Abstract
Background
We studies the expression of Coronin 1c and F-actin protein in breast cancer and explored their relationship with breast cancer metastasis.
Material/Methods
A total of 210 breast cancer tissues and adjacent normal tissues were collected from January 2013 to December 2014. The expressions of Coronin 1c and F-actin were detected by immunohistochemistry and Western blotting. We analyzed the relationship between Coronin 1c and F-actin and clinical data of breast cancer.
Results
The expressions of Coronin 1c and F-actin in breast cancer tissues were positively correlated (r=0.926, P<0.05) and were significantly higher than those in normal tissues (P<0.05). The Coronin 1c and F-actin expressions were not correlated with age, tumor size, ER expression, or PR expression in breast cancer patients (P>0.05), but were significantly correlated with HER-2 expression, histological grade, lymph node metastasis, molecular classification, and TNM (P<0.05). The expression of HER-2 in breast cancer tissues was positively correlated with the expression of Coronin 1c (r=0.706, P<0.05) and F-actin 1c, while F-actin protein in breast cancer tissues with lymph node metastasis was significantly higher than in those without lymph node metastasis (P<0.05).
Conclusions
Coronin 1c protein and F-actin protein are highly expressed in breast cancer and their expression may be related to the metastasis of breast cancer cells.
MeSH Keywords: Immunohistochemistry, Lymphatic Metastasis, Triple Negative Breast Neoplasms
Background
Breast cancer is one of the most common malignancies in China, accounting for about 17.0% of all cases of cancer. It is the most common cancer in women and is the sixth leading cause of death [1]. Although breast cancer death rates declined in recent years with the development of screening and comprehensive treatment of breast cancer, there are about 71 000 deaths per year in women and the death rate is high largely due to cancer metastasis [2]. Studies [3,4] have shown that metastasis of cancer cells to lymph nodes, distal tissues, or organs is one of the major causes of death in breast cancer patients. The survival rate of breast cancer patients with metastasis of cancer cells is also significantly reduced [5]. Therefore, studying the specific molecular mechanisms of breast cancer cell metastasis is of great importance to improve the clinical treatment and prolong the survival of patients with breast cancer.
Many studies have revealed [6] that overexpression of Coronin 1c can promote the invasion and migration of malignant glioma [7], liver cancer [8], and gastric cancer [9], while inhibition the expression of Coronin 1c effectively decreases invasion and migration of lung cancer [10] and triple-negative breast cancer [11]. Wang et al. [12] showed that the mitotic kinase Aurora-A promotes breast cancer cell migration and metastasis by inducing Coronin and Fibrous actin (F-actin) protein expression. However, no studies have reported the expression of Coronin 1c and F-actin in breast cancer tissues. In the present study, we assessed the expression of Coronin 1c and F-actin in breast cancer tissues using Western blotting and immunohistochemistry, analyzed the relationship between their expression and clinical data of breast cancer, and explored their significance in breast cancer.
Material and Methods
Clinical specimens
A total of 210 pairs of tumor tissues and adjacent non-cancerous tissues (≥5 cm away from tumor tissue) were collected from January 2013 to December 2014 in Cangzhou Central Hospital: 15 cases were lobular carcinoma, 32 cases were ductal carcinoma in situ, and 163 cases were invasive carcinoma.
Inclusion criteria were: age ≥18 years old; clinically and pathologically confirmed breast cancer; complete clinical data; and patients who were informed of the study, and signed informed consent reviewed by the Cangzhou Central Hospital Ethics Association.
Exclusion criteria were: non-primary breast cancer lesions due to the transfer of other malignant tumors; with congenital, acquired, or iatrogenic immunodeficiency disease; lymph node metastasis or distant metastasis uncertain in breast cancer patients; preoperative radiotherapy or chemotherapy; patients with poor health who could not tolerate surgery or other related tests; poor postoperative mental state, personal conditions, or poor prognosis; combined with other malignant tumors, severe cardiovascular and cerebrovascular disease, or severe liver and kidney dysfunction; patients who could not complete the 2-year follow-up after surgery or clear cause of death; and postoperative death due to other diseases or accidents.
