Abstract
Acute retinal arterial ischemia, which includes transient monocular vision loss (TMVL), branch retinal artery occlusion (BRAO), central retinal artery occlusion (CRAO) and ophthalmic artery occlusion (OAO), is most commonly the consequence of an embolic phenomenon from the ipsilateral carotid artery, heart or aortic arch, leading to partial or complete occlusion of the central retinal artery (CRA) or its branches. Acute retinal arterial ischemia is the ocular equivalent of acute cerebral ischemia and is an ophthalmic and medical emergency. Patients with acute retinal arterial ischemia are at a high risk of having further vascular events, such as subsequent strokes and myocardial infarctions (MIs). Therefore, prompt diagnosis and urgent referral to appropriate specialists and centers is necessary for further work-up (such as brain magnetic resonance imaging with diffusion weighted imaging, vascular imaging, and cardiac monitoring and imaging) and potential treatment of an urgent etiology (e.g., carotid dissection or critical carotid artery stenosis). Since there are no proven, effective treatments to improve visual outcome following permanent retinal arterial ischemia (central or branch retinal artery occlusion), treatment must focus on secondary prevention measures to decrease the likelihood of subsequent ischemic events.
Keywords: Central retinal artery occlusion (CRAO), stroke, ischemia, management, treatment, thrombolysis
Introduction
Acute retinal arterial ischemia, which includes vascular transient monocular vision loss (TMVL or retinal transient ischemic attack), branch retinal arterial occlusion (BRAO), central retinal artery occlusion (CRAO) and ophthalmic artery occlusion (OAO), is a classic cause of acute painless monocular vision loss. Acute retinal arterial ischemia can be caused by any process that interrupts blood flow through the central retinal artery (CRA). The CRA, which originates from the ophthalmic artery (the first intracranial branch off the internal carotid artery), mainly supplies blood to the inner retina, including the macula and fovea.
TMVL is caused by transient occlusion of the CRA or its branches and causes unilateral vision loss typically lasting several minutes, followed by spontaneous recovery of vision without detectable permanent functional visual deficits (1–3). Conversely, BRAO and CRAO are caused by longer-lasting partial or complete occlusion of the CRA or its branches, leading to permanent visual dysfunction [decreased visual acuity and/or visual field deficits (4–6)]. Typically, CRAO produces severe visual dysfunction (very poor visual acuity and/or severely constricted visual field), while BRAO produces less severe visual dysfunction (4,7).
The cilioretinal artery, which is present in 15–30% of the population, originates from the posterior ciliary circulation, not the CRA. Therefore, the cilioretinal artery is not affected in CRAO and central visual acuity can be near normal (20/50 or better) if the cilioretinal artery supplies part of the macula and the fovea (8–10). However, the affected eye will have severely impaired peripheral vision (9,11).
Incidence of acute retinal ischemia
TMVL is the most common form of acute retinal arterial ischemia; the incidence of TMVL has been estimated to be approximately 14 per 100,000 people per year (12,13), while the incidence of CRAO is approximately 1–2 in 100,000. The incidence of acute retinal arterial ischemia increases with age, likely due to an increased prevalence of cardiovascular disease associated with aging; the incidence of CRAO has been estimated to be as high as 10 in 100,000 in patients over 80 years old (14,15) and CRAO accounts for approximately 1 in 10,000 outpatient ophthalmology visits (14,16,17).
Etiology
Acute retinal ischemia can be broadly classified as arteritic, i.e. due to vasculitis, or non-arteritic, i.e. not due to vasculitis. Except for the paragraph in this section which briefly discusses giant cell arteritis (GCA; temporal arteritis) the terms TMVL, OAO, CRAO, and BRAO will be used to refer to the non-arteritic forms of acute retinal arterial ischemia.
The most common cause of acute retinal arterial ischemia is an embolus from a distant source, similar to anterior circulation cerebral infarctions. Retinal emboli most commonly originate from the ipsilateral carotid artery, followed by the aortic arch and the heart. Hypercoagulable states, vasculitis (e.g., GCA) and certain ocular and systemic diseases are less common.
Atheromatous stenosis of the ipsilateral internal carotid artery is the most common cause of acute retinal arterial ischemia. However, in any patient, especially a young patient, who presents with a CRAO associated with facial/ neck pain or headache, an ipsilateral carotid artery dissection needs to be strongly considered (18,19). Often, a Horner syndrome is present on the same side as the CRAO (19). Carotid artery dissection can occur spontaneously or may be precipitated by neck trauma or chiropractic neck manipulation. Therefore, a thorough history is necessary to determine if any neck trauma/manipulation occurred prior to the onset of vision loss and to determine if there is a family history of vascular disease, such as fibromuscular dysplasia, that may predispose the patient to arterial dissections (20). Although all patients with a CRAO should have vascular imaging of the carotid arteries and aortic arch, a CT angiogram or MR angiogram should be obtained urgently to evaluate the extracranial and intracranial carotid arteries in any patient suspected of or at increased risk of having a carotid artery dissection.
