure in patients that underwent surgical resection followed by postoperative SRS. Atalar et al. found that 21 patients out 165 patients (12.7%) developed LMD after a median of 5 months following postoperative SRS [38]. The median survival following LMD was 6 months, with a range of 1.1–36.7 months. Patients with metastatic breast cancer to the brain had an increased risk for developing LMD after postoperative SRS compared to those patients with non-breast metastasis (24 vs 9%, p = 0.004). Other studies have reported an incidence of 8% for the development of LMD in patients treated with post-resection SRS [17,39]. Future prospective studies must continue to explore the incidence of LMD in patients with brain metastasis treated with surgery and SRS because a higher incidence would limit the utility of this treatment approach. In these cases, WBRT may be favored.
Complications following postoperative SRS
Limited evidence is available regarding complications and toxicities associated with surgery plus postoperative SRS. In an extensive review of several case series, the authors found that complications were reported in 10% of patients (range of 0–33%) [40–43]. The most commonly reported complications following SRS were radiation-related edema and radionecrosis. Many of these complications were successfully managed with corticosteroids. The Brennan et al. Phase II study discussed previously reported that 17.5% (n = 7) patients developed radionecrosis (three of the seven surgical cavities had received a dose of 15 Gy and four surgical cavities had received 18 Gy) [27]. Other complications following postoperative SRS that have been reported include RTOG grade 2 radiation CNS toxicity, hydrocephalus, meningitis, and motor weaknesses [40].
Ongoing randomized clinical trials
Multiple clinical trials are currently underway that will provide insights into the efficacy and toxicities of using postoperative SRS. The North Central Cancer Treatment Group N107C (ClinicalTrials.gov identifier: NCT01372774, RTOG 1270) randomizes patients (estimated enrollment: 192 patients) who underwent resection of their brain metastasis to WBRT or SRS to the resection bed. Patients eligible for this study have four or fewer brain metastases with one being resected. The resection cavity must be 5 cm or less in diameter. Dose to the resection bed is 12–20 Gy depending on the volume of the resection bed. WBRT dose is 30 Gy in ten fractions or 37.5 Gy in 15 fractions. Unresected brain metastases can receive SRS boost. The primary goal is to determine if there is improved overall survival and less neurocognitive progression with SRS to the resection bed.
A Phase I trial (ClinicalTrials.gov identifier: NCT01395407) at Emory University is currently enrolling approximately 54 patients in a dose escalation trial assessing treatment toxicity following radiosurgery in patients with resected brain metastases. The primary outcome is to assess maximum tolerated dose for postoperative SRS to the surgical cavity based on the RTOG CNS toxicity scale at 4 months post-SRS. Secondary outcomes include: local control, distant control, neurocognitive outcomes, and quality of life. This study will include three investigational arms: group 1 will be patients with a resection cavity volume up to 4.2 cc (0–2 cm diameter) who will receive a dose escalation of 21, 23, 25 Gy; group 2 will be patients with a resection cavity volume >4.2 cc and ≤14.1 cc (2–3 cm diameter) who will receive a dose escalation of 18, 20, 22 Gy; and group 3 will be patients with a resection cavity volume >14.1 cc and ≤ 35 cc (3–4 cm diameter), who will receive a dose escalation of 15, 17 and 19 Gy.
A randomized controlled Phase III clinical trial at MD Anderson Cancer Center will enroll approximately 132 patients (ClinicalTrials.gov identifier: NCT00950001) to assess the efficacy of postoperative SRS in patients with brain metastasis. The primary endpoint is time to local recurrence, which will be assessed at three time points: after a total of 39 events occur; after 77 events occur; and after at least 115 events occur. Two arms will be investigated: group 1 will receive stereotactic radiosurgery to the resected cavity group 2 will be observed with routine MRI scan post-resection only.
Another Phase III trial (ClinicalTrials.gov identifier: NCT01535209) at Curie Memorial Cancer Center, Institute of Oncology in Poland will enroll approximately 100 patients to compare postoperative SRS of the resected cavity versus WBRT after surgical resection of a single brain metastasis. The primary end point of this study is failure-free survival. Secondary outcomes include: overall survival, quality of life, time to local progression, and time to regional intracranial progression. Patients will be randomly assigned to one of two arms: the control arm will receive WBRT (30 Gy in ten fractions over 12 days to whole brain) and the experimental arm will receive SRS (18 Gy in one fraction for resection cavity <2 cm in maximum diameter, 15 Gy in one fraction for resection cavity 2.1–3 cm in maximum diameter, 15 Gy in one fraction or 25 Gy in five fractions over 5 days for resection cavity 3.1–4 cm in maximum diameter, 25 Gy in five fractions over 5 days for resection cavity >4 cm in maximum diameter).
