Abstract
Functional bowel disorder patients can suffer from chronic abdominal pain, likely due to visceral hypersensitivity to mechanical stimuli. As there is only a limited understanding of the basis of chronic visceral hypersensitivity (CVH), drug-based management strategies are ill defined, vary considerably, and include NSAIDs, opioids, and even anticonvulsants. We previously reported that the 1.1 subtype of the voltage-gated sodium (NaV; NaV1.1) channel family regulates the excitability of sensory nerve fibers that transmit a mechanical pain message to the spinal cord. Herein, we investigated whether this channel subtype also underlies the abdominal pain that occurs with CVH. We demonstrate that NaV1.1 is functionally upregulated under CVH conditions and that inhibiting channel function reduces mechanical pain in 3 mechanistically distinct mouse models of chronic pain. In particular, we use a small molecule to show that selective NaV1.1 inhibition (a) decreases sodium currents in colon-innervating dorsal root ganglion neurons, (b) reduces colonic nociceptor mechanical responses, and (c) normalizes the enhanced visceromotor response to distension observed in 2 mouse models of irritable bowel syndrome. These results provide support for a relationship between NaV1.1 and chronic abdominal pain associated with functional bowel disorders.
Keywords: Gastroenterology, Neuroscience
Keywords: Ion channels, Pain
The Nav1.1 subtype of the voltage-gated sodium channel family regulates abdominal pain that occurs with chronic visceral hypersensitivity.
Introduction
Functional bowel disorders (FBDs) such as irritable bowel syndrome (IBS), constipation, diarrhea, and abdominal bloating occur worldwide, and effective treatment is a major unmet clinical need in gastroenterology (1). Typically, FBDs are associated with alterations in bowel habits that result in a substantially decreased quality of life. Because of their prevalence, they are also a considerable drain on health care resources (2). People suffering from FBDs can have an array of symptoms. In particular, most common and observed in ≥40% of the IBS patient population (3) is chronic abdominal pain. A prominent hypothesis for the etiology of this abdominal pain is mechanical hypersensitivity of sensory fibers that innervate the gut (4–8). Although a range of channels and receptors have been implicated (9–20), our understanding of chronic visceral hypersensitivity (CVH) is still poor. Not surprisingly, therefore, pharmacological management of chronic abdominal pain is nonspecific and ranges from NSAIDs to opioids, without or with adjuvant analgesics (21). Anticonvulsants such as gabapentin and pregabalin show promise to treat CVH, but there is insufficient data to support their effectiveness in FBD patients, and all use is considered off-label (22).
Cell membrane–embedded voltage-gated sodium (NaV) channels regulate cellular excitability and initiate action potentials in the peripheral, central, and enteric nervous systems (23–27). A subset of the 9 NaV channel subtypes is expressed in the enteric nervous system, and mutations can lead to gastrointestinal disorders, including constipation and diarrhea (10, 28). The discovery that NaV1.1 can regulate the excitability of sensory nerve fibers that mediate mechanical pain (29) led to our hypothesis that this channel subtype may also underlie the development of abdominal pain in FBD states. In our previous studies, consistent with this, a spider toxin (Hm1a) that activates NaV1.1 increased mechanically evoked spiking in high-threshold colonic nociceptors in gut-nerve preparations from healthy control mice (29). Furthermore, baseline mechanosensory responses of colonic afferents from CVH mice were significantly increased compared with healthy control mice, and application of Hm1a enhanced mechanically evoked spiking beyond this already elevated level. Finally, in contrast to healthy control animals, toxin application evoked a pronounced increase in the electrical excitability of colonic dorsal root ganglion (DRG) neurons from CVH mice, suggesting that NaV1.1 channels are functionally upregulated in CVH states (29). Taken together, these experiments suggest that inhibiting NaV1.1 function could reduce chronic abdominal pain related to FBDs. This hypothesis remains to be tested, since we previously employed a nonspecific NaV channel inhibitor in isolated mouse colonic afferents, not in behavioral experiments (29).
Here, we administered a selective NaV1.1 inhibitor— 1-(phenylmethyl)-1H-1,2,3-triazole-4-carboxamide-5-methyl (Compound B) (30) — in a mouse model for peripheral afferent–mediated mechanical pain and 2 CVH paradigms in order to test whether mechanical hypersensitivity can be alleviated. We demonstrate that NaV1.1 inhibition significantly reduces mechanical pain in CVH states, thereby substantiating an important contribution of this NaV channel subtype to FBD-associated chronic abdominal pain.
Results
Pharmacological target screen.
Compound B (100 μM) was previously shown to inhibit NaV1.1 opening by depolarizing its conductance-voltage (G-V) relationship, whereas other channel gating properties were unaffected (30). Here, we tested compound susceptibility of additional NaV channel subtypes that have also been found in gut nerves (10, 18, 31). When applying 100 μM Compound B to NaV1.5, NaV1.7, and NaV1.8, none of the channels showed an altered G-V relationship (Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.121000DS1). At 1 μM, Compound B still inhibited NaV1.1 activation similar to 100 μM, which is likely to be a saturating concentration. To further investigate potential nonspecific activity of Compound B at 100 μM, we carried out a compound safety screen on 44 commonly tested targets (Supplemental Table 1). With 1 exception, Compound B did not interact with any of the receptors, ion channels, or transporters. Of the enzymes, only phosphodiesterase 4D isoform 2 (PDE4D2) shows a mildly significant inhibitory response to 100 μM Compound B. This protein has 3′,5′-cyclic-AMP phosphodiesterase activity and degrades cAMP, a signal transduction molecule in various cell types. PDE4 inhibitors are known to possess procognitive, neuroprotective, and antiinflammatory effects. Emetic effects caused by PDE4 inhibition have also been reported (32) but were not observed in our experiments.
Pharmacokinetic profile of Compound B.
To make optimal use of our animal models, we determined the pharmacokinetic (PK) profile of Compound B. We found that the molecule was metabolically stable in mouse and human plasma over a period of 60 minutes (Supplemental Figure 2). Additionally, in both mouse and human liver microsomal incubations fortified with NADPH, the compound was stable (>95% remaining), suggesting a resistance to CYP-450–dependent oxidation. A positive control with testosterone was completely metabolized, confirming assay validity (Supplemental Figure 2).
We next examined the plasma PK, brain, and CSF distribution of Compound B in male C57BL/6J mice. Following a single i.v. administration, Compound B (10 mg/kg) showed low plasma clearance (~11 ml/min/kg) with an elimination half-life of ~1.5 hours (Supplemental Figure 3 and Supplemental Table 2). The brain/plasma concentration ratio ranged between 0.6 and 1.7, and the CSF/plasma concentration ratio ranged between 0.3 and 0.5. After a single i.p. dose administration of Compound B (10 mg/kg), plasma, CSF, and brain concentrations were detected up to 24 hours with a time at maximum (Tmax) of 0.5 hours. The brain/plasma concentration ratio ranged from 0.8–1.8, and the CSF/plasma concentration ratio fluctuated between 0.3 and 0.8. A single s.c. dose administration allowed detection of Compound B in plasma, CSF, and brain concentrations up to 8 hours with a Tmax of 1 hour. The brain/plasma ratio ranged between 0.8 and 1.2, and the CSF/plasma ratio was found to be between 0.3 and 0.5. Following a single per os (p.o.) administration of the compound, plasma, CSF, and brain concentrations were detected up to 8 hours with a Tmax of 0.5 hours in plasma and 0.25 hours in the brain and CSF. The brain/plasma ratio ranged between 0.8 and 1.2, whereas the CSF/plasma ratio oscillated between 0.1 and 0.7. The oral solution bioavailability was 79%.
Toxicity.
To examine the possibility of adverse side effects interfering with the interpretation of our animal model experiments, we performed a comprehensive toxicity study. In Phase A, single i.v. doses of 10, 25, 50, or 100 mg/kg Compound B were well tolerated in male and female Sprague Dawley rats. There were no treatment-related effects on survival or clinical signs such as body/organ weight changes, food consumption discrepancies, or visible inflammation. In Phase B, daily i.v. doses of 25, 50, or 100 mg/kg Compound B over the course of 7 days were well tolerated in male and female Sprague Dawley rats. There were no consistent or treatment-related effects on endpoints, including survival, clinical signs, body weight, clinical chemistry, blood chemistry, hematology, urinalysis parameters, absolute or relative organ weights, or histopathology.
Compound B reduces peripheral nerve injury–induced mechanical hypersensitivity.
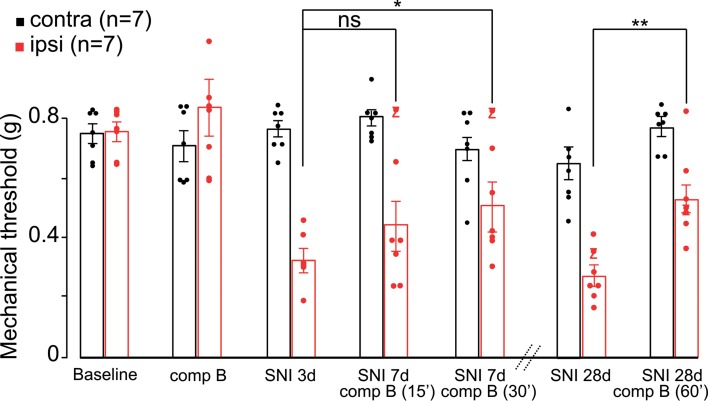
We previously demonstrated that spider toxin–mediated (Hm1a-mediated) activation of NaV1.1 in peripheral sensory neurons elicits robust pain behaviors (29). Interestingly, profound mechanical but not thermal hypersensitivity was produced by Hm1a without neurogenic inflammation, indicating that Hm1a does not target unmyelinated peptidergic nociceptors (29). Conversely, partially eliminating NaV1.1 from sensory neurons using a genetic approach significantly attenuated the toxin-evoked pain behaviors, indicating that this channel subtype regulates the excitability of primary afferent fibers that mediate mechanical pain. Before embarking on FBD mouse model trials, we sought to corroborate the contribution of NaV1.1 to peripheral afferent-mediated mechanical pain. In these studies, we tested whether inhibiting NaV1.1 with Compound B can ameliorate the hypersensitivity that occurs in a mouse model of neuropathic pain. For this, we used the spared nerve injury (SNI) model in which 2 of 3 branches of the sciatic nerve are transected. SNI mice rapidly exhibited profound and long-lasting mechanical hypersensitivity in response to von Frey hairs (vfh; see Methods). As expected, 3 days after SNI, we recorded a significant reduction (~57%) of the mechanical thresholds leading to mechanical hypersensitivity, ipsilateral to the injury side (Figure 1). At 7 days, this mechanical hypersensitivity was significantly reduced 30 minutes after systemic injection of a single dose of Compound B (i.p., 60 mg/kg). Contralateral mechanical thresholds were unaffected by the compound. One month after SNI, mice received another single dose of Compound B, and again, we recorded a significant reduction in mechanical hypersensitivity ipsilateral to the injury side (i.p., 60 mg/kg; Figure 1). Importantly, the same dose did not affect baseline thresholds, nor did it impair motor functions in naive mice, despite the compound penetrating the blood-brain barrier (Supplemental Figure 4; rotarod test). Taken together, these results show that NaV1.1 contributes, at least in part, to the mechanical hypersensitivity that develops after peripheral nerve injury.
Figure 1. Pharmacological blockade of NaV1.1 is antinociceptive.
Systemic administration of Compound B (comp B; 60 mg/kg) has no effect on baseline mechanical thresholds of naive mice (baseline, 0.760 ± 0.03 g, vs. Compound B, 0.836 ± 0.105 g, 2-way ANOVA, P = 0.501, n = 7). Three days after spared nerve injury (SNI), mice exhibit mechanical hypersensitivity (~57%) ipsilateral to the injury (baseline, 0.760 ± 0.03 g, vs. SNI, 0.330 ± 0.034 g, 2-way ANOVA, P = 0.0001). A systemic injection of a single dose of Compound B (i.p., 60 mg/kg) significantly reduces mechanical hypersensitivity 30 minutes (i.p., 60 mg/kg; 0.515 ± 0.072 g, 2-way ANOVA, P = 0.039, n = 7), but not 15 minutes after injection. Contralateral mechanical thresholds were unaffected by the compound (0.703 ± 0.051 g). One month after SNI, mice still exhibited mechanical hypersensitivity ipsilateral to the injury. Again, a single dose of Compound B (i.p., 60 mg/kg) significantly reduced mechanical hypersensitivity (60 minutes; SNI baseline, 0.277 ± 0.031 g, vs. Compound B, 0.530 ± 0.061 g, 2-way ANOVA, P = 0.003, n = 7). Data are presented as mean ± SEM with *P ≤ 0.05 and **P ≤ 0.005.
Sodium current recordings in colon-innervating DRG neurons.
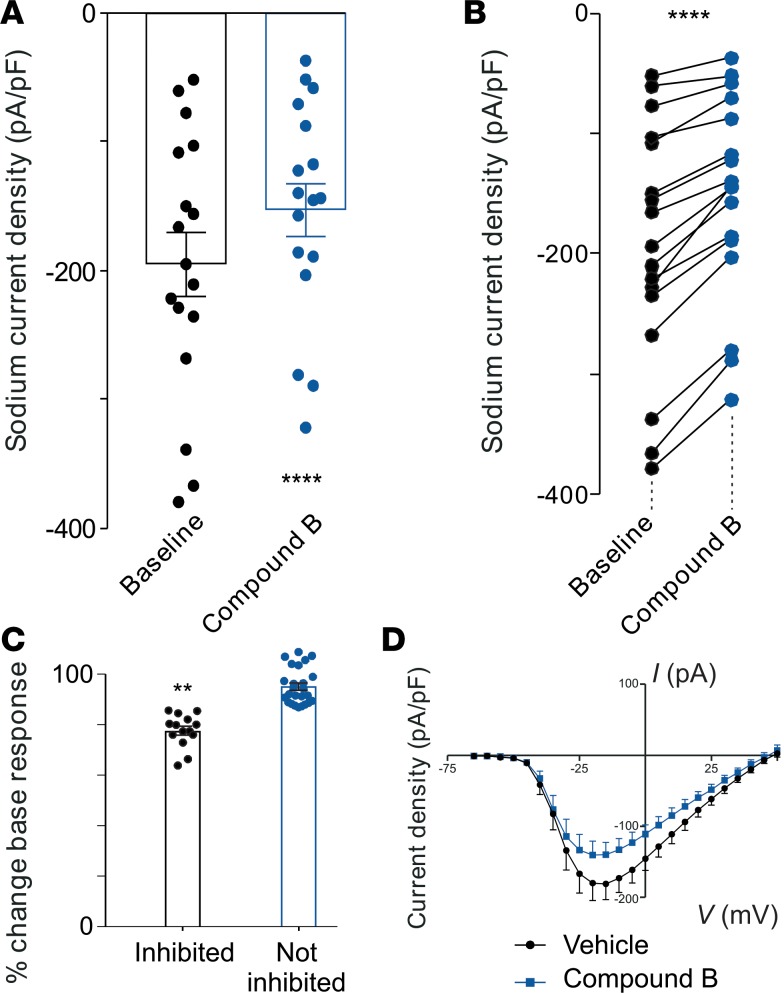
In addition to eliciting behaviors indicative of pain, Hm1a-induced activation of NaV1.1 also evokes neuronal hypersensitivity in a subpopulation of retrograde-labeled colon-innervating DRG neurons (29). Here, we determined if the inhibitory effect of Compound B on NaV1.1 reduces peak sodium current density in subpopulations of these neurons (Figure 2 and Supplemental Figure 5). We found that Compound B inhibited sodium currents from 17 of 39 (44%) colonic-innervating DRG neurons tested and caused an overall ~23% decrease in peak sodium currents in affected neurons, likely originating from inhibiting the NaV1.1 current component, whereas other NaV channel subtypes are unaffected.
Figure 2. Compound B reduces sodium currents in colon-innervating DRG neurons.
(A) Group data showing that sodium current density (pA/pF) in a population of colon-innervating DRG neurons was reduced when applying Compound B (100 μM). ****P < 0.0001, n = 14 neurons, paired t test. (B) Individual data from that the group data presented in A. ****P < 0.0001, n = 17 neurons, paired t test.(C) Compound B caused ~23% decrease in peak sodium currents in affected neurons (**P < 0.001, Mann Whitney U test). (D) Current-voltage (I-V) plots of sodium current density before (vehicle; black) and after (blue) Compound B application (100 μM) in inhibited colon-innervating DRG neurons. Data represent ± SEM.
Colonic nociceptor recordings.
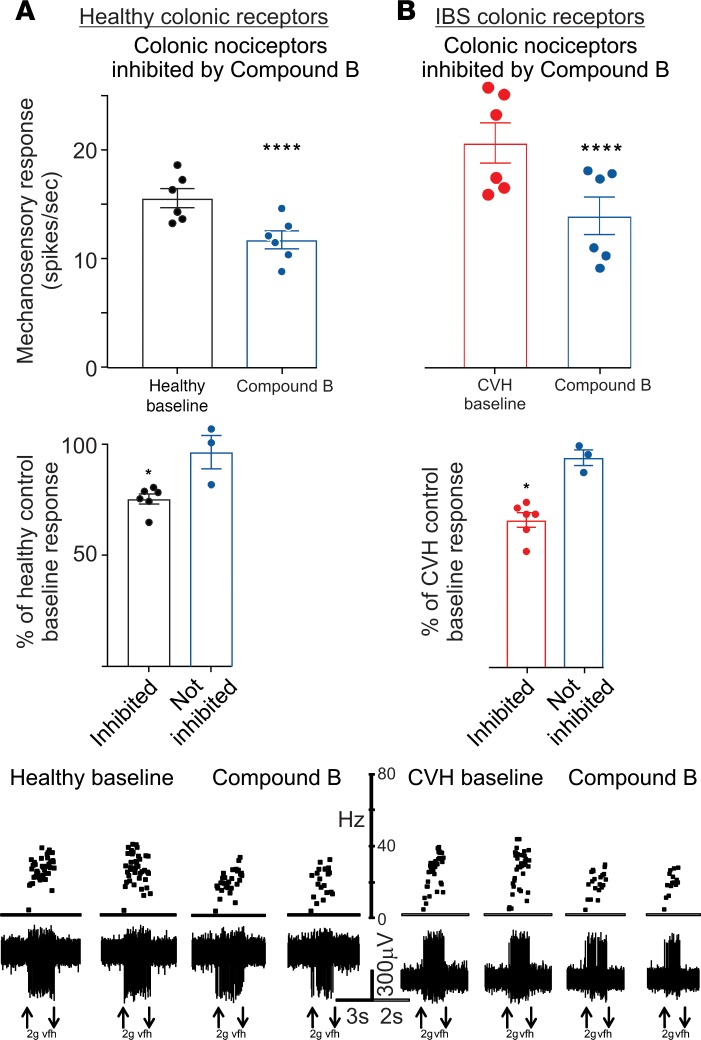
We showed that the NaV1.1 activator Hm1a also induces mechanical hypersensitivity of colonic nociceptive afferents, an effect that is enhanced in a mouse model of IBS induced by intracolonic (i.c.) administration of trinitrobenzenesulfonic acid (TNBS) (29). Therefore, we tested whether NaV1.1 inhibition by Compound B reduces colonic nociceptor mechanical responses in both healthy and CVH states (Figure 3). A substantial population of colonic nociceptors (6 of 9) from healthy mice was inhibited by application of Compound B, reducing responses by ~25% relative to baseline responses in affected afferents (Figure 3 and Supplemental Figure 6). In CVH mice, 75% of colonic nociceptors were inhibited by Compound B, and their response was reduced by ~35% relative to CVH baseline responses. In both control and CVH mice, Hm1a (100 nM) was unable to overcome Compound B inhibition of colonic nociceptor mechanical responses (Supplemental Figure 7). Based on these findings, we next asked whether NaV1.1 inhibition also reduces behavioral measures of mechanical hypersensitivity in representative FBD animal models.
Figure 3. Effect of Compound B on colonic nociceptive afferents.
(A) Top panel: Application of 100 μM Compound B inhibited a large subpopulation of colonic nociceptors from healthy control mice (n = 6, ****P < 0.0001, paired t test). Middle panel: In inhibited afferents, Compound B reduced responses to ~75% of healthy control baseline levels. *P < 0.05, unpaired t test. Lower panel: Representative examples of ex vivo healthy control colonic nociceptor recordings showing nociceptors in the absence and presence of Compound B. *P < 0.05, paired t test. (B) Top panel: In an IBS mouse model of TNBS-induced chronic visceral hypersensitivity (CVH), Compound B (100 μM) inhibited a large subpopulation of CVH colonic nociceptors (n = 6, ****P < 0.0001, paired t test). Middle panel: In inhibited afferents, Compound B reduced responses to ~67% of CVH baseline levels. Lower panel: Representative examples of ex vivo CVH colonic nociceptor recordings showing nociceptors in the absence and presence of Compound B. vfh, von Frey hair. The vfh with upward arrow indicates start of the application, and a downward arrow signifies removal.
Visceral motor reflex (VMR) in response to colorectal distension (CRD).
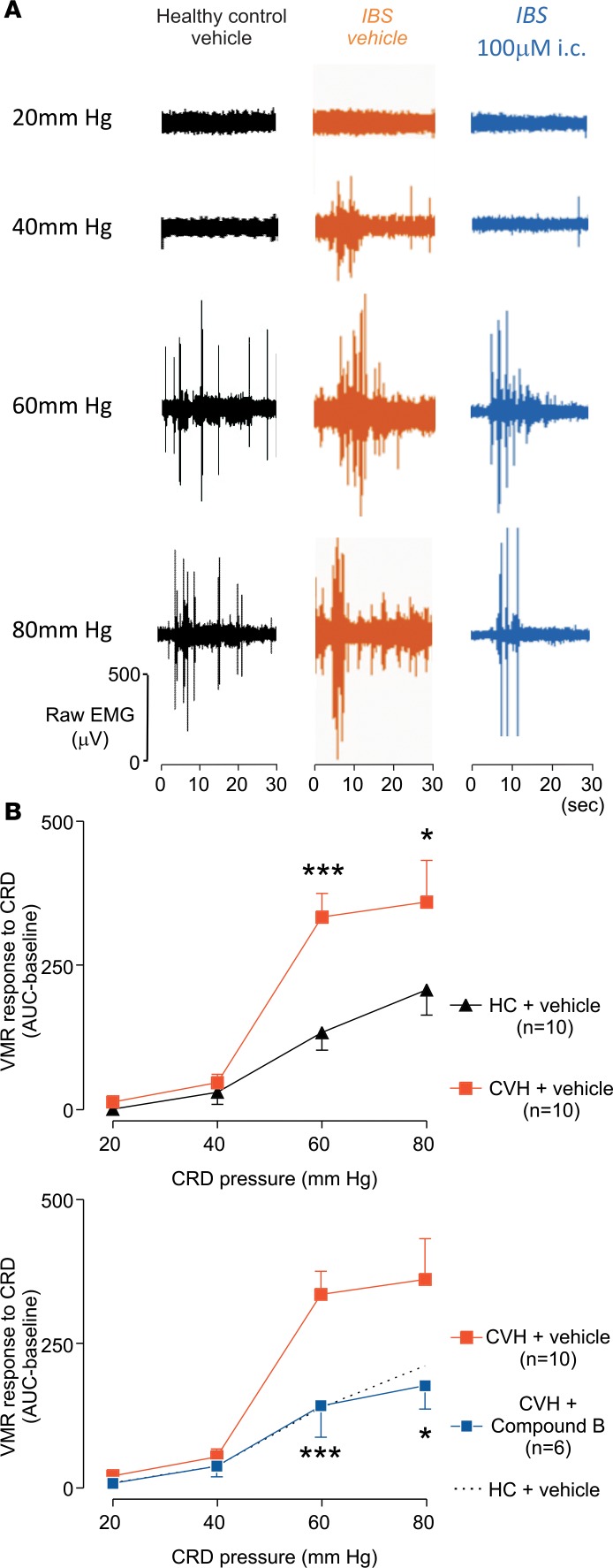
In these studies, we monitored the VMR to CRD in 2 commonly used mouse models of IBS representing FBDs. The VMR technique involves placement of a flexible inflatable balloon wrapped around a pliable catheter into the descending colon (33). To quantify the effect of balloon pressures (in mmHg), we measured abdominal electromyogram (EMG) activity of the abdominal musculature via surgically implanted electrodes. As expected, IBS mice with TNBS-evoked CVH displayed significantly enhanced VMRs compared with healthy control animals. I.c. administration of Compound B (100μM) (i.e., directly applied to the peripheral endings of the colonic nociceptors) to IBS mice reduced VMRs to CRD to healthy control levels (Figure 4). Compared with healthy control mice, colonic compliance was unaltered in CVH mice or in CVH mice that were administered 100 μM Compound B (Supplemental Figure 8), suggesting that changes in the VMR to CRD are not due to variations in smooth muscle function. These findings indicate that pharmacologically inhibiting NaV1.1 could be a viable approach to reverse visceral hypersensitivity in vivo.
Figure 4. Effect of intracolonic administration of Compound B on VMR in an IBS mouse model of TNBS-induced CVH.
(A) Representative EMG recordings at increasing colorectal distension pressures (mmHg) in healthy control mice with intracolonically (i.c.) administered vehicle (black), or IBS mice i.c. administered with vehicle (orange) or Compound B 100 μM (blue), 30 minutes before recordings. (B) Upper panel: Group data showing that IBS mice with CVH display increased VMRs (visceromotor reflexes) to CRD (colorectal distension) compared with healthy control mice, particularly at a distension pressure of 60 mmHg (***P < 0.001) and 80 mmHg (*P < 0.05). Lower panel: I.c. Compound B administration significantly reduced the VMR to CRD in IBS mice, normalizing responses to healthy control levels; 60 mmHg (***P < 0.001) and 80 mmHg (*P < 0.05). Significance of differences were analyzed by the Generalized Estimating Equation (GEE), followed by the Least Significant Difference (LSD) post hoc test. HC, healthy control; CVH, chronic visceral hypersensitivity.
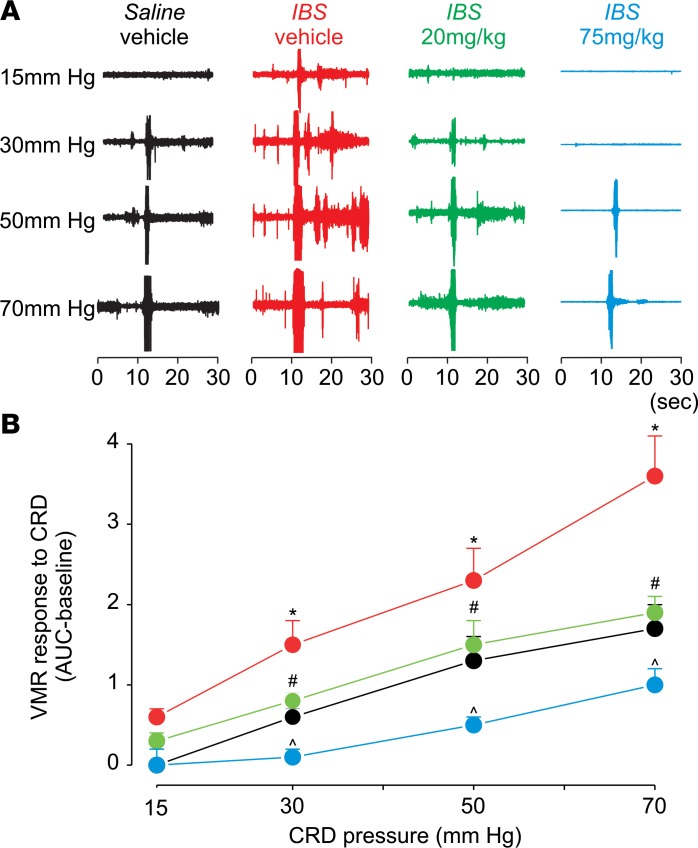
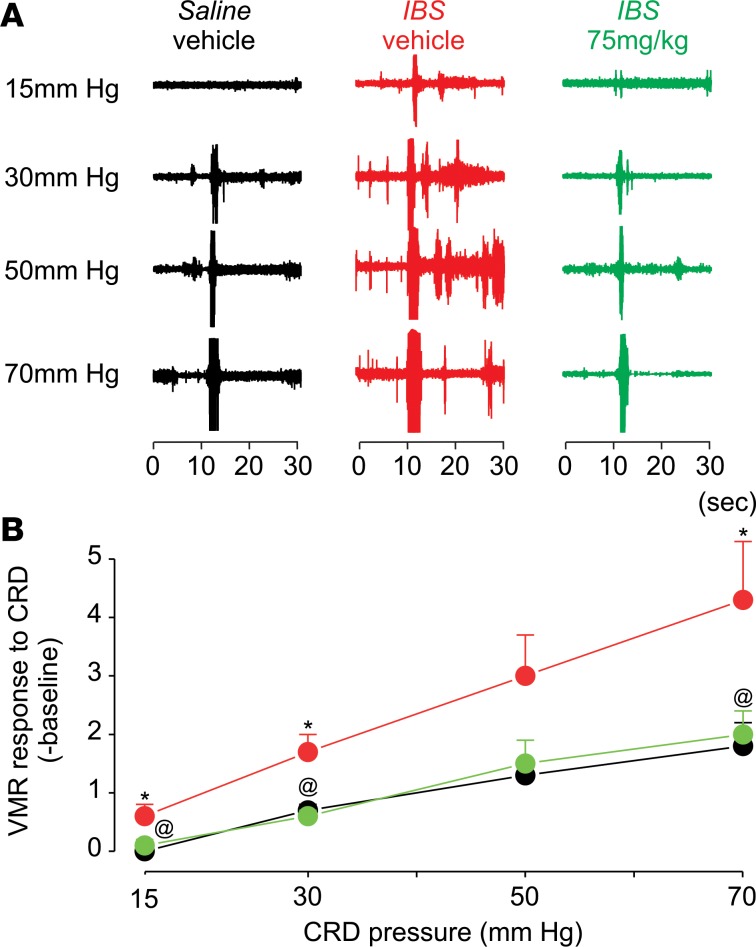
In a second experiment, we systemically applied Compound B at 2 doses (i.p.) in control and acetic acid–evoked IBS mice and then measured the VMR to CRD. At 20 mg/kg, acute treatment with Compound B in IBS mice returned pain thresholds to those of the saline-injected healthy controls (Figure 5). At a higher dose (75 mg/kg), Compound B reduced mechanical pain thresholds in IBS mice to below the level of control mice. Based on the PK experiments, Compound B absorption via s.c. injection leads to a lower peak concentration in plasma with a longer Tmax of 1 hour. Therefore, higher concentrations may be needed to normalize mechanical pain thresholds in CVH mice. Indeed, acute treatment with Compound B at a dose of 75 mg/kg (s.c.) significantly reduced the increased pain thresholds in CVH mice to a level noted in saline-injected controls (Figure 6). Together, these in vivo experiments suggest that NaV1.1 inhibition can be an effective approach to reduce mechanical hypersensitivity associated with CVH states.
Figure 5. Effect (i.p.) of Compound B in an IBS mouse model of acetic acid–induced CVH.
(A) Shown is the effect of Compound B (i.p. injection) at 20 and 75 mg/kg as measured by VMR response to CRD. Acute treatment at a dose of 20 mg/kg normalized the increased pain sensitivity in IBS mice, whereas 75 mg/kg compound reduced pain sensitivity in IBS mice to a level that is lower than control mice. (B) Two-way ANOVA showed main effect of treatment F(3,76) = 31.93, P < 0.001; main effect of pressure F(3,76) = 44.09, P < 0.001; interaction of treatment × pressure F(9,76) = 2.4, P = 0.017. Data (n = 7) are presented as mean ± SEM. *P < 0.05, significantly different from saline-vehicle at the same pressure; #P < 0.05, significantly different at the same pressure from IBS vehicle; and ^P < 0.05, significantly different from saline-vehicle and IBS-vehicle at the same pressure (Student Newman-Keuls post hoc test). F represents F statistic obtained after 2-way ANOVA to test whether the means between 2 populations are significantly different.
Figure 6. Effect (s.c.) of Compound B in an acetic acid–induced IBS mouse model.
(A and B) Effect on hyperalgesia of s.c. Compound B treatment in an IBS mouse model measured by VMR response to CRD. Data are presented as mean ± SEM. *P < 0.05, significantly different from saline-vehicle at same pressure; @P < 0.05, significantly different from IBS-vehicle (Student Newman-Keuls post hoc test). Two-way ANOVA analysis showed the main effect of treatment F(2,56) = 14.02, P < 0.001; main effect of pressure F(3,56) = 17.61, P < 0.001; interaction of treatment x pressure F(6,56) = 1.13, P = 0.35 (n = 7); these P values were obtained from the 2-way ANOVA test for the data shown in the figure.
Discussion
FBDs represent a major clinical problem in gastroenterology, with a large population of patients experiencing chronic abdominal pain secondary to visceral hypersensitivity to mechanical stimuli (1). Unfortunately, the mechanisms underlying CVH are unclear, and pharmacological management of chronic abdominal pain is therefore challenging (34–37). Accumulating evidence implicates a subset of NaV channel subtypes in normal gut sensory function and in the development of mechanical hypersensitivity and pain associated with injury (10, 23, 25, 29).
Together with previously reported observations (29), the results presented here suggest that NaV1.1 is functionally upregulated under CVH conditions (Figure 4) and that pharmacologically inhibiting this channel subtype can reduce CVH in 2 mechanically distinct mouse IBS models (Figure 4–6) without altering colonic compliance (Supplemental Figure 8). We show that Compound B, a NaV1.1-targeting inhibitor of channel gating (30), reduces colonic nociceptor mechanical responses and can reduce the significantly enhanced VMRs in 2 mouse models of IBS to levels that are observed in healthy control animals. Importantly, as Compound B has ~79% oral bioavailability, without noticeable signs of toxicity upon acute or chronic dosing, our findings suggest a possible pathway toward the design of novel NaV1.1-inhibiting therapeutics for FBD-related visceral pain (Supplemental Figure 3 and Supplemental Table 2). Although extrapolating preclinical findings in rodents to human chronic conditions such as IBS is challenging, the ability to target the etiology at the level of the peripheral afferent may have many advantages. Therefore, it is worth exploring whether synthesis of compound derivatives that do not penetrate the blood-brain barrier could avoid potential adverse side effects that might occur when even higher but even more effective doses are introduced.
It is also of interest that mutations in NaV1.1 have been linked to an array of epilepsy phenotypes (38). Via a subtle action on NaV1.1 function, Compound B increases the threshold to action potential initiation in hippocampal neurons, thereby reducing the frequency of seizures in various animal models (30). Given the role of NaV1.1 in both epilepsy and CVH, it is therefore reasonable to assume that a subset of epilepsy patients may be prone to FBDs. Indeed, an observational study on 65 people with epilepsy showed a significantly increased prevalence of IBS compared with controls (39). Conversely, a large-scale study found that IBS patients had greater cumulative incidence of epilepsy compared with the control cohort (40). As people with epilepsy are not routinely screened for FBDs, appropriate treatment may be delayed; it is also conceivable that gastrointestinal complaints may be erroneously attributed to administration of antiepileptic drugs. Alternatively, the central contribution of NaV1.1 in both disorders may provide a rationale to administer clinically used anticonvulsants in FBD patients to treat CVH.
Methods
Two-electrode voltage-clamp recording from Xenopus oocytes.
Human NaV1.5 (hNaV1.5), hNaV1.7, and hNaV1.8 (SCN5a, SCN9a, and SCN10a, respectively) and hβ1 (SCN1b) clones were obtained from OriGene Technologies Inc. and expressed in Xenopus laevis oocytes (Xenopus 1). The DNA sequence of all constructs was confirmed by automated DNA sequencing. RNA was synthesized using T7 polymerase (Invitrogen). Channels were expressed with hβ1 in a 1:5 molar ratio, and currents were studied following incubation for 1–4 days after cRNA injection (incubated at 17°C in [mM] 96 NaCl, 2 KCl, 5 HEPES, 1 MgCl2, 1.8 CaCl2, and 50 μg/ml gentamycin [pH 7.6 with NaOH]) using 2-electrode voltage-clamp recording techniques (OC-725C, Warner Instruments). Data were filtered at 4 kHz and digitized at 20 kHz using pClamp10 (Molecular Devices). Microelectrode resistances were 0.5–1 MΩ when filled with 3M KCl. The external recording solution (ND100) contained (mM) 100 NaCl, 5 HEPES, 1 MgCl2, and 1.8 CaCl2 (pH 7.6 with NaOH). All experiments were performed at ~22°C. Leak and background conductances were subtracted by blocking NaV channels with 10 μM tetrodotoxin. Chemicals were obtained from MilliporeSigma, unless otherwise stated. Voltage-activation relationships were obtained by measuring peak currents and calculating conductance (G). Compound B was dissolved in DMSO (~5 mg/ml stock) and diluted to 100 μM with ND100. Oocytes were incubated in solutions containing 100 μM drug (final DMSO concentration of ≤1%) for 1 hour. Data analysis was performed using Microsoft Excel and Origin 8 (OriginLab).
Compound safety screen on 44 targets.
A saturating Compound B concentration (100 μM, LifeTein) was tested on 44 targets using a binding/competition assay with scintillation counting. Method and target are shown in Supplemental Table 1. The study was carried out by Eurofins Pharma Discovery Services.
Metabolic stability of Compound B in plasma and liver microsomes.
Plasma stability was evaluated using plasma from mice and humans as described previously (41, 42). Briefly, Compound B (10 μM) was spiked in plasma and incubated in an orbital shaker at 37°C. At predetermined times, aliquots of the mixture, in triplicate, were removed and the reaction quenched by the addition of 3× the volume of ice-cold acetonitrile spiked with the internal standard losartan (500 nM). The samples were vortexed for 30 seconds and centrifuged at 12,000 g for 10 minutes. Compound disappearance was monitored over time using a Liquid chromatography–tandem mass spectrometry (LC/MS/MS) method. Phase I metabolic stability assay was conducted in mouse and human liver microsomes as described previously (43). The reaction was carried out with 100 mM potassium phosphate buffer, pH 7.4, in the presence of a NADPH regenerating system (1.3 mM NADPH, 3.3 mM glucose 6-phosphate, 3.3 mM MgCl2, 0.4 U/ml glucose-6-phosphate dehydrogenase, 50 μM sodium citrate). Reactions in triplicate were initiated by the addition of liver microsomes to the incubation mixture (compound final concentration was 10 μM; 0.5 mg/ml microsomes). Negative controls in the absence of NADPH were performed to determine the specific cofactor–free degradation. Testosterone was used as a positive control. Chromatographic analysis was performed using an Accela ultra high–performance system consisting of an analytical pump and an autosampler coupled with a TSQ Vantage mass spectrometer (Thermo Fisher Scientific). Separation of analyte was achieved at ambient temperature using an Agilent Eclipse Plus column (100 × 2.1 mm i.d.) packed with a 1.8 μm C18 stationary phase. The mobile phase used was composed of 0.1% formic acid in acetonitrile and 0.1% formic acid in H2O with gradient elution.
PK profiling of Compound B.
Healthy male C57BL/6J mice (8–12 weeks old) weighing between 20–35 g were obtained from In Vivo Biosciences. Temperature and humidity were maintained at 22°C ± 3°C and 40%–70%, respectively, and illumination was controlled to give a sequence of 12-hour light and 12-hour dark cycle. Animals were administered with compound solution formulation (10 mg/kg) prepared in 40% w/v hydroxypropyl-β-cyclodextrin in normal saline through p.o., i.p., i.v., and s.c. route. A group of 12 mice was used in each study. Blood samples were collected under light isoflurane anesthesia at 0.1, 0.5, 1, 2, 4, and 8 hours in labeled micro centrifuge tube containing K2EDTA as anticoagulant. Immediately after blood collection, plasma was harvested by centrifugation and stored at –70°C until bioanalysis. Following blood collection, animals were euthanized by CO2 asphyxiation, and brain and CSF were collected at each time point. Collected brain was dipped in 20 ml fresh phosphate buffer saline (pH 7.4) 3 times and dried on blotted paper. Brain was weighed and homogenized using ice-cold phosphate buffer saline (pH 7.4), and homogenates were stored below –70°C until bioanalysis. Total homogenate volume was 3× the brain weight. All samples were processed for analysis by protein precipitation using acetonitrile and analyzed with a fit-for-purpose LC/MS/MS method (lower limit of quantification [LLOQ] = 5 ng/ml in plasma, brain, and CSF).
Compound toxicity study in rats.
This study consisted of 2 phases: A and B. In Phase A, the maximum tolerable dose (MTD) of the NaV1.1 compound was determined. In Phase B, the dose range of the compound was investigated together with the toxicokinetic (TK) profile. Phase A consisted of 4 treatment groups of 3 male/female Sprague Dawley rats (Envigo) dosed with the compound orally, once daily for 5 days at 10 ml/kg. Phase A rats were treated with 10 mg/kg, 25 mg/kg, 50 mg/kg, and 100 mg/kg compound. Phase B consisted of 3 compound treatment groups of 5 male and 5 female — and 1 vehicle control group of 3 male and 3 female — Sprague Dawley rats dosed orally once daily for 7 days at 10 ml/kg. Phase B rats were treated with 25 mg/kg, 50mg/kg, and 100 mg/kg compound, and the group of 3 males/females received the vehicle, 15% DMSO, 35% PEG 400, and 50% sterile water and served as the vehicle control. Phase B included a TK cohort with 6 males/females in each treatment group and 3 males/females in the vehicle control group that were bled at 6 time points following dosing on study day 1 and day 7. Body and organ weights were collected, as well as urine for urinalysis. Food consumption was also monitored. Necropsies allowed 43 tissue types to be collected for clinical pathology examination. Tissues examined include adrenal mammary gland with skin, aorta ovaries with oviduct, brain, pancreas, cecum Peyer’s patches, colon, pituitary gland, duodenum, prostate, epididymis, rectum, esophagus, salivary gland (mandibular), eyes with optic nerve, skeletal muscle (thigh) with sciatic nerve, femur with BM (articular surface of the distal end to include femorotibial joint), seminal vesicles with coagulating glands, heart, spinal cord (cervical, thoracic, and lumbar), ileum, spleen, sternum, jejunum, stomach, kidney, testes, lacrimal gland, thymus, larynx, thyroid with parathyroids, tongue, liver (sections from 2 lobes), trachea, lung with bronchi, urinary bladder, lymph node (mandibular), uterus with cervix, lymph node (mesenteric), and vagina. Animals were obtained from Envigo and were housed in an environmentally controlled room that maintained temperatures of 20°C–24°C and a relative humidity of 30%–70% with a 12-hour light/12-hour dark cycle. Animals were group housed based on group/sex designation, except for overnight urine collection, when animals were individually housed in metabolic caging for no more than 18 hours. The animals had ad libitum access to drinking water and to Rodent Diet 2916 (Harlan TEKLAD). The animals were acclimated for 48 or 25 days (Phase A and Phase B, respectively) prior to dosing.
Behavioral analysis in the SNI model of neuropathic pain.
For these experiments, we used adult, male C57BL/6J mice from The Jackson Laboratory. Mice were anesthetized with isoflurane (2.0%). After skin and muscle incision at the level of the popliteal fossa, we tightly ligated the sural and superficial peroneal branches of the sciatic nerve with 8-0 silk sutures (Ethicon), leaving the tibial nerve intact. Next, the ligated branches were transected distal to the ligature, and ~2.0 mm of each distal nerve stump was removed. Particular care was taken not to stretch or contact the intact spared branch. The overlying muscle and skin were sutured, and the animals were allowed to recover and then returned to their cages. We assessed mechanical sensitivity in this mouse model of neuropathic pain (44) by placing animals on an elevated wire mesh grid and stimulating the hind paw with vfh. We used an up-down paradigm (45) to define threshold. Animals were tested 3 times, once every other day before surgery to determine baseline threshold and once 3 days after surgery to assess the magnitude of the mechanical hypersensitivity. On day 7, mice received an i.p. injection of Compound B (60 mg/kg), and behavioral testing was performed 15, 30, and 60 minutes after the injection. On day 28 after SNI, mice received a single dose (60 mg/kg) of Compound B, and mechanical thresholds were measured 1 hour after. For behavioral tests, the investigator was blind to treatment. Motor performance of the mice injected with Compound B (60 mg/kg) was evaluated with the rotarod test.
Retrograde labeling to identify colonic neurons in DRG and dissociated DRG cell culture.
Healthy, male C57BL/6J mice (The Jackson Laboratory) of 16 weeks were anesthetized with isoflurane, and — following midline laparotomy — five 2-μl injections of a fluorescent retrograde neuronal tracer (cholera toxin subunit B conjugated to AlexaFluor-488, Thermo Fisher Scientific) were made subserosally within the wall of the descending colon. Mice were administered analgesic (buprenorphine; 0.4 mg/10 kg s.c.) following completion of the surgery. Four days after tracer injection, mice were culled by CO2 inhalation, and DRG from thoracolumbar (T10-L1) and lumbosacral spinal levels (L5-S1) were surgically removed. DRGs were digested with 4 mg/ml collagenase II (GIBCO, Invitrogen) and 4 mg/ml dispase (GIBCO) for 30 minutes at 37˚C, followed by 4 mg/ml collagenase II for 10 minutes at 37˚C. Neurons were mechanically dissociated into a single-cell suspension via trituration through fire-polished Pasteur pipettes. Neurons were resuspended in DMEM (GIBCO) containing 10% FCS (Invitrogen), 2 mM L-glutamine (GIBCO), 100 μM MEM nonessential amino acids (GIBCO), and 100 mg/ml penicillin/streptomycin (Invitrogen). Neurons were spot-plated on 15-mm coverslips coated with poly-D-lysine (800 μg/ml) and laminin (20 μg/ml) and maintained in an incubator at 37˚C in 5% CO2.
Neuronal whole-cell electrophysiological recordings.
Male C57BL/6J mice (The Jackson Laboratory) were used in all experiments. Twenty to 48 hours after plating, whole-cell recordings were made from fluorescent colon-innervating DRG neurons using fire-polished glass electrodes with a resistance of 0.7–2 MΩ (≥75% of series resistance was compensated). All recordings were performed at room temperature (20°C–22°C). Signals were amplified with an Axopatch 200A amplifier, digitized with a Digidata 1322A, recorded using pCLAMP 9 software (Molecular Devices), sampled at 20kHz, filtered at 5kHz, and analyzed in Clampfit 10.7 (Molecular Devices) and GraphPad Prism 7. Voltage-clamp intracellular solution contained (in mM) 60 CsF; 45 CsCl; 2 MgCl2; 5 EGTA-Na; 10 HEPES-Cs; 30 TEA-Cl; and 2 MgATP adjusted to pH 7.2 with CsOH, 280 mOsm. Extracellular solution contained (in mM) 70 NaCl; 50 NMDG; 40 TEA-Cl; 4 CsCl; 2 MgCl2; 2 CaCl2; 10 HEPES; and 5 Glucose adjusted to pH 7.4, approximately 300 mOsm. Current-voltage (INa-V) relationships were determined by application of a prepulse to –100 mV (100 ms), followed by a series of step pulses from –65 mV to +60 mV (5 mV increments [100 ms]), before returning to hold at –70 mV (repetition interval of 3 sec, P/8 leak subtraction). Neurons inhibited by Compound B (LifeTein) were determined as a >15% reduction from baseline response. Extracellular (bath) solution containing Compound B at 100 μM (2-minute incubation) was applied with a gravity-driven multibarrel perfusion system positioned within ~1mm of the neuron.
Colonic nociceptor afferent recordings.
Male C57BL/6J mice (The Jackson Laboratory) were used in all experiments. In vitro single-unit extracellular recordings of action potential discharge were made of splanchnic colonic afferents from healthy control or IBS mice using standard protocols (12, 29, 46, 47). Baseline mechanosensitivity was determined in response to a 3-second application of a 2 g vfh probe to the afferent receptive field. This process was repeated 3–4 times, separated each time by 10 seconds. Mechanosensitivity was then retested after application of Compound B (100 μM). Afferents were considered inhibited by Compound B if we recorded a >10% reduction from baseline response. In some instances, data are presented as percentage change from baseline. This value was calculated as the percentage change in mechanosensitivity of individual afferents between the baseline responses compared with the respective mechanical responses following compound addition. This difference is then averaged across all afferents to obtain a final mean ± SEM of percentage change in response from baseline. The spider toxin Hm1a was purified as previously described (29).
Animal models of FBDs.
Male C57BL/6J mice (The Jackson Laboratory) were used in all experiments. In a first assay, colitis was induced by administration of TNBS as described previously (46, 47). Briefly, 13-week-old anesthetized mice were administered an i.c. enema of 0.1 ml TNBS (3.8 mg per mouse in 30% EtOH) via a polyethylene catheter. Histological examination of mucosal architecture, cellular infiltrate, crypt abscesses, and goblet cell depletion confirmed that TNBS induced significant damage by day 3 after treatment, largely recovered by day 7, and fully recovered at 28 days. High-threshold nociceptor recordings at the 28-day time point revealed significant mechanical hypersensitivity and lower mechanical activation thresholds. Based on these properties, we consider these mice an IBS model of TNBS-induced CVH.
An abdominal EMG allows assessment of visceral sensitivity in vivo in fully awake animals. Under isoflurane anesthesia, the bare endings of 2 Teflon-coated stainless-steel wires (Advent Research Materials Ltd.) were sutured into the right abdominal muscle, tunnelled s.c., and then exteriorized at the base of the neck for future access. At the end of the surgery, mice received prophylactic antibiotic (Baytril; 5 mg/kg s.c.) and analgesic (buprenorphine; 0.09 mg/kg s.c.), were housed individually, and were allowed to recover for at least 3 days before assessment of the VMR. On the day of VMR assessment, mice were briefly anesthetized using isoflurane and received a 100 μl enema of vehicle (sterile saline) or Compound B (100 μM). A lubricated balloon (2.5 cm length) was gently introduced through the anus and inserted into the colorectum, up to 0.25 cm past the anal verge. The balloon catheter was secured to the base of the tail and connected to a barostat (Isobar 3, G&J Electronics) for graded and pressure-controlled balloon distension. Mice were allowed to recover from anesthesia in a restrainer for 15 minutes prior to initiation of the distension sequence. Distensions were applied at 20, 40, 60, and 80 mmHg (20-second duration) at a 4-minute interval. The last distension was performed 30 minutes after i.c. treatment. The EMG electrodes were relayed to a data acquisition system, and the signal was recorded (NL100AK headstage), amplified (NL104), filtered (NL 125/126, Neurolog, Digitimer Ltd., bandpass 50–5000 Hz), and digitized (CED 1401, Cambridge Electronic Design) to a PC for off-line analysis using Spike2 (Cambridge Electronic Design). The analogue EMG signal was rectified and integrated. To quantify the magnitude of the VMR at each distension pressure, the AUC during the distension (20 seconds) was corrected for the baseline activity (AUC predistension, 20 seconds). After the final distension, the mice were killed by cervical dislocation. Colonic compliance was assessed by applying graded volumes (40–200 μl, 20-second duration) to the balloon in the colorectum of fully awake mice, while recording the corresponding colorectal pressure as described previously.
In a second assay, pain sensitivity was measured by VMR in response to CRD. As opposed to TNBS in the first assay, acetic acid was used to evoke mechanical hypersensitivity (48). A sterilized multistranded, Teflon-insulated, 40-gauge stainless steel wire (Cooner Wire) was implanted in the external oblique muscle and then s.c. tunneled through the back and fixed on the neck skin. One week after surgery, a balloon (made from 3 cm2 polyethylene membrane and attached to soft Tygon [PE60] tubing) was implanted into the colorectum. To test the VMR, the mouse was placed in a restrainer and allowed to adapt for 30–45 minutes. CRD was induced by applying pressure of 15, 30, 45, and 70 mmHg for 10 seconds each. There were at least 4-minute intervals between stimulations. The EMG was recorded from 2 externalized electrodes implanted in the external oblique muscle 20 seconds before, 10 seconds during, and 20 seconds after CRD. The EMG in the 10 seconds before (baseline) and during CRD were analyzed using CED 1401 plus and Spike 2 software. The AUC of the EMG 10 seconds before and during the CRD was measured. CRD response data were normalized to baseline. We used VMR response to CRD to determine the effect of Compound B on visceral hypersensitivity in this mouse model of IBS. This model was generated by colorectal infusion of 20 μl 0.5% acetic acid or saline (control) on postnatal days 9–12. The mice were weaned at 3 weeks. At 8 weeks of age, we conducted 2 studies. First, IBS mice (n = 6) were treated with Compound B (75 mg/kg, 10 ml/kg) or vehicle (20% m/V 2-hydroxypropyl-β-cyclodextrin in saline) s.c. A group of mice (neonatal saline treated) was used together with the vehicle as control. In the second study, IBS mice were treated with vehicle, 20 or 75 mg/kg of Compound B i.p. alongside a control group that was saline treated. Thirty minutes after Compound B treatment, EMG responses to CRD were recorded. Analysis methods are specified in the appropriate sections. For these behavioral tests, the investigator was blind to treatment.
Statistics.
PK parameters were calculated using the noncompartmental analysis tool of Phoenix WinNonlin (Version 6.3). For rat toxicity tests, animals were randomized but assigned a unique identification number. For Phase A, data were analyzed by parametric 1-way ANOVA if normally distributed and variances were homogeneous. Post hoc analysis was conducted by making all possible comparisons among the treatment groups with the Holm-Sidak test. If the assumptions of parametric analysis were not met, data were analyzed by Kruskal-Wallis 1-way ANOVA on ranks. Dunn’s test was used to assess any post hoc differences by comparisons among all groups if the Kruskal-Wallis 1-way ANOVA was significant. For Phase B, data were analyzed by 1-way ANOVA followed by Dunnett’s test if data were normally distributed and variances were homogeneous. Post hoc comparisons with Dunnett’s test were only employed if treatment effect in the 1-way ANOVA was significant. If the assumptions of parametric analysis were not met, data were analyzed by Kruskal-Wallis 1-way ANOVA on ranks. Dunn’s test was used to assess any post hoc differences among groups if the Kruskal-Wallis 1-way ANOVA was significant. For all statistical procedures, differences were considered significant if P < 0.05. All statistical analyses were performed with SigmaPlot 13.0 (Build 13.0.0.83), Systat Software Inc. For electrophysiological recordings, data are presented as mean ± SEM and analyzed using GraphPad Prism 7. More details about statistical methods used can be found in the text. If used, Student’s t test was 2-tailed. For IBS mouse model experiments, data were statistically analyzed by generalized estimating equations followed by LSD post hoc tests when appropriate using SPSS 23.0. Analysis was typically carried out in GraphPad Prism 7 Software.
Study approval.
PK profiling of Compound B was conducted at Sai Life Sciences Limited in accordance with Study Plan SAIDMPK/PK-16-05-213 and the guidelines of the Institutional Animal Ethics Committee (IAEC). Rat toxicity study was carried out by Sobran Inc. with an approved IACUC protocol, number SOB-051-2017. Behavioral analysis in the SNI model of neuropathic pain were approved by the UCSF IACUC. The Animal Ethics Committees of The Flinders University, The University of Adelaide, and the South Australian Health and Medical Research Institute (SAHMRI) approved experiments involving animals in FBD studies. The Animal Care and Use Committee of Johns Hopkins University approved the relevant IBS experiments.
Author contributions
JS, JC, AE, QL, JB, JG, AD, GYR, RR, BSS, AB, PJP, SMB, and FB designed the study. GFK provided Hm1a. JS, JC, AE, QL, JB, JG, AD, GYR, RR, BSS, AB, PJP, SMB, and FB generated or analyzed the data. JC, AE, LG, GYR, AD, and SMB designed, performed, and analyzed studies relating to colonic afferent, colon-innervating DRG neuron patch clamp and VMR to CRD studies from healthy control mice and the IBS mouse model of TNBS-induced CVH. BSS, AB, PJP, SMB, and FB wrote the manuscript.
Supplementary Material
Acknowledgments
This work was supported by a Ruth Kirschstein NIH predoctoral Fellowship (F31NS084646 to JG), a Department of Defense (DoD) National Defense Science and Engineering Graduate (NDSEG) Fellowship (JS), the Maryland Innovation Initiative (MII) Tedco (FB), the Abell Foundation (FB), a Blaustein Pain Research grant to FB (Johns Hopkins University), NIH R35 NS097306 to AIB, the Amos Food Body and Mind Center at Johns Hopkins University, a National Health and Medical Research Council of Australia (NHMRC) RD, Wright Biomedical Research Fellowship APP1126378 to SMB, and NHMRC Australia Project grants 1083480, 1139366, and 1140297 to SMB. We would like to thank Jiachen Chu (Johns Hopkins University) for helpful comments, Andrew Escayg (Emory University) for help with the PK profiling, and Katie Hamel (UCSF) for performing some of the behavioral studies.
Version 1. 06/07/2018
Electronic publication
Footnotes
Conflict of interest: FB is an inventor on a patent for Compound B (US Patent and Trademark Office, 9505727).
Reference information: JCI Insight. 2018;3(11):e121000. https://doi.org/10.1172/jci.insight.121000.
Contributor Information
Juan Salvatierra, Email: jjstierra@gmail.com.
Joel Castro, Email: joel.castro@health.sa.gov.au.
Andelain Erickson, Email: andelain.erickson@adelaide.edu.au.
Qian Li, Email: qli43@jhmi.edu.
Joao Braz, Email: bjoao@phy.ucsf.edu.
John Gilchrist, Email: johnmichael.gilchrist@ucsf.edu.
Luke Grundy, Email: luke.grundy@adelaide.edu.au.
Annemie Deiteren, Email: adeitere@its.jnj.com.
Rana Rais, Email: rrais2@jhmi.edu.
Allan Basbaum, Email: Allan.Basbaum@ucsf.edu.
Frank Bosmans, Email: fbosmans@gmail.com.
References
- 1.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313(9):949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 2.Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24(1):21–37. doi: 10.2165/00019053-200624010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med. 2008;358(16):1692–1699. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer AD, Aziz Q. Gut pain & visceral hypersensitivity. Br J Pain. 2013;7(1):39–47. doi: 10.1177/2049463713479229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farzaei MH, Bahramsoltani R, Abdollahi M, Rahimi R. The Role of Visceral Hypersensitivity in Irritable Bowel Syndrome: Pharmacological Targets and Novel Treatments. J Neurogastroenterol Motil. 2016;22(4):558–574. doi: 10.5056/jnm16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbar A, Walters JR, Ghosh S. Review article: visceral hypersensitivity in irritable bowel syndrome: molecular mechanisms and therapeutic agents. Aliment Pharmacol Ther. 2009;30(5):423–435. doi: 10.1111/j.1365-2036.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- 7.Azpiroz F, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19(1 Suppl):62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 8.Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol. 2014;11(10):611–627. doi: 10.1038/nrgastro.2014.103. [DOI] [PubMed] [Google Scholar]
- 9.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57(7):923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyder A, et al. Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology. 2014;146(7):1659–1668. doi: 10.1053/j.gastro.2014.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brierley SM, et al. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137(6):2084–2095.e3. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro J, et al. α-Conotoxin Vc1.1 inhibits human dorsal root ganglion neuroexcitability and mouse colonic nociception via GABAB receptors. Gut. 2017;66(6):1083–1094. doi: 10.1136/gutjnl-2015-310971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135(3):937–946. doi: 10.1053/j.gastro.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Cenac N, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117(3):636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henström M, D’Amato M. Genetics of irritable bowel syndrome. Mol Cell Pediatr. 2016;3(1):7. doi: 10.1186/s40348-016-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hockley JR, Winchester WJ, Bulmer DC. The voltage-gated sodium channel NaV 1.9 in visceral pain. Neurogastroenterol Motil. 2016;28(3):316–326. doi: 10.1111/nmo.12698. [DOI] [PubMed] [Google Scholar]
- 17.Hughes PA, et al. Increased κ-opioid receptor expression and function during chronic visceral hypersensitivity. Gut. 2014;63(7):1199–1200. doi: 10.1136/gutjnl-2013-306240. [DOI] [PubMed] [Google Scholar]
- 18.Laird JM, Souslova V, Wood JN, Cervero F. Deficits in visceral pain and referred hyperalgesia in Nav1.8 (SNS/PN3)-null mice. J Neurosci. 2002;22(19):8352–8356. doi: 10.1523/JNEUROSCI.22-19-08352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marger F, et al. T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci USA. 2011;108(27):11268–11273. doi: 10.1073/pnas.1100869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page AJ, et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54(10):1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Defrees DN, Bailey J. Irritable Bowel Syndrome: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Prim Care. 2017;44(4):655–671. doi: 10.1016/j.pop.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Lacy BE, Weiser K, De Lee R. The treatment of irritable bowel syndrome. Therap Adv Gastroenterol. 2009;2(4):221–238. doi: 10.1177/1756283X09104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahern CA, Payandeh J, Bosmans F, Chanda B. The hitchhiker’s guide to the voltage-gated sodium channel galaxy. J Gen Physiol. 2016;147(1):1–24. doi: 10.1085/jgp.201511492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao Y, Wang B, Kunze W. Characterization of myenteric sensory neurons in the mouse small intestine. J Neurophysiol. 2006;96(3):998–1010. doi: 10.1152/jn.00204.2006. [DOI] [PubMed] [Google Scholar]
- 25.Osorio N, Korogod S, Delmas P. Specialized functions of Nav1.5 and Nav1.9 channels in electrogenesis of myenteric neurons in intact mouse ganglia. J Neurosci. 2014;34(15):5233–5244. doi: 10.1523/JNEUROSCI.0057-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copel C, Clerc N, Osorio N, Delmas P, Mazet B. The Nav1.9 channel regulates colonic motility in mice. Front Neurosci. 2013;7:58. doi: 10.3389/fnins.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson A, et al. Voltage-gated sodium channels: (NaV )igating the field to determine their contribution to visceral nociception. J Physiol (Lond) 2018;596(5):785–807. doi: 10.1113/JP273461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods CG, Babiker MO, Horrocks I, Tolmie J, Kurth I. The phenotype of congenital insensitivity to pain due to the NaV1.9 variant p.L811P. Eur J Hum Genet. 2015;23(10):1434. doi: 10.1038/ejhg.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osteen JD, et al. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature. 2016;534(7608):494–499. doi: 10.1038/nature17976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilchrist J, et al. Nav1.1 modulation by a novel triazole compound attenuates epileptic seizures in rodents. ACS Chem Biol. 2014;9(5):1204–1212. doi: 10.1021/cb500108p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheeler DW, Lee MC, Harrison EK, Menon DK, Woods CG. Case Report: Neuropathic pain in a patient with congenital insensitivity to pain. F1000Res. 2014;3:135. doi: 10.12688/f1000research.2642.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15(14):1688–1698. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- 33.Whitehead WE, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98(5 Pt 1):1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 34.Bradesi S, Herman J, Mayer EA. Visceral analgesics: drugs with a great potential in functional disorders? Curr Opin Pharmacol. 2008;8(6):697–703. doi: 10.1016/j.coph.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drossman DA. Beyond tricyclics: new ideas for treating patients with painful and refractory functional gastrointestinal symptoms. Am J Gastroenterol. 2009;104(12):2897–2902. doi: 10.1038/ajg.2009.341. [DOI] [PubMed] [Google Scholar]
- 36.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367–378. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 37.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007;56(9):1218–1225. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol (Lond) 2010;588(Pt 11):1849–1859. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camara-Lemarroy CR, Escobedo-Zúñiga N, Ortiz-Zacarias D, Peña-Avendaño J, Villarreal-Garza E, Díaz-Torres MA. Prevalence and impact of irritable bowel syndrome in people with epilepsy. Epilepsy Behav. 2016;63:29–33. doi: 10.1016/j.yebeh.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 40.Chen CH, Lin CL, Kao CH. Irritable Bowel Syndrome Increases the Risk of Epilepsy: A Population-Based Study. Medicine (Baltimore) 2015;94(36):e1497. doi: 10.1097/MD.0000000000001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedelcovych MT, et al. N-(Pivaloyloxy)alkoxy-carbonyl Prodrugs of the Glutamine Antagonist 6-Diazo-5-oxo-l-norleucine (DON) as a Potential Treatment for HIV Associated Neurocognitive Disorders. J Med Chem. 2017;60(16):7186–7198. doi: 10.1021/acs.jmedchem.7b00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rais R, et al. Discovery of 6-Diazo-5-oxo-l-norleucine (DON) Prodrugs with Enhanced CSF Delivery in Monkeys: A Potential Treatment for Glioblastoma. J Med Chem. 2016;59(18):8621–8633. doi: 10.1021/acs.jmedchem.6b01069. [DOI] [PubMed] [Google Scholar]
- 43.Zou MF, et al. Structure-Activity Relationship Studies on a Series of 3α-[Bis(4-fluorophenyl)methoxy]tropanes and 3α-[Bis(4-fluorophenyl)methylamino]tropanes As Novel Atypical Dopamine Transporter (DAT) Inhibitors for the Treatment of Cocaine Use Disorders. J Med Chem. 2017;60(24):10172–10187. doi: 10.1021/acs.jmedchem.7b01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shields SD, Eckert WA, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4(8):465–470. doi: 10.1067/S1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 45.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 46.Castro J, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3’,5’-monophosphate. Gastroenterology. 2013;145(6):1334–46.e1. doi: 10.1053/j.gastro.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 47.de Araujo AD, et al. Selenoether oxytocin analogues have analgesic properties in a mouse model of chronic abdominal pain. Nat Commun. 2014;5:3165. doi: 10.1038/ncomms4165. [DOI] [PubMed] [Google Scholar]
- 48.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132(2):615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.