Abstract
Background
The aspartate aminotransferase-to-platelet ratio index (APRI) has been correlated with clinical outcome in patients with hepatocellular carcinoma (HCC), but controversial results were obtained with previous studies. This study was aimed to evaluate the prognostic value of the APRI in patients with HCC.
Materials and methods
A literature survey was conducted by searching PubMed, Web of Science, Cochrane library, Embase, Wanfang, and National Knowledge Infrastructure for publications released prior to March 1, 2018. Pooled hazard ratios (HRs) with 95% CIs were calculated to assess the association between the APRI and HCC prognosis using Stata SE 12.0 software.
Results
Analysis was performed on a total of 15 articles that included 5,051 patients. The pooled results showed that APRI was significantly associated with overall survival for patients with HCC (HR =1.62, 95% CI: 1.23–2.01). Furthermore, HCC patients with higher APRI were at significantly greater risk of short recurrence-free survival (HR =1.83, 95% CI: 1.48–2.18) and poor disease-free survival (HR =1.46, 95% CI: 1.26–1.66).
Conclusions
APRI could serve as a promising and noninvasive marker for predicting HCC prognosis.
Keywords: aspartate aminotransferase-to-platelet ratio index, hepatocellular carcinoma, prognosis, meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related death globally.1,2 Despite great advances in early diagnosis and treatments, the prognosis, especially the long-term survival, remains dissatisfactory in patients with HCC.3–6 Additionally, the prognostic markers of HCC have not been completely elucidated. Established prognostic factors can contribute to predicting the survival and relapse for HCC patients as well as guiding their clinical management.
Recently, several serum markers that can be used as noninvasive tools have been identified in human cancers, including HCC.7–10 Among them, there has been great interest in the aspartate aminotransferase-to-platelet ratio index (APRI) because it is an inexpensive and feasible test that can be used for daily oncologic practice.
It has been reported that APRI might be a candidate as a prognostic biomarker in HCC.11–20 However, there were shortcomings in the current clinical studies because they were of limited sample sizes, and some results regarding the clinical value of APRI in HCC were inconsistent and debatable.14,21,22 Therefore, we conducted this systematic review and meta-analysis based on all relevant studies for a better understanding of the relationship between APRI and prognosis of HCC patients.
Materials and methods
Search strategy and study selection
A comprehensive literature search was performed in several electronic databases to retrieve the eligible studies before March 1, 2018. The online databases included PubMed, Web of Science, Cochrane library, Embase, Wanfang, and National Knowledge Infrastructure. The combined key words and search terms were as follows: (aspartate aminotransferase-to-platelet ratio index OR aspartate aminotransferase/platelet count ratio index OR AST-to-platelet ratio index OR AST/PLT ratio index OR APRI) AND (liver cancer OR HCC OR hepatocellular carcinoma OR liver neoplasms OR hepatic tumor). We also manually reviewed the references in retrieved papers for potential studies.
Publications with full text that met the following criteria were considered to be eligible and were included in this meta-analysis: 1) studies concerned with the prognostic impact of APRI in primary HCC, 2) a definite cutoff value of APRI was given, 3) the hazard ratio (HR) with 95% CI for prognosis was available, and 4) patients with HCC were divided into two groups according to the APRI value.
Data extraction and quality assessment
The collected information from all studies included name of the first author, study country, year of publication, included time, sample size, number of male and female patients, age distribution, survival type, follow-up period, cutoff value for APRI, cutoff selection, treatment methods, tumor stage, HR, and the corresponding 95% CI. HRs and the corresponding 95% CIs were directly extracted from the original article if a study reported the HRs and 95% CIs in univariate and/or multivariate analysis. Otherwise, Engauge Digitizer software (version 4.1) was used to estimate the HR from the Kaplan–Meier curve.
All included studies were assessed by the Newcastle–Ottawa quality assessment scale (NOS). In this method, NOS scores ranged from 0 (lowest) to 9 (highest) points. A study with an NOS score ≥6 was considered to be of high quality.
Statistical analysis
In this meta-analysis, statistical analyses for pooled HRs were executed using Stata 12.0 software (Stata, College Station, TX, USA).
For measuring the heterogeneity among research studies, Cochran’s Q test and Higgins I-squared statistic were applied. A probability value of PQ <0.05 or I2 >50%indicated that heterogeneity existed. If heterogeneity was present, the random-effects model was used to calculate the integrated HRs. If there was no significant heterogeneity across studies, the fixed-effects model was adopted.
Funnel plot and Begg’s test were used to judge the potential publication bias. Sensitivity analysis was applied to evaluate the robustness of the pooled results. All P-values <0.05 were regarded as statistically significant.
Results
The detailed steps of the potential literature search are shown in Figure 1. Based on the selection criteria for the eligible studies, high-quality articles (including 17 studies) written in English were identified in this meta-analysis.11–25 A total of 5,051 patients with HCC were enrolled. All included studies were retrospective and reported the relationship between the APRI value and HCC prognosis. The detailed characteristics of all included studies are summarized in Table 1.
Figure 1.
The flowchart of literature selection.
Table 1.
Main characteristics of all included studies
| Study/Year | Country | Included time | No. (M/F) | Age (Years) | Survival type | Follow-up (Months) | Cutoff value | Cutoff selection | Treatment methods | Stage | MVA | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Huang HH, et al 201011 | China | 1990–2002 | 76 (64/12) | Median 57 | OS;RFS | Median 77.0±50.7 | 0.47 | ROC analysis | With surgery | Child-Pugh A/B | OS-Yes, RFS-No | 6 |
| Kao W, et al 201112 | China | 2002–2007 | 190 (121/69) | Mean 67.4 | OS;RFS | Median 30.7±17.5 | 1 | NA | No surgery | Child-Puge A/B | Yes | 7 |
| Shen SL, et al 201413 | China | 2006–2009 | 332 (292/40) | Mean 49.82 | OS;DFS | 1-80 | 0.62 | ROC analysis | With surgery | Edmonson stage: I–II–III–IV | Yes | 8 |
| Pang Q-1, et al 201514 | China | 2002–2012 | 172 (139/33) | Mean 53.52 | OS;DFS | Median 46 | 1.23 | ROC analysis | With surgery | No extrahepatic spread | Yes | 7 |
| Pang Q-2, et al 201514 | China | 2002–2012 | 191 (159/32) | Mean 54.12 | OS;DFS | Median 40 | 1.79 | ROC analysis | No surgery | No extrahepatic spread | Yes | 7 |
| Pang Q, et al 201515 | China | 2002–2012 | 172 (139/33) | Mean 53.5 | RFS | Median 52 | 1.94 | ROC analysis | With surgery | No extrahepatic spread | Yes | 6 |
| Okamura Y, et al 201616 | Japan | 2002–2014 | 140 (115/25) | Median 71 | OS;RFS | Median 38.9 | 0.544 | ROC analysis | With surgery | UICC: I–II–III | No | 7 |
| Chuang HA, et al 201617 | Korea | 2005–2013 | 98 (70/28) | Mean 60.5 | RFS | Median 40 | 1.38 | ROC analysis | No surgery | Modified UICC: I–II–III | Yes | 6 |
| Ji F, et al 201618 | China | 2006–2009 | 321 (285/36) | Mean 51 | OS;DFS | 1-96 | 1.68 | ROC analysis | With surgery | TNM stage: I–II–III | Yes | 8 |
| Liu Y, et al 201619 | China | 2004–2011 | 223 (189/34) | Median 54 | RFS | Median 26.1 | 0.23 | ROC analysis | With surgery | TNM stage: I–II–III–IV | Yes | 7 |
| Peng W, et al 201620 | China | 2007–2013 | 244 (213/31) | Mean 50 | OS;RFS | 1-80 | 1 | ROC analysis | With surgery | No extrahepatic metastasis | Yes | 8 |
| Teng W, et al 201721 | China | 2010–2013 | 153 (82/71) | Median 64.1 | OS;RFS | NA | 2 | NA | No surgery | BCLC stage:0/A/B/C | Yes | 7 |
| Toyoda H, et al 201722 | Japan | 1997–2016 | 1669 (1181/488) | Mean 68.7 | OS;RFS | 1-240 | 1.2 | ROC analysis | With surgery | BCLC stage:0/A/B/C/D | OS-Yes, RFS-No | 8 |
| Sarkar J, et al 201723 | USA | 1993–2014 | 94 (71/23) | Mean 62 | OS | >60 | 0.5 | NA | Mixed | NA | No | 6 |
| Yang HJ, et al 201724 | China | 2004–2010 | 661 (574/87) | NA | OS;DFS | 1-60 | 0.25 | ROC analysis | With surgery | BCLC stage:0/A/B/C | Yes | 8 |
| Tang T-1, et al 201825 | China | 2005–2013 | 158 (136/22) | NA | OS | 1-40 | 0.4 | X-tile plots | No surgery | BCLC stage:B | No | 7 |
| Tang T-2, et al 201825 | China | 2005–2014 | 157 (135/22) | NA | OS | 1-35 | 0.4 | X-tile plots | No surgery | BCLC stage:B | No | 7 |
Note: All the studies were retrospective.