Protein expression detection by immunohistochemistry
Paraffin sections of the surgically resected tumor tissues and para-cancerous tissues were prepared according to conventional method, and the expression of Coronin 1c and F-actin protein was detected by immunohistochemical staining according to the instructions of the VECTASTAIN® Elite® ABC Kit (Vector Laboratories, USA). Firstly, the paraffin sections were placed at 60°C for 2 h, deparaffinized, hydrated with xylene and ethanol, and washed with PBS and double-distilled water for staining retrieval nuclear antigen. Secondly, the cells were stained with Coronin 1C antibody (D-9) (Santa Cruz, USA) and F-actin antibody (Abcam, UK) as a primary antibody (PBS instead of primary antibody as a negative control) overnight at 4°C with goat anti-rabbit IgG H & L (HRP) (Abcam, UK) for 2 h. Finally, 5 fields were selected for each section. Results of immunohistochemistry were evaluated according to the method of Yueming Zhang [13]. Coronin 1c protein localized in the cytoplasm and F-actin protein was present on the cell membrane. A score more than 4 was considered to be high expression in the specimen.
Protein expression detection by western blotting
Frozen tumor tissues and adjacent normal tissues were removed and total protein in the tissues was extracted according to the instructions of the total protein extraction kit (Beyotime Biotechnology, China). Total protein was determined by the BCA protein concentration determination kit (Beyotime Biotechnology, China). We loaded 75 μg of total protein on SDS-PAGE electrophoresis (120 V, 1.5 h) and then transferred it onto a PVDF membrane (Amersham Biosciences, UK) by wet transfer (400 mA, 1.5 h). The PVDF membrane was washed with TBST, blocked, incubated with primary antibody overnight at 4°C (Coronin 1C antibody, F-actin antibody and GAPDH antibody [Abcam, UK]). Then, membrane was washed with TBST and incubated with secondary antibody (Goat Anti-Rabbit IgG H&L [HRP]) at room temperature for 1 h. The protein bands were visualized with an ECL Western blotting substrate kit after washing. The expression of the target protein was analyzed using Image J software and the gray value of target bands and corresponding GAPDH band was used as the quantitative index of the target protein.
Statistical analysis
The data were analyzed using SPSS version 20.0. We used the chi-square test for comparison between groups (%) and the paired t test for comparison of data between groups (mean ±SEM). For normally distributed data, Spearman’s correlation between the 2 variables was used. The difference was considered statistically significant when a p-value was less than 0.05.
Results
Clinical data of 210 breast cancer cases
Our study included 210 breast cancer patients: 122 cases age ≤50 years and 88 cases age >50 years, among which 43 cases had a diameter greater than 2 cm and 43 cases had a diameter ≤2 cm, 145 cases were ER-positive and 65 cases were ER-negative, 106 cases were PR-positive and 104 cases were PR-negative, 101 cases were proto-oncogene human epidermal growth factor receptor 2 (HER-2)-positive and 109 cases were negative, 85 cases had lymph node metastasis and 125 cases did not have lymph node metastasis. Histological grades I, II, and III were determined in 43 cases, 95 cases and 72 cases, respectively. There were127 cases with TNM stage I–II and 83 cases with stage III. Clinical manifestations of LuminalA, LuminalB, HER-2 overexpression, and Basal-like type were found in 78 cases, 73 cases, 29 cases, and 30 cases, respectively.
Protein expression of Coronin 1c and F-actin detection by immunohistochemistry
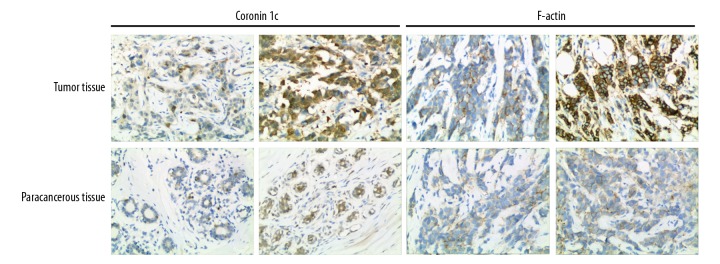
Immunohistochemical staining and scoring were performed on 210 pairs of tumor tissues and adjacent non-cancerous tissues. The results showed that: (1) Coronin 1c protein had high expression in tumor tissues in 90 cases and low expression in 120 cases. Immunohistochemical score was 3.34±1.76. The expression of Coronin 1c protein was low in all the adjacent tissues and the immunohistochemical score was 1.07±0.84; (2) The expression of F-actin protein in tumor tissues was high in 76 cases (N=134). The immunohistochemical score was 3.08±1.58. The expression of F-actin protein was low in all the adjacent tissues. The immunohistochemical score was 1.17±0.94. Results are shown in Figure 1.
