Abstract
Although the technique of pars plana vitrectomy (PPV) develops rapidly, scleral buckling (SB) has several advantages over PPV for rhegmatogenous retinal detachment (RRD), including early visual rehabilitation and prevention of cataract progression. It is recommended to select the primary procedure for RRD by considering the advantages and disadvantages of each procedure based on the patient status. The vitreous body status affects the features of RRD. Vitreous liquefaction is an age-dependent process, resulting in the development of posterior vitreous detachment (PVD). RRD is usually associated with PVD, typically presenting with a retinal tear, strong vitreoretinal traction, and bullous detachment. In contrast, RRD may develop without PVD, and typically presents with a small atrophic hole, shallow detachment, and slow progression. RRD with less liquefied vitreous and no PVD can be managed successfully with SB alone even in the presence of subretinal strand as less liquefied vitreous acts as bio-tamponade blocking fluid passage. The strong traction induced by PVD and bullous detachment in an eye with extensively liquefied vitreous reduces the success rate of SB. PPV is gaining popularity as the primary procedure for RRD, especially in eyes with retinal tears, PVD, or pseudophakia. Nevertheless, SB remains the preferred procedure in young phakic patients without PVD.
Keywords: age, myopia, posterior vitreous detachment, rhegmatogenous retinal detachment, scleral buckling, vitreous
Introduction
Rhegmatogenous retinal detachment (RRD) is a potentially blinding disease characterized by the separation of the inner neurosensory retina from the outer retinal pigment epithelium because of a break in the structural integrity of the sensory retina.1 Primary RRD results from formation of a retinal break, vitreoretinal traction, and entry of the liquefied vitreous through the break.2 The prevalence of RRD ranges from 6.3 to 17.9 per 100,000, with the highest incidence in people who are in their sixties.1,3 Before the era of scleral buckling (SB), most RRD progressed to complete retinal detachment and resulted in the loss of vision of the affected eye. In the 1950s, SB was introduced, which allowed performance of surgical treatment for RRD.4 Even after the advent of pars plana vitrectomy (PPV), which was introduced as a new treatment option by Robert Machemer,5 SB had been the standard technique for RRD for several decades, and PPV was considered as a supplemental procedure to SB in complicated cases, such as proliferative vitreoretinopathy (PVR). Evolution of vitrectomy machines and related instruments has significantly increased the application of PPV in recent years.6–10
There have been several clinical trials comparing the two methods.11–16 The scleral buckling vs primary vitrectomy in RRD (SPR) study16 was the largest randomized clinical trial, and it showed that anatomic and functional outcomes of the two methods were comparable. Apparently, PPV has become more popular as the primary procedure for management of RRD. SB is sometimes considered an uncomfortable outdated operation for the surgeon compared to PPV, as it required more anesthesia and repeated taking on and off the indirect ophthalmoscope. In addition, SB might induce change of refractive errors or diplopia postoperatively. Nevertheless, SB has apparent merits over PPV in selected cases.
The purpose of this review was to summarize the latest reports on the management of RRD and to suggest management guidelines for choosing a surgical method in patients with RRDs.
Pathogenesis of RRD
Vitreous humor is liquefied with aging; hyaluronic acid and collagen molecular networks change, and vitreous fibers aggregate (vitreous syneresis).17 The rate of vitreous liquefaction is affected by various ocular conditions, including genetic abnormality,18,19 myopia, previous intraocular surgery,20,21 inflammation,22 or trauma.20,23 The vitreous may be liquefied congenitally in genetic disorders, such as the Stickler’s syndrome and Wagner syndrome; in such cases, the vitreous can be observed as an optically empty space.18,19 Although the status of the liquefied vitreous can be evaluated using biomicroscopy and optical coherence tomography (OCT),24 there is no objective grading system for clinical use. It is even more difficult to assess the degree of vitreous liquefaction in an eye with RRD because the liquefied vitreous migrates into the subretinal space. The height of the retinal detachment can be a clue: bullous detachment implies more liquefaction and shallow detachment less liquefaction.
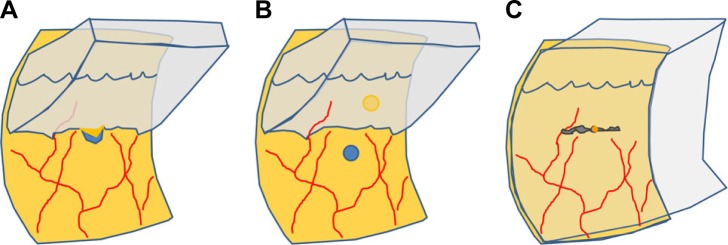
With aging, the vitreous attachments to the retina weaken; this results in posterior vitreous detachment (PVD), in which the space between the detached vitreous and retina is filled with liquefied vitreous.25 PVD causes contraction of the formed vitreous with forward displacement of the vitreous toward the base, which generates traction on the peripheral retina, and occasionally this may result in a retinal tear and subsequent RRD.25 The development of RRD typically involves the convergence of three factors: PVD, one or more full-thickness breaks in the retina, and passage of fluid from the vitreous cavity through the retinal breaks into the potential subretinal space.26 However, RRD without PVD can also develop, and it is characterized by a small atrophic hole, chronic progression, and shallow detachment27,28 (Figure 1); in such cases, spontaneous resolution of RRD may occur.29
Figure 1.
Two patterns of RRD based on the presence of PVD.
Notes: (A) RRD in an eye with PVD has strong traction, retinal tears, and more liquefied vitreous humor. (B) RRD in an eye without PVD has the characteristics of a small atrophic hole, less liquefied vitreous, and shallow detachment.
Abbreviations: PVD, posterior vitreous detachment; RRD, rhegmatogenous retinal detachment; SRF, subretinal fluid.
The vitreous degenerates with age, and myopia,20 cataract surgery, and other intraocular interventions may accelerate this process.20,21 Although PVD occurs as an acute event, it is a consequence of lifelong vitreous liquefaction and highly age-dependent. The incidence of PVD is less than 10% in people younger than 60 years, 27% in the seventh decade of life, and 63% in those in the eighth decade.30 PVD is defined as the separation of the posterior cortical vitreous from the internal limiting membrane. Complete PVD (stage IV) refers to the separation of the posterior cortical vitreous from the optic nerve head.31 Although PVD can be assessed using biomicroscopy or ultrasonography, OCT is the best method to confirm the development of PVD.20,32,33 Presence of adhesions between the posterior cortical vitreous and optic nerve head on OCT indicates that PVD has not occurred yet. The status of the detached retina interferes with reliable evaluation of PVD in an OCT image, and there is no published report on the evaluation of PVD using OCT in patients with RRD.
A retinal break can be classified into an operculum tear, a horse-shoe tear, or an atrophic hole. Based on the presence of PVD, the shape of retinal break can vary (Figure 2). In eyes with PVD, a retinal break tends to have a larger size. The vitreous may continue to adhere to the anterior margin of the retinal tear, which has a shape of a horse shoe (Figure 2A). When the retinal tissue adhering to the vitreous is separated from the remaining retina completely, the break usually has a round shape and is called operculum tear (Figure 2B). A retinal break in eyes without PVD tends to be small and forms an atrophic hole in most cases (Figure 2C).27
Figure 2.
Different patterns of a retinal break in rhegmatogenous retinal detachment associated with PVD.
Notes: (A) A horse-shoe tear has vitreoretinal adhesion on the anterior margin of the break. This retinal break can be seen in eyes with PVD. (B) An operculum tear has the margin separated from the vitreous; this tear can also be found in eyes with PVD. (C) An atrophic hole is not related to PVD. Vitreous humor may be less liquefied, and vitreous traction is not always strong.
Abbreviation: PVD, posterior vitreous detachment.
Phakic eyes
RRD can be repaired using either SB or PPV, and the choice depends on various factors. Generally, PPV has several advantages, including less pain and better management of vitreous pathology, and better management of multiple, large, or posterior breaks. On the other hand, advantages of SB include prevention of cataract progression, early visual rehabilitation, and absence of position restriction after the operation.
Many prospective and retrospective studies have reported the outcomes of the two methods in phakic eyes.7,16,34–38 A multicenter, prospective clinical trial16 showed that visual outcomes of SB were superior to those of PPV and there is no significant difference between PPV and SB with respect to the single surgery success rate (SSSR). Most of the published articles have reported similar results in the two methods: 74%–94% with SB vs 75%–96% with PPV.7,16,34–38 Despite noninferior outcomes of SB in these reports, it appears that the recent development in PPV-related technology has made PPV more popular as the primary procedure in the management of phakic RRD.10,39
The presence of the lens is a limitation during the performance of PPV. The lens limits the visualization of the periphery as well as the range of the instrument movement, and progression of postoperative cataract impairs visual recovery. However, use of a wide-viewing system and a combined surgery with phacoemulsification can overcome these disadvantages. Recently, the SSSR of PPV has been reported to be 95% or more.6,7,9,40 We have also reported a retrospective study evaluating the recent advances in PPV, which showed that PPV was superior to SB in older patients in terms of SSSR and visual outcomes.7
Nevertheless, SB is still advantageous with respect to the avoidance of postoperative cataract and achievement of early visual recovery; this is especially important for young patients, for whom loss of accommodation would significantly affect the visual function.
Pseudophakia
The structure of the vitreous in a pseudophakic eye is different from that in a phakic eye. Patients with pseudophakic RRD tends to be older (58–73 years old).16,41,42 Cataract surgery removes the lens volume, which induces changes in the vitreous structure. PVD was observed to occur in 73% of patients within 6 months after cataract surgery.20 Based on the above findings, patients with pseudophakic RRD are more likely to have advanced vitreous liquefaction and PVD.
Capsular opacity in pseudophakia hinders the observation of the periphery. It is difficult to find a small break in the periphery using an indirect ophthalmoscope, which has a low magnifying power. Performance of PPV using a wide-angle viewing system mounted on a microscope provides definite advantages in the management of pseudophakic RRD with respect to peripheral visualization at higher magnification by using the zoom function of the microscope.
The superior outcomes of PPV against SB have been reported consistently in pseudophakic RRD.16,41–43 In the SPR study, the SSSR of SB was 53.4%, which was lower than 72.0% of PPV in pseudophakia.16 These success rates appeared quite low because of the strict criteria compared to the real-world data. Other studies reported that the SSSR of SB was 76%–83%, which were lower than 84%–94% of PPV.41,42 In addition, the SPR study reported that the SSSR of SB was lower in pseudophakic RRD than phakic RRD (63.6%).16 Pseudophakia is a poor prognostic factor in the management of RRD using SB,16 but not when using PPV.44 There is no need to concern cataract progression in such eyes. PPV is widely accepted as the primary treatment option for pseudophakic RRD.
High myopia
High myopia is generally defined by a refractive error of −6 diopters or more or an axial length exceeding 26 mm. The prevalence of high myopia is higher in East Asia.45 The prevalence of RRD is higher in highly myopic eyes because of the alterations in the vitreous.46 An highly myopic eye has a long axial length with thin sclera, develops premature vitreous liquefaction and earlier PVD, and has the complex vitreous–retinal interfaces.20,46–48 Although these factors are considered to be disadvantageous for SB, they are for PPV as well. The standard instruments may be too short to reach the posterior pole.49 The residual vitreous cortex may be attached to the retina even in eyes with apparent complete PVD.50 The thin vitreous cortex is difficult to remove completely, and might be related to recurrence and development of PVR.
The success rate of SB in subjects with high myopic RRD did not appear to be worse than the general success rate. The SSSR of SB in RRD in highly myopic eyes is 84.8%–86.3%,51–54 and for RRD including both non-high myopic and high myopic eyes is 73.7%–88.8%.8,16,41,55,56 These studies had patients with different baseline characteristics and cannot be compared directly. The ages of the subjects should especially be considered. The average age was 33.2–35.9 in the studies among patients with high myopia,51–53 which was younger than 38.9–61.3 in the studies among patients with RRD including non-high myopia and high myopia.8,16,41,55,56 It suggests that SB was performed in younger age in high myopic patients. We previously evaluated the prognostic factors for SB in patients with high myopia.54 Axial length did not have a significant impact on SSSR, whereas age was a significant prognostic factor. As a patient got older by 1 year, the SSSR decreased 1.086 times. The SSSR in patients with high myopia was comparable to that in patients including non-high myopic and high myopic eyes; this observation was probably related to younger age of the subjects, implying that the degree of vitreous liquefaction may be a critical factor. Accordingly, SB remains a good treatment option for high myopic RRD, which tends to develop in young patients who still have phakic eyes.
Proliferative vitreoretinopathy
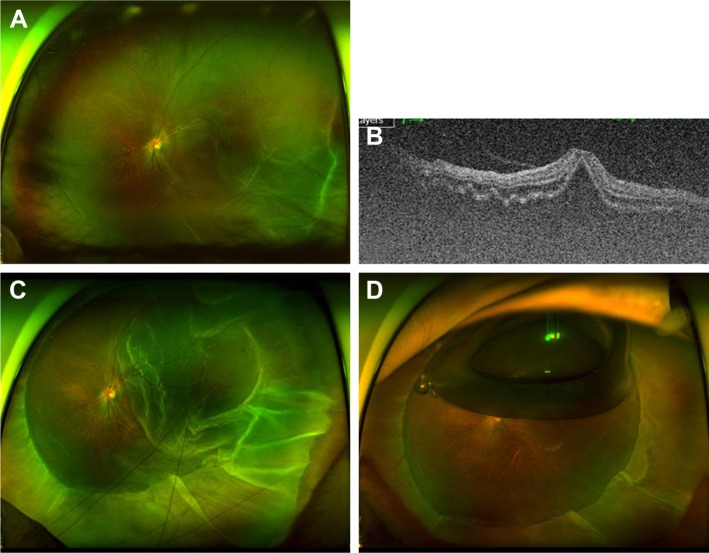
It is challenging to manage RRD combined with severe PVR using SB alone, and PPV or PPV combined with SB should be considered as the procedure of choice.57,58 One of the exceptions is chronic RRDs with subretinal strand. Although subretinal strand is classified as grade C PVR,57 the surgical outcomes of SB in eyes with subretinal strands have been reported to be excellent.59,60 Yao et al59 have suggested that patients with chronic RRD with subretinal strands are good candidates to undergo SB alone. The high success rate of SB in this group of RRD may be related to the degree of vitreous liquefaction. In two cohort studies,59,60 the patients shared common baseline characteristics of young age (26.7 and 26.5 years), shallow retinal detachment, and small retinal breaks.
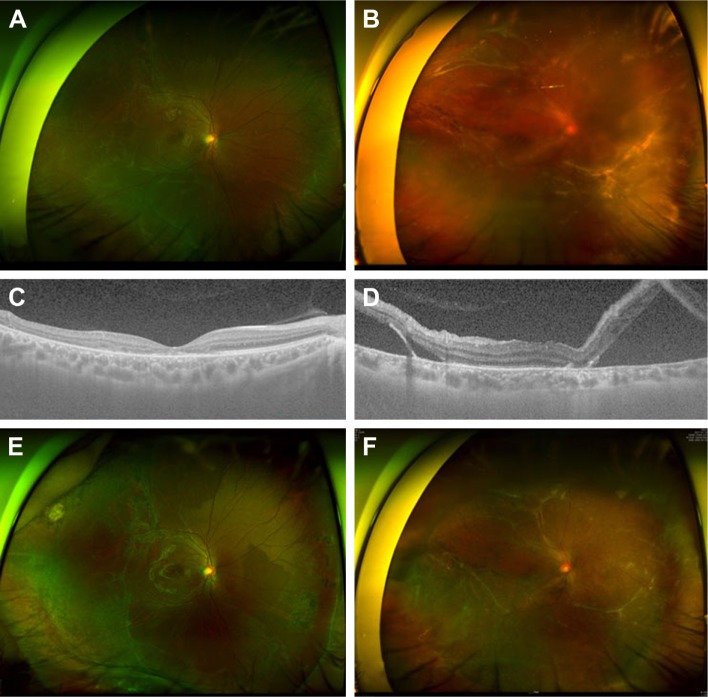
Two cases with chronic RRD with subretinal strands and atrophic holes are presented in Figure 3. Both had chronic RRD with small atrophic holes, and their ages were 26 and 32 years. They did not have PVD on OCT imaging (Figure 3B and D), and RRD was successfully treated using scleral encircling only (Figure 3E and F).
Figure 3.
Chronic rhegmatogenous retinal detachment with subretinal strand.
Notes: (A and B) Preoperative wide photographs depicting the common characteristics of the small atrophic hole and shallow retinal detachment, which are indicators of a less liquefied vitreous. (C and D) Posterior vitreous detachment was not observed in preoperative or postoperative optical coherence tomography imaging. (E and F) The retina was attached after scleral encircling alone without pars plana vitrectomy despite extensive subretinal strand.
Even if the subretinal strand is complicated, SB should be considered as a primary treatment option for RRD in young patients who have less liquefied vitreous and do not have PVD.
Vitreous status and SB surgery
The selection of the method to treat RRD depends on various factors, including the surgeon’s preference. Generally, SB is considered as an ideal procedure for RRD in young age, in phakic eyes, and in limited retinal detachment, particularly with a hole in the lattice or an inferior location.61–63
The purpose of SB is to reduce the vitreous traction and close the retinal break. During SB, the sclera is pressed inward so that the vitreous traction is lessened to close the retinal break. Accurate placement of the buckle element with an adequate height is the key factor for a successful SB surgery. The actual procedure is highly dependent on the surgeon, and the buckling effect is determined by the surgeon’s preference and skill regarding the buckle element selection and suture tightness. A high buckle effect may reduce vitreous traction by a high amount, but it may be associated with complications, including development of astigmatism, scleral cheese-wiring, and a fish-mouth configuration. A weak effect may result in surgical failure. Because PVD is associated with a stronger traction and a larger tear, a high and wide buckling effect is commonly required. On the other hand, a lower and narrower SB can adequately induce reattachment of the retina in RRD without PVD.
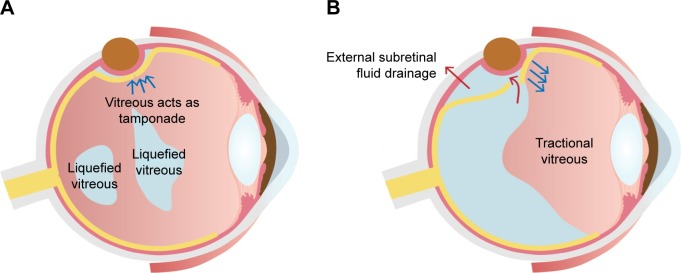
In addition to the previously mentioned variables, vitreous liquefaction and PVD seem to be important factors in SB for RRD. Presence of PVD affects the development of RRD, the shape of the retinal break, and the difficulty of SB surgery. Vitreous liquefaction can impact not only the progression of retinal detachment but also the closure of the retinal break and drainage of subretinal fluid (SRF). SB is suitable for RRD with less liquefied vitreous humor without PVD (Figure 4A), whereas SB alone is not suitable for RRDs with fluidic vitreous humor and PVD (Figure 4B). The vitreous body would be an important factor in the closure of the retinal breaks during SB because it plays a role of an intraocular tamponade to block the passage of fluid55 (Figure 4A). The formed vitreous can be called a “bio-tamponade”. A retinal break is closed effectively by SB without additional procedures in RRD associated with the less liquefied vitreous. On the contrary, traction to the retinal break associated with PVD and vitreous liquefaction may prevent break closure (Figure 4B), and external drainage of the SRF or injection of gas is required sometimes (Figure 5).
Figure 4.
Different effects of SB according to the vitreous status.
Notes: (A) The figure depicts the effects of SB in RRD with less liquefied vitreous and no PVD. Because the tractional force is not strong and the size of the retinal break is small, a small narrow buckle effect can adequately close the break. Less liquefied vitreous may play a role as tamponade (bio-tamponade) to close the retinal break. (B) The figure shows that RRD in eyes with PVD and fluidic vitreous humor. Because RRD with PVD has strong vitreous traction and large retinal tear, high and wide buckle effect is usually required. Because the bio-tamponade effect of the formed vitreous is lacking, external drainage of subretinal fluid or gas tamponade may be needed to close the retinal tear.
Abbreviations: PVD, posterior vitreous detachment; RRD, rhegmatogenous retinal detachment; SB, scleral buckling.
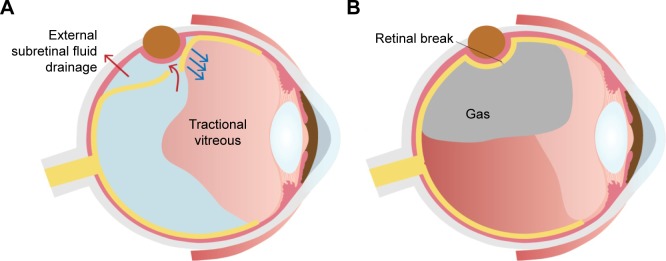
Figure 5.
Intraocular gas tamponade can be an effective adjuvant for scleral buckling when external drainage of SRF fails to close the break because of the extensively liquefied vitreous.
Notes: (A) When the liquefied vitreous enters the subretinal space and is drained externally, the retinal break cannot be closed using external drainage of SRF. (B) Injection of intraocular gas is required to close the retinal tear by preventing the flow of the liquefied vitreous into the subretinal space.
Abbreviation: SRF, subretinal fluid.
The role of the adjunctive procedures is still limited in patients with extensive vitreous liquefaction and a large tear, because the liquefied vitreous may migrate through the retinal break, and the vitreous can be removed externally with SRF, causing severe hypotony (Figure 5A). A case in Figure 6C is an example of a failure to remove the SRF. External drainage of SRF resulted in drainage of the liquefied vitreous and hypotony without lowering the height of the detachment. Intravitreal injection of a balanced salt solution did not improve the situation, and intraoperative reattachment of the retina failed.
Figure 6.
A case of RRD with fluidic vitreous humor without PVD. A 12-year-old girl presented with visual loss for 3 days.
Notes: (A) RRD with small atrophic hole was noticed on the inferotemporal quadrant. (B) Preoperative optical coherent tomography showed vitreous–fovea adhesion (no PVD). (C) Although scleral encircling was performed with a high buckle effect, the retinal hole was not closed. External drainage also failed because the liquefied vitreous entered the subretinal space during the drainage. (D) After intravitreal injection of C3F8 (0.3 cc), the retina was reattached.
Abbreviations: PVD, posterior vitreous detachment; RRD, rhegmatogenous retinal detachment.
As mentioned above, it is difficult to assess the degree of vitreous liquefaction in an eye with RRD. Because vitreous liquefaction is an age-dependent process, the patient’s age can be used as an indirect indicator for vitreous liquefaction. In a comparison SSSR among retrospective studies with similar populations and designs, it was observed that the success rate was lower in patients with older age groups. Kobashi et al38 reported 93.7% of SSSR in patients with mean age of 43.3 years, Wong et al8 reported 88.8% in 47.3 years old, and Park et al7 reported 77.8% in 54.4 years old. We compared the results of SB between two age groups: 35 years or older vs younger than 35 years.55 The SSSR of SB was 92.3% in the younger age group (average, 23.2 years) and 79.0% in the older age group (average, 62.0 years). The clinical characteristics were remarkably different. The younger group was correlated with slow progression, small break, and higher SSSR, whereas the older group had the opposite findings.55 These results can be explained by the fact that the younger group represented RRD with less liquefied vitreous humor and the older group represented more liquefied vitreous. SB is still considered as the primary treatment option in young patients based on a recent study of trend in the nationwide insurance claims in South Korea although the application of PPV had gradually increased.39
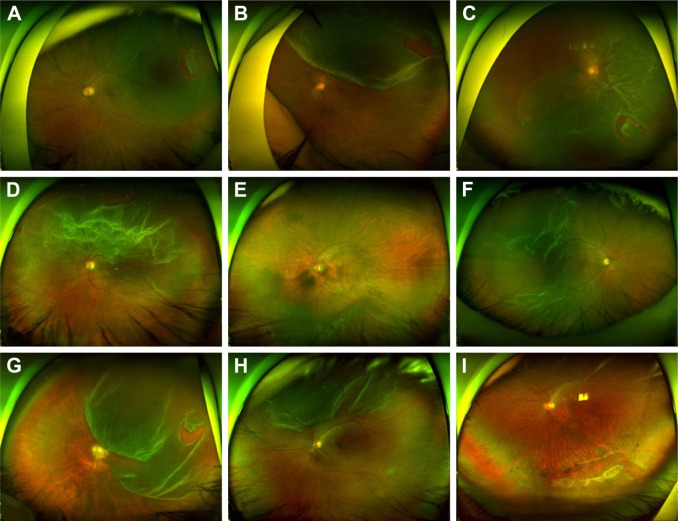
Vitreous liquefaction and PVD are not necessarily correlated. Rarely, vitreous may be significantly liquefied even without PVD. Figure 6 depicts an example, in which a 12-year-old girl presented with RRD. She had no history of trauma or uveitis, and myopia was −2 diopters. Although the vitreopapillary adhesion was confirmed by OCT examination (Figure 6B), the vitreous was liquefied extensively. The detached retina fluttered in the vitreous cavity and RRD progressed rapidly. The encircling procedure failed to close the small hole even with external drainage of the SRF (Figure 6C). After intravitreal injection of C3F8, the retinal hole was closed to achieve reattachment of the retina (Figure 6D). Conversely, some patients have RRD with PVD and less liquefied vitreous humor. They usually have relatively shallow retinal detachments and small breaks (Figure 7E and I).
Figure 7.
Cases of rhegmatogenous retinal detachment less suitable for SB alone. PVD, bullous retinal detachment, and a relatively large retinal tear are noted.
Notes: (A–H) Pars plana vitrectomy was performed as the primary operation, and the retina was reattached. (I) The retina was not attached after the SB procedure. Reattachment was achieved after the pars plana vitrectomy. (A–I) PVD was confirmed using preoperative optical coherent tomography and intraoperative vitreous staining using triamcinolone.
Abbreviations: PVD, posterior vitreous detachment; SB, scleral buckling.
Patient selection and perspective
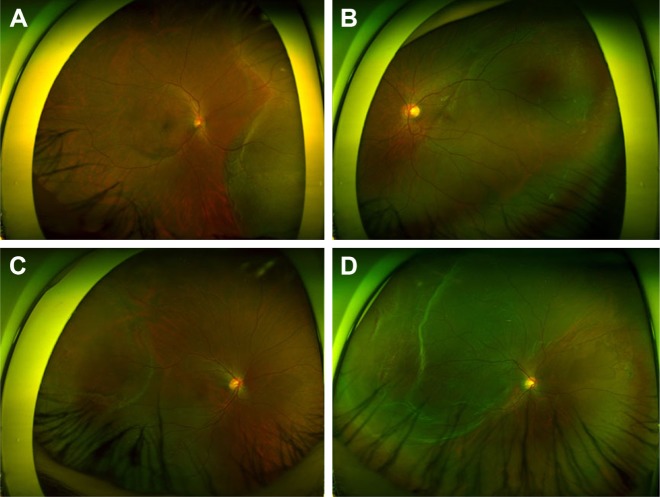
The best candidate for SB in the management of RRD is a young patient without PVD and less liquefied vitreous (Figure 8). This group is characterized by younger age, small atrophic hole, slow progression, and shallow detachment. In RRD with less liquefied vitreous humor and no PVD, the tractional force is so low that the size of retinal break is usually small and detachment progression is slow (Figure 8); in such eyes, peripheral and localized RRD is usually asymptomatic and usually found by accident. RRDs progress in a stop-and-go fashion, which leaves demarcation lines. In cases with localized RRD, barrier laser photocoagulation may be sufficient to prevent the progression. Even in cases with involvement of the fovea, emergent operation is not necessary because early intervention seldom results in remarkable improvement of vision. Because the formed vitreous works as a bio-tamponade (Figure 4A), the success rate of SB alone is excellent, even in cases with grade C PVR associated with a complicated subretinal strand.59,60 If the subretinal strand is widely invasive, scleral encircling should be considered (Figure 3). Shallow SRF may persist for quite a long time along the subretinal strand, of which removal is not necessarily indicated unless the SRF increases so much that opens the break causing a recurrence of RRD.
Figure 8.
Cases that are good candidates for scleral buckling.
Notes: (A–D) Four cases share the characteristics of having no posterior vitreous detachment, shallow retinal detachment, less liquefied vitreous, and a small atrophic hole. Adhesion between the vitreous and optic disc head was confirmed using preoperative and postoperative optical coherent tomography in all cases.
SB is less preferred for RRD with fluidic vitreous humor and PVD (Figure 7A–H). RRD with PVD is associated with strong vitreous traction that causes a large retinal tear and rapid progression. Vitreous liquefaction is usually advanced in these eyes, and retinal detachment is usually bullous. Early intervention is indicated when the fovea is spared or involved recently. A large retinal tear with strong vitreous traction requires high and large buckle effect. The bio-tamponade effect of the vitreous is weak, and external drainage of the SRF or gas tamponade may be needed (Figure 5). PPV is preferred as the primary operation in this kind of RRD.
PPV is recommended for severe PVR, media opacity, or posterior breaks difficult to be covered with SB.64 However, the risk of cataract progression should be considered in a young phakic patient. When performing combined phacoemulsification and PPV, postoperative anisometropia must also be considered in patients with high myopia. Although PPV is generally preferred as the more comfortable procedure for the surgeon, RRD with multiple breaks can be treated using SB or encircling successfully when accurate marking and buckle placement are performed for every break.
Conclusion
In summary, SB has clear advantages over PPV and will remain as a primary treatment option in selected cases, although the advancement of PPV techniques has yielded excellent outcomes. SB has a high success rate, especially in young and phakic patients, even in subjects with high myopia. Vitreous status appears to be important regarding reattachment with SB for RRD. For the intermediate cases between the above extreme groups, the selection of the primary procedure is at the treating surgeon’s discretion. A thorough examination of the vitreous status will provide the information for determining the surgical plan: SB vs PPV.
Footnotes
Disclosure
Lee JE is a consultant for Allergan, Bayer, and Novartis, and received honorarium from Alcon, Allergan, Bayer, and Novartis. The other authors report no conflicts of interest in this work.
References
- 1.Park SJ, Choi NK, Choi NK, Park KH, Woo SJ. Five year nationwide incidence of rhegmatogenous retinal detachment requiring surgery in Korea. PLoS One. 2013;8(11):e80174. doi: 10.1371/journal.pone.0080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitry D, Fleck BW, Wright AF, Campbell H, Charteris DG. Pathogenesis of rhegmatogenous retinal detachment: predisposing anatomy and cell biology. Retina. 2010;30(10):1561–1572. doi: 10.1097/IAE.0b013e3181f669e6. [DOI] [PubMed] [Google Scholar]
- 3.Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010;94(6):678–684. doi: 10.1136/bjo.2009.157727. [DOI] [PubMed] [Google Scholar]
- 4.Schepens CL, Okamura ID, Brockhurst RJ. The scleral buckling procedures. I. Surgical techniques and management. AMA Arch Ophthalmol. 1957;58(6):797–811. doi: 10.1001/archopht.1957.00940010819003. [DOI] [PubMed] [Google Scholar]
- 5.Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(4):813–820. [PubMed] [Google Scholar]
- 6.Pak KY, Lee SJ, Kwon HJ, Park SW, Byon IS, Lee JE. Exclusive use of air as gas tamponade in rhegmatogenous retinal detachment. J Ophthalmol. 2017;2017:1341948. doi: 10.1155/2017/1341948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SW, Kwon HJ, Kim HY, Byon IS, Lee JE, Oum BS. Comparison of scleral buckling and vitrectomy using wide angle viewing system for rhegmatogenous retinal detachment in patients older than 35 years. BMC Ophthalmol. 2015;15:121. doi: 10.1186/s12886-015-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CW, Wong WL, Yeo IY, et al. Trends and factors related to outcomes for primary rhegmatogenous retinal detachment surgery in a large Asian tertiary eye center. Retina. 2014;34(4):684–692. doi: 10.1097/IAE.0b013e3182a48900. [DOI] [PubMed] [Google Scholar]
- 9.Schneider EW, Geraets RL, Johnson MW. Pars plana vitrectomy without adjuvant procedures for repair of primary rhegmatogenous retinal detachment. Retina. 2012;32(2):213–219. doi: 10.1097/IAE.0b013e3182278b29. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz SG, Flynn HW. Pars plana vitrectomy for primary rhegmatogenous retinal detachment. Clin Ophthalmol. 2008;2(1):57–63. doi: 10.2147/opth.s1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feltgen N, Heimann H, Hoerauf H, Walter P, Hilgers RD, Heussen N, Writing Group For The SPR Study Investigators Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment study (SPR study): risk assessment of anatomical outcome. SPR study report no. 7. Acta Ophthalmol. 2013;91(3):282–287. doi: 10.1111/j.1755-3768.2011.02344.x. [DOI] [PubMed] [Google Scholar]
- 12.Heussen N, Feltgen N, Walter P, Hoerauf H, Hilgers RD, Heimann H, SPR Study Group Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment study (SPR Study): predictive factors for functional outcome. Study report no. 6. Graefes Arch Clin Exp Ophthalmol. 2011;249(8):1129–1136. doi: 10.1007/s00417-011-1619-7. [DOI] [PubMed] [Google Scholar]
- 13.Heussen N, Hilgers RD, Heimann H, Collins L, Grisanti S, SPR study group Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment study (SPR study): multiple-event analysis of risk factors for reoperations. SPR Study report no. 4. Acta Ophthalmol. 2011;89(7):622–628. doi: 10.1111/j.1755-3768.2009.01766.x. [DOI] [PubMed] [Google Scholar]
- 14.Feltgen N, Weiss C, Wolf S, Ottenberg D, Heimann H, SPR Study Group Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment study (SPR Study): recruitment list evaluation. Study report no. 2. Graefes Arch Clin Exp Ophthalmol. 2007;245(6):803–809. doi: 10.1007/s00417-006-0399-y. [DOI] [PubMed] [Google Scholar]
- 15.Heimann H, Hellmich M, Bornfeld N, Bartz-Schmidt KU, Hilgers RD, Foerster MH. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment (SPR Study): design issues and implications. SPR Study report no. 1. Graefes Arch Clin Exp Ophthalmol. 2001;239(8):567–574. doi: 10.1007/s004170100319. [DOI] [PubMed] [Google Scholar]
- 16.Heimann H, Bartz-Schmidt KU, Bornfeld N, Weiss C, Hilgers RD, Foerster MH, Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment Study Group Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114(12):2142–2154. doi: 10.1016/j.ophtha.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Spitzer MS, Januschowski K. Aging and age-related changes of the vitreous body. Ophthalmologe. 2015;112552(7):554–558. doi: 10.1007/s00347-015-0031-9. [DOI] [PubMed] [Google Scholar]
- 18.Snead MP, Mcninch AM, Poulson AV, et al. Stickler syndrome, ocular-only variants and a key diagnostic role for the ophthalmologist. Eye. 2011;25(11):1389–1400. doi: 10.1038/eye.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meredith SP, Richards AJ, Flanagan DW, Scott JD, Poulson AV, Snead MP. Clinical characterisation and molecular analysis of Wagner syndrome. Br J Ophthalmol. 2007;91(5):655–659. doi: 10.1136/bjo.2006.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degirmenci C, Afrashi F, Mentes J, Oztas Z, Nalcaci S, Akkin C. Evaluation of posterior vitreous detachment after uneventful phacoemulsification surgery by optical coherence tomography and ultrasonography. Clin Exp Optom. 2017;100(1):49–53. doi: 10.1111/cxo.12417. [DOI] [PubMed] [Google Scholar]
- 21.Geck U, Pustolla N, Baraki H, Atili A, Feltgen N, Hoerauf H. Posterior vitreous detachment following intravitreal drug injection. Graefes Arch Clin Exp Ophthalmol. 2013;251(7):1691–1695. doi: 10.1007/s00417-013-2266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogan MJ. Inflammation and its effect on the vitreous. Trans Ophthalmol Soc U K. 1975;95(3):378–381. [PubMed] [Google Scholar]
- 23.Raymond LA, Choromokos E, Bibler LW, Spaulding AG, Alexander DW, Kao WW. Change in vitreous collagen after penetrating injury. Ophthalmic Res. 1985;17(2):102–105. doi: 10.1159/000265358. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Kishi S, Itakura H, Ikeda F, Akiyama H. Posterior precortical vitreous pockets and connecting channels in children on swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55(4):2412–2416. doi: 10.1167/iovs.14-13967. [DOI] [PubMed] [Google Scholar]
- 25.Grossniklaus HE, Nickerson JM, Edelhauser HF, Bergman LA, Berglin L. Anatomic alterations in aging and age-related diseases of the eye. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF23–27. doi: 10.1167/iovs.13-12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Amico DJ. Clinical practice. Primary retinal detachment. N Engl J Med. 2008;359(22):2346–2354. doi: 10.1056/NEJMcp0804591. [DOI] [PubMed] [Google Scholar]
- 27.Mitry D, Singh J, Yorston D, et al. The predisposing pathology and clinical characteristics in the Scottish retinal detachment study. Ophthalmology. 2011;118(7):1429–1434. doi: 10.1016/j.ophtha.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 28.Williams KM, Dogramaci M, Williamson TH. Retrospective study of rhegmatogenous retinal detachments secondary to round retinal holes. Eur J Ophthalmol. 2012;22(4):635–640. doi: 10.5301/ejo.5000080. [DOI] [PubMed] [Google Scholar]
- 29.Park JY, Shin MK, Park SW, Byon IS, Lee JE. Two cases of acute spontaneous resolution in macula-off rhegmatogenous retinal detachment. J Korean Ophthalmol Soc. 2015;56(3):466–470. [Google Scholar]
- 30.Foos RY, Wheeler NC. Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982;89(12):1502–1512. doi: 10.1016/s0161-6420(82)34610-2. [DOI] [PubMed] [Google Scholar]
- 31.Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149382(3):371–382. doi: 10.1016/j.ajo.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Ripandelli G, Coppé AM, Parisi V, Olzi D, Scassa C, Chiaravalloti A, Stirpe M. Posterior vitreous detachment and retinal detachment after cataract surgery. Ophthalmology. 2007;114(4):692–697. doi: 10.1016/j.ophtha.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 33.Höhn F, Mirshahi A, Hattenbach LO. Optical coherence tomography for diagnosis of posterior vitreous detachment at the macular region. Eur J Ophthalmol. 2009;19(3):442–447. doi: 10.1177/112067210901900319. [DOI] [PubMed] [Google Scholar]
- 34.Azad RV, Chanana B, Sharma YR, Vohra R. Primary vitrectomy versus conventional retinal detachment surgery in phakic rhegmatogenous retinal detachment. Acta Ophthalmol Scand. 2007;85(5):540–545. doi: 10.1111/j.1600-0420.2007.00888.x. [DOI] [PubMed] [Google Scholar]
- 35.Koriyama M, Nishimura T, Matsubara T, Taomoto M, Takahashi K, Matsumura M. Prospective study comparing the effectiveness of scleral buckling to vitreous surgery for rhegmatogenous retinal detachment. Jpn J Ophthalmol. 2007;51(5):360–367. doi: 10.1007/s10384-007-0463-0. [DOI] [PubMed] [Google Scholar]
- 36.Oshima Y, Yamanishi S, Sawa M, Motokura M, Harino S, Emi K. Two-year follow-up study comparing primary vitrectomy with scleral buckling for macula-off rhegmatogenous retinal detachment. Jpn J Ophthalmol. 2000;44(5):538–549. doi: 10.1016/s0021-5155(00)00205-7. [DOI] [PubMed] [Google Scholar]
- 37.Miki D, Hida T, Hotta K, Shinoda K, Hirakata A. Comparison of scleral buckling and vitrectomy for retinal detachment resulting from flap tears in superior quadrants. Jpn J Ophthalmol. 2001;45(2):187–191. doi: 10.1016/s0021-5155(00)00377-4. [DOI] [PubMed] [Google Scholar]
- 38.Kobashi H, Takano M, Yanagita T, Shiratani T, Wang G, Hoshi K, Shimizu K. Scleral buckling and pars plana vitrectomy for rhegmatogenous retinal detachment: an analysis of 542 eyes. Curr Eye Res. 2014;39(2):204–211. doi: 10.3109/02713683.2013.838270. [DOI] [PubMed] [Google Scholar]
- 39.Park SJ, Cho SC, Choi NK, Park KH, Woo SJ. Age, sex, and time-specific trends in surgical approaches for rhegmatogenous retinal detachment: a nationwide, population-based study using the National Claim Registry. Retina. 2017;37(12):2326–2333. doi: 10.1097/IAE.0000000000001485. [DOI] [PubMed] [Google Scholar]
- 40.Otsuka K, Imai H, Fujii A, et al. Comparison of 25- and 27-Gauge Pars Plana Vitrectomy in Repairing Primary Rhegmatogenous Retinal Detachment. J Ophthalmol. 2018;2018:7643174. doi: 10.1155/2018/7643174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma YR, Karunanithi S, Azad RV, Vohra R, Pal N, Singh DV, Chandra P. Functional and anatomic outcome of scleral buckling versus primary vitrectomy in pseudophakic retinal detachment. Acta Ophthalmol Scand. 2005;83(3):293–297. doi: 10.1111/j.1600-0420.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- 42.Brazitikos PD, Androudi S, Christen WG, Stangos NT. Primary pars plana vitrectomy versus scleral buckle surgery for the treatment of pseudophakic retinal detachment: a randomized clinical trial. Retina. 2005;25(8):957–964. doi: 10.1097/00006982-200512000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Arya AV, Emerson JW, Engelbert M, Hagedorn CL, Adelman RA. Surgical management of pseudophakic retinal detachments: a meta-analysis. Ophthalmology. 2006;113(10):1724–1733. doi: 10.1016/j.ophtha.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 44.Lee SJ, Kwon HJ, Park KY, Park SW, Byon IS, Lee JE. Prognostic factors of anatomical success in microincisional vitrectomy for rhegmatogenous retinal detachment. J Korean Ophthalmol Soc. 2016;57(10):1613–1618. [Google Scholar]
- 45.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Stirpe M, Heimann K. Vitreous changes and retinal detachment in highly myopic eyes. Eur J Ophthalmol. 1996;6(1):50–58. doi: 10.1177/112067219600600111. [DOI] [PubMed] [Google Scholar]
- 47.Fisher YL, Slakter JS, Friedman RA, Yannuzzi LA. Kinetic ultrasound evaluation of the posterior vitreoretinal interface. Ophthalmology. 1991;98(7):1135–1138. doi: 10.1016/s0161-6420(91)32166-3. [DOI] [PubMed] [Google Scholar]
- 48.Jaffe NS. Complications of acute posterior vitreous detachment. Arch Ophthalmol. 1968;79(5):568–571. doi: 10.1001/archopht.1968.03850040570012. [DOI] [PubMed] [Google Scholar]
- 49.Singh A, Fawzi AA, Stewart JM. Limitation of 25-gauge vitrectomy instrumentation in highly myopic eyes. Ophthalmic Surg Lasers Imaging. 2007;38(5):437–438. doi: 10.3928/15428877-20070901-17. [DOI] [PubMed] [Google Scholar]
- 50.Philippakis E, Couturier A, Gaucher D, Gualino V, Massin P, Gaudric A, Tadayoni R. Posterior vitreous detachment in highly myopic eyes undergoing vitrectomy. Retina. 2016;36(6):1070–1075. doi: 10.1097/IAE.0000000000000857. [DOI] [PubMed] [Google Scholar]
- 51.Kwok AK, Cheng LL, Tse MW, Cheung EY, Lam DS. Outcomes of primary rhegmatogenous retinal detachment in myopes of five or more diopters. Ophthalmic Surg Lasers. 2002;33(3):188–194. [PubMed] [Google Scholar]
- 52.Bernheim D, Rouberol F, Palombi K, Albrieux M, Romanet JP, Chiquet C. Comparative prospective study of rhegmatogenous retinal detachments in phakic or pseudophakic patients with high myopia. Retina. 2013;33(10):2039–2048. doi: 10.1097/IAE.0b013e31828992ac. [DOI] [PubMed] [Google Scholar]
- 53.Cheng SF, Yang CH, Lee CH, et al. Anatomical and functional outcome of surgery of primary rhegmatogenous retinal detachment in high myopic eyes. Eye. 2008;22(1):70–76. doi: 10.1038/sj.eye.6702527. [DOI] [PubMed] [Google Scholar]
- 54.Kwon H, Shin MK, Park SW, Byon IS, Lee JE, Oum BS. Prognostic factors of anatomical success in scleral buckling for high myopic rhegmatogenous retinal detachment. J Korean Ophthalmol Soc. 2016;57(10):1586–1691. [Google Scholar]
- 55.Park SW, Kwon HJ, Byon IS, Lee JE, Oum BS. Impact of age on scleral buckling surgery for rhegmatogenous retinal detachment. Korean J Ophthalmol. 2017;31(4):328–335. doi: 10.3341/kjo.2016.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koh TH, Choi MJ, Cho SW, Lee TG, Lee JH. Scleral Buckling and Primary Vitrectomy in Simple Rhegmatogenous Retinal Detachment. J Korean Ophthalmol Soc. 2010;51(3):366–371. [Google Scholar]
- 57.Pastor JC. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998;43(1):3–18. doi: 10.1016/s0039-6257(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 58.Storey P, Alshareef R, Khuthaila M, London N, Leiby B, DeCroos C, Kaiser R, Wills PVR Study Group Pars plana vitrectomy and scleral buckle versus pars plana vitrectomy alone for patients with rhegmatogenous retinal detachment at high risk for proliferative vitreoretinopathy. Retina. 2014;34(10):1945–1951. doi: 10.1097/IAE.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 59.Yao Y, Jiang L, Wang ZJ, Zhang MN. Scleral buckling procedures for longstanding or chronic rhegmatogenous retinal detachment with subretinal proliferation. Ophthalmology. 2006;113(5):821–825. doi: 10.1016/j.ophtha.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Ghasemi Falavarjani K, Alemzadeh SA, Modarres M, et al. Scleral buckling surgery for rhegmatogenous retinal detachment with subretinal proliferation. Eye. 2015;29(4):509–514. doi: 10.1038/eye.2014.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Häring G, Wiechens B. Long-term results after scleral buckling surgery in uncomplicated juvenile retinal detachment without proliferative vitreoretinopathy. Retina. 1998;18(6):501–505. doi: 10.1097/00006982-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Lincoff H, Kreissig I. Extraocular repeat surgery of retinal detachment. A minimal approach. Ophthalmology. 1996;103(10):1586–1592. doi: 10.1016/s0161-6420(96)30459-4. [DOI] [PubMed] [Google Scholar]
- 63.Tillery WV, Lucier AC. Round atrophic holes in lattice degeneration – an important cause of phakic retinal detachment. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81(3 Pt 1):509–518. [PubMed] [Google Scholar]
- 64.Ryan EH, Mittra RA. Scleral buckling vs vitrectomy: the continued role for scleral buckling in the vitrectomy era. Arch Ophthalmol. 2010;128(9):1202–1205. doi: 10.1001/archophthalmol.2010.192. [DOI] [PubMed] [Google Scholar]