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; DFS, disease-free survival; MVA, multivariate analysis; NA, not available; NOS, Newcastle–Ottawa quality assessment scale; OS, overall survival; RFS, recurrence-free survival; ROC, receiver operating characteristic curve; TNM, tumor-node-metastasis; UICC, Union for International Cancer Control.
Relationship between APRI and overall survival in HCC
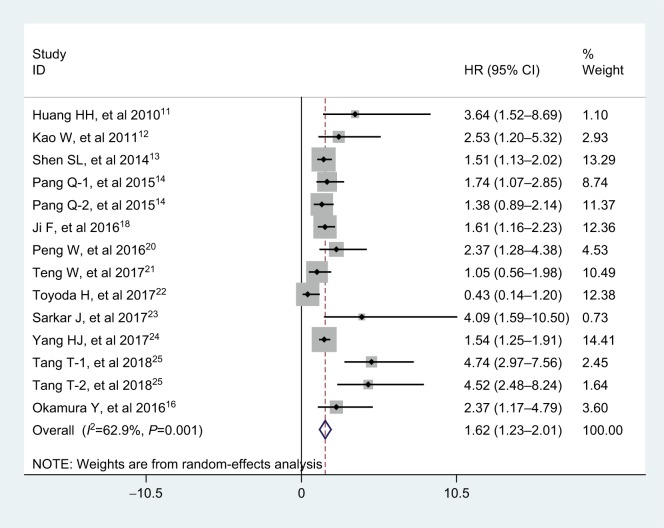
A total of 12 articles (including 14 studies) with 4,558 subjects reported the relationship between APRI and overall survival (OS) in patients with HCC. Considering the significant heterogeneity among these articles (I2=62.9%, PQ =0.001), a random-effects model was applied. As shown in Figure 2, the overall results indicated that elevated APRI predicted a poor outcome for OS of HCC (HR =1.62, 95% CI: 1.23–2.01).
Figure 2.
Forest plot for the relationship between APRI and OS.
Abbreviations: APRI, aminotransferase-to-platelet ratio index; HR, hazard ratio; OS, overall survival.
Exploratory subgroup analyses were performed according to analysis type (multivariate analysis and univariate analysis), cutoff value selection (receiver operating characteristic curve and Others), and treatments (With surgery, No surgery, and Mixed). Table 2 shows that the calculated pooled HR values were significantly >1.0 in those subgroup analyses. Interestingly, the APRI could be an independent predictor of OS in patients with HCC (HR =1.41, 95% CI: 1.08–1.73, P<0.001).
Table 2.
Results of subgroup analysis of pooled HRs of OS of HCC patients with high APRI
| Stratified analysis | No. of studies | No. of patients | Pooled HR (95% CI) | P-value | I2(%) | PQ |
|---|---|---|---|---|---|---|
| Analysis type | ||||||
| MVA | 10 | 4,009 | 1.41 (1.08–1.73) | <0.001 | 55.1 | 0.018 |
| UVA | 4 | 549 | 3.59 (2.32–4.87) | <0.001 | 5.2 | 0.367 |
| Cutoff value selection | ||||||
| ROC analysis | 9 | 3,806 | 1.46 (1.10–1.81) | <0.001 | 58.1 | 0.014 |
| Others | 5 | 752 | 3.08 (1.23–4.92) | 0.001 | 73.9 | 0.004 |
| Treatments | ||||||
| No surgery | 5 | 849 | 2.24 (1.14–3.33) | <0.001 | 72.5 | 0.006 |
| With surgery | 8 | 3,615 | 1.48 (1.07–1.90) | <0.001 | 63.3 | 0.008 |
| Mixed | 1 | 94 | 4.09 (1.59–10.50) | <0.001 | NA | NA |
Note: Relationship between APRI and RFS in HCC.
Abbreviations: APRI, aminotransferase-to-platelet ratio index; HCC, hepatocellular carcinoma; HR, hazard ratio; MVA, multivariate analysis; NA, not available; OS, overall survival; RFS, recurrence-free survival; ROC, receiver operating characteristic curve; UVA, univariate analysis.
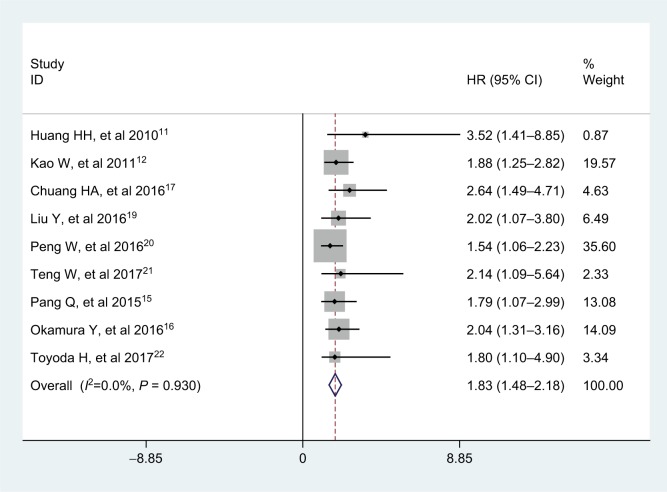
Nine studies with a total of 2,965 HCC patients reported the prognostic value of APRI for recurrence-free survival (RFS). No significant heterogeneity was observed among studies (I2=0.0%, PQ =0.930), and the fixed-effects model was adopted. The pooled HR was 1.83 (95% CI: 1.48–2.18, P<0.001; Figure 3), indicating that high APRI was an unfa-vorable factor for RFS in HCC. In addition, we found that the analysis types, cutoff value selections, and treatments did not affect the prognostic predictor of RFS in HCC patients from the subgroup analyses (Table 3).
Figure 3.
Forest plot for the relationship between APRI and RFS.
Abbreviations: APRI, aminotransferase-to-platelet ratio index; HR, hazard ratio; RFS, recurrence-free survival.
Table 3.
Results of subgroup analysis of pooled HRs of RFS of HCC patients with high APRI
| Stratified analysis | No. of studies | No. of patients | Pooled HR (95% CI) | P-value | I2(%) | PQ |
|---|---|---|---|---|---|---|
| Analysis type | ||||||
| MVA | 6 | 1,080 | 1.78 (1.39–2.16) | <0.001 | 0.0 | 0.848 |
| UVA | 3 | 1,885 | 2.07 (1.26–2.88) | <0.001 | 0.0 | 0.716 |
| Cutoff value selection | ||||||
| ROC analysis | 7 | 2,622 | 1.81 (1.42–2.20) | <0.001 | 0.0 | 0.811 |
| Others | 2 | 343 | 1.90 (1.16–2.65) | 0.001 | 0.0 | 0.832 |
| Treatments | ||||||
| No surgery | 3 | 441 | 2.03 (1.36–2.71) | <0.001 | 0.0 | 0.703 |
| With surgery | 6 | 2,524 | 1.76 (1.35–2.16) | <0.001 | 0.0 | 0.862 |
Note: Relationship between APRI and DFS in HCC.
Abbreviations: APRI, aminotransferase-to-platelet ratio index; DFS, disease-free survival; HCC, hepatocellular carcinoma; HR, hazard ratio; MVA, multivariate analysis; RFS, recurrence-free survival; ROC, receiver operating characteristic curve; UVA, univariate analysis.
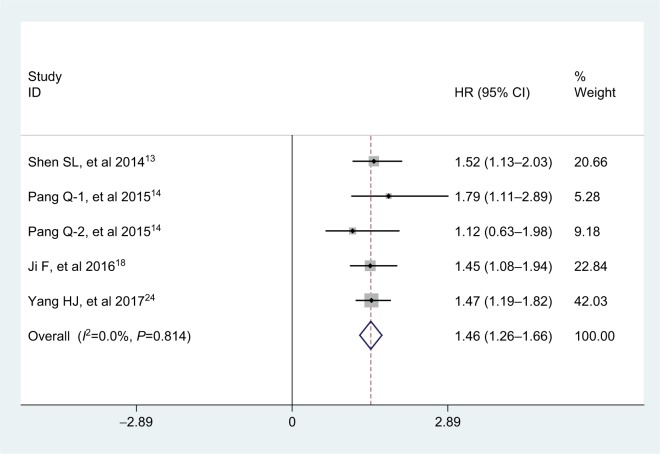
Five studies, consisting of 1,677 patients, explored the association between APRI and disease-free survival (DFS) in multivariate analysis. There was no obvious heterogeneity across studies (I2=0.0%, PQ =0.814), and therefore, the fixed-effects model was used. Analysis revealed the pooled HR of 1.46 with 95% CI: 1.26–1.66 (P<0.001), which showed that APRI was an independent predictive factor of DFS in patients with HCC (Figure 4).
Figure 4.
Forest plot for the relationship between APRI and DFS.
Abbreviations: APRI, aminotransferase-to-platelet ratio index; DFS, disease-free survival; HR, hazard ratio.
Publication bias
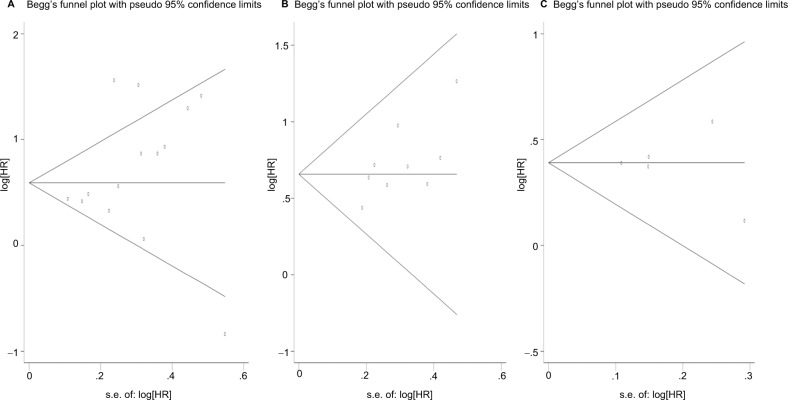
Funnel plot and Begg’s test both suggested no evidence of publication bias for OS, RFS, and DFS (Pr Begg’s test>|z|=0.228 for OS; Pr Begg’s test>|z|=0.118 for RFS; Pr Begg’s test>|z|=1.000 for DFS; Figure 5).
Figure 5.
Funnel plots for publication bias test.
Note: (A) For OS; (B) for RFS; (C) for DFS.
Abbreviations: DFS, disease-free survival; HR, hazard ratio; OS, overall survival; RFS, recurrence-free survival.
Sensitivity analysis
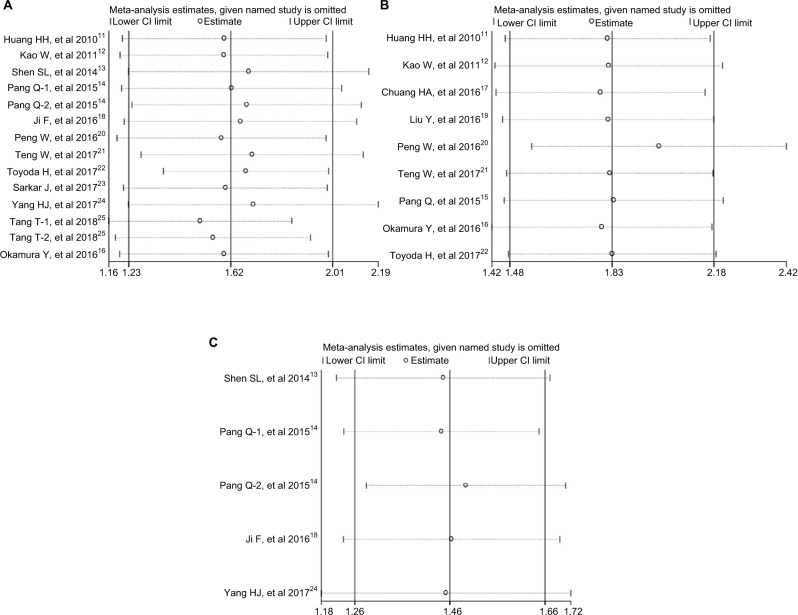
Sensitivity analysis was performed to assess the potential impact of each individual study on the overall results. The results showed that any single study had little influence on the pooled results (Figure 6), thus indicating that our results were relatively stable and credible.
Figure 6.
Sensitivity analysis of the relationship between APRI and HCC prognosis.
Note: (A) For OS; (B) for RFS; (C) for DFS.
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; DFS, disease-free survival; HCC, hepatocellular carcinoma; OS, overall survival; RFS, recurrence-free survival.
Discussion
As a noninvasive scoring marker, APRI consists of two routinely available clinical and laboratory variables, aspartate aminotransferase (AST) and platelets (PLT), and can be calculated using the following formula: (AST/the upper limit of normal value)×100: PLT (109/L).26 It was firstly proposed as a simple, straightforward test to assess liver fibrosis stage and liver function reserve.27–29 In-depth studies determined that APRI also had potential prognostic value and could be used as a stable marker to predict the outcome of patients with HCC.11–13 It has been known that AST and PLT play an important role in tumor progression and are associated with prognosis in several human cancers, including HCC.15,30,31 APRI is a combination of the above two predictors, and it is low in cost and more easily detectable. Its clinical predictive value could be enhanced due to its stability and reliability.
The exact mechanisms governing the prognostic values of APRI still remain unclear; however, there are some possible explanations.24,25 HCC patients with a high APRI value frequently have elevated AST levels, and the level of AST could represent the cirrhosis level or the injury to the hepa-tocytes, and could also reflect liver stress or damage arising from progressive liver fibrosis or reactivation of hepatitis virus replication. All of these are major contributors to liver carcinogenesis.32–34 In contrast, many patients with HCC and elevated APRI often have a low PLT level. Progressive destruction of an enlarged spleen and progressive liver fibrosis can often lead to low preoperative PLT,35–37 and there were also close relationships between low preoperative PLT and great risk of major liver-related complications.37,38 All of these factors and events are closely linked to poor clinical outcomes in HCC patients.
To the best of our knowledge, this is the first meta-analysis that comprehensively assesses the prognostic value of APRI in HCC. In the present meta-analysis, a total of 15 published articles with 5,051 HCC patients were collected. When the out comes from all available studies were combined and pooled, we found that an elevated APRI was significantly associated with poor OS (HR =1.62, 95% CI: 1.23–2.01) in HCC, although there was heterogeneity. Furthermore, the subgroup analyses revealed that APRI could be an independent prognostic factor of OS in patients with HCC. For the relationship between APRI and secondary endpoints, the combined results showed that APRI might act as a prognostic indicator for RFS in HCC (HR =1.83, 95% CI: 1.48–2.18), and the subgroup analyses also confirmed the prognostic value of APRI for RFS in HCC. Furthermore, HCC patients with high APRI had a worse DFS when compared with that of patients with low APRI (HR =1.46, 95% CI: 1.26–1.66). Thus, the APRI might represent a predictive biomarker with great clinical utility in HCC patients.
Nevertheless, there are some limitations existing in the meta-analysis that should be carefully interpreted. Firstly, all studies enrolled were retrospective, and the number of published studies included was not sufficiently large for a more detailed subgroup analysis. Secondly, because the majority of studies included were from Asian countries (China, Japan, and Korea), additional clinical studies from other countries are required. Thirdly, four of 14 studies for OS only reported HRs in the univariate analysis, which might cause a bias toward overestimation of the prognostic role of APRI. In addition, some heterogeneity was observed among studies for OS, which was probably due to factors such as the adjusted multivariate analysis of studies with different factors or different start time to follow-up or diverse therapies applied. In addition, there are some other factors that could influence AST and PLT, such as antiviral drugs and other accompanying diseases of the patients, and this should also be noted. Finally, the cutoff value for defining high or low APRI differed in studies, and it is essential that they be unified before APRI can be utilized in clinical prognostication for HCC.
In summary, the results of our study suggest that high APRI indicated poor prognosis in HCC patients, and APRI could serve as a cost-effective, significant biomarker for predicting HCC survival outcome. Considering the limitations mentioned above, larger well-designed clinical studies with more diverse populations are warranted in the future to validate our findings.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62(6):394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 3.Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol. 2018;43(1):13–25. doi: 10.1007/s00261-017-1209-1. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Lin H, Wang XY, et al. Predictive value of microRNA-143 in evaluating the prognosis of patients with hepatocellular carcinoma. Cancer Biomark. 2017;19(3):257–262. doi: 10.3233/CBM-160357. [DOI] [PubMed] [Google Scholar]
- 5.Yang XP, Zhou LX, Yang QJ, et al. Diagnostic and prognostic roles of serum vitronectin in hepatitis B-related hepatocellular carcinoma. Cancer Biomark. 2016;17(3):271–279. doi: 10.3233/CBM-160639. [DOI] [PubMed] [Google Scholar]
- 6.Chen CJ. Global elimination of viral hepatitis and hepatocellular carcinoma: opportunities and challenges. Gut. 2018;67(4):595–598. doi: 10.1136/gutjnl-2017-315407. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Attar BM, Fuentes HE, Jaiswal P, Tafur AJ. Evaluation of the prognostic value of platelet to lymphocyte ratio in patients with hepa-tocellular carcinoma. J Gastrointest Oncol. 2017;8(6):1065–1071. doi: 10.21037/jgo.2017.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao T, Cui L, Li A. The significance of RDW in patients with hepatocellular carcinoma after radical resection. Cancer Biomark. 2016;16(4):507–512. doi: 10.3233/CBM-160591. [DOI] [PubMed] [Google Scholar]
- 9.Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):971–978. doi: 10.1016/j.ctrv.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Lin H, Jiao X, Yu B, et al. Clinical significance of serum 14-3-3 beta in patients with hepatocellular carcinoma. Cancer Biomark. 2017;20(2):143–150. doi: 10.3233/CBM-160533. [DOI] [PubMed] [Google Scholar]
- 11.Hung HH, Su CW, Lai CR, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010;4(4):691–699. doi: 10.1007/s12072-010-9213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao WY, Chiou YY, Hung HH, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol. 2011;23(6):528–536. doi: 10.1097/MEG.0b013e328346d529. [DOI] [PubMed] [Google Scholar]
- 13.Shen SL, Fu SJ, Chen B, et al. Preoperative aspartate aminotransfer-ase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2014;21(12):3802–3809. doi: 10.1245/s10434-014-3771-x. [DOI] [PubMed] [Google Scholar]
- 14.Pang Q, Zhang JY, Xu XS, et al. The prognostic values of 12 cirrhosis-relative noninvasive models in patients with hepatocellular carcinoma. Scand J Clin Lab Invest. 2015;75(1):73–84. doi: 10.3109/00365513.2014.981759. [DOI] [PubMed] [Google Scholar]
- 15.Pang Q, Zhang JY, Xu XS, et al. Significance of platelet count and platelet-based models for hepatocellular carcinoma recurrence. World J Gastroenterol. 2015;21(18):5607–5621. doi: 10.3748/wjg.v21.i18.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamura Y, Ashida R, Yamamoto Y, et al. The FIB-4 index is a significant prognostic factor in patients with non-B non-C hepatocellular carcinoma after curative surgery. Langenbecks Arch Surg. 2016;401(2):195–203. doi: 10.1007/s00423-016-1389-0. [DOI] [PubMed] [Google Scholar]
- 17.Chung HA, Kim JH, Hwang Y, et al. Noninvasive fibrosis marker can predict recurrence of hepatocellular carcinoma after radiofrequency ablation. Saudi J Gastroenterol. 2016;22(1):57–63. doi: 10.4103/1319-3767.173760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji F, Liang Y, Fu SJ, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate amino-transferase/platelet count ratio index (APRI) BMC Cancer. 2016;16:137. doi: 10.1186/s12885-016-2189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Wang ZX, Cao Y, et al. Preoperative inflammation-based markers predict early and late recurrence of hepatocellular carcinoma after curative hepatectomy. Hepatobiliary Pancreat Dis Int. 2016;15(3):266–274. doi: 10.1016/s1499-3872(16)60094-2. [DOI] [PubMed] [Google Scholar]
- 20.Peng W, Li C, Wen TF, et al. Postoperative aspartate aminotransferase to platelet ratio index change predicts prognosis for hepatocellular carcinoma. Medicine. 2016;95(30):e4160. doi: 10.1097/MD.0000000000004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng W, Hsieh YC, Lui KW, et al. Eradication of hepatitis C virus profoundly prolongs survival in hepatocellular carcinoma patients receiving transarterial chemoembolization. J Viral Hepat. 2017;24(12):1160–1167. doi: 10.1111/jvh.12745. [DOI] [PubMed] [Google Scholar]
- 22.Toyoda H, Kumada T, Tada T, et al. Differences in the impact of prognostic factors for hepatocellular carcinoma over time. Cancer Sci. 2017;108(12):2438–2444. doi: 10.1111/cas.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar J, Deleon T, Wong LL. MELD score and AST-to-platelet ratio index (APRI) predict long-term survival in patients with a small hepa-tocellular carcinoma following non-transplant therapies: a pilot study. Hepatoma Res. 2017;3:79–85. doi: 10.20517/2394-5079.2017.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang HJ, Jiang JH, Yang YT, et al. Stratified aspartate aminotransferase-to-platelet ratio index accurately predicts survival in hepatocellular carcinoma patients undergoing curative liver resection. Tumour Biol. 2017;39(3) doi: 10.1177/1010428317695944. 1010428317695944. [DOI] [PubMed] [Google Scholar]
- 25.Tang T, Qiu JL, Li GW, et al. Aspartate aminotransferase-to-platelet ratio predicts response to transarterial chemoembolisation and prognosis in hepatocellular carcinoma patients. Clin Radiol. 2018;73(3):259–265. doi: 10.1016/j.crad.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Wai CT, Cheng CL, Wee A, et al. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int. 2006;26(6):666–672. doi: 10.1111/j.1478-3231.2006.01287.x. [DOI] [PubMed] [Google Scholar]
- 27.Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. doi: 10.1186/1471-230X-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate amino-transferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46(3):912–921. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 29.Elsebae MM, Abu-Zekri NB. A study of the effect of splenectomy on hepatic functional reserve and structural damage in patients with chronic hepatitis C virus infection by non-invasive serum markers. A prospective study. Int J Surg. 2008;6(5):362–366. doi: 10.1016/j.ijsu.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Chen SL, Xue N, Wu MT, et al. Influence of preoperative serum aspartate aminotransferase (AST) level on the prognosis of patients with non-small cell lung cancer. Int J Mol Sci. 2016;17(9) doi: 10.3390/ijms17091474. pii:E1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan G, Gao F, Chen J, et al. Nomogram prediction of individual prognosis of patients with hepatocellular carcinoma. BMC Cancer. 2017;17(1):91. doi: 10.1186/s12885-017-3062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamimoto Y, Horiuchi S, Tanase S, Morino Y. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology. 1985;5(3):367–375. doi: 10.1002/hep.1840050305. [DOI] [PubMed] [Google Scholar]
- 33.Kumada T, Toyoda H, Kiriyama S, et al. Predictive value of tumor markers for hepatocarcinogenesis in patients with hepatitis C virus. J Gastroenterol. 2011;46(4):e44. doi: 10.1007/s00535-010-0349-7. [DOI] [PubMed] [Google Scholar]
- 34.Kim HC, Nam CM, Jee SH, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. Br Med J. 2004;328(7446):983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toghill PJ, Green S, Ferguson F. Platelet dynamics in chronic liver disease with special reference to the role of the spleen. J Clin Pathol. 1977;30(4):367–371. doi: 10.1136/jcp.30.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mccormick PA, Murphy KM. Splenomegaly, hypersplenism and coagulation abnormalities in liver disease. Baillieres Best Pract Res Clin Gastroenterol. 2000;14(6):1009–1031. doi: 10.1053/bega.2000.0144. [DOI] [PubMed] [Google Scholar]
- 37.Tomimaru Y, Eguchi H, Gotoh K, et al. Platelet count is more useful for predicting posthepatectomy liver failure at surgery for hepatocel-lular carcinoma than indocyanine green clearance test. J Surg Oncol. 2016;113(5):565–569. doi: 10.1002/jso.24166. [DOI] [PubMed] [Google Scholar]
- 38.Maithel SK, Kneuertz PJ, Kooby DA, et al. Importance of low preoperative platelet count in selecting patients for resection of hepa-tocellular carcinoma: a multi-institutional analysis. J Am Coll Surg. 2011;212(4):638–648. doi: 10.1016/j.jamcollsurg.2011.01.004. discussion 648–650. [DOI] [PMC free article] [PubMed] [Google Scholar]