Figure 1.
Protein expression of Coronin 1c and F-actin detection by immunohistochemistry.
The protein expression of Coronin 1c and F-actin in 210 cases of breast cancer were detected by Western blotting and analyzed by Spearman correlation methods. The results showed that the expression of Coronin 1c and F-actin were positively correlated in breast cancer tissues (r=0.929, P<0.05). Results are shown in Figure 2.
Figure 2.
Relationship between Coronin 1c breast cancer and F-actin protein.
Relationship between Coronin 1c and F-actin protein expression and clinical data of breast cancer patients
Among 210 cases of breast cancer, Coronin 1c protein had high expression in 90 cases and low expression in 120 cases, while F-actin protein had high expression in 76 cases and low expression in 134 cases. The relationship between Coronin 1c and F-actin protein expression and clinical data of breast cancer patients was analyzed. The results showed that the expression of Coronin 1c and F-actin was not correlated with age, tumor size, ER expression, or PR expression in breast cancer patients (P>0.05), but were significantly correlated with the expression of HER-2, histological grade, lymph node metastasis type, and TNM staging (P<0.05). Results are shown in Table 1.
Table 1.
Relationship between Coronin 1c and F-actin protein expression and clinical data of breast cancer patients.
| Items | Coronin 1c | χ2 | P | F-actin | χ2 | P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High expression (n) | Low expression (n) | High expression (n) | Low expression (n) | |||||||||||||
| Age (years) | ||||||||||||||||
| ≤50 | 41 | 47 | 0.862 | 0.353 | 30 | 58 | 0.289 | 0.591 | ||||||||
| >50 | 49 | 73 | 46 | 76 | ||||||||||||
| Tumor size (cm) | ||||||||||||||||
| ≤2 | 16 | 27 | 0.704 | 0.401 | 18 | 25 | 0.753 | 0.386 | ||||||||
| >2 | 74 | 93 | 58 | 109 | ||||||||||||
| ER | ||||||||||||||||
| Positive | 60 | 85 | 0.418 | 0.518 | 49 | 96 | 1.166 | 0.280 | ||||||||