Although less common causes of acute retinal ischemia, thrombosis of the CRA or its branches from a hypercoagulable state or vasculitis (such as in GCA) must be considered if a detailed evaluation fails to find an embolic source (9,21–26). Indeed, in any patient over 50 years old who presents with acute retinal ischemia and has systemic symptoms, such as temporal headaches, temporal artery tenderness on palpation, or associated jaw pain, GCA needs to be considered urgently. Evaluation for GCA would include inflammatory biomarkers, such as an erythrocyte sedimentation rate (ESR), platelet count, and a C-reactive protein (CRP). Irrespective of laboratory results, if there is a high clinical suspicion for GCA, a temporal artery biopsy should be performed (8,9) and treatment with high dose intravenous (IV) steroids followed by a slow oral prednisone taper (beginning at 1 mg/kg) should be started to prevent further vision loss and systemic complications (27,28).
In addition, certain ocular conditions, such as acutely elevated intraocular pressure, seen in certain forms of glaucoma, are associated with development of CRAO (29,30). Ocular compression may precipitate acute retinal arterial ischemia in some patients undergoing spine surgery in the prone position (31,32). In rare instances, acute retinal arterial ischemia has been reported to occur after dental or facial cosmetic procedures, following inadvertent injection of drugs or filling materials into a facial vessel (33).
Risk factors
The risk factors and etiologies for acute retinal arterial ischemia are similar to the risk factors and etiologies for cerebrovascular ischemic events (e.g., hypertension, atherosclerosis, and diabetes) (8,15,24,34–37). In the EAGLE trial, a large multi-centered European trial designed to assess the efficacy of intra-arterial fibrinolysis for the treatment of CRAO, 77 patients with CRAO were included. Of the 77 patients, 56 (73%) had arterial hypertension, 31 (40%) had at least 70% stenosis of a carotid artery (the majority had ipsilateral carotid artery stenosis), 17 (22%) had coronary artery disease, 15 (19%) had atrial fibrillation, and 13 (17%) had valvular heart disease. Despite the majority of patients having known cardiovascular disease, 78% of the patients had at least 1 new cardiovascular risk factor identified at the time of the CRAO (34).
Since the risk factors and the etiologies are similar for acute retinal arterial ischemia and cerebral ischemia, the American Heart Association (AHA) and American Stroke Association (ASA) consider acute retinal ischemia to be the equivalent of acute cerebral ischemia. In a consensus statement in 2013, the AHA and ASA defined central nervous system infarction (stroke) as “brain, spinal cord, or retinal cell death attributable to ischemia, based on neuropathological, neuroimaging, and/or clinical evidence of permanent injury” (38). Therefore, TMVL is considered the retinal equivalent of a cerebral transient ischemic attack (TIA) and BRAO, CRAO and OAO are considered the retinal equivalent of cerebral ischemia (stroke) (37,38).
Diagnosis of acute retinal ischemia
Acute retinal arterial ischemia typically presents as sudden, painless, visual acuity loss and/or visual field loss in the affected eye. A range of visual disturbances, from “graying” or “dimming” of vision to complete loss of vision (12,39), has been reported with TMVL. Vision loss associated with TMVL typically lasts minutes, but rarely may last an hour or more (12,40). Since the vision returns to normal following the episode of vision loss, the examination in the majority of cases of TMVL does not reveal any abnormalities. Therefore, the diagnosis and potential etiology of TMVL is based on a thorough history (12,39,41).
In contrast to TMVL, BRAO and CRAO produce permanent visual dysfunction (loss of visual acuity and/or visual field). Visual acuity following a BRAO or CRAO can range from near normal to counting fingers or worse (4,7,42). In a study of 260 eyes with CRAO (6), 74% had a presenting visual acuity of counting fingers or worse; the majority with better visual acuity had a perfused or partially perfused fovea due to a patent cilioretinal artery. The decrease in color vision is commensurate with the decrease in visual acuity. A relative afferent pupillary defect is present in the affected eye. Findings on dilated funduscopic examination of the retina include retinal edema of the ischemic retina (evident as retinal whitening) (Figures 1,2), the presence of a cherry red spot (a pink or red fovea due to the presence of normal underlying choroidal circulation in the fovea which has a very thin nerve fiber layer) in CRAO, slow segmental blood flow in retinal arterioles (known as “box-carring”), attenuation of retinal arterioles, and a normal optic nerve (8,43). In addition, emboli in the CRA or its branches may be seen. If the ophthalmic artery is occluded, there is no cherry red spot and optic disc edema will be present. Visible retinal findings may not be present or obvious in the acute setting and may take several hours to develop (8,25,43). Optical coherence tomography (OCT), OCT angiography (OCTA) or fluorescein angiogram (FA) can be performed in unclear cases to aid in the diagnosis of a retinal artery occlusion (CRAO or BRAO) or when the expected examination findings are very subtle or absent. Delayed or absent retinal arteriolar blood flow or retinal perfusion (25,44) can be seen on FA (Figure 2) and OCTA. Retinal damage from poor blood flow through the CRA, seen acutely as retinal edema and later as a disruption or thinning of inner retinal layers (45), can be detected by OCT.
