Abstract
Introduction
The physiologic safety of devices and materials intended for clinical implantation should be evaluated. This study, a logical extension of our previous work, aimed to investigate the safety of a novel contraceptive device, the copper/low-density polyethylene nanocomposite intrauterine device (nano-Cu/LDPE IUD), through studies of its potential toxicity after acute and subchronic administration in mice and rats.
Methods
For the acute toxicity study, single 50 mL/kg doses of nano-Cu/LDPE IUD extracts were administered to mice via intravenous or intraperitoneal injection. General behavioral adverse effects, mortality, and body weights were evaluated for up to 72 hours. In the 13-week subchronic toxicity study, the nano-Cu/LDPE composite with 10-fold higher than the standard clinical dose was implanted subcutaneously into the dorsal skin of Wistar rats. The control group underwent a sham procedure without material insertion.
Results
During all acute study observation times, the biologic reactions of the mice in the nano-Cu/LDPE group did not differ from those observed in the control group. The groups did not differ statistically in terms of body weight gain, and no macroscopic changes were observed in any organs. In the subchronic study, no clinical signs of toxicity or mortality were observed in either the nano-Cu/LDPE or control group during the 13-week period. The nano-Cu/LDPE composite did not cause any alterations in body weight, food consumption, hematologic and biochemical parameters, or organ weight relative to the control for any observed sample group. Histopathologic examinations of the organs revealed normal architecture, indicating that the inserted material did not cause morphologic disturbances in the rats.
Conclusion
Overall, the results indicate that the nano-Cu/LDPE IUD did not induce systemic toxicity under experimental conditions of the recommended standard practices, suggesting that the novel material IUD is safe and feasible for future contraceptive applications.
Keywords: intrauterine device, copper/low-density polyethylene nanocomposite intrauterine device, acute toxicity, subchronic toxicity
Introduction
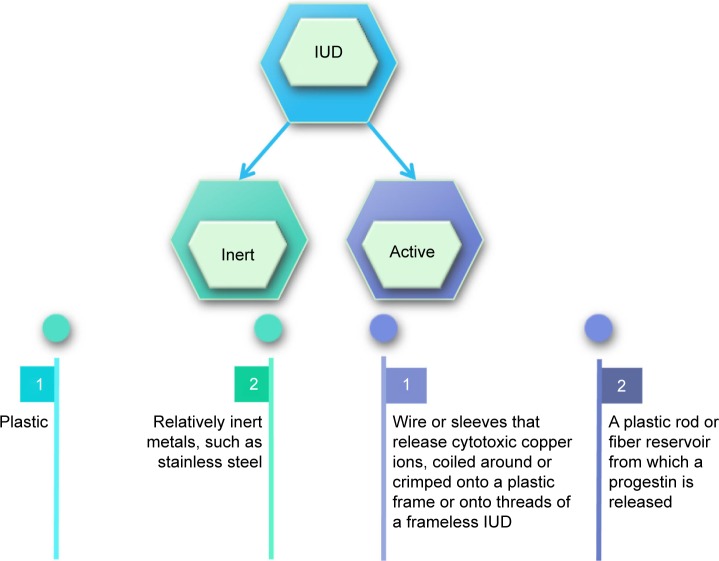
Since the beginning of the modern era of intrauterine contraception in 1959, intrauterine devices (IUDs) have become the world’s most prevalent reversible contraceptive method, with approximately 175 million current users.1 Of these, nonhormonal IUDs have become the most widely used. IUDs can be divided into two groups based on the contraceptive mechanism: inert IUDs and active IUDs (Figure 1).2 Inert IUDs have been discontinued because of their poor contraceptive effects. Active IUDs mainly comprise copper IUDs and hormonal IUDs.
Figure 1.
Types of IUDs.
Abbreviation: IUD, intrauterine device.
Copper-containing intrauterine devices (Cu-IUDs), which are safe, economical, long-lasting and effective, are increasingly used for population control worldwide.3–5 The enhanced contraceptive abilities of these devices are commonly attributed to their spermicidal activity; specifically, the activities of spermatozoa are inhibited by cupric ion biotoxicity. Additionally, Cu-IUDs alter endometrial cell metabolism and biochemistry to inhibit embryo implantation after fertilization.6 Despite satisfactory contraceptive efficacy and very wide utilization, the clinical use of existing Cu-IUDs is complicated by persistent side effects, including irregular and heavy bleeding, spotting, and pelvic pain.7 These side effects are most likely related to the burst release of cupric ions during the first few months of use. Therefore, controlling the process of cupric ion release and thus preventing such a burst initially after insertion should be considered to maximize contraceptive efficacy and minimize adverse effects.8
Recent decades have seen rapid advances in nanotechnology in many scientific fields (Figure 2). The characteristics of nanomaterials have contributed to notable biomedical achievements. In our previous studies, our research used nanotechnology in an attempt to decrease the side effects caused by Cu-IUDs and invented a new type of copper/low-density polyethylene nanocomposite IUD (nano-Cu/LDPE IUD, Patent No ZL200610124658.9). Details of the preparation of this IUD have been described elsewhere.9–11 Briefly, the mechanical properties, corrosion behavior, and prospective life-span of the nano-Cu/LDPE IUD were superior to those of an existing Cu-IUD.11,12 Additionally, experiments to determine the contraceptive effectiveness, side effects, and safety of the nano-Cu IUD in rats, mice, rabbits, and rhesus monkeys demonstrated a high level of efficacy with low side effects and relative safety.8,13–17 In summary, previous studies of this novel composite IUD material indicate that it can greatly improve the performance of IUDs.
Figure 2.
Application of nanotechnology.
Abbreviation: Cu-LDPE IUD, copper/low-density polyethylene nanocomposite intrauterine device.
In recent years, biologic evaluations of biomaterials and medical devices have become increasingly globally standardized concurrent with the publication of the International Organization for Standardization (ISO) 10993 standard for biomaterial and medical device testing. As the nano-Cu/LDPE IUD is a novel substitute and implanted medical device, it is necessary to evaluate its safety through experimental protocols conducted according to regulatory standards. The results from previous biologic evaluations (cytotoxicity, skin irritation and sensitization, and implantation) indicate that the novel nano-Cu/LDPE IUD was highly likely to be histocompatible.18 However, systematic research regarding the possible toxicity of the nano-Cu/LDPE IUD has not yet been conducted.
The present study aimed to evaluate the systemic toxicity of this novel nano-Cu/LDPE IUD. Systemic toxicity is a potential adverse effect of medical devices.19 Generalized effects, as well as organ and organ system effects, can result from the absorption, distribution, and metabolism of device leachates or materials to parts of the body with which they are not in direct contact. Therefore, toxicologic evaluations are performed in animal models to determine the safety of drugs and implantable medical products for human use. The present study, in which the potential toxicity of the novel nano-Cu/LDPE IUD was evaluated after acute and subchronic systemic assays, was conducted as part of an investigation to evaluate the safety of this device. The results may help us to determine whether the nanocomposite IUD meets the safety standards for implantable medical devices.