| Negative | 30 | 35 | 27 | 38 | ||||||||||||
| PR | ||||||||||||||||
| Positive | 46 | 60 | 0.025 | 0.873 | 36 | 70 | 0.460 | 0.498 | ||||||||
| Negative | 44 | 60 | 40 | 64 | ||||||||||||
| HER-2 | ||||||||||||||||
| Positive | 55 | 46 | 10.689 | 0.001 | 46 | 55 | 7.376 | 0.007 | ||||||||
| Negative | 35 | 74 | 30 | 79 | ||||||||||||
| Histology classification | ||||||||||||||||
| I | 12 | 31 | 6.472 | 0.039 | 10 | 33 | 6.101 | 0.047 | ||||||||
| II | 42 | 43 | 33 | 62 | ||||||||||||
| III | 36 | 36 | 33 | 39 | ||||||||||||
| Lymph node metastasis | ||||||||||||||||
| Metastasis | 51 | 34 | 17.136 | 0.000 | 41 | 44 | 10.712 | 0.001 | ||||||||
| Non-metastasis | 39 | 86 | 35 | 90 | ||||||||||||
| Molecular classification | ||||||||||||||||
| Luminal A | 23 | 55 | 14.637 | 0.002 | 20 | 58 | 10.578 | 0.014 | ||||||||
| Luminal B | 35 | 38 | 29 | 44 | ||||||||||||
| HER-2 (+) | 20 | 9 | 17 | 12 | ||||||||||||
| Basal-like | 12 | 18 | 10 | 20 | ||||||||||||
| TNM stage | ||||||||||||||||
| I+II | 47 | 80 | 4.489 | 0.034 | 39 | 88 | 4.181 | 0.041 | ||||||||
| III | 43 | 40 | 37 | 46 | ||||||||||||
Relationship between Coronin 1c and F-actin protein expression and molecular types of breast cancer
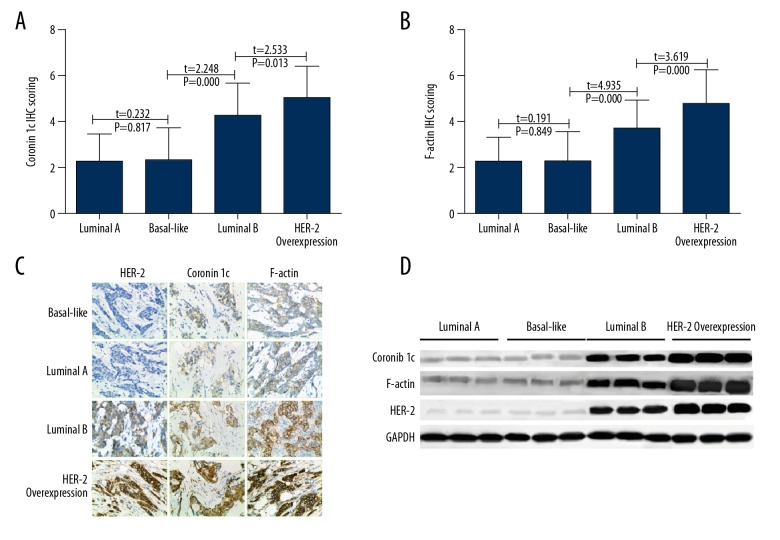
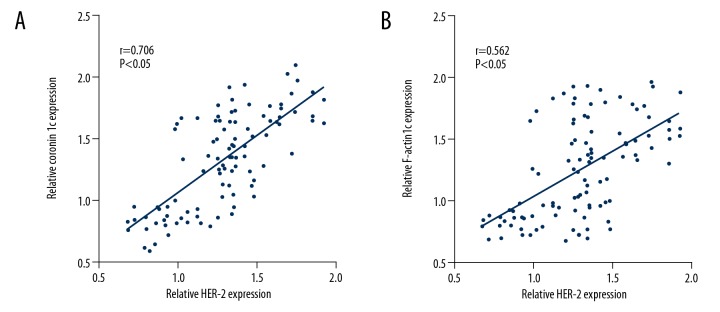
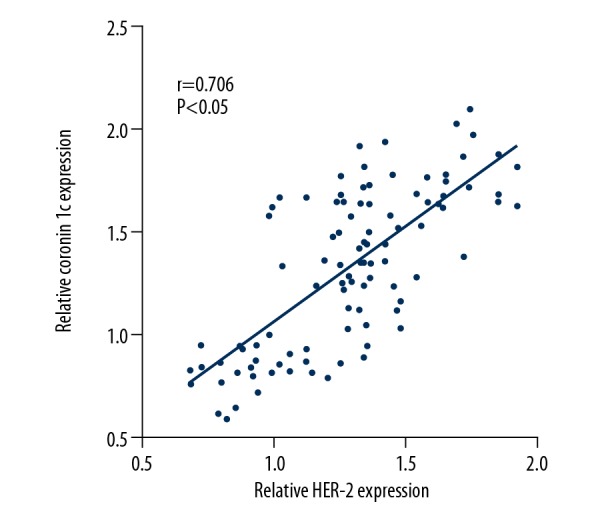
The expression of Coronin 1c and F-actin in breast cancer tissues with different molecular types was analyzed. The results showed that the expressions of Coronin 1c in Luminal A, Basal-like, Luminal B, and HER-2 overexpression breast cancer tissues were 2.27±1.23, 2.33±1.42, 4.27±1.44, and 5.07±1.41, respectively. The immunohistochemical scores of F-actin protein were 2.22±1.34, 2.27±1.31, 3.67±1.31, and 4.76±1.50, respectively. Results are shown in Figure 3. HER-2 protein expression was positively correlated with Coronin 1c (r=0.706, P<0.05) and F-actin (r=0.562, P<0.05) in 102 cases. Results are shown in Figure 4.