Conclusion & future perspective
Most of the studies that have examined surgical resection plus SRS for the management of brain metastasis have been retrospective. While multiple studies have demonstrated efficacy in postoperative SRS in preventing local recurrence to treated sites, rates of new regional intracranial recurrence have been significant [21]. The lack of prospective randomized data and the heterogeneity and relatively small sample size of the currently published studies makes it difficult to make conclusions regarding the utility of postoperative SRS. Ongoing clinical trials are prospectively examining local control rates, survival rates, toxicity, neurocognitive outcomes and quality of life in patients with brain metastasis undergoing surgery and postoperative SRS. Current questions center around the ideal candidates for postoperative SRS versus whole brain radiation. This includes histology, tumor location, tumor size, and expected prognosis. Leptomeningeal disease remains a concern as discussed above. Prospective studies may show that tumor abutting dural disease in the posterior fossa or breast cancer histology may be at risk for leptomeningeal disease and are not good candidates for SRS to the resection bed. Other questions that remain unanswered include determining ideal margins and optimal dose and fractionation, if fractionation may reduce toxicities. While NCCTG N107C uses single fraction radiosurgery, other studies have included up to 5 fractions of radiosurgery particularly for larger resection beds. Margins for radiosurgery for resected brain metastases have become an area of interest due to the results from Stanford University [26,29]. This has also been investigated at Duke University for intact brain metastases (ClinicalTrials.gov identifier: NCT01017497). In the meantime, many institutions will continue to consider SRS for brain metastasis resection cavity in order to avoid upfront WBRT, although, WBRT remains the standard of care based on the Patchell et al. study. Results from clinical trials that are currently underway will help refine the postoperative management of brain metastasis.
Footnotes
Financial & competing interests disclosure
S Chao has received honoraria from Varian Speakers Bureau. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Bradley KA, Mehta MP. Management of brain metastases. Semin. Oncol. 2004;31(5):693–701. doi: 10.1053/j.seminoncol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA. The management of brain metastases. Cancer Treat. Rev. 2003;29(6):533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 3.Siu TL, Jeffree RL, Fuller JW. Current strategies in the surgical management of cerebral metastases: an evidence-based review. J. Clin. Neurosci. 2011;18(11):1429–1434. doi: 10.1016/j.jocn.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Soffietti R, Rudā R, Mutani R. Management of brain metastases. J. Neurol. 2002;249(10):1357–1369. doi: 10.1007/s00415-002-0870-6. [DOI] [PubMed] [Google Scholar]
- 5.Soffietti R, Rudà R, Trevisan E. Brain metastases: current management and new developments. Curr. Opin. Oncol. 2008;20(6):676–684. doi: 10.1097/CCO.0b013e32831186fe. [DOI] [PubMed] [Google Scholar]
- 6.Suh JH. Stereotactic Radiosurgery for the Management of Brain Metastases. N. Engl. J. Med. 2010;362(12):1119–1127. doi: 10.1056/NEJMct0806951. [DOI] [PubMed] [Google Scholar]
- 7.Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract. Radiat. Oncol. 2012;2(3):210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990;322(8):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 9.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 10.Shibamoto Y, Baba F, Oda K, et al. Incidence of brain atrophy and decline in mini-mental state examination score after whole-brain radiotherapy in patients with brain metastases: a prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2008;72(4):1168–1173. doi: 10.1016/j.ijrobp.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 11.Marsh JC, Gielda BT, Herskovic AM, Abrams RA. Cognitive sparing during the administration of whole brain radiotherapy and prophylactic cranial irradiation: current concepts and approaches. J. Oncol. 2010;2010:198208. doi: 10.1155/2010/198208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 14.Roberge D, Parney I, Brown PD. Radiosurgery to the postoperative surgical cavity: who needs evidence? Int. J. Radiat. Oncol. Biol. Phys. 2012;83(2):486–493. doi: 10.1016/j.ijrobp.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Hwang SW, Abozed MM, Hale A, et al. Adjuvant Gamma Knife radiosurgery following surgical resection of brain metastases: a 9-year retrospective cohort study. J. Neurooncol. 2010;98(1):77–82. doi: 10.1007/s11060-009-0051-x. [DOI] [PubMed] [Google Scholar]
- 16.Iwai Y, Yamanaka Kazuhiro, Yasui Toshihiro. Boost radiosurgery for treatment of brain metastases after surgical resections. Surg. Neurol. 2008;69(2):181–186. doi: 10.1016/j.surneu.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Jensen CA, Chan MD, McCoy TP, et al. Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis: clinical article. J. Neurosurg. 2011;114(6):1585–1591. doi: 10.3171/2010.11.JNS10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagannathan J, Yen C-P, Ray DK, et al. Gamma Knife radiosurgery to the surgical cavity following resection of brain metastases: clinical article. J. Neurosurg. 2009;111(3):431–438. doi: 10.3171/2008.11.JNS08818. [DOI] [PubMed] [Google Scholar]
- 19.Minniti G, Esposito V, Clarke E, et al. Multidose stereotactic radiosurgery (9 Gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2013;86(4):623–629. doi: 10.1016/j.ijrobp.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Hartford AC, Paravati AJ, Spire WJ, et al. Postoperative stereotactic radiosurgery without whole-brain radiation therapy for brain metastases: potential role of preoperative tumor size. Int. J. Radiat. Oncol. Biol. Phys. 2013;85(3):650–655. doi: 10.1016/j.ijrobp.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Do L, Pezner R, Radany E, Liu A, Staud C, Badie B. Resection followed by stereotactic radiosurgery to resection cavity for intracranial metastases. Int. J. Radiat. Oncol. Biol. Phys. 2009;73(2):486–491. doi: 10.1016/j.ijrobp.2008.04.070. [DOI] [PubMed] [Google Scholar]
- 22.Quigley MR, Fuhrer R, Karlovits S, Karlovits B, Johnson M. Single session stereotactic radiosurgery boost to the post-operative site in lieu of whole brain radiation in metastatic brain disease. J. Neurooncol. 2008;87(3):327–332. doi: 10.1007/s11060-007-9515-z. [DOI] [PubMed] [Google Scholar]
- 23.Kelly PJ, Lin YB, Yu AY, et al. Stereotactic irradiation of the postoperative resection cavity for brain metastasis: a frameless linear accelerator-based case series and review of the technique. Int. J. Radiat. Oncol. Biol. Phys. 2012;82(1):95–101. doi: 10.1016/j.ijrobp.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 24.Karlovits BJ, Quigley MR, Karlovits SM, et al. Stereotactic radiosurgery boost to the resection bed for oligometastatic brain disease: challenging the tradition of adjuvant whole-brain radiotherapy. Neurosurg. Focus. 2009;27(6):E7. doi: 10.3171/2009.9.FOCUS09191. [DOI] [PubMed] [Google Scholar]
- 25.Soltys SG, Adler JR, Lipani JD, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2008;70(1):187–193. doi: 10.1016/j.ijrobp.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 26.Choi CYH, Chang SD, Gibbs IC, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases: prospective evaluation of target margin on tumor control. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(2):336–342. doi: 10.1016/j.ijrobp.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Brennan C, Yang TJ, Hilden P, et al. A Phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2014;88(1):130–136. doi: 10.1016/j.ijrobp.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Majority of the studies currently reported assessing postoperative stereotactic radiosurgery (SRS) have been limited to retrospective studies. This article describes a Phase II clinical trial of SRS following resection (2013).
- 28.Limbrick DD, Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg. Neurol. 2009;71(3):280–288. doi: 10.1016/j.surneu.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Nataf F, Schlienger M, Liu Z, et al. Radiosurgery with or without a 2-mm margin for 93 single brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2008;70(3):766–772. doi: 10.1016/j.ijrobp.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Atalar B, Choi CYH, Harsh GR, et al. Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery. 2013;72(2):180–185. doi: 10.1227/NEU.0b013e31827b99f3. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis LA, Simmons NE, Bellerive M, et al. Tumor bed dynamics after surgical resection of brain metastases: implications for postoperative radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(4):943–948. doi: 10.1016/j.ijrobp.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 32.Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005;56(5):1021–1034. [PubMed] [Google Scholar]
- 33.Stark AM, Tscheslog H, Buhl R, Held-Feindt J, Mehdorn HM. Surgical treatment for brain metastases: prognostic factors and survival in 177 patients. Neurosurg. Rev. 2005;28(2):115–119. doi: 10.1007/s10143-004-0364-3. [DOI] [PubMed] [Google Scholar]
- 34.Hatiboglu MA, Wildrick DM, Sawaya R. The role of surgical resection in patients with brain metastases. Ecancermedicalscience. 2013;7:308. doi: 10.3332/ecancer.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. J. Neurosurg. 1993;79(2):210–216. doi: 10.3171/jns.1993.79.2.0210. [DOI] [PubMed] [Google Scholar]
- 36.Pollock BE, Brown PD, Foote RL, Stafford SL, Schomberg PJ. Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease. J. Neurooncol. 2003;61(1):73–80. doi: 10.1023/a:1021262218151. [DOI] [PubMed] [Google Scholar]
- 37.Groves MD. Leptomeningeal disease. Neurosurg. Clin. N. Am. 2011;22(1):67–78. doi: 10.1016/j.nec.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Atalar B, Modlin LA, Choi CYH, et al. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2013;87(4):713–718. doi: 10.1016/j.ijrobp.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Robbins JR, Ryu S, Kalkanis S, et al. Radiosurgery to the surgical cavity as adjuvant therapy for resected brain metastasis. Neurosurgery. 2012;71(5):937–943. doi: 10.1227/NEU.0b013e31826909f2. [DOI] [PubMed] [Google Scholar]
- 40.Gans JH, Raper DMS, Shah AH, et al. The role of radiosurgery to the tumor bed after resection of brain metastases. Neurosurgery. 2013;72(3):317–326. doi: 10.1227/NEU.0b013e31827fcd60. [DOI] [PubMed] [Google Scholar]
- 41.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2010;77(4):996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011;6(1):48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers LR, Rock JP, Sills AK, et al. Results of a Phase II trial of the GliaSite radiation therapy system for the treatment of newly diagnosed, resected single brain metastases. J. Neurosurg. 2006;105(3):375–384. doi: 10.3171/jns.2006.105.3.375. [DOI] [PubMed] [Google Scholar]