Materials and methods
Experimental animals
Sexually mature Kunming mice (body weight: 17–23 g; SPF grade, Certificate No 0013938) and Wistar rats (body weight: 180–240 g; SPF grade, Certificate No 0013936) were obtained from the Experimental Animal Center of Shandong Lukang Pharmaceutical Co., Ltd. and acclimatized in the laboratory for 1 week before starting the experiment. The animals were individually housed and maintained under standard conditions (12-hour light/dark cycle, 22°C ± 1°C, 50%–60% relative humidity) and provided a conventional laboratory diet and unrestricted supply of drinking water. All animal experiments were conducted in compliance with the local Ethics Committee and were approved by the Institutional Review Board at Shandong Quality Inspection Center for Medical Devices. Systemic toxicity studies were performed in accordance with the ISO 10993-11 standard. This section of ISO 10993 specifies the requirements for and gives guidance on the procedures to be followed when evaluating the potential for medical device materials to cause adverse systemic reactions.19
Materials
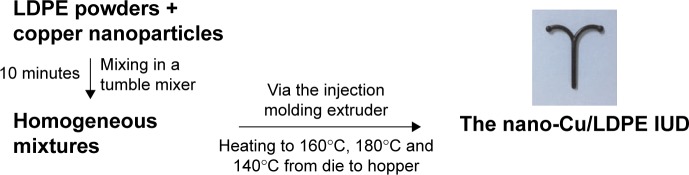
The nano-Cu/LDPE IUDs were provided by the Department of Materials Science and Engineering of Huazhong University of Science and Technology. The nanosized copper particles were generated using our own patented techniques (ie, hybrid induction and laser heating evaporation condensation method) and had a mean diameter of approximately 50 nm and purity >99.9%. The process used to prepare the nano-Cu/LDPE IUD was introduced in our previous work (Figure 3).8 The nano-Cu/LDPE IUD comprises a pair of symmetrical transverse arms with a total length of 28 mm and a longitudinal stem with a total height of 30 mm. Both the transverse arms and the longitudinal stem have a diameter of 2.0 mm. The preparation of reference materials for this test was based on the standard ISO 10993-12.20
Figure 3.
The flow chart of the nano-Cu/LDPE IUD.
Abbreviation: Cu/LDPE IUD, copper/low-density polyethylene nanocomposite intrauterine device.
Acute systemic toxicity
Twenty mice were used in the evaluation of extracts from the nano-Cu/LDPE IUDs administered via two different injection procedures (intravenous and intraperitoneal). Extracts prepared in a 0.9% sodium chloride solution and cottonseed oil were used for intravenous and intraperitoneal injection, respectively. The material extracts were prepared at a ratio of 1.25 cm2 of sample material per 1 mL of extract vehicle and were soaked for 24 hours at 37°C. Ten mice were used per type of injection: five for the nano-Cu/LDPE group and five for the control group. Mice in the nano-Cu/LDPE group were injected with the material extracts at a dose of 50 mL/kg body weight. Mice in the control group were injected with the same volume of blank extract vehicle. All tested animals were observed at 4, 24, 48, and 72 hours after injection, and symptoms of slight, moderate, or marked toxicity and death were recorded. The animals’ body weights were also measured and recorded. During the observation period, if none of the animals in the nano-Cu/LDPE group exhibited significantly greater biologic reactivity than the animals in the control group, the sample would be considered to meet the requirements of this assay.
Subchronic toxicity
Upon initiating the experiment, 20 Wistar rats were randomly allocated to two groups: the nano-Cu/LDPE group (n=10, five males and five females) and sham-operated control group (n=10, five males and five females). Group allocation was based on the body weights measured immediately before the experiment. Rats in the nano-Cu/LDPE group were anesthetized with 10% chloral hydrate (3 mg/kg via intraperitoneal injection). The 12.6 mm length of nano-Cu/LDPE composite material, equivalent to 10-fold the human dose, was inserted subcutaneously into the dorsal skin. Rats in the control group underwent the same procedure without subcutaneous dorsal material insertion. Each rat was treated for 13 weeks.
Clinical observations, body weight, and food consumption
All animals were observed twice daily for clinical signs of toxicity and the clinical conditions were recorded. The observed signs included but were not limited to changes in skin, fur, eyes, and mucous membranes; occurrence of secretions and excretions and autonomic activity (eg, lacrimation, pupil size, piloerection, and unusual respiratory pattern). Changes in gait, posture, and responses to handling, as well as the presence of clonic or tonic movements, stereotypy, or bizarre behavior (eg, self-mutilation, walking backwards) were also recorded. The mean weekly body weight and food consumption were calculated for each rat throughout the test period.
Urinalysis, hematology, and clinical biochemistry
On the 89th day, each rat was placed in a metabolism cage for 24 hours for urine collection. The urine color was observed and the volume was recorded. The following parameters were analyzed using the Urine Test Strip Analyzer (Combi-Scan 500, Analyticon Biotechnologies AG, Lichtenfels, Germany): pH, specific gravity, nitrite, glucose (GLU), ketones, urobilinogen, and bilirubin. Blood samples were collected on the 91st day after an overnight fast. The following hematology assessments were conducted using an automated hematology analyzer (MEK-6318, Nihon Kohden, Tokyo, Japan): white blood cell count, red blood cell count, hemoglobin concentration, hematocrit, percent of neutrophilic granulocytes, percent of lymphocytes, percent of monocytes, and platelet count. The prothrombin time and activated partial thromboplastin time for blood coagulation were analyzed using a blood coagulation analyzer (CA-530, Sysmex, Kobe, Japan). Serum biochemical parameters, including alanine aminotransferase, total protein, albumin, total bilirubin, aspartate aminotransferase, total cholesterol, blood urea nitrogen, creatinine, GLU, sodium (Na), potassium (K), and chloride (Cl), were evaluated using an automated biochemical analyzer (7020, Hitachi, Tokyo, Japan).
Pathology
All animals were necropsied, and descriptions of all macroscopic abnormalities were recorded at the scheduled termination. After removing peripheral fat, the following organs from each rat were weighed: brain, heart, thymus, thyroid, liver, lung, spleen, intestine, pancreas, kidney (left, right), adrenal gland (left, right), testis (left, right), epididymis (left, right), ovaries (left, right), and uterus. Relative organ weights were calculated according to the formula: Relative organ weight (%) = organ wt (g)/body weight (g)×100. The peripheral oral cavity, cranial cavity, and all tissues and organs in the thoracic and abdominal cavity were examined visually for any abnormalities, which were recorded. All of the above-mentioned collected organs and tissues were fixed in 10% neutral buffered formalin, routinely processed, embedded in paraffin, sectioned at a thickness of 3–5 µm, and stained with H&E for microscopic examination. Tissue sections were observed using an optical microscope (Olympus, Tokyo, Japan).
Statistical analysis
All data are presented as means ± SD. Body weights, hematology and serum biochemistry parameters, and relative organ weights were compared between the nano-Cu/LDPE and control groups using the independent-samples t-test and SPSS 20 statistical software (SPSS Inc., Chicago, IL, USA). Male and female rats were evaluated separately. A P-value of <0.05 was considered statistically significant.
Results
Acute toxicity
During all observation times, no differences in biologic reactions were observed between mice in the nano-Cu/LDPE group and those in the control group. No other abnormal behaviors or deaths were observed. The two groups did not differ statistically in terms of body weight gain (P>0.05) (Table 1). No alterations were observed in the macroscopic examination of the organs in all animals.
Table 1.
Body weight changes of mice in acute toxicity study (intravenous injection) (A) and (intraperitoneal injection) (B)
| A
| |||
|---|---|---|---|
| Group | n | Body weight (g)
|
|
| Before testing | 72 hours | ||
| Nano-Cu/LDPE | 5 | 18.0±1.00 | 21.8±1.64 |
| Control | 5 | 18.2±0.84 | 23.4±0.55 |
|
| |||
| B | |||
|
| |||
| Group | n |
Body weight (g)
|
|
| Before testing | 72 hours | ||
|
| |||
| Nano-Cu/LDPE | 5 | 17.8±0.84 | 23.4±0.89 |
| Control | 5 | 18.4±0.89 | 22.6±1.34 |
Note: Values expressed as mean ± SD.
Abbreviation: Cu/LDPE, copper/low-density polyethylene nanocomposite.
Subchronic toxicity
Clinical observations, body weight, and food consumption
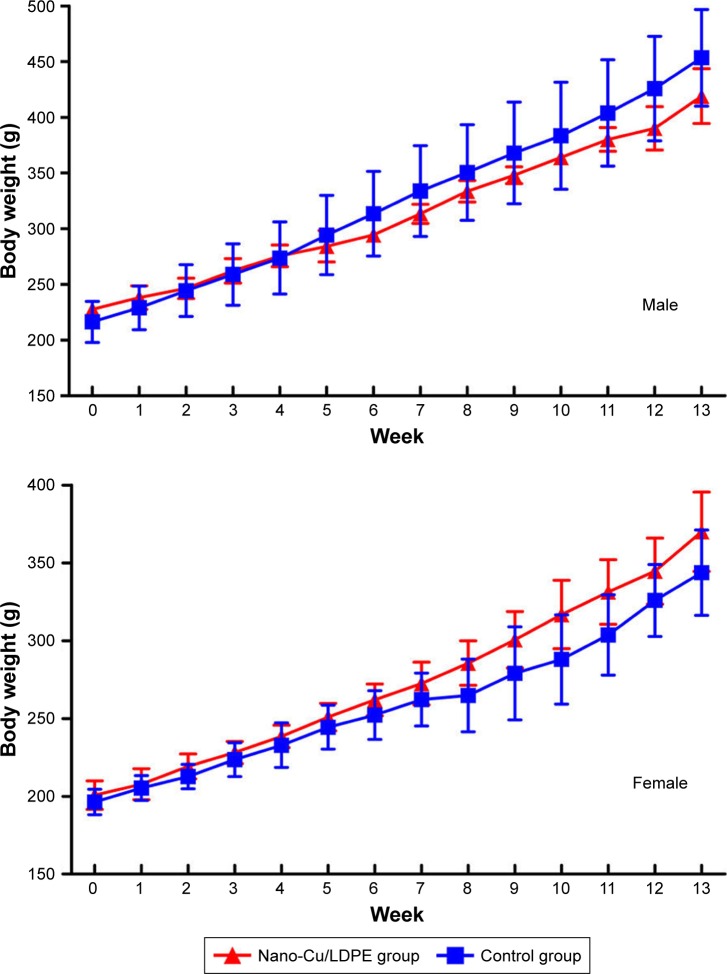
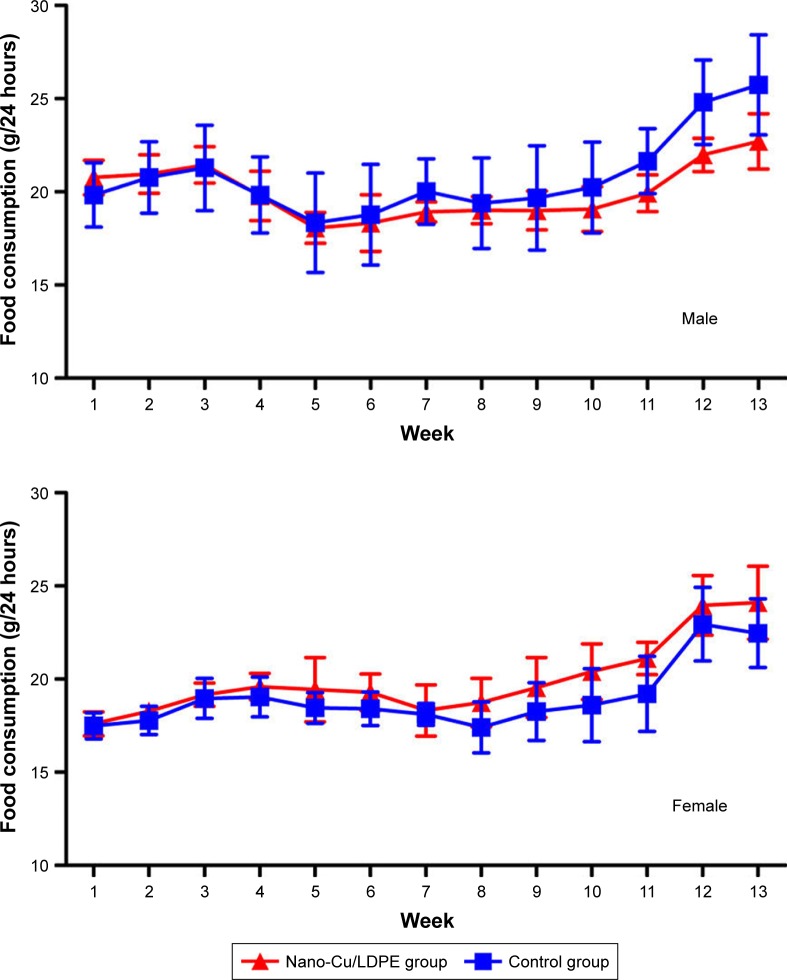
No clinical signs of toxicity or mortality were observed in the nano-Cu/LDPE and control groups during the 90-day study. The body weight and body weight gain for all groups in the 13-week trial were comparable to the control group values. No significant differences were noted in any male or female test groups relative to their corresponding control groups (P>0.05). The body weight curves for male and female rats during the 13-week period are shown in Figure 4. The mean weekly food consumption values for all groups of male and female rats were generally comparable with the control groups. Similarly, there were no statistical changes relative to the control in any observed sample group (P>0.05) (Figure 5).
Figure 4.
Body weight changes of male and female Wistar rats in subchronic study.
Abbreviation: Cu/LDPE, copper/low-density polyethylene nanocomposite.
Figure 5.
Food consumption changes of male and female Wistar rats in subchronic study.
Abbreviation: Cu/LDPE, copper/low-density polyethylene nanocomposite.
Urinalysis, hematologic and biochemical analysis
Semiquantitative urinary parameters, such as the urine volume, pH, specific gravity, GLU, bilirubin, and urobilinogen, did not reveal any relevant changes at the end of the study (data not shown). Hematologic data are shown in Table 2. Blood coagulation tests and biochemical analysis data are shown in Tables 3 and 4. Compared to the control group, no statistically significant changes were detected in any parameters in the sample groups (P>0.05).
Table 2.
Hematology values of male rats (A) and female rats (B) in subchronic toxicity study
| A
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | n | WBC (×109/L) | RBC (×1012/L) | HGB (g/L) | HCT (%) | NEUT (%) | LY (%) | MO (%) | PLT (×109/L) |
| Nano-Cu/LDPE | 5 | 11.5±1.3 | 7.7±0.9 | 145.4±18.2 | 40.9±4.5 | 21.3±4.1 | 66.0±2.8 | 12.8±4.6 | 435.2±7.6 |
| Control | 5 | 12.2±1.0 | 7.5±0.5 | 145.4±5.8 | 41.9±2.3 | 22.8±5.0 | 61.7±3.6 | 15.5±3.8 | 442.6±18.6 |
|
| |||||||||
| B | |||||||||
|
| |||||||||
| Group | n | WBC (×109/L) | RBC (×1012/L) | HGB (g/L) | HCT (%) | NEUT (%) | LY (%) | MO (%) | PLT (×109/L) |
|
| |||||||||
| Nano-Cu/LDPE | 5 | 9.2±0.3 | 6.8±0.3 | 142.6±7.1 | 42.2±2.7 | 17.3±1.9 | 69.7±1.9 | 13.0±2.6 | 416.2±20.4 |
| Control | 5 | 9.2±0.6 | 7.1±0.4 | 142.0±4.7 | 41.0±1.9 | 17.6±2.7 | 68.0±2.2 | 14.3±2.3 | 425.0±8.5 |
Note: Values expressed as mean ± SD.
Abbreviations: Cu/LDPE, copper/low-density polyethylene nanocomposite; HCT, hematocrit; HGB, hemoglobin concentration; LY, percent of lymphocyte; MO, percent of monocyte; NEUT, percent of neutrophilic granulocyte; PLT, platelet count; RBC, red blood cell count; WBC, white blood cell count.
Table 3.
Blood coagulation time of male rats (A) and female rats (B) in subchronic toxicity study
| A
| |||
|---|---|---|---|
| Group | n | PT (seconds) | APTT (seconds) |
| Nano-Cu/LDPE | 5 | 7.88±0.31 | 21.34±0.86 |
| Control | 5 | 7.98±0.18 | 21.22±1.32 |
|
| |||
| B | |||
|
| |||
| Group | n | PT (seconds) | APTT (seconds) |
|
| |||
| Nano-Cu/LDPE | 5 | 7.84±0.38 | 21.60±1.17 |
| Control | 5 | 7.80±0.20 | 21.94±1.04 |
Note: Values expressed as mean ± SD.
Abbreviations: APTT, activated partial thromboplastin time; Cu/LDPE, copper/low-density polyethylene nanocomposite; PT, prothrombin time.
Table 4.
Clinical biochemistry values of male rats (A) and female rats (B) in subchronic toxicity study
| A
| ||
|---|---|---|
| Parameters | Nano-Cu/LDPE group (n=5) | Control group (n=5) |
| ALT (U/L) | 47.6±3.58 | 50.5±6.20 |
| TP (g/L) | 80.3±3.54 | 81.3±2.61 |
| ALB (g/L) | 30.4±1.06 | 29.1±1.18 |
| TBIL (µmol/L) | 1.48±0.08 | 1.36±0.21 |
| AST (U/L) | 195.6±40.5 | 163.8±11.0 |
| T-CHO (mmol/L) | 1.56±0.32 | 1.58±0.08 |
| BUN (mmol/L) | 6.06±0.63 | 6.10±0.56 |
| Cr (µmol/L) | 23.8±1.53 | 23.62±1.49 |
| GLU (mmol/L) | 5.08±0.23 | 5.76±0.44 |
| Na (mmol/L) | 145.1±1.5 | 144.8±1.3 |
| K (mmol/L) | 6.16±0.29 | 6.28±0.45 |
| Cl (mmol/L) | 106.9±0.9 | 106.5±1.4 |
|
| ||
| B | ||
|
| ||
| Parameters | Nano-Cu/LDPE group (n=5) | Control group (n=5) |
|
| ||
| ALT (U/L) | 44.8±6.57 | 44.4±2.30 |
| TP (g/L) | 70.2±3.97 | 74.4±4.03 |
| ALB (g/L) | 34.0±1.68 | 31.9±1.92 |
| TBIL (µmol/L) | 1.26±0.23 | 1.28±0.18 |
| AST (U/L) | 166.2±22.7 | 177.7±29.3 |
| T-CHO (mmol/L) | 1.48±0.13 | 1.24±0.17 |
| BUN (mmol/L) | 6.32±1.04 | 6.48±0.97 |
| Cr (µmol/L) | 24.48±2.27 | 23.32±2.10 |
| GLU (mmol/L) | 5.76±0.84 | 5.22±0.85 |
| Na (mmol/L) | 145.3±0.7 | 144.2±2.6 |
| K (mmol/L) | 5.66±0.80 | 5.66±0.62 |
| Cl (mmol/L) | 106.8±0.9 | 106.5±0.7 |
Note: Values expressed as mean ± SD.
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cl, chloride; Cr, creatinine; Cu/LDPE, copper/low-density polyethylene nanocomposite; GLU, glucose; K, potassium; Na, sodium; TBIL, total bilirubin; T-CHO, total cholesterol; TP, total protein.
Pathology
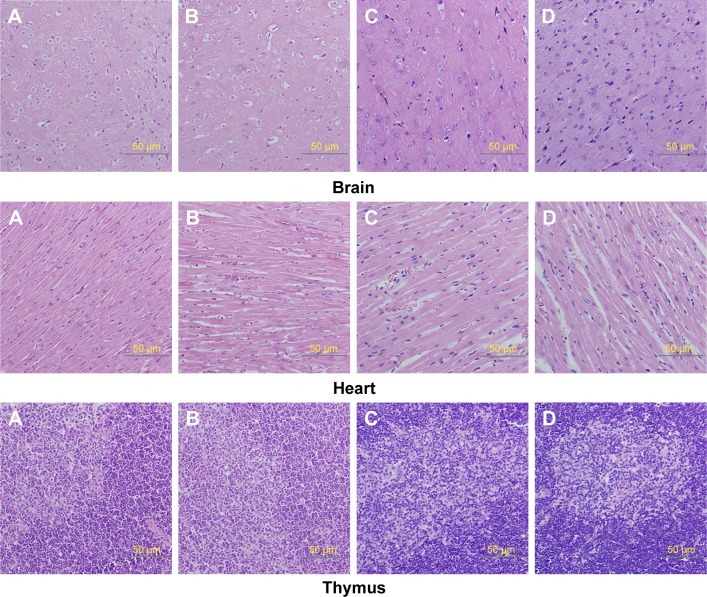
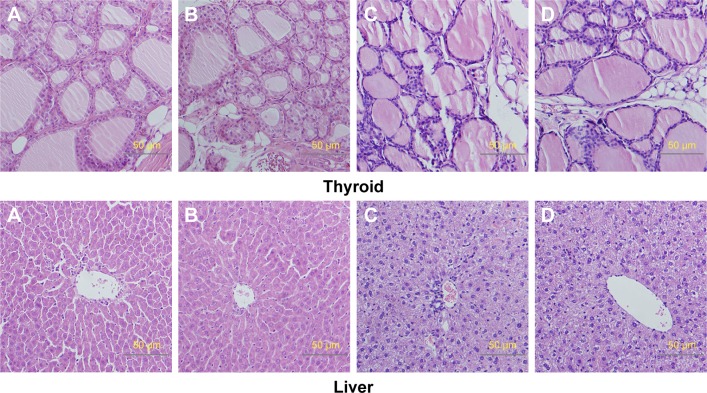
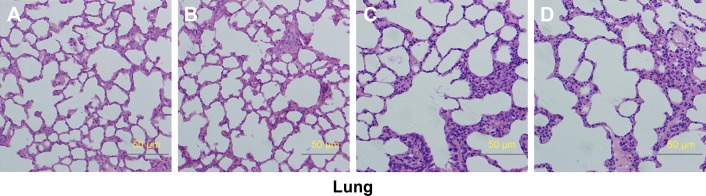
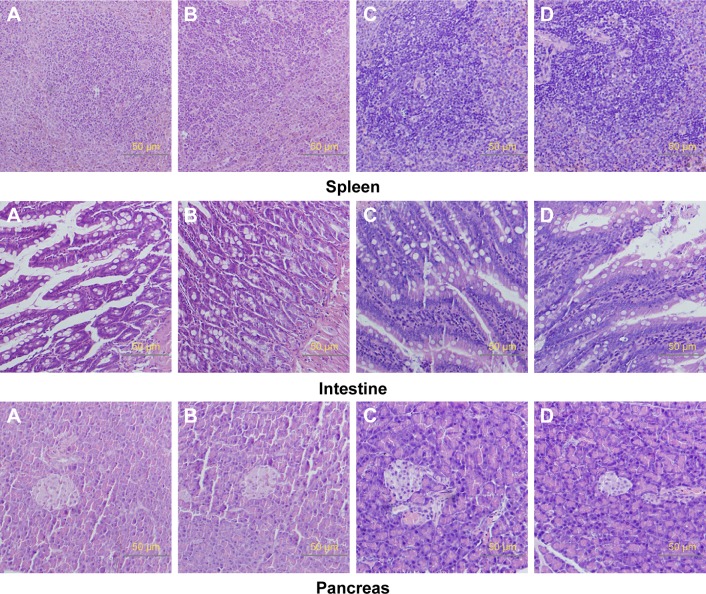
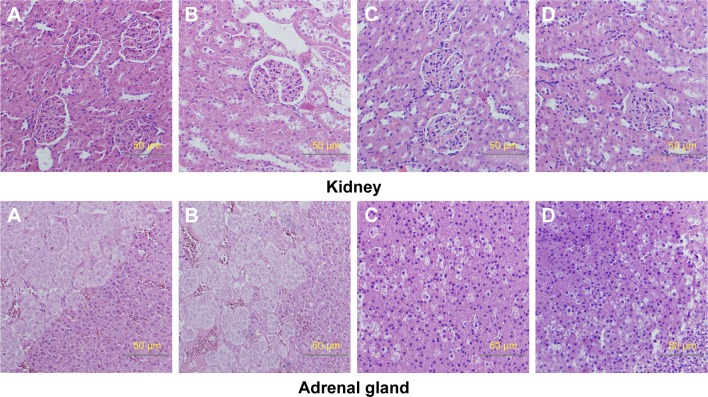
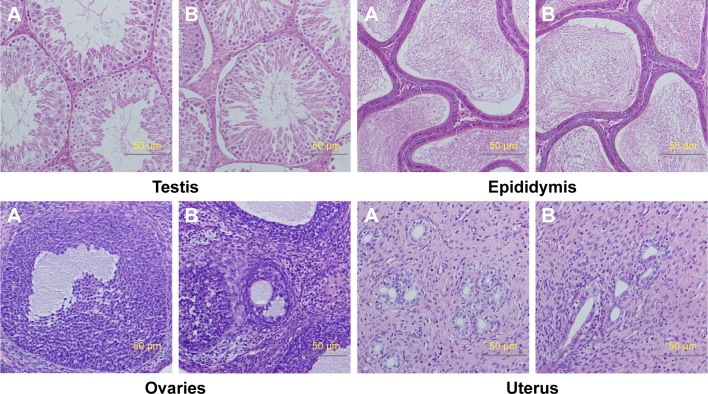
Relative organ weights are shown in Table 5. No significant differences were observed in the relative organ weights among animals of either sex after 13 weeks (P>0.05). Gross necropsy revealed no abnormalities in rats from the nano-Cu/LDPE group. Finally, organs from animals in both groups were subjected to a microscopic examination. Histopathologic examinations of the brain, heart, and thymus (Figure 6), thyroid, liver, and lung (Figure 7), spleen, intestine, and pancreas (Figure 8), kidney and adrenal gland (Figure 9), and testis, epididymis, ovaries, and uterus (Figure 10) revealed normal architecture and indicated a lack of morphologic disturbances in the treated animals.
Table 5.
Relative organ weights of male rats (A) and female rats (B) in subchronic toxicity study
| A
| ||
|---|---|---|
| Parameters | Nano-Cu/LDPE group (n=5) | Control group (n=5) |
| Brain (%) | 0.421±0.057 | 0.457±0.042 |
| Heart (%) | 0.305±0.047 | 0.350±0.039 |
| Thymus (%) | 0.154±0.028 | 0.164±0.029 |
| Liver (%) | 2.537±0.362 | 3.038±0.051 |
| Spleen (%) | 0.249±0.041 | 0.320±0.048 |
| Kidney (%) | 0.584±0.044 | 0.618±0.048 |
| Adrenal gland (%) | 0.013±0.001 | 0.013±0.002 |
| Testes (%) | 0.733±0.092 | 0.850±0.080 |
|
| ||
| B | ||
|
| ||
| Parameters | Nano-Cu/LDPE group (n=5) | Control group (n=5) |
|
| ||
| Brain (%) | 0.475±0.043 | 0.489±0.058 |
| Heart (%) | 0.300±0.030 | 0.311±0.032 |
| Thymus (%) | 0.163±0.050 | 0.162±0.012 |
| Liver (%) | 2.691±0.629 | 2.782±0.445 |
| Spleen (%) | 0.304±0.054 | 0.327±0.030 |
| Kidney (%) | 0.560±0.022 | 0.552±0.065 |
| Adrenal gland (%) | 0.025±0.001 | 0.022±0.005 |
| Ovaries (%) | 0.065±0.006 | 0.642±0.004 |
| Uterus (%) | 0.177±0.010 | 0.204±0.013 |
Note: Values expressed as mean ± SD.
Abbreviation: nano-Cu/LDPE, copper/low-density polyethylene nanocomposite.
Figure 6.
Histologic sections of brain, heart, and thymus.
Notes: (A) Male rats of nano-Cu/LDPE group. (B) Male rats of control group. (C) Female rats of nano-Cu/LDPE group. (D) Female rats of control group. No statistically significant changes were detected in any groups.
Abbreviation: Cu/LDPE, copper/low-density polyethylene nanocomposite.
Figure 7.
Histologic sections of thyroid, liver, and lung.
Notes: (A) Male rats of nano-Cu/LDPE group. (B) Male rats of control group. (C) Female rats of nano-Cu/LDPE group. (D) Female rats of control group. No abnormalities were detected in any groups.
Abbreviation: Cu/LDPE, copper/low-density polyethylene nanocomposite.
Figure 8.
Histologic sections of spleen, intestine, and pancreas.
Notes: (A) Male rats of nano-Cu/LDPE group. (B) Male rats of control group. (C) Female rats of nano-Cu/LDPE group. (D) Female rats of control group. No abnormalities were detected in any groups.
Abbreviation: Cu/LDPE, copper/low-density polyethylene nanocomposite.
Figure 9.
Histologic sections of kidney and adrenal gland.
Notes: (A) Male rats of nano-Cu/LDPE group. (B) Male rats of control group. (C) Female rats of nano-Cu/LDPE group. (D) Female rats of control group. No abnormalities were detected in any groups.
Abbreviation: Cu/LDPE, copper/low-density polyethylene nanocomposite.
Figure 10.
Histologic sections of testis, epididymis, ovaries, and uterus.
Notes: (A) The nano-Cu/LDPE group. (B) The control group. No abnormalities were detected in any groups.
Abbreviation: Cu/LDPE, copper/low-density polyethylene nanocomposite.
Discussion
The potential adverse effects of medical products on tissues and organs other than those in contact with the product are evaluated through systemic toxicity studies. Although the valid extrapolation of the results of these studies to humans is limited, the results can provide useful information regarding permissible human exposure. One section of ISO 10993 specifies the requirements for evaluating the potential for medical device materials to cause adverse systemic reactions and gives guidance on the procedures to be followed.19 The results of the present acute systemic toxicity experiment indicated that no mortality or evidence of any systemic toxicity was observed in any animal exposed to the extract liquids according to the recommendation specified by the systematic international guideline. The subchronic toxicity of nano-Cu/LDPE IUD was assessed by implanting Wistar rats with an amount of nanocomposite equivalent to 10-fold the human dose. No abnormal clinical observations, significant differences in serum chemistry or hematology parameters, or gross necropsy or histopathology abnormalities were noted in any of the animals. These results demonstrate that under experimental conditions and at the indicated doses, nano-Cu/LDPE IUD did not induce systemic toxicity in animals.
The contraceptive effect of a Cu-IUD depends mainly on the release of cupric ions via the corrosion of copper. However, the excess release of cupric ions causes cytotoxicity and increases the risk of adverse effects.21–23 The findings from several in vitro and in vivo assays provide evidence that cupric ions can interact directly with macromolecules to modify conformational structures, thus causing site-specific damage and indirectly catalyzing the formation of reactive oxygen species (ROS) via the Fenton/Haber–Weiss reaction to cause various cellular alterations.24,25 Previously, a study by Araya demonstrated that sperm motility decreased to nearly zero following exposure to a cupric ion concentration of approximately 8×10−6 mol/L (0.5 µg/mL) for roughly 20 minutes.26 This suggests that a cupric ion concentration of 1.0 µg/mL is sufficient to inhibit the motility of human sperm in a simulated uterine solution, even if half of the released cupric ions were absorbed by the endometrium in vivo. Accordingly, 1.0 µg/mL can be considered the minimal cupric ion concentration required to ensure contraceptive efficacy of the IUD in clinical use. However, the cupric ion concentrations released from existing Cu-IUDs in uterine fluid range from 3.9 to 19.1 µg/mL, or much higher than the minimal concentration required to ensure contraceptive efficacy.6 Similar to the existing Cu-IUD, the novel nano-Cu/LDPE IUD must release the minimum concentration of cupric ions to decrease the risk of adverse effects and ensure contraceptive efficacy.
Compared to conventional Cu-IUDs, nano-Cu/LDPE IUDs have unique characteristics. First, the frame comprises an LDPE matrix, and copper nanoparticles are distributed uniformly in the composite. On one hand, LDPE exhibits remarkable biocompatibility with the human body and is widely used as an implantable material. On the other hand, the dispersal of nanoparticles throughout a polymer matrix has attracted considerable attention because of its superior properties relative to a neat polymer, including enhanced thermal stability, antimicrobial properties, and gas barrier properties.27–30 The rate of cupric ions’ release from a nano-Cu/LDPE IUD is determined by the internal structure and functional groups on the surface of the nano-Cu/LDPE IUD polymer matrix. The interior space provides an osmotic passage for cupric ions and corrosion mediators and can effectively control the corrosion rate by separating the copper nanoparticles within the matrix. This was confirmed by the prophase of study in which the material was shown to release cupric ions at a steady rate in simulated uterine solution, in contrast to the burst release of cupric ions from conventional Cu-IUDs.31,32 Our previous research demonstrated that the average cupric ion concentrations released by the nano-Cu/LDPE IUD in simulated uterine solution are approximately 4.5482 µg/mL within 60 days and 4.4958 µg/mL within 120 days.11 In other words, the cupric ion concentration released by the nano-Cu/LDPE IUD in uterine fluid is apparently lower than that released by existing Cu-IUDs. Furthermore, the results from a cytotoxicity assay indicated that the nano-Cu/LDPE IUD was nontoxic or mildly cytotoxic at all incubation periods and concentrations. Accordingly, these results met the requirements to ensure contraceptive efficacy.18 In contrast to these findings, the currently available TCu220C IUD has more serious cytotoxic effects than those of the novel composite IUD. In this sense, the low level of cupric ions released by the nano-Cu/LDPE IUD induced little systemic toxicity in animals in the present study.
The nano-Cu/LDPE IUD also packages the copper nanoparticles in a framework composed of organic material, thus avoiding direct contact between metal and the endometrium. Here, cupric ions transformed by copper nanoparticles, rather than the nanoparticles themselves, act on the endometrium. The mechanism underlying the release of cupric ions from the nano-Cu/LDPE IUD can be explained as follows.9–11 1) The simulated uterine solution enters the nano-Cu/LDPE composite via spaces in the sample. 2) The copper nanoparticles dispersed homogeneously in the LDPE matrix convert to Cu ions upon contact with the simulated uterine solution. 3) The concentration gradient of cupric ions from the inside to the outside of the sample allows the diffusion of cupric ions into the external solution. Cupric ions thus diffuse through the polymer matrix by traveling through holes inside the LDPE matrix. As the diffusion rate of cupric ions in the LDPE matrix is lower than that in solution, the rate of cupric ion release from the nano-Cu/LDPE IUD is considered a stable slow-release process. This type of controlled cupric ion-release system can avoid the wide spectrum of effects produced by overexposure.
In recent years, the public has increasingly expressed concerns about the effects of nanoparticles on health. Nano-particles have a large surface area per mass, which directly increases the risk of toxicity via reactive surface atoms and molecules.33 The mechanisms of copper nanoparticle-mediated toxicity involve the generation of ROS, which can damage most biologic molecules, including DNA, protein, and lipids, and consequently damage cellular membranes and various organelles.34,35 Moreover, a study in which mice were acutely exposed to copper nanoparticles or microparticles found that only the former induced severe impairment of the kidney, liver, and spleen.36 A further study revealed a strong correlation of the size/specific surface area of copper nanoparticles with toxicity, and a study of mice exposed to copper nanoparticles observed metabolic alkalosis and copper accumulation in the kidneys.37 Yet another study observed overt toxicity in male Wistar rats that had been orally administered 200 mg/kg of copper nanoparticles daily for 5 days. Here, the kidney, a target organ, contained widespread necrosis of the renal proximal tubule cells. In a previous study, we showed that exposure to copper nanoparticles for 14 days can cause ovarian injury in rats. These findings only imply that exposure to copper nanoparticles should be a matter of concern.38 Overall, caution should be exercised when applying copper nanoparticles in various areas.
The toxicity associated with copper nanoparticles has been verified using conventional toxicologic parameters, including body weight, clinical chemistry, and histopathology.39 However, the results of the present systemic toxicity analysis found no symptoms of toxicity in any animal exposed to the nano-Cu/LDPE IUD. A histopathologic examination of the liver, spleen, kidneys, and ovaries at the end of the study revealed normal architecture, with no indication of morphologic disturbances. The unique characteristics of the nano-Cu/LDPE IUD might explain this lack of any systemic reaction. As shown in our previous work, the nano-Cu/LDPE composite is a simple hybrid of copper nanoparticles and pure LDPE wherein almost all of the copper nanoparticles are enveloped by LDPE. After the copper nanoparticles within the nanocomposite are corroded in the uterine solution, LDPE remains the outer layer of the nanocomposite.17,40 In addition, the results from a cytotoxicity assay in which the membranes of L929 mouse fibroblasts were cultured with nano-Cu/LDPE IUD extracts for 5 days are integral. The morphologies of these L929 mouse fibroblasts were near normal according to light microscopy and scanning electron microscopy evaluations.18 Therefore, the copper nanoparticles appear to have little effect on the surface condition of the nano-Cu/LDPE IUD.
In conclusion, the results presented in this study indicate the absence of systemic toxicity in response to acute and subchronic exposure to the nano-Cu/LDPE IUD under the test conditions. These findings suggest that an IUD comprising this novel material would be safe and feasible for future applications as a contraceptive device. However, further preclinical toxicologic studies must be conducted according to the ISO-recommended protocols for evaluating the toxicity of a medical device. Additional studies of the reproductive toxicity and genotoxicity associated with the nano-Cu/LDPE IUD are already in progress to assess the ultimate suitability of this device for end-use human applications.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no 81671507) and the National Key Research and Development Program (grant no 2016YFC1000903).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sivin I, Batár I. State-of-the-art of non-hormonal methods of contraception: III. Intrauterine devices. Eur J Contracept Reprod Health Care. 2010;15(2):96–112. doi: 10.3109/13625180903519885. [DOI] [PubMed] [Google Scholar]
- 2.Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol. 2002;187(6):1699–1708. doi: 10.1067/mob.2002.128091. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Altmann DR. Socio-demographic determinants of intrauterine device use and failure in China. Hum Reprod. 2002;17(5):1226–1232. doi: 10.1093/humrep/17.5.1226. [DOI] [PubMed] [Google Scholar]
- 4.Hubacher D, Lara-Ricalde R, Taylor DJ, Guerra-Infante F, Guzmán-Rodríguez R. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345(8):561–567. doi: 10.1056/NEJMoa010438. [DOI] [PubMed] [Google Scholar]
- 5.Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception. 2007;75(6 Suppl):S70–S75. doi: 10.1016/j.contraception.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Arancibia V, Peña C, Allen HE, Lagos G. Characterization of copper in uterine fluids of patients who use the copper T-380A intrauterine device. Clin Chim Acta. 2003;332(1–2):69–78. doi: 10.1016/s0009-8981(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Li J, Li HG, Li JX, Xie CS, Zhu CH. Comparative study on contraceptive efficacy and clinical performance of the copper/low-density polyethylene nanocomposite IUD and the copper T220C IUD. Contraception. 2008;78(4):319–323. doi: 10.1016/j.contraception.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Hu LX, Wang H, Rao M, et al. Alterations in the endometrium of rats, rabbits, and Macaca mulatta that received an implantation of copper/low-density polyethylene nanocomposite. Int J Nanomed. 2014;9:1127–1138. doi: 10.2147/IJN.S56756. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Cai S, Xia X, Xie C. Research on Cu2+ transformations of Cu and its oxides particles with different sizes in the simulated uterine solution. Corros Sci. 2005;47(4):1039–1047. [Google Scholar]
- 10.Xia X, Xie C, Cai S, Yang Z, Yang X. Corrosion characteristics of copper microparticles and copper nanoparticles in distilled water. Corros Sci. 2006;48(12):3924–3932. [Google Scholar]
- 11.Xia X, Tang Y, Xie C, Wang Y, Cai S, Zhu C. An approach to give prospective life-span of the copper/low-density-polyethylene nanocomposite intrauterine device. J Mater Sci Mater Med. 2011;22(7):1773–1781. doi: 10.1007/s10856-011-4347-y. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Xia X, Wang Y, Xie C. Study on the mechanical properties of Cu/LDPE composite IUDs. Contraception. 2011;83(3):255–262. doi: 10.1016/j.contraception.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Liu HF, Liu ZL, Xie CS, Yu J, Zhu CH. The antifertility effectiveness of copper/low-density polyethylene nanocomposite and its influence on the endometrial environment in rats. Contraception. 2007;75(2):157–161. doi: 10.1016/j.contraception.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Di HH, Zhu CH, Xie CS, Xia XP, Zhen B, Liu ZL. Influence of nano-Cu/LDPE-IUD on ultrastructure of rabbit endometrium. Reprod Contracept. 2006;26(10):584–588. [Google Scholar]
- 15.Zhen B, Zhu CH, Xie CS, et al. Impact of nano-Cu/LDPE-IUD on the level of t-PA, PAF, PGE2 in the uterine fluid of Macaca mulatta. Reprod Contracept. 2006;26(8):467–471. [Google Scholar]
- 16.Li J, Liu Z, Li S, et al. Correlative investigation of copper/low-density polyethylene nanocomposite on the endometrial angiogenesis in rats. Front Med China. 2007;1(4):1–4. doi: 10.1007/s11684-007-0078-3. [DOI] [PubMed] [Google Scholar]
- 17.Xia X, Xie C, Zhu C, Cai S, Yang X. Effect of implanted Cu/low-density polyethylene nanocomposite on the morphology of endometrium in the mouse. Fertil Steril. 2007;88(2):472–478. doi: 10.1016/j.fertnstert.2006.11.122. [DOI] [PubMed] [Google Scholar]
- 18.Hu LX, He J, Hou L, et al. Biological evaluation of the copper/low-density polyethylene nanocomposite intrauterine device. PLoS One. 2013;8(9):e74128. doi: 10.1371/journal.pone.0074128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Organization for Standardization ISO 10993-11. Biological evaluation of medical devices – part 11: Tests for systemic toxicity. 2006. [Accessed August 15, 2018]. Available from: https://www.iso.org/standard/35977.html.
- 20.International Organization for Standardization ISO 10993-12. Biological evaluation of medical devices – part 12: Sample preparation and reference materials. 2007. [Accessed August 15, 2018]. Available from: https://www.iso.org/standard/53468.html.
- 21.Patchen L, Berggren EK. Use of the copper T380A intrauterine device by adolescent mothers: continuation and method failure. J Pediatr Adolesc Gynecol. 2011;24(2):71–73. doi: 10.1016/j.jpag.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaislasuo J, Suhonen S, Gissler M, Lahteenmaki P, Heikinheimo O. Uterine perforation caused by intrauterine devices: clinical course and treatment. Hum Reprod. 2013;28(6):1546–1551. doi: 10.1093/humrep/det074. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez F, Grillo C, Schilardi P, et al. Decrease in cytotoxicity of copper-based intrauterine devices (IUD) pretreated with 6-mercaptopurine and pterin as biocompatible corrosion inhibitors. ACS Appl Mater Interfaces. 2013;5(2):249–255. doi: 10.1021/am3025307. [DOI] [PubMed] [Google Scholar]
- 24.Letelier ME, Lepe AM, Faúndez M, et al. Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem Biol Interact. 2005;151(2):71–82. doi: 10.1016/j.cbi.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Moriwaki H, Osborne MR, Phillips DH. Effects of mixing metal ions on oxidative DNA damage mediated by a Fenton-type reduction. Toxicol In Vitro. 2008;22(1):36–44. doi: 10.1016/j.tiv.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Araya R, Gómez-Mora H, Vera R, Bastidas JM. Human spermatozoa motility analysis in a Ringer’s solution containing cupric ions. Contraception. 2003;67(2):161–163. doi: 10.1016/s0010-7824(02)00477-8. [DOI] [PubMed] [Google Scholar]
- 27.Beigzadeh Ghelejlu S, Esmaiili M, Almasi H. Characterization of chitosan–nanoclay bionanocomposite active films containing milk thistle extract. Int J Biol Macromol. 2016;86:613–621. doi: 10.1016/j.ijbiomac.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Hu CY, Zhao Q, Shi YJ, Zhong HN. Migration of copper from nanocopper/LDPE composite films. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016;33(11):1741–1749. doi: 10.1080/19440049.2016.1237779. [DOI] [PubMed] [Google Scholar]
- 29.Dealba-Montero I, Guajardo-Pacheco J, Morales-Sánchez E, et al. Antimicrobial properties of copper nanoparticles and amino acid chelated copper nanoparticles produced by using a soya extract. Bioinorg Chem Appl. 2017;2017(2):6. doi: 10.1155/2017/1064918. Article ID 1064918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashfaq M, Verma N, Khan S. Copper/zinc bimetal nanoparticles-dispersed carbon nanofibers: a novel potential antibiotic material. Mater Sci Eng C Mater Biol Appl. 2016;59:938–947. doi: 10.1016/j.msec.2015.10.079. [DOI] [PubMed] [Google Scholar]
- 31.Grillo CA, Reigosa MA, de Mele MA. Does over-exposure to copper ions released from metallic copper induce cytotoxic and genotoxic effects on mammalian cells? Contraception. 2010;81(4):343–349. doi: 10.1016/j.contraception.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Cai S, Xia X, Xie C. Corrosion behavior of copper/LDPE nanocomposites in simulated uterine solution. Biomaterials. 2005;26(15):2671–2676. doi: 10.1016/j.biomaterials.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nano-level. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser J-P, Zuin S, Wick P. Is nanotechnology revolutionizing the paint and lacquer industry? A critical opinion. Sci Total Environ. 2013;442:282–289. doi: 10.1016/j.scitotenv.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Zheng JL, Yuan SS, Wu CW, Lv ZM. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio) Aquat Toxicol. 2016;180:36–44. doi: 10.1016/j.aquatox.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Meng H, Xing G, et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett. 2006;163(2):109–120. doi: 10.1016/j.toxlet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Meng H, Chen Z, Xing G, et al. Ultrahigh reactivity provokes nano-toxicity: explanation of oral toxicity of nano-copper particles. Toxicol Lett. 2007;175(1–3):102–110. doi: 10.1016/j.toxlet.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Hu S, Rao M, et al. Copper nanoparticle-induced ovarian injury, follicular atresia, apoptosis, and gene expression alterations in female rats. Int J Nanomed. 2017;12:5959–5971. doi: 10.2147/IJN.S139215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao M, Liu H. Gene expression profiling of nephrotoxicity from copper nanoparticles in rats after repeated oral administration. Environ Toxicol Pharmacol. 2012;34(1):67–80. doi: 10.1016/j.etap.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Xia X, Cai S, Xie C. Preparation, structure and thermal stability of Cu/LDPE nanocomposites. Mater Chem Phys. 2006;95(1):122–129. [Google Scholar]