Figure 3.
Coronin 1c and F-actin protein expression in molecular types of breast cancer. (A) IHC scoring of Coronin 1c in different tissues. (B) IHC scoring of F-actin in different tissues. (C) IHC of HER-2, Coronin 1c, and F-actin in different tissues. (D) Western blot results of HER-2, Coronin 1c, and F-actin in different tissues.
Figure 4.
Relationship between Coronin 1c and F-actin protein expression and HER-2 protein expression. (A) HER-2 protein expression correlated with Coronin 1c. (B) HER-2 protein expression correlated with F-actin.
Relationship between Coronin 1c and F-actin protein expression and breast cancer cell metastasis
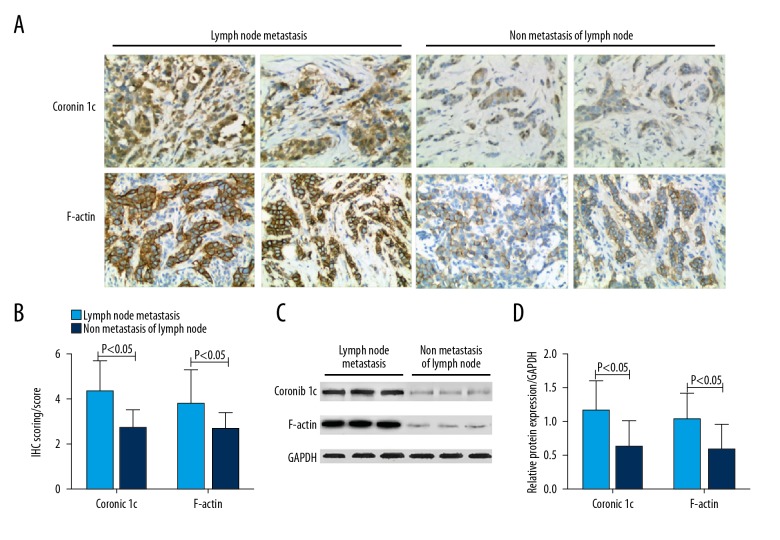
Among 210 cases of breast cancer, 85 cases showed lymph node metastasis while 125 cases did not show lymph node metastasis. Immunohistochemistry showed that scores of Coronin 1c and F-actin protein in breast cancer patients with lymph node metastasis were 4.32±1.66 and 3.72±1.71, respectively. Scores of non-metastatic lymph nodes were 2.68±1.50 and 2.65±1.33 (P<0.05). Western blotting showed that the relative expression of Coronin 1c and F-actin in patients in lymph node metastasis were 1.15±0.56 and 1.02±0.51, which were significantly higher than in those without lymph node metastases (0.62±0.48 and 0.59±0.43, respectively) (P<0.05) Results are shown in Figure 5.
Figure 5.
Relationship between Coronin 1c and F-actin protein expression and breast cancer cell metastasis. (A) IHC of Coronin 1c and F-actin in lymph node metastasis and non-metastasis of lymph node tissues. (B) IHC scoring of Coronin 1c and F-actin in lymph node metastasis and non-metastasis of lymph node tissues. (C) Western blot analysis of Coronin 1c and F-actin in lymph node metastasis and non-metastasis of lymph node tissues. (D) Statistical analysis of Western blot results of Coronin 1c and F-actin in lymph node metastasis and non-metastasis of lymph node tissues.
Discussion
Tumor invasion and metastasis of tumor cells are the major causes of death in breast cancer patients. Tumor invasion and metastasis is a multi-step process. Cell migration is a prerequisite for invasion and metastasis of tumor cells. Cytoskeletal movement is required for cell migration. F-actin is composed of the cytoskeletal protein and monomer actin hydrolysis and assembly and plays an important role in mediating cell migration [14]. The Coronin family consists of a series of proteins that have received increasing research attention in recent years. They participate in the invasion and metastasis of tumor cells. Coronin 1c protein is one of the most widely studied proteins in the Coronin family. Studies [15,16] showed that Coronin 1c N-terminal, C-terminal, and the entire C-terminal WD40 sequences have binding sites with F-actin, and Coronin 1c binds and regulates F-actin protein activity to mediate tumor cell migration.