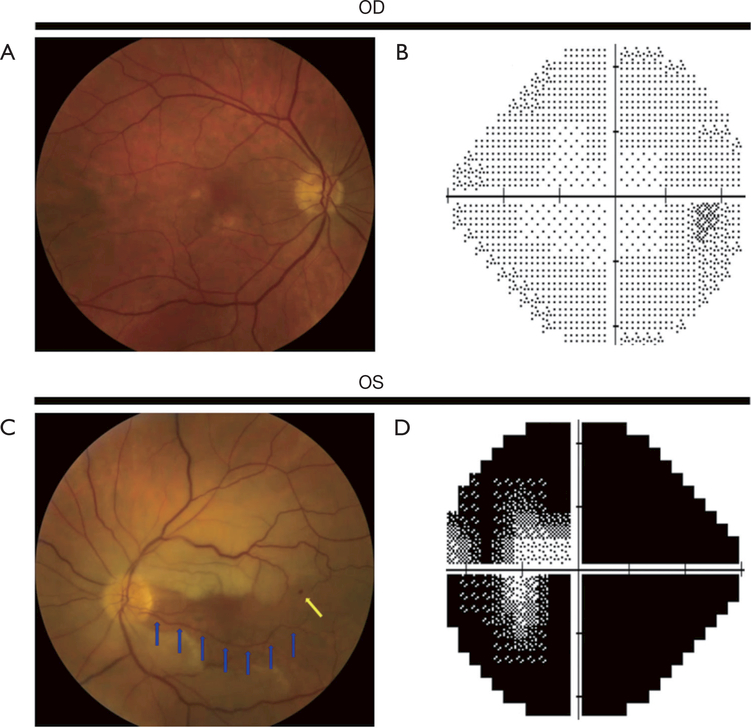
Figure 1.
Left central retinal artery occlusion (CRAO) with sparing of the cilioretinal artery. (A) Color fundus photograph of the normal right eye; (B) normal Humphrey visual field from the right eye; (C) color fundus photograph of the left eye showing a CRAO with a perfused cilioretinal artery (blue arrows). The remainder of the retinal arterioles is attenuated. There is a small retinal hemorrhage near the end of the superotemporal arcade (yellow arrow). Compared to the right eye, the fundus of the left eye has a white hue, indicative of inner retinal edema. Visual acuity in the left eye was 20/40. (D) Left eye Humphrey visual field showing marked visual field deficits despite near normal visual acuity, due to the patent cilioretinal artery, in the left eye.
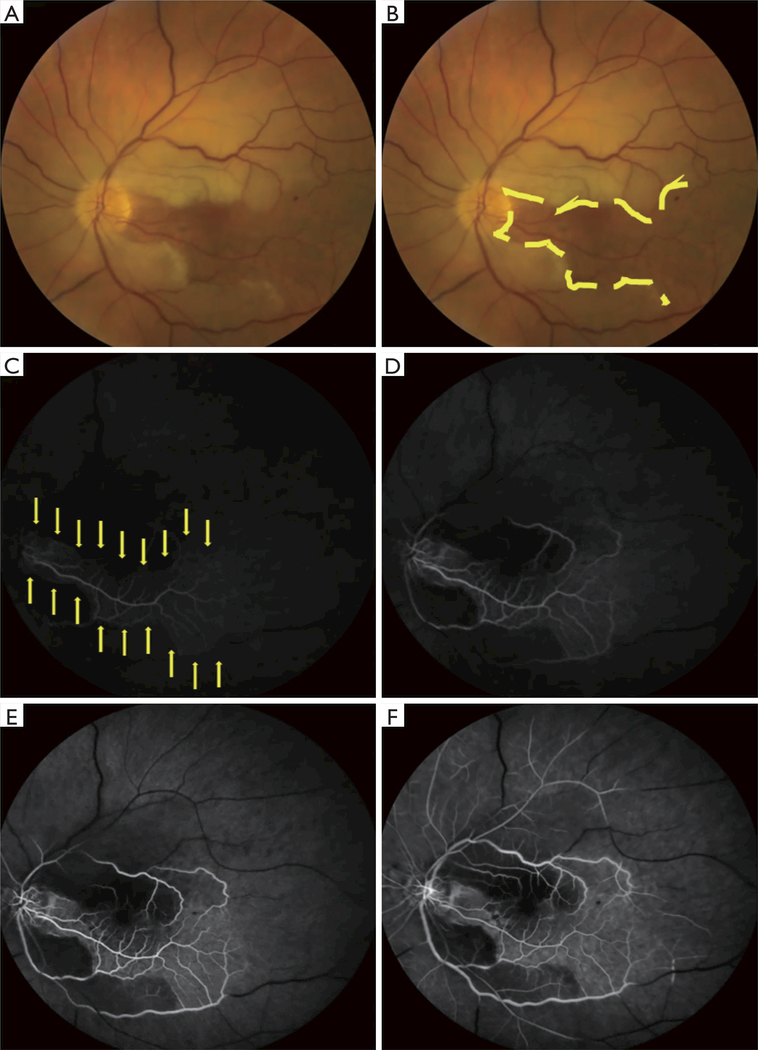
Figure 2.
Left central retinal artery occlusion (CRAO) with sparing of the cilioretinal artery. Color fundus photographs (A,B) and retinal fluorescein angiography (C,D,E,F) in acute left CRAO with cilioretinal artery sparing (same patient as Figure 1). A. Color fundus photograph of the left eye showing a CRAO with cilioretinal artery sparing. The papillomacular bundle is perfused by a patent cilioretinal artery. (B) Same photograph as in (A) outlining the area perfused by the cilioretinal arteries (area contained within the yellow lines). Retinal edema is seen outside of the area perfused by the cilioretinal arteries. (C,D,E,F) Fluorescein angiogram of the left eye taken 21 seconds (C), 24 seconds (D), 28 seconds (E), and 1 minute and 5 seconds (F) after injection of fluorescein dye. There is minimal fluorescent signal in the papillomacular bundle (C, area outlined by yellow arrows) and no appreciable fluorescent signal outside of the papillomacular bundle 21 seconds after fluorescein dye injection. One minute after injection of fluorescein dye (F), there continue to be large vascular segments without fluorescent signal (black segments of retinal vessels) and almost no retinal perfusion outside of the papillomacular bundle.
Morbidity and mortality
Visual prognosis
OAO and CRAO are classically associated with severe visual acuity and/or visual field loss in the affected eye, while BRAO is classically associated with less severe visual dysfunction than CRAO (4,6,7,42). The visual outcome in BRAO and CRAO is variable and is dependent on a number of factors, including the length of time the CRA or its branches are occluded, the type of embolus, and, in CRAO, the presence of a patent cilioretinal artery (11). In a study of 244 patients with CRAO, initial visual acuity of counting fingers or worse was seen in 93.2% without a cilioretinal artery and 60% with a cilioretinal artery not reaching the fovea, while an initial visual acuity of 20/40 or better was seen in 20% of patients with a cilioretinal artery and only one patient without a cilioretinal artery. In patients whose initially visual acuity was counting fingers or worse, some improvement in visual acuity occurred in 47% with a cilioretinal artery and 16% without a cilioretinal artery. Final visual acuity worsened compared to initial visual acuity in 6% with a cilioretinal artery and 8% without a cilioretinal artery (6,11). In patients who presented with a central scotoma on visual field testing, approximately 20– 25% had improvement in their central scotoma, irrespective of the presence of a cilioretinal artery (6). Although the presence of a cilioretinal artery is associated with a higher likelihood of improvement in visual acuity, spontaneous improvement in visual function does not occur in the majority of patients with a CRAO; they continue to have profound visual dysfunction in the involved eye.
Although, visual acuity is typically better in BRAO than in CRAO, visual acuity of at least counting fingers has been reported in BRAO (4,7,42). In a study of 212 eyes with BRAO, 79.5% (124/156) of patients with a non-arteritic BRAO were found to have an initial visual acuity of 20/40 or better (42). In addition to better initial visual acuity, patients with a BRAO are more likely to have improvement in visual acuity and visual field defects; indeed, most patients with BRAO retain or improve to a visual acuity of 20/40 or better (4,7,42).
The most important determinant of final visual outcome likely is the length of time the CRA or its branches are occluded. No retinal damage was detected with occlusion of the CRA for up to approximately 100 minutes in a nonhuman primate model of CRAO. However, occlusion of the CRA for 100 to 240 minutes produced variable amounts of permanent retinal dysfunction. After approximately 240 minutes of occlusion, massive, irreversible retinal damage occurred (5,46). These studies strongly suggest that the duration of retinal ischemia correlates with the likelihood for improvement in visual function following acute retinal arterial ischemia. Therefore, similar to an acute cerebral infarction, there likely is a discrete time frame following acute retinal arterial ischemia when restoration of retinal blood flow may have a beneficial effect on visual outcome. Therefore, the sooner a diagnosis is made and retinal blood flow re-established, theoretically the better the chance for visual recovery (5,11,46).
Ophthalmic complications
The development of ocular neovascularization, which can occur as early as 2 weeks following acute retinal arterial ischemia, is a known complication of acute retinal arterial ischemia and can lead to further severe vision loss (47). Although ocular neovascularization can occur following a BRAO, it is much more common following a CRAO (48,49). Anterior segment neovascularization can cause neovascular glaucoma and lead to ocular pain, markedly elevated intraocular pressure, and rapidly deteriorating visual acuity. In addition, retinal neovascularization can cause vitreous hemorrhages and/or tractional retinal detachments. To attempt to prevent further vision loss, referral to a vitreoretinal specialist is necessary if ocular neovascularization is detected.
Systemic considerations
CRAO and, to a lesser extent, BRAO are associated with a high degree of morbidity and mortality due to permanent, severe vision loss, as well as the immediate and long-term ocular and systemic risks associated with acute retinal ischemia (35,50–54). Fortunately, both CRAO and BRAO are often monocular disorders. Therefore, they are less likely to cause significant physical disability compared with cerebral ischemia (55). However, both CRAO and BRAO can cause markedly decreased visual acuity and/ or reduced visual field leading to decreased quality of life, independence, and possibly institutional care (50). Due to their visual dysfunction, patients with severe vision loss from acute retinal arterial ischemia are also at higher risk for falls and subsequent hip fractures, further decreasing independence and quality of life.
In addition to visual impairment, acute retinal ischemia is associated with a higher incidence of systemic morbidity. Patients with acute retinal arterial ischemia have a higher incidence of having had a recent prior non-ocular ischemic event [cerebral infarction or myocardial infarction (MI)] and have a higher risk of a subsequent cerebral infarction or MI. The risk of a subsequent MI or cerebral infarction is highest within the first week following acute retinal ischemia (24,30,34–36,53,54,56,57) and may remain higher than in the general population for up to 10 years following the CRAO or BRAO (54,58). In a population based study in Taiwan, the risk of stroke was 2.7 times higher within the first 3 years in patients following a CRAO compared to matched controls, with the highest stroke incidence occurring in the first month following the CRAO (58). In the EAGLE study, 5 of 77 CRAO patients had a stroke within the first month following the CRAO and 4 of those 5 patients had significant carotid artery stenosis ipsilateral to the CRAO (34). During the first year following a CRAO the stroke rate has been reported to be as high as 13% and may be as much as 10 times higher than in the general population for up to 3.5 years following the CRAO.
In addition to an increased stroke risk, cardiovascular mortality (vascular death or MI) is also higher than in the general population following a CRAO (34,51,53).
Acute retinal arterial ischemia may therefore be a sign of underlying systemic disease placing patients at a significantly higher risk for subsequent cardiovascular or cerebral ischemia than the general population, further increasing patient mortality by decreasing quality of life and patient independence.
Management
The acute management of TMVL, the ocular equivalent of a TIA, is similar to that of patients with permanent retinal ischemia (59). Therefore, the specific management of TMVL will not be discussed separately in this review and the guidelines for the management of permanent retinal ischemia (BRAO and CRAO) should be followed for patients presenting with TMVL.
Since the AHA, the ASA, and numerous international stroke agencies have classified CRAO and BRAO as stroke equivalents, patients with an acute CRAO or BRAO must be evaluated similarly to patients with acute cerebral ischemia. Therefore, acute retinal ischemia is an ophthalmologic and medical emergency; patients with acute retinal ischemia should be immediately referred to the nearest certified stroke center for a detailed evaluation to determine the cause/source of the acute retinal ischemia and for secondary prevention of further ischemic complications (MI or cerebral infarction) (6,22,53,54,57,59,60).
In multiple recent studies it was reported that approximately 15% to 25% of patients with acute retinal ischemia have concurrent acute small cerebral infarctions on diffusion weighted imaging-MRI (DWI-MRI) (8,21,37,52,59,61–64), despite the lack of other focal neurologic deficits at the time of presentation to suggest acute cerebral ischemia. Silent infarctions bear a high risk of future stroke; patients with acute silent cerebral infarctions and acute retinal ischemia most often have a major etiology identified, usually requiring urgent treatment to prevent a subsequent stroke (52,61,63,64). Therefore, all patients with acute retinal ischemia, even in the absence of other focal neurologic deficits, should have an immediate DWI-MRI of the brain to assess for concurrent cerebral ischemia.
Since the most common cause of acute retinal ischemia is occlusion of the CRA or one of its branches by an embolus, the work-up in patients with acute retinal ischemia must focus on determining whether an underlying source of emboli exists. Vascular imaging of the carotid arteries and aortic arch should be performed urgently, usually with an MRA of the head and neck performed concurrently with the brain DWI-MRI or with a CTA, depending on local available resources. Cardiac evaluation, including blood pressure monitoring, EKG, echocardiogram (ideally transesophageal echocardiogram with a bubble contrast study), and cardiac monitoring (which can replace Holter monitoring when work-up is performed over 24 hours in a stroke center) should be performed in all patients with acute retinal ischemia (34).
Further evaluation is warranted in younger patients without an identified embolic cause. Evaluation should include testing for hypercoagulable conditions (e.g., factor V Leiden deficiency, protein C and S deficiency, hyperhomocysteinemia, anti-phospholipid antibodies, thrombocytosis, prothrombin gene mutations, anti-thrombin deficiency, and hyperviscosity syndrome), rheumatologic systemic inflammatory disorders, vascular occlusive disorders (e.g., sickle cell disease), use of illicit substances (intravenous drugs and cocaine) and use of certain medications (e.g., nasal vasoconstrictive agents) (29,65).
Current practice patterns
Despite the AHA, ASA, and numerous international stroke agencies strongly recommending urgent evaluation at a certified stroke center for patients with acute retinal ischemia, many ophthalmologists and, to a lesser extent, neurologists do not appropriately refer patients with acute retinal ischemia (59,66–68). In a 2009 survey of ophthalmologists in the state of Georgia in the USA, only approximately 35% of ophthalmologists reported referring patients with an acute CRAO to the emergency department for further work-up and risk stratification (67). In a 2017 survey of vitreoretinal specialists and neurologists in the USA, only 18% of vitreoretinal specialists compared with 75% of neurologists reported that they would recommend admission to a stroke unit or ER referral for a 52-year-old patient with an acute embolic retinal artery occlusion that occurred less than 12 hours prior to evaluation (66). In the same study, only 46% of neurologists and 8% of vitreoretinal specialists reported that they would pursue a hospital-based work-up for an acute retinal artery occlusion that occurred 24–48 hours prior to evaluation (66).
Immediate evaluation of patients with acute retinal arterial occlusion is necessary to obtain a thorough workup searching for etiologies for the acute retinal ischemia, to treat any urgent etiologies, such as critical stenosis of the ipsilateral carotid artery or atrial fibrillation, and to institute secondary prevention measures to attempt to minimize the likelihood of subsequent ocular, cardiovascular, or cerebral ischemic events (60). Patients with acute retinal arterial ischemia should be evaluated immediately in an emergency care center affiliated with a certified stroke center, allowing the patient to receive an effective, rapid workup and consultation with a stroke neurologist. The work-up typically is performed within 24 hours and the patient may be discharged with appropriate follow-up with a stroke neurologist and secondary prevention measures or may be admitted to a stroke unit for immediate treatment of a major etiology, depending on the results of the initial workup.
Treatment of acute retinal ischemia (CRAO or BRAO)
Treatment of a CRAO or BRAO can be divided into acute treatment, directed at resolving the BRAO/ CRAO and improving visual outcome, and secondary prevention of subsequent ischemic events. Although multiple interventions have been attempted to restore ocular perfusion and improve visual outcome following a BRAO/CRAO (3,8,9,22, 25,69–71), unfortunately no current therapy has been shown to improve visual outcome beyond what is expected based upon the natural history of BRAO/CRAO (3,71). Theoretically, the chance to improve visual function should increase as the duration of retinal ischemia decreases (i.e., the sooner blood flow returns to the retina, the better the chance to improve visual function) (5,6,46,72). Based upon primate studies of acute retinal ischemia, treatment likely would need to be administered within 3 hours of vision loss to prevent permanent retinal ischemia, similar to the recommendations for acute cerebral ischemia. Although treatments administered between 6 and 12 hours from the onset of vision loss may have some beneficial effects on visual function, it is extremely unlikely that any improvement in visual function would be obtained from interventions occurring 12 or more hours after the onset of vision loss. This limits the value of many of the published studies, since treatment in those studies was often administered more than 12 hours after the occurrence of vision loss. Until treatment shows improvement in visual function beyond what is expected based on the natural history of acute retinal ischemia, the treatment focus must remain centered on secondary prevention of subsequent systemic ischemic events.
Conservative therapies
A number of conservative therapies (also referred to as “classic” or “conventional” therapies) have been attempted to restore blood flow to the retina and improve visual function following a BRAO or CRAO, including attempting to physically dislodge an embolus, increasing retinal artery perfusion pressure, and increasing blood oxygen tension.
Therapies aimed at physically dislodging the embolus include ocular massage, which increases retinal artery perfusion pressure via retinal arteriolar dilatation and decreased IOP (17,22,73), and the use of an Nd:YAG laser to physically dislodge a visible embolus (74,75). The use of ocular massage, either alone or in combination with IOP lowering medications, has not been shown to significantly improve visual function following acute retinal ischemia (17,22,76). Nd:YAG embolysis for the treatment of acute retinal ischemia is controversial and is not considered the standard of care; it is associated with significant side effects, such as vitreous hemorrhage and creation of false aneurysms in the CRA (74,75).
In addition to ocular massage, other therapies aimed at increasing retinal artery perfusion have also been attempted, such as the use of IOP lowering medications (17,77) and anterior chamber paracentesis (44,78). Similar to ocular massage, these treatments have not been shown to improve visual outcome following acute retinal ischemia (17,69,76,78,79).
Therapies focused on increasing retinal arteriolar dilatation and decreasing oxygen-induced vasoconstriction of retinal vasculature, such as hyperventilating, inhalation of carbogen (a mixture of 95% oxygen and 5% carbon dioxide) and the use of certain medications (isosorbide dinitrate and pentoxifylline), have also been attempted to improve visual outcome following acute retinal ischemia (17,78,80–84). Unfortunately, there is no evidence in the literature showing that these therapies improve visual outcome in CRAO patients (78).
Hyperbaric oxygen, which causes an increase in the concentration of soluble oxygen in the blood, has also been suggested as a potential treatment for acute retinal ischemia (85,86). Similar to the above therapies, there is no evidence in the literature to suggest that hyperbaric oxygen improves visual outcome in patients beyond what is expected based upon the natural history of acute retinal ischemia (87,88).
Thrombolysis
Thrombolytics, such as urokinase, streptokinase, and tissue plasminogen activator (tPA), convert plasminogen to plasmin and lead to dissolution of fibrin-based clots (22,24,25). Since fibrin-based clots are believed to be the most common type of clot in acute retinal ischemia and because of the use and efficacy of thrombolytics in acute cerebral ischemia, thrombolytics have been used in the treatment of CRAO. However, no standard treatment protocol exists for the use of thrombolytics in acute retinal ischemia. Therefore, most clinicians base their decision to use thrombolytics in acute retinal ischemia on established stroke protocols. Unfortunately, a reliable improvement in visual function has not been demonstrated in patients treated with thrombolytics following acute retinal ischemia (89,90); no significant improvement in visual function following administration of tPA was reported in a number of retrospective reviews and observational studies (91–93), however other observational studies, case reports, and retrospective reviews have suggested that visual function improved following administration of tPA (94–101). It is possible that the low rate of vision improvement reported in most studies is related to the time between onset of visual symptoms and the administration of thrombolytics (72,96,101–105); in most studies, thrombolytics were administered more than 12 hours after vision loss. However, a recent study examining the data from five retrospective case series of patients diagnosed with CRAO and treated with either urokinase (45 patients) or tPA (73 patients) failed to find a relationship between improvement in visual function and the time from onset of visual symptoms to treatment (106). In addition to the low rate of vision improvement following the use of thrombolytics, the use of thrombolytics for acute retinal arterial ischemia has been associated with a high rate of complications (89). Therefore, the decision to treat patients presenting with acute retinal ischemia with thrombolytics should be made on a case by case basis, after carefully considering the potential risks and benefits of thrombolytic therapy.
In the EAGLE trial (107), the effect of intra-arterial tPA was compared to conservative therapy (daily ASA, hemodilution, IOP lowering medications, ocular massage, and IV heparin) in patients with an acute CRAO (less than 20 hours from onset of vision loss). Localized intra-arterial tPA into the ophthalmic artery was administered in 42 (51.2%) patients (107). Unfortunately, this study failed to find a statistically significant improvement in visual acuity in patients treated with tPA compared to patients treated with conservative therapy; 3 or more lines of improvement in visual acuity was reported in 57% of patients in the thrombolysis group and 60% of patients in the conservative treatment group. However, adverse reactions, including headaches, hemiparesis, post-procedural hemorrhages, intracranial hemorrhages, epistaxis, and oral hemorrhages, were more common in the thrombolysis group (occurring in 37.1% of patients) than in the conservative treatment group (occurring in 4.3% of patients). Due to the increased incidence of adverse events and the lack of significant improvement in visual function in the tPA group, the study was stopped prematurely at the first interim analysis (107).
Based on the current literature, it is extremely difficult to suggest therapeutic guidelines for the use of thrombolytics in acute retinal ischemia. This is mainly due to the heterogeneity in study designs (varying drug regimens, timing of medication administration following acute retinal ischemia), the heterogenicity in study endpoints, the lack of visual function improvement following administration of tPA in randomized, controlled studies, the equivocal effectiveness of tPA in improving visual outcome in retrospective and observational studies, and the increased risk of adverse events associated with the administration of thrombolytics (102). Therefore, further studies are necessary to determine the optimal timing and dosage of thrombolytic therapy and to determine if earlier treatment of patients with thrombolysis produces a significant improvement in visual function compared to conservative therapy (108). Unfortunately, the rarity of acute retinal ischemia and the delay in patient care (59,68,109–111) are some of the multiple barriers that exist to designing and performing such studies.
In summary, acute retinal arterial ischemia is the equivalent of acute cerebral ischemia (TMVL is the equivalent of a TIA and BRAO/CRAO are the equivalent of a stroke) and therefore represents an ophthalmologic and medical emergency. Given the lack of evidence in the literature to support an improvement in visual function with either conservative or thrombolytic therapy, the management of these patients must focus on determining an etiology for the acute retinal ischemia, treating any urgent etiologies, identifying concomitant acute cerebral infarctions, and attempting to prevent subsequent ischemic events (acute MI, vascular death, and cerebral infarction) by optimizing known cardiovascular risk factors and identifying undiagnosed cardiovascular risk factors. Since patients with acute vision loss are likely to present initially to an eye care specialist (ophthalmologist or optometrist), it is imperative that, once the diagnosis of acute retinal ischemia is made, these patients are immediately referred to the nearest emergency care center affiliated with a certified stroke center. Optimal management of patients with acute retinal ischemia requires collaboration between stroke neurologists to perform the acute work-up, to optimize known cardiovascular risk factors, and to monitor for any evidence of further systemic ischemic events; and ophthalmologists, to monitor for the presence of secondary ocular complications, such as neovascularization, which may cause further visual impairment and loss of independence, and to monitor for any subsequent retinal ischemic events.
Acknowledgements
Funding: V Biousse and NJ Newman are supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness Inc., New York, by NIH/NEI core grant P30-EY006360 (Department of Ophthalmology, Emory University School of Medicine), and by NIH/NINDS (RO1NSO89694). M Dattilo is supported by the NEI sponsored teaching grant T32-EY007092 (Department of Ophthalmology). V Biousse received research support form NIH/PHS (UL1-RR025008). NJ Newman is a recipient of the Research to Prevent Blindness Lew R. Wasserman Merit Award.
Footnotes
Conflicts of Interest: V Biousse and NJ Newman are consultants for GenSight Biologics. NJ Newman is a consultant for Santhera Pharmaceuticals. M Dattilo has no conflicts of interest to declare.
References
- 1.Marshall J, Meadows S. The natural history of amaurosis fugax. Brain 1968;91:419–34. [DOI] [PubMed] [Google Scholar]
- 2.Tippin J, Corbett JJ, Kerber RE, et al. Amaurosis fugax and ocular infarction in adolescents and young adults. Ann Neurol 1989;26:69–77. [DOI] [PubMed] [Google Scholar]
- 3.Vodopivec I, Cestari DM, Rizzo JF 3rd. Management of Transient Monocular Vision Loss and Retinal Artery Occlusions. Semin Ophthalmol 2017;32:125–33. [DOI] [PubMed] [Google Scholar]
- 4.Mason JO 3rd, Shah AA, Vail RS, et al. Branch retinal artery occlusion: visual prognosis. Am J Ophthalmol 2008;146:455–7. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. Retinal survival time. Exp Eye Res 2004;78:723–36. [DOI] [PubMed] [Google Scholar]
- 6.Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol 2005;140:376–91. [DOI] [PubMed] [Google Scholar]
- 7.Yuzurihara D, Iijima H. Visual outcome in central retinal and branch retinal artery occlusion. Jpn J Ophthalmol 2004;48:490–2. [DOI] [PubMed] [Google Scholar]
- 8.Biousse V, Newman N. Retinal and optic nerve ischemia. Continuum (Minneap Minn) 2014;20:838–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cugati S, Varma DD, Chen CS, et al. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol 2013;15:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorentzen SE. Incidence of cilioretinal arteries. Acta Ophthalmol (Copenh) 1970;48:518–24. [DOI] [PubMed] [Google Scholar]
- 11.Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res 2014;41:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawlor M, Perry R, Hunt BJ, et al. Strokes and vision: The management of ischemic arterial disease affecting the retina and occipital lobe. Surv Ophthalmol 2015;60:296–309. [DOI] [PubMed] [Google Scholar]
- 13.Andersen CU, Marquardsen J, Mikkelsen B, et al. Amaurosis fugax in a Danish community: a prospective study. Stroke 1988;19:196–9. [DOI] [PubMed] [Google Scholar]
- 14.Park SJ, Choi NK, Seo KH, et al. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology 2014;121:1933–8. [DOI] [PubMed] [Google Scholar]
- 15.Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology 2009;116:1928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leavitt JA, Larson TA, Hodge DO, et al. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol 2011;152:820–3 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol 1999;128:733–8. [DOI] [PubMed] [Google Scholar]
- 18.Patel M, Shah G, Davies JB, et al. Re-evaluating our perspective on retinal artery occlusion from carotid dissection: a report of three cases and review of the literature. Ophthalmic Surg Lasers Imaging Retina 2013;44:555–60. [DOI] [PubMed] [Google Scholar]
- 19.Biousse V, Touboul PJ, D’Anglejan-Chatillon J, et al. Ophthalmologic manifestations of internal carotid artery dissection. Am J Ophthalmol 1998;126:565–77. [DOI] [PubMed] [Google Scholar]
- 20.Narula N, Kadian-Dodov D, Olin JW. Fibromuscular Dysplasia: Contemporary Concepts and Future Directions. Prog Cardiovasc Dis 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt D, Hetzel A, Geibel-Zehender A, et al. Systemic diseases in non-inflammatory branch and central retinal artery occlusion--an overview of 416 patients. Eur J Med Res 2007;12:595–603. [PubMed] [Google Scholar]
- 22.Chen CS, Lee AW. Management of acute central retinal artery occlusion. Nat Clin Pract Neurol 2008;4:376–83. [DOI] [PubMed] [Google Scholar]
- 23.Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res 2011;30:359–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudkin AK, Lee AW, Chen CS. Vascular risk factors for central retinal artery occlusion. Eye (Lond) 2010;24:678–81. [DOI] [PubMed] [Google Scholar]
- 25.Varma DD, Cugati S, Lee AW, et al. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond) 2013;27:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fineman MS, Savino PJ, Federman JL, et al. Branch retinal artery occlusion as the initial sign of giant cell arteritis. Am J Ophthalmol 1996;122:428–30. [DOI] [PubMed] [Google Scholar]
- 27.Bossert M, Prati C, Balblanc JC, et al. Aortic involvement in giant cell arteritis: current data. Joint Bone Spine 2011;78:246–51. [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta B, Borg FA, Hassan N, et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology (Oxford) 2010;49:1594–7. [DOI] [PubMed] [Google Scholar]
- 29.Brown GC, Magargal LE, Shields JA, et al. Retinal arterial obstruction in children and young adults. Ophthalmology 1981;88:18–25. [DOI] [PubMed] [Google Scholar]
- 30.Bruno A, Jones WL, Austin JK, et al. Vascular outcome in men with asymptomatic retinal cholesterol emboli. A cohort study. Ann Intern Med 1995;122:249–53. [DOI] [PubMed] [Google Scholar]
- 31.Chang SH, Miller NR. The incidence of vision loss due to perioperative ischemic optic neuropathy associated with spine surgery: the Johns Hopkins Hospital Experience. Spine (Phila Pa 1976) 2005;30:1299–302. [DOI] [PubMed] [Google Scholar]
- 32.Sys J, Michielsen J, Mertens E, et al. Central retinal artery occlusion after spinal surgery. Eur Spine J 1996;5:74–5. [DOI] [PubMed] [Google Scholar]
- 33.Park SW, Woo SJ, Park KH, et al. Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol 2012;154:653–62 e1. [DOI] [PubMed] [Google Scholar]
- 34.Callizo J, Feltgen N, Pantenburg S, et al. Cardiovascular Risk Factors in Central Retinal Artery Occlusion: Results of a Prospective and Standardized Medical Examination. Ophthalmology 2015;122:1881–8. [DOI] [PubMed] [Google Scholar]
- 35.Douglas DJ, Schuler JJ, Buchbinder D, et al. The association of central retinal artery occlusion and extracranial carotid artery disease. Ann Surg 1988;208:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein R, Klein BE, Moss SE, et al. Retinal emboli and cardiovascular disease: the Beaver Dam Eye Study. Arch Ophthalmol 2003;121:1446–51. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka K, Uehara T, Kimura K, et al. Comparison of Clinical Characteristics among Subtypes of Visual Symptoms in Patients with Transient Ischemic Attack: Analysis of the PROspective Multicenter registry to Identify Subsequent cardiovascular Events after TIA (PROMISE-TIA) Registry. J Stroke Cerebrovasc Dis 2018;27:1711–6. [DOI] [PubMed] [Google Scholar]
- 38.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petzold A, Islam N, Hu HH, et al. Embolic and nonembolic transient monocular visual field loss: a clinicopathologic review. Surv Ophthalmol 2013;58:42–62. [DOI] [PubMed] [Google Scholar]
- 40.Current management of amaurosis fugax. The Amaurosis Fugax Study Group. Stroke 1990;21:201–8. [DOI] [PubMed] [Google Scholar]
- 41.Pula JH, Kwan K, Yuen CA, et al. Update on the evaluation of transient vision loss. Clin Ophthalmol 2016;10:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayreh SS, Podhajsky PA, Zimmerman MB. Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology 2009;116:1188–94 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina 2007;27:276–89. [DOI] [PubMed] [Google Scholar]
- 44.Beatty S, Au Eong KG. Acute occlusion of the retinal arteries: current concepts and recent advances in diagnosis and management. J Accid Emerg Med 2000;17:324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shinoda K, Yamada K, Matsumoto CS, et al. Changes in retinal thickness are correlated with alterations of electroretinogram in eyes with central retinal artery occlusion. Graefes Arch Clin Exp Ