SUMMARY
The therapeutic paradigm of gliomas is changing from a general approach towards an individualized and targeted approach. Accordingly, the search for prognostic and predictive biomarkers, as well as the demand for quantitative, feasible and robust methods for biomarker analysis increases. We find that software classifiers can identify and quantify the expression of a given biomarker within different subcellular compartments and that such classifiers can exclude frequently occurring nontumor cells, thereby avoiding potential bias. The use of a quantitative approach provides a continuous measurement of the expression, allowing establishment of new cut-points and identification of patients with specific prognoses. However, some pitfalls must be noted. This article focuses on benefits and pitfalls of novel approaches for quantifying protein biomarkers in gliomas.
KEYWORDS: biomarker, classifier, digital pathology, glioblastoma, glioma, quantification, sampling, slide scanning
Practice points.
Future individualized treatment of glioma patients will most likely be dependent on implementation of novel biomarkers.
By novel quantitative approaches conventional pathologist-based scoring can be replaced by digitized and hence more unbiased approaches.
Software classifiers identifying nuclei, cytoplasm and membranes can quantify biomarkers in gliomas.
Nontumor cells in gliomas are a crucial pitfall in glioma biomarker studies. Biomarker expression in these cells can be excluded by staining approaches and trained software classifiers.
Immunofluorescence is a promising approach in biomarker research. This approach enables more precise quantification of biomarkers as well as co-localization of biomarkers.
Background
The prognosis remains poor in the majority of glioma patients, but it has been noticed that patients respond differently to treatment, and that some patients become long-term survivors [1–4]. In order to provide more individualized and targeted treatment, more precise knowledge about glioma biology and the clinical presentation of gliomas and glioma patients is needed. Identification of prognostic and predictive markers has thus become an area of considerable interest.
Many different proteins [5–7], mRNAs [8,9] and mutations [10,11] have been proposed as being prognostic in gliomas, but currently only few biomarkers are used routinely in the clinical setting. With the increasing number of biomarkers, more feasible and robust methods are needed.
The vast majority of protein biomarker studies have applied chromogenic immunohistochemical (IHC) methods, but in 2002, Rao et al. showed that quantitative fluorescence image analysis could be used on formalin-fixed paraffin-embedded archival material [12]. Simultaneously, automated quantitative analysis was shown to provide an objective, continuous variable for measuring protein expression by immunofluorescence (IF) signals. This method was subsequently used in several studies regarding solid cancers [13–15], where it was shown to match or even exceed pathologist-based scoring regarding identification of patients with a specific prognosis [13].
As the accessibility of both conventional chromogenic and fluorescence slide scanners, as well as software systems for image analysis have increased, digital quantification has become a new possibility in biomarker research and daily pathology. This also applies to glioma research, where it has been shown that the use of quantitative automated equipment is a feasible approach [16].
This article focuses on novel approaches for quantifying protein biomarkers in gliomas – approaches that, according to our experience, are valuable in biomarker studies – and may be valuable in daily pathology.
Digital quantification versus pathologist-based scoring
A common approach for identification of putative protein biomarkers is IHC followed by semi-quantitative scoring of the chromogenic staining reactions by a pathologist [17,18]. IHC is often easy and quickly performed, but it also contains possible bias due to differences between laboratories [17–20]. Moreover, oversaturation of chromogenic-based IHC reactions, semi-quantitative scoring systems, as well as inter- and intra-observer variability make quantification difficult or even impossible [20,21].
Although the use of digitized approaches do not avoid all the pitfalls known to bias the interpretation of IHC, it has been shown to exceed the pathologist’s ability to reproducibly score on a continuous scale, discriminate between subtle low-level staining differences, and accurately score expression within subcellular compartments [13]. However, introducing a new technique poses new challenges. Previous studies used semi-quantitative approaches, and results obtained by quantitative measurements may complicate the comparison of previously reported studies and future studies. Moreover, in order to obtain high data quality it is important to introduce new steps; for example, a step to assure that a sufficiently high percentage of the tissue is sampled in order to obtain reliable and quantitative data. This step is necessary, since sampling of only a minor part of the tumor area would provide under/over-estimation of the expression of a given marker. Including a step with test sampling using increasing sampling fractions reveal how high a percentage of the tissue it is necessary to sample to obtain constant measurements. The heterogeneity of gliomas is thus taken into account and bias on this basis is avoided. However, heterogeneously expressed biomarkers such as Ki-67 may require high sampling fractions to obtain constant measurements. This procedure including the design of the classifiers are all time consuming, but necessary.
In Figure 1, an example of the work flow is shown illustrating how biomarkers can be digitized and quantified. We have used such approaches for a series of nuclear, cytoplasmic and membrane markers (Table 1) in order to study different aspects of glioma biology. These markers have been suggested to be related to chemoresistance, tumor aggressiveness, tumor stemness or other aspects of glioma biology. Some of the markers have been investigated by our group in terms of prognostic and predictive potential using patient cohorts and some markers have been a part of experimental in vitro studies using cultured glioma spheroids. The biomarker examples included in the present article, such as, c-Met, HIF1α and GLUT1, have been chosen to illustrate how both cytoplasmic, nuclear or membrane marker can be quantified by a digital approach.
Figure 1. . Quantitation of a biomarker implies several steps before the final data is obtained, as illustrated in this figure.