In our study, we found that the expressions of Coronin 1c and F-actin in breast cancer tissues were significantly higher than those in adjacent tissues (P<0.05) and there was a positive correlation between the expressions of Coronin 1c and F-actin in breast cancer tissues. Earlier studies by Moreno et al. confirmed that Coronin 1c overexpressed in tumor cells could promote the tumor cell EMT process by promoting the expression of Snail, Slug, and E47 proteins in tumor cells to enhance invasion and migration [17]. In addition, recent studies on Coronin 1c also showed that Coronin 1c protein was highly expressed in malignant glioma, liver cancer, gastric cancer, lung cancer, and triple-negative breast cancer tumor cells or tissues. Its high expression was closely correlated with tumor cell invasion and metastasis [10,11]. Promoting tumor cell migration by Coronin 1c was closely related to F-actin. Like other Coronin family members, Coronin 1c regulates the actin network by stimulating cell motility and binding to F-actin. However, unlike other Coronin family members, there are 3 binding sites for Coronin 1c on F-actin, which affect not only F-actin binding but also F-actin self-domains interaction to influence aggregation activity [15]. Research showed that Coronin 1C affects the aggregation activity of F-actin protein by inhibiting the activity of Arp2/3, accelerating the F-actin depolymerization mediated by cofilin, and the interaction of its own domain, thus affecting the cell migration ability [18,19].
Further analysis of the relationship between Coronin 1c and F-actin protein expression and clinical data of breast cancer found that Coronin 1c and F-actin protein expressions were only correlated with breast cancer patients with HER-2 expression, histological grade, lymph node metastasis, molecular typing, and TNM staging (P<0.05). HER-2 locates on chromosome 17q12-21.32. It encodes a transmembrane receptor-like protein with a molecular weight of 185 000 and possesses tyrosine kinase activity. It is an independent factor of breast cancer prognosis [20]. In breast cancer cells [21], when HER-2 gene in cancer cells is highly expressed, excessive HER-2 protein is produced and presents on the cell membrane to stimulate the rapid growth and increases the invasiveness of cancer cells. Therefore, HER-2-positive breast cancer patients have a worse prognosis and are more prone to recurrence and metastasis, with shorter survival.
In our study, we found that the expressions of Coronin 1c and F-actin in 2 HER-2-negative breast cancer patients were significantly lower than those in HER-2 positive patients. In HER-2-positive breast cancer patients, Coronin 1c and F-actin protein expression and HER-2 protein expression were positively correlated with the clinical features of patients with HER-2-positive breast cancer, suggesting that Coronin 1c and F-actin protein expressions are associated with breast cancer metastasis. Further analysis found that Coronin 1c and F-actin protein expressions in lymph node metastatic breast cancer were significantly higher than those in patients without lymph node metastasis. In vitro Experiments have confirmed [12] that the mitotic kinase Aurora-A enhances the migration and metastasis of MCF-10A, MCF-7, SK-BR-3, and MDA-MB-435 in breast cancer cells by promoting the expression of Coronin and F-actin protein.
Conclusions
Coronin 1c and F-actin proteins in breast cancer tissues were highly expressed and their expression may be related to the metastasis of breast cancer cells.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;1:10. doi: 10.21147/j.issn.1000-9604.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuo T, Chen W. [Advances in research on population-based female breast cancer survival in China]. Chinese Journal of Clinical Oncology. 2016 [in Chinese] [Google Scholar]
- 3.Res C. Correction: Review article on aggresomes in cancer. Cancer Res. 2009;69:4092. [Google Scholar]
- 4.Setonrogers S. Epithelial-mesenchymal transition: Untangling EMT’s functions. Nat Rev Cancer. 2016;16:1. doi: 10.1038/nrc.2015.6. [DOI] [PubMed] [Google Scholar]
- 5.Aleskandarany MA, Sonbul SN, Mukherjee A, et al. Molecular mechanisms underlying lymphovascular invasion in invasive breast cancer. Pathobiology. 2015;82:113–23. doi: 10.1159/000433583. [DOI] [PubMed] [Google Scholar]
- 6.Wu L, Hou JX, Peng CW, et al. [Increased coronin-1C expression is related to hepatocellular carcinoma invasion and metastasis]. Zhonghua gan zang bing za zhi. 2010;18:516. doi: 10.3760/cma.j.issn.1007-3418.2010.07.011. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 7.Mondol AS, Tonks NK, Kamata T. Nox4 redox regulation of PTP1B contributes to the proliferation and migration of glioblastoma cells by modulating tyrosine phosphorylation of coronin-1C. Free Radic Biol Med. 2014;67:285–91. doi: 10.1016/j.freeradbiomed.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, Peng CW, Hou JX, et al. Coronin-1C is a novel biomarker for hepatocellular carcinoma invasive progression identified by proteomics analysis and clinical validation. J Exp Clin Cancer Res. 2010;29:17. doi: 10.1186/1756-9966-29-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi S, Shang Y, Gui R, et al. Coronin3 regulates gastric cancer invasion and metastasis by interacting with Arp2. Cancer Biol Ther. 2014;15:1163–73. doi: 10.4161/cbt.29501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mataki H, Enokida H, Chiyomaru T, et al. Downregulation of the microRNA-1/133a cluster enhances cancer cell migration and invasion in lung-squamous cell carcinoma via regulation of Coronin1C. J Hum Genet. 2015;60:53–61. doi: 10.1038/jhg.2014.111. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Tsouko E, Jonsson P, et al. miR-206 inhibits cell migration through direct targeting of the actin-binding protein Coronin 1C in triple-negative breast cancer. Mol Oncol. 2014;8:1690–702. doi: 10.1016/j.molonc.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LH, Xiang J, Yan M, et al. The mitotic kinase Aurora-A induces mammary cell migration and breast cancer metastasis by activating the Cofilin-F-actin pathway. Cancer Res. 2010;70:9118–28. doi: 10.1158/0008-5472.CAN-10-1246. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, You L, Chen J, et al. Expression of kinesin family member 3B is associated with poor prognosis in epithelial ovarian cancer patients. International Journal of Clinical and Experimental Pathology. 2017;10:2834–42. [Google Scholar]
- 14.Villanueva J, Gimenez-Molina Y, Viniegra S, et al. F-actin cytoskeleton and the fate of organelles in chromaffin cells. J Neurochem. 2016;137:860–66. doi: 10.1111/jnc.13560. [DOI] [PubMed] [Google Scholar]
- 15.Jodoin JN, Martin AC. Epithelial contractility: a crowning achievement. Dev Cell. 2016;37:3–4. doi: 10.1016/j.devcel.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Mikati MA, Breitsprecher D, Jansen S, et al. Coronin enhances actin filament severing by recruiting cofilin to filament sides and altering F-actin conformation. J Mol Biol. 2015;427:3137–47. doi: 10.1016/j.jmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MorenoBueno G, Cubillo E, Sarrió D, et al. Genetic profiling of epithelial cells expressing e-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543–56. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- 18.Abella JV, Galloni C, Pernier J, et al. Isoform diversity in the Arp2/3 complex determines actin filament dynamics. Nat Cell Biol. 2016;18:76. doi: 10.1038/ncb3286. [DOI] [PubMed] [Google Scholar]
- 19.Williamson RC, Cowell CA, Reville T, et al. Coronin-1C protein and caveolin protein provide constitutive and inducible mechanisms of Rac1 protein trafficking. J Biol Chem. 2015;290:15437–49. doi: 10.1074/jbc.M115.640367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackisch C, Kim SB, Semiglazov V, et al. Trastuzumab, HER2, breast cancer. Ann Oncol. 2015;26:320–25. doi: 10.1093/annonc/mdu524. [DOI] [PubMed] [Google Scholar]
- 21.Leung HW, Leung J-H, Chan AL. Efficacy and safety of a combination of HER2-targeted agents as first-line treatment for metastatic HER2-positive breast cancer: a network meta-analysis. Exp Opin Drug Saf. 2018;17:1–7. doi: 10.1080/14740338.2018.1394454. [DOI] [PubMed] [Google Scholar]