Acquisition of sample images for the analysis is based on manually outlining the regions of interest on scanned slides. Then systematic random sampling within the ROI is performed. A crucial part of the process is training of a software classifier, which is able to identify the biomarker of interest. Classification of images, postprocessing, and generation of output variables are performed in the subsequent processes followed by data analysis.
IF: Immunofluorescence; IHC: Immunohistochemistry; ROI: Region of interest.
Table 1. . Summary of markers identified by software-based classifiers in the present article.
| Marker | Function | Localization | Ref. |
|---|---|---|---|
| APNG | Enzyme that initiates base excision repair at N3-methyladenine and N7-methyl guanine DNA adducts | Nucleus | [33,34] |
| ASMA | Intracellular contractile filament expressed by vessel smooth muscle cells and pericytes (collectively mural cells) | Cytoplasm | [47] |
| CD31 | Transmembrane protein expressed by endothelial cells. Involved in angiogenesis, leukocyte migration and integrin activation | Membrane | [46] |
| CD45 | Transmembrane glycoprotein expressed in leukocytes. Involved in immunological functions | Membrane | [44,45] |
| c-Met | Receptor tyrosine kinase involved in tumor growth and angiogenesis | Cytoplasm, membrane | [29–31] |
| GLUT1 | Protein facilitating transport of glucose across the plasma membrane | Membrane | [32] |
| GLUT3 | Protein facilitating transport of glucose across the plasma membrane. Expressed in neurons and thus designated as the neuronal glucose transporter | Membrane | [32] |
| HIF1α | Transcription factor mediating the acute hypoxic response | Nucleus | [25,26] |
| IBA-1 | Calcium-binding adaptor protein expressed by microglia and macrophages. Upregulated upon cell activation | Cytoplasm | [43] |
| JAM-A | Cell–cell adhesion protein expressed in brain tumor stem-like cells | Cytoplasm, membrane | [27,28] |
| Ki-67 | Protein expressed in proliferative cells during the active phases of the cell cycle | Nucleus | [22–24] |
| MGMT | Ubiquitously expressed DNA repair protein, removing methylation at the O6-position of guanine | Nucleus | [10,45,46,48–52] |
| Musashi-1 | Neural RNA-binding protein expressed by neural stem cells/progenitor cells and brain tumor stem-like cells | Nucleus | [16] |
APNG: Alkylpurine-DNA-N-glycolase; ASMA: α-smooth muscle actin; CD: Cluster of differentiation; GLUT: Glucose transporter type; HIF: Hypoxia inducible factor; IBA-1: Ionized calcium-binding adapter molecule 1; JAM-A: Junctional adhesion molecule 1; MGMT: O6-methylguanine-DNA methyltransferase.
Quantification of chromogenic IHC stainings
When preparing a quantitative evaluation of chromogenic IHC stainings, histological slides can be digitized using slide scanners (Figure 1). Using the digitized slides, software classifiers can be designed and trained by the observer, for example, a pathologist using certain software image analysis features to identify certain patterns or levels of staining. The classifier training procedure depends on the type of software. It may imply selection of appropriate color bands, based on the ability of classification algorithms to identify areas with positive and negative staining, for example, in the nuclei. Areas containing background staining or other structures are omitted simultaneously. The classifier is thus trained to recognize and correctly classify, for example, tumor nuclei within an area containing positive as well as negative tumor nuclei. This step is repeated in different tumor areas and in tissue from tumors with different histology in order to train the classifier to recognize nuclei with different morphology. The final approval of a classifier relies on whether the classifier is able to recognize and correctly classify, for example, tumor nuclei. This approval is made by the observer. The classifier will therefore never be better than the observer at recognizing nuclei but the intraobserver variation from, for example, day to day is eliminated. Approval of the classifier by a group of observers may help eliminate interobserver variability as well. Other types of bias influencing the immune reaction such as fixation time or specificity of antibody clones need the same attention whether the biomarkers are subjected to manual scoring or quantification by a software classifier.
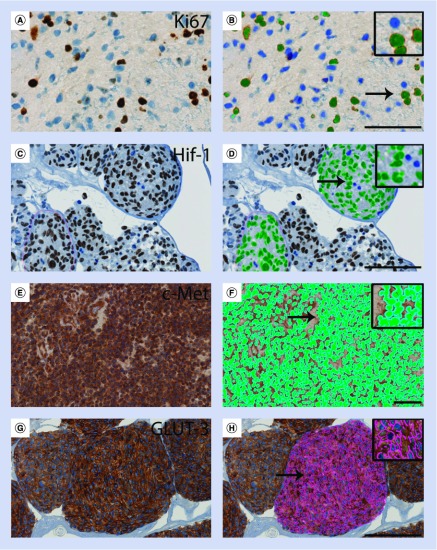
When digital quantification is used, images are sorted to exclude those with an insufficient amount of vital tumor tissue, areas with staining artefacts, areas mainly composed of vessels, normal brain tissue, unspecific background staining and necrotic areas. Different types of software output variables such as staining intensity, staining area or the area fraction of a biomarker within different cellular compartments are selected and the images are finally classified. The proliferation marker Ki-67 [22–24] and the hypoxia inducible factor HIF1α [25,26] provide examples of nuclear markers expressed in glioma tissue or glioma spheroids, which can be detected by such trained classifiers (Figure 2A–D).
Figure 2. . Computer-based software classifiers can be trained to identify immunohistochemical staining reactions.
Immunohistochemical stainings in (A–D) nuclei, (E & F) in the cytoplasm/membranes and (G & H) in the membranes. Analyzing expression of the nuclear marker Ki-67 in (A) a glioblastoma sample, positive cells with brown nuclei (green) and negative unstained nuclei (blue) were easily identified by (B) the classifier. Analyzing HIF-1 nuclear staining in (C) cultured glioblastoma spheroids, positive cell nuclei (green) and negative unstained nuclei (blue) were easily identified as well (D). For analysis of the cytoplasmic/membrane labeling obtained, for example, with c-Met in (E) glioblastoma samples, we trained a classifier to identify the staining located to the cytoplasm/membrane by growing a perinuclear area (light blue) around the nuclei (green) (F). For analysis of the membrane marker GLUT-3 in glioblastoma spheroids (G), the trained classifier easily identified the membranes (pink) (H). All stainings shown are performed on histological sections of formalin-fixed paraffin-embedded material. Scale bar: (A & B) 50 μm and (C–H) 100 μm.
When the expression of a cytoplasmic biomarker or biomarkers with a combined cytoplasmic/membrane localization is analyzed, the classifier can be trained to grow a defined perimeter around all nuclei. In these defined areas, representing the cytoplasm, staining intensity can be measured. Examples of combined cytoplasmic/membrane markers, which has been successfully analyzed using this approach, are the junctional adhesion molecule-A (JAM-A) [27,28] and c-Met (Figure 2E & F) [29–31]. We measured the expression of JAM-A as a continuous variable followed by an optimal cut-point analysis. The analysis identified an optimal cut-point, which showed that the 55% of the patients with the lowest expression of JAM-A had the best prognosis [27].
In some image analysis softwares, identification of membrane labeling is a build-in feature that only requires the user to define an appropriate input color band, the desired sensitivity and outputs of interest. The membrane is then detected based of morphological features. Using this approach we have successfully analyzed GLUT1 and GLUT3 (Figure 2G & H) [32] in glioma spheroids in experimental studies.
Quantification of immunofluorescence stainings
IF is another commonly used approach in glioma research. In many papers IF is used to demonstrate co-localization of multiple biomarkers using fluorescent tags in different colors. The combination of IF, automated image acquisition and software analysis tools makes it possible to identify the expression of one or more biomarkers on a quantitative level in large cohorts of patients [16]. This is possible by fast image acquisition with slide scanners or multistage microscopes since this equipment has reduced the problems with fading of fluorescence, although it is still an important issue, even with the more stable fluorochromes of today. Our experiences with fluorescence-based quantification of biomarkers, as presented in the present article, are based on experiences with multistage microscopes.
We have based our digital fluorescence classifiers on the RGB three color model, where it is possible to measure the intensity of three different colors; red, green and blue, for each pixel. When we investigated Musashi-1, areas with a blue intensity above 120 and a red intensity below 60 were classified as nuclei. Areas of a blue intensity below 120, and a red intensity above 60 were classified as Musashi-1-positive cytoplasm and areas with both a blue intensity above 120 and a red intensity above 60 were classified as Musashi-1-positive nuclei. In this particular classifier, all levels of green intensity were accepted. Approval of the used intensities was conducted in collaboration between the authors. We are aware that the selection criteria may be biased and that different users would accept different intensity levels as acceptable. However, the exact intensity levels from different classifiers can be transferred to other groups who can verify the results on their own material. We recommend the use of the same classifier thresholds across all images of patient cohorts including different types and grades of gliomas with different histologies.
Automated quantitative analysis is an approach used in many papers [13–15]. This approach depends on a set of algorithms designed to identify biomarkers expressed in tumor cells. Tissue is stained using a tumor-specific tag, which recognize all tumor cells and a binary mask is then created, each pixel representing tumor or nontumor cells. Glial fibrillary astrocytic protein (GFAP) has been considered a tumor-specific tag in gliomas, but it is not reliable since some glioblastomas lose expression of GFAP and since some nontumor cells, for example, reactive astrocytes, may express GFAP. We have investigated other markers CD56, vimentin and S100, as potential markers of glioma tumor cells, but all markers labeled nontumor cells or only a subgroup of tumor cells or tumors. Mutational-specific antibodies have some value but, for example, only a part of gliomas harbor the IDH-1 R132 mutation giving the antibody recognizing this specific mutated version of the protein only limited value as a tumor-specific tag. Unfortunately, the lack of tumor-specific tags complicates the recognition of tumor cells in samples from gliomas compared with other tumor types.
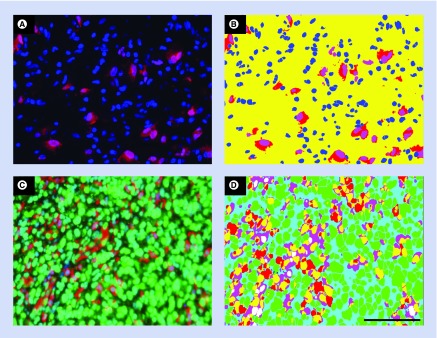
We earlier investigated the expression of Musashi-1 [16] without using a tumor-specific tag. In order to prevent bias introduced by sample sizes, we therefore constructed an area fraction of positive tissue by using the total area of each image frame. Using a fluorescent staining protocol and a specific Musashi-1 classifier (Figure 3A & B), we identified the expression of Musashi-1 in both the nuclei and in the cytoplasm. Neither the nuclear nor the cytoplasmic expression of Musashi-1 was prognostic, when patients were dichotomized at the median. However, in an explorative analysis, we showed that a subgroup of patients with glioblastoma multiforme and a high level of Musashi-1 had a better outcome with a median overall survival of 15 months compared with a median overall survival of 10 months in the group with low Musahi-1. This analysis was only possible due to the continuous measurements of the expression of Musashi-1. To our knowledge, our study was the first glioma study identifying the expression of Musashi-1 or other biomarkers in different subcellular compartments. This is an important option for some biomarkers as it, for example, has been shown previously that colon cancer patients with high nuclear expression of β-catenin do worse than those with high membrane expression [13].
Figure 3. . Computer-based software classifiers can be trained to identify immunofluorescence staining reactions in the nuclei and in the cytoplasm.
Musashi-1 immunofluorescence staining (red) in a glioblastoma sample (A) was easily identified by a trained classifier identifying negative nuclei (blue), Musashi-1-positive nuclei (pink) and Musashi-1 positive cytoplasm (red) (B). Analyzing alkylpurine-DNA-N-glycosylase (APNG) using immunofluorescence staining (green) in a glioblastoma sample where nontumor cells (red) were excluded from the analysis (C). The classifier was trained to label the tissue as follows: APNG-positive tumor cell nuclei (green), nontumor cell nuclei (white), tumor cell area to intimately related to red staining to be evaluated as safe tumor cell area (pink), nontumor cell area (yellow), background (turquoise) (D). All stainings shown are performed on histological sections of formalin-fixed paraffin-embedded material.
(A & B) modified from [16] with kind permission from Springer Science and Business Media.
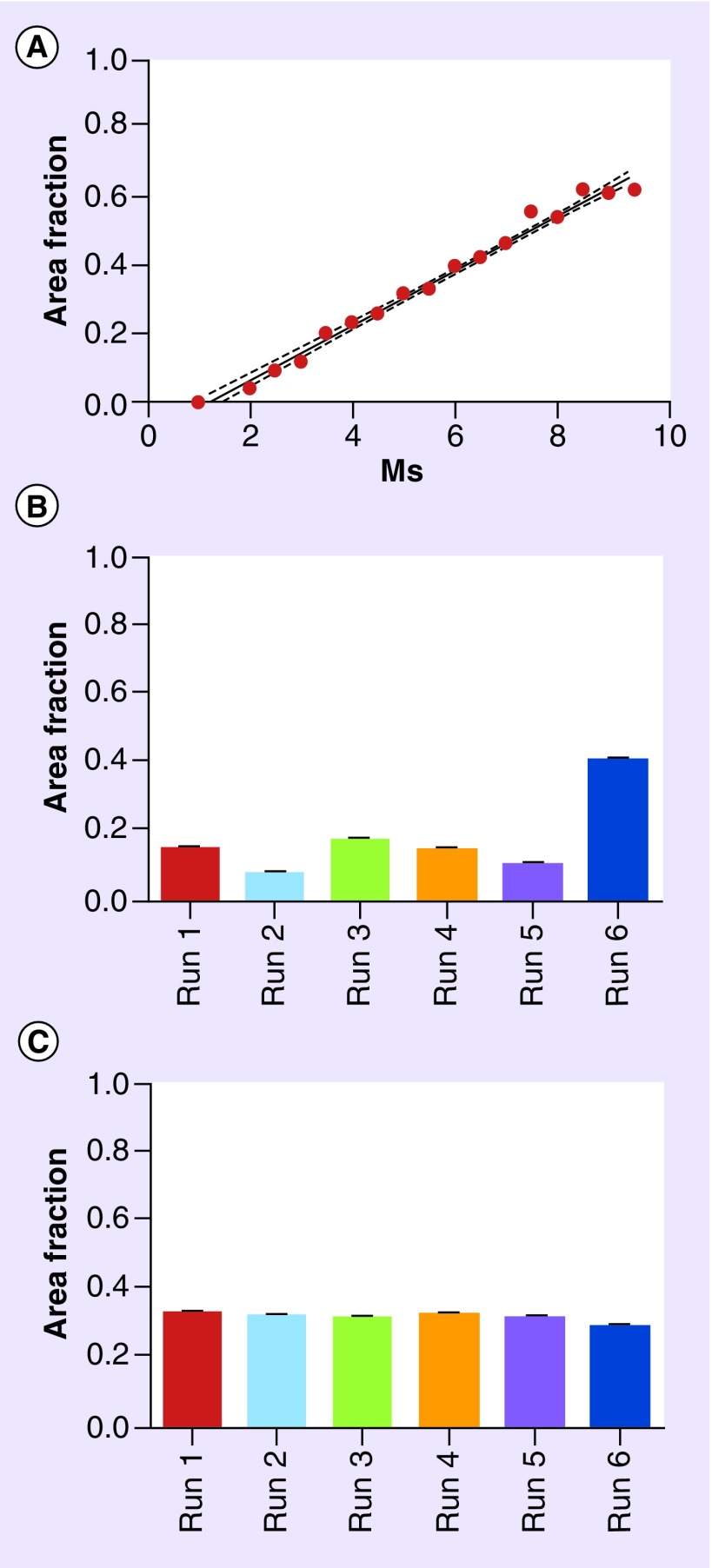
Although quantitative fluorescence may provide a more precise evaluation of the expression of some biomarkers, this more quantitative approach reveals that the technical aspect of variation of staining intensity between staining runs preferably should be taken into account. To illustrate this aspect, we measured the expression of APNG, an enzyme involved in DNA repair [33,34]. We trained a classifier to measure APNG expression in tumor cell nuclei and to exclude nontumor cells expressing APNG (Figure 3C–D). Using this staining, we found variation in APNG staining intensity in tumor cell nuclei between runs and accordingly, we established a correlation curve between exposure time and the area fraction for calibration (Figure 4A). Using this calibration curve, we showed that calibration between runs is possible resulting in almost identical measurements on identical tissues (Figure 4B & C). Extending this to biomarker analysis on a patient cohort, the exposure time for each run should be adjusted before sampling based on a homogenous-positive control tissue and a calibration curve. Elimination of run-to-run variation is important in quantitative studies of patient cohorts in order to allow comparison of output variables across the cohort and to secure robust cut-off levels. Technically, this is not an option for chromogenic staining reactions but in our hands the run-to-run variation is low for chromogenic stainings, which is most likely due to the intense brown staining reactions obtained by these stainings.
Figure 4. . In quantitative immunofluorescence assays, variation in staining intensities between staining runs can be eliminated by calibration of exposure time.
The figure illustrates the quantitative data on alkylpurine-DNA-N-glycosylase (APNG) expression in tumor cells, excluding nontumor cells as illustrated in Figure 3C & D. (A) Calibration curve with linear correlation between exposure time (ms) and the area fraction of APNG-positive tumor cells in glioblastoma tissue. (B) The area fraction of APNG for six individual runs before calibration. An exposure time of 4.5 ms was used for all runs. Note the variation in the area fraction in adjacent glioblastoma tissue sections between runs (p < 0.0001). (C) After calibration the same area fraction of APNG was measured in adjacent glioblastoma tissue sections from six individual runs (p = 0.46). The exposure time was as follows when calibrations were used: run 1: 7 ms; run 2: 8 ms; run 3: 6 ms; run 4: 7 ms; run 5: 7.5 ms; and run 6: 3.5 ms. Statistic analysis in (B) and (C) was performed using analysis if variance and p < 0.05 was regarded as significant.
Biomarker analysis taking nontumor cells into account
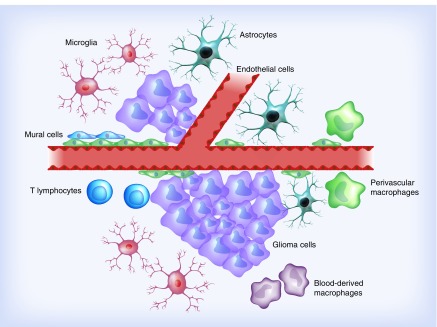
Gliomas, and especially glioblastoma multiforme, unveil a rich heterogeneous microenvironment including various cell types. Specialized cells such as lymphocytes, monocytes/macrophages, and progenitors to endothelial and mural cells are recruited to the site of the tumor resulting in a massively infiltrated brain parenchyma (Figure 5) [35–38]. As several of these cells express some of the previously proposed prognostic markers in gliomas [39–42], their presence may introduce crucial bias in biomarker studies.
Figure 5. . Cellular heterogeneity of glioblastomas.
The tumor cells are surrounded by nontumor cells such as tumor-associated microglia, macrophages and lymphocytes. Moreover, vessels are numerous in many glioblastomas. The rich glioma microenvironment needs to be taken into consideration when the expression of a biomarker is evaluated.
We are currently focusing on the proliferation marker Ki-67, which is widely used in pathology, since it may have a potential in predicting malignant behavior of a given tumor. In gliomas, both tumor cells and nontumor cells contribute to the proliferation index but the contribution from nontumor cells is difficult to determine, thereby introducing bias. We used Iba-1 [43], CD45 [44,45], CD31 [46] and ASMA [47] as markers to exclude microglia and macrophages (Iba-1), leukocytes (CD45) and vessels (CD31 and ASMA) from the analysis. This was performed by combining a Ki-67 IHC staining (brown) with an IHC staining (red) containing a cocktail of antibodies identifying these markers (Figure 6A). A classifier was designed to detect the brown Ki-67-positive nuclei representing tumor cells and to exclude Ki-67-positive nuclei surrounded by a red staining representing nontumor cells (Figure 6B). Based on this, the fraction of proliferating tumor cells could easily be calculated. The prognostic potential of this approach remains to be clarified, but we consider such approaches as important steps towards more intelligent biomarker analysis. However, it should be noticed that the suggested antibody cocktail does not exclude biomarker expression in reactive astrocytes emphasizing that identification of tumor-specific tags would be of high value.
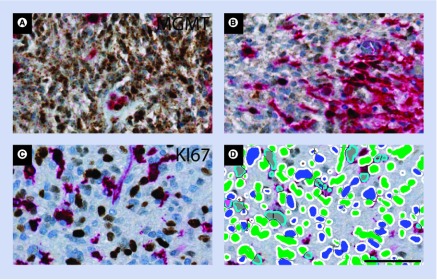
Figure 6. . Chromogenic immunohistochemical stainings can be designed for identification of the marker of interest, while excluding at the same time nontumor cells from the analysis by labeling them with an additional tag.
Using the nuclear marker Ki-67 (brown) in a glioblastoma sample together with a cocktail of antibodies (red), Ki-67-positive nontumor cells can be excluded (A). The antibody cocktail contain four antibodies used to exclude microglia and macrophages (Iba-1), leucocytes (CD45) and vessels (CD31 and ASMA).The trained software classifier identifies positive tumor cell nuclei (blue), double-labeled cells (gray), as well as nonstained cells (green) (B). To further illustrate the value of this approach in glioblastomas, we developed a staining, which identifies O6-methylguanine DNA-methyltransferase (MGMT)-positive tumor cells (brown), while a cocktail of antibodies at the same time identifies MGMT-positive macrophages/microglia, leukocytes, and vessels as well (red) (C & D). This avoids interpreting MGMT expression by nontumor cells (brown–red) as MGMT expression in tumor cells (C). All stainings shown are performed on histological sections of formalin-fixed paraffin-embedded glioblastoma tissue. Scale bar: 50 μm.
Another biomarker, where we used this approach and where nontumor cells may introduce severe bias is O6-methylguanine DNA-methyltransferase (MGMT). The prognostic importance of MGMT methylation in gliomas has been investigated by several groups, but the results were based on different techniques [10,45,48,49]. The use of different detection methods provided different results [50], and previously IHC studies have shown some prognostic potential of MGMT [46,51,52], but other groups have not been able to confirm the results [53,54]. Based on trials in elderly glioblastoma patients [55,56], MGMT methylation status is currently being used in some countries as a predictive marker allocating the patients to radiation or chemotherapy. Therefore, there is a need for a detection method, which can be performed by most laboratories (e.g., IHC). Having such clinical implications, it is of crucial importance to avoid false-positive tumors. As MGMT is expressed in both tumor cells and nontumor cells, we propose that exclusion of nontumor cells may improve future analysis of this biomarker (Figure 6C & D).
Conclusion & future perspective
With digital slide scanners and training of software classifiers, the future holds promising perspectives for quantitative biomarker studies in gliomas. It is often difficult to manually score biomarker expression in gliomas, and the digital approach is a major step forward towards a more unbiased approach obtaining a continuous measure of biomarkers with software not influenced by knowledge of the diagnosis. The approach can be improved further by exclusion of nontumor cells from the analysis. As some biomarkers may be differentially expressed in tumor and nontumor cells, it is important to avoid false-negative or -positive tumors. This is crucial in terms of using protein biomarkers such as MGMT as predictive markers guiding clinical decisions on type of therapy. We encourage the glioma field to implement these strategies in future studies of prognostic and predictive biomarkers. Since access to slide scanners and image analysis software is becoming easier in pathology, more biomarker studies can be performed in this way. Moreover, the results, based on robust quantitative cut-off values, can more easily be implemented in daily pathology, thereby strengthening the clinical relevance of a digital biomarker approach.
Acknowledgements
The authors would like to thank technicians Helle Wohlleben and Tanja Dreehsen Højgaard for expert assistance with the immunohistochemical and immunofluorescence stainings.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J. Clin. Oncol. 2012;31(3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013;31(3):344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised Phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Dahlrot RH, Hermansen SK, Hansen S, Kristensen BW. What is the clinical value of cancer stem cell markers in gliomas? Int. J. Clin. Exp. Pathol. 2013;6(3):334–348. [PMC free article] [PubMed] [Google Scholar]
- 6.Wei KC, Huang CY, Chen PY, et al. Evaluation of the prognostic value of CD44 in glioblastoma multiforme. Anticancer Res. 2010;30(1):253–259. [PubMed] [Google Scholar]
- 7.Preusser M, Hoeftberger R, Woehrer A, et al. Prognostic value of Ki67 index in anaplastic oligodendroglial tumours – a translational study of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Histopathology. 2012;60(6):885–894. doi: 10.1111/j.1365-2559.2011.04134.x. [DOI] [PubMed] [Google Scholar]
- 8.Hermansen SK, Dahlrot RH, Nielsen BS, Hansen S, Kristensen BW. MiR-21 expression in the tumor cell compartment holds unfavorable prognostic value in gliomas. J. Neurooncol. 2013;111(1):71–81. doi: 10.1007/s11060-012-0992-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhi F, Chen X, Wang S, et al. The use of hsa-miR-21, hsa-miR-181b and hsa-miR-106a as prognostic indicators of astrocytoma. Eur. J. Cancer (Oxford) 2010;46(9):1640–1649. doi: 10.1016/j.ejca.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 11.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science (NY) 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao J, Seligson D, Hemstreet GP. Protein expression analysis using quantitative fluorescence image analysis on tissue microarray slides. Biotechniques. 2002;32(4):924–926. doi: 10.2144/02324pt04. 928–930, 932. [DOI] [PubMed] [Google Scholar]; •• The expression of BRCA1 was measured on tissue microarrays with formalin-fixed and paraffin-embedded breast cancer tissue using quantitative immunofluorescence image analysis.
- 13.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat. Med. 2002;8(11):1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]; •• Development of automated and quantitative immunofluorescence analysis of tissue microarrays. The authors showed that automated continuous measurements matched or even exceeded pathologist-based scoring regarding identification of patients with a specific prognosis.
- 14.Giltnane JM, Ryden L, Cregger M, Bendahl PO, Jirstrom K, Rimm DL. Quantitative measurement of epidermal growth factor receptor is a negative predictive factor for tamoxifen response in hormone receptor positive premenopausal breast cancer. J. Clin. Oncol. 2007;25(21):3007–3014. doi: 10.1200/JCO.2006.08.9938. [DOI] [PubMed] [Google Scholar]
- 15.Gustavson MD, Molinaro AM, Tedeschi G, Camp RL, Rimm DL. AQUA analysis of thymidylate synthase reveals localization to be a key prognostic biomarker in 2 large cohorts of colorectal carcinoma. Arch. Pathol. Lab. Med. 2008;132(11):1746–1752. doi: 10.5858/132.11.1746. [DOI] [PubMed] [Google Scholar]
- 16.Dahlrot RH, Hansen S, Herrstedt J, Schroder HD, Hjelmborg J, Kristensen BW. Prognostic value of Musashi-1 in gliomas. J. Neurooncol. 2013;115(3):453–461. doi: 10.1007/s11060-013-1246-8. [DOI] [PubMed] [Google Scholar]; •• The first study using automated and quantitative immunofluorescence analysis of a biomarker in gliomas. In this study, whole slide sections from a glioma cohort were used.
- 17.Cregger M, Berger AJ, Rimm DL. Immunohistochemistry and quantitative analysis of protein expression. Arch. Pathol. Lab. Med. 2006;130(7):1026–1030. doi: 10.5858/2006-130-1026-IAQAOP. [DOI] [PubMed] [Google Scholar]
- 18.Taylor CR. The total test approach to standardization of immunohistochemistry. Arch. Pathol. Lab. Med. 2000;124(7):945–951. doi: 10.5858/2000-124-0945-TTTATS. [DOI] [PubMed] [Google Scholar]
- 19.De Marzo AM, Fedor HH, Gage WR, Rubin MA. Inadequate formalin fixation decreases reliability of p27 immunohistochemical staining: probing optimal fixation time using high-density tissue microarrays. Hum. Pathol. 2002;33(7):756–760. doi: 10.1053/hupa.2002.126187. [DOI] [PubMed] [Google Scholar]
- 20.Riechers A, Bosserhoff AK. Pitfalls in immunohistochemistry – a recent example. Int. J. Clin. Exp. Pathol. 2012;5(2):137–139. [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor CR, Levenson RM. Quantification of immunohistochemistry – issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006;49(4):411–424. doi: 10.1111/j.1365-2559.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- 22.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Dowsett M, Nielsen TO, A’hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J. Natl Cancer Instit. 2011;103(22):1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 25.Heddleston JM, Li Z, Mclendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8(20):3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Bao S, Wu Q, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15(6):501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lathia JD, Li M, Sinyuk M, et al. High-throughput flow cytometry screening reveals a role for junctional adhesion molecule a as a cancer stem cell maintenance factor. Cell Rep. 2014;6(1):117–129. doi: 10.1016/j.celrep.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mcsherry EA, Brennan K, Hudson L, Hill AD, Hopkins AM. Breast cancer cell migration is regulated through junctional adhesion molecule-A-mediated activation of Rap1 GTPase. Breast Cancer Res. 2011;13(2):R31. doi: 10.1186/bcr2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong DS, Song SY, Kim DH, et al. Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115(1):140–148. doi: 10.1002/cncr.23972. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Fu Y, Xu S, et al. c-Met expression is associated with time to recurrence in patients with glioblastoma multiforme. J. Clin. Neurosci. 2011;18(1):119–121. doi: 10.1016/j.jocn.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Olmez OF, Cubukcu E, Evrensel T, et al. The immunohistochemical expression of c-Met is an independent predictor of survival in patients with glioblastoma multiforme. Clin. Transl. Oncol. 2014;16(2):173–177. doi: 10.1007/s12094-013-1059-4. [DOI] [PubMed] [Google Scholar]
- 32.Mobasheri A, Richardson S, Mobasheri R, Shakibaei M, Hoyland JA. Hypoxia inducible factor-1 and facilitative glucose transporters GLUT1 and GLUT3: putative molecular components of the oxygen and glucose sensing apparatus in articular chondrocytes. Histol. Histopathol. 2005;20(4):1327–1338. doi: 10.14670/HH-20.1327. [DOI] [PubMed] [Google Scholar]
- 33.Agnihotri S, Gajadhar AS, Ternamian C, et al. Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J. Clin. Invest. 2012;122(1):253–266. doi: 10.1172/JCI59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu C, Tu Y, Yuan J, et al. Aberrant expression of N-methylpurine-DNA glycosylase influences patient survival in malignant gliomas. J. Biomed. Biotechnol. 2012;2012:760679. doi: 10.1155/2012/760679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59(8):1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]; • Summarizes the importance of the microenvironment in gliomas. They report that microglial cells may represent up to 30% of a brain tumor mass.
- 36.Alves TR, Lima FR, Kahn SA, et al. Glioblastoma cells: a heterogeneous and fatal tumor interacting with the parenchyma. Life Sci. 2011;89(15–16):532–539. doi: 10.1016/j.lfs.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 37.Jones TS, Holland EC. Standard of care therapy for malignant glioma and its effect on tumor and stromal cells. Oncogene. 2012;31(16):1995–2006. doi: 10.1038/onc.2011.398. [DOI] [PubMed] [Google Scholar]
- 38.Lorger M. Tumor microenvironment in the brain. Cancers. 2012;4(1):218–243. doi: 10.3390/cancers4010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasai K, Nodagashira M, Nishihara H, et al. Careful exclusion of non-neoplastic brain components is required for an appropriate evaluation of O6-methylguanine-DNA methyltransferase status in glioma: relationship between immunohistochemistry and methylation analysis. Am. J. Surg. Pathol. 2008;32(8):1220–1227. doi: 10.1097/PAS.0b013e318164c3f0. [DOI] [PubMed] [Google Scholar]; • Shows that gliomas contain various types of non-neoplastic cells expressing O6-methylguanine-DNA methyltransferase. Recommendation of exclusion of non-neoplastic cells from analysis.
- 40.Nakasu S, Fukami T, Baba K, Matsuda M. Immunohistochemical study for O6-methylguanine-DNA methyltransferase in the non-neoplastic and neoplastic components of gliomas. J. Neurooncol. 2004;70(3):333–340. doi: 10.1007/s11060-004-9170-6. [DOI] [PubMed] [Google Scholar]
- 41.Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992;52(19):5334–5341. [PubMed] [Google Scholar]
- 42.Sugawara K, Kurihara H, Negishi M, et al. Nestin as a marker for proliferative endothelium in gliomas. Lab Invest. 2002;82(3):345–351. doi: 10.1038/labinvest.3780428. [DOI] [PubMed] [Google Scholar]
- 43.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. 1998;57(1):1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 44.Burke E, Grobler M, Elderfield K, et al. Double-labelling immunohistochemistry for MGMT and a ‘cocktail’ of non-tumourous elements is a reliable, quick and easy technique for inferring methylation status in glioblastomas and other primary brain tumours. Acta Neuropathol. Commun. 2013;1(1):1–10. doi: 10.1186/2051-5960-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe R, Nakasu Y, Tashiro H, et al. O6-methylguanine DNA methyltransferase expression in tumor cells predicts outcome of radiotherapy plus concomitant and adjuvant temozolomide therapy in patients with primary glioblastoma. Brain Tumor Pathol. 2011;28(2):127–135. doi: 10.1007/s10014-011-0022-8. [DOI] [PubMed] [Google Scholar]
- 46.Hsu CY, Lin SC, Ho HL, et al. Exclusion of histiocytes/endothelial cells and using endothelial cells as internal reference are crucial for interpretation of MGMT immunohistochemistry in glioblastoma. Am. J. Surg. Pathol. 2013;37(2):264–271. doi: 10.1097/PAS.0b013e318267b061. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Liu J. Tumor stroma as targets for cancer therapy. Pharmacol. Ther. 2013;137(2):200–215. doi: 10.1016/j.pharmthera.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esteller M, Gaidano G, Goodman SN, et al. Hypermethylation of the DNA repair gene O(6)-methylguanine DNA methyltransferase and survival of patients with diffuse large B-cell lymphoma. J. Natl Cancer Instit. 2002;94(1):26–32. doi: 10.1093/jnci/94.1.26. [DOI] [PubMed] [Google Scholar]
- 49.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N. Engl. J. Med. 2000;343(19):1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 50.Karayan-Tapon L, Quillien V, Guilhot J, et al. Prognostic value of O6-methylguanine-DNA methyltransferase status in glioblastoma patients, assessed by five different methods. J. Neurooncol. 2010;97(3):311–322. doi: 10.1007/s11060-009-0031-1. [DOI] [PubMed] [Google Scholar]
- 51.Lalezari S, Chou AP, Tran A, et al. Combined analysis of O6-methylguanine-DNA methyltransferase protein expression and promoter methylation provides optimized prognostication of glioblastoma outcome. Neurooncology. 2013;15(3):370–381. doi: 10.1093/neuonc/nos308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quillien V, Lavenu A, Karayan-Tapon L, et al. Comparative assessment of 5 methods (methylation-specific polymerase chain reaction, MethyLight, pyrosequencing, methylation-sensitive high-resolution melting, and immunohistochemistry) to analyze O6-methylguanine-DNA-methyltranferase in a series of 100 glioblastoma patients. Cancer. 2012;118(17):4201–4211. doi: 10.1002/cncr.27392. [DOI] [PubMed] [Google Scholar]
- 53.Brell M, Tortosa A, Verger E, et al. Prognostic significance of O6-methylguanine-DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplastic gliomas. Clin. Cancer Res. 2005;11(14):5167–5174. doi: 10.1158/1078-0432.CCR-05-0230. [DOI] [PubMed] [Google Scholar]
- 54.Preusser M, Charles Janzer R, Felsberg J, et al. Anti-O6-methylguanine-methyltransferase (MGMT) immunohistochemistry in glioblastoma multiforme: observer variability and lack of association with patient survival impede its use as clinical biomarker. Brain Pathol. (Zurich) 2008;18(4):520–532. doi: 10.1111/j.1750-3639.2008.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, Phase 3 trial. Lancet Oncol. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 56.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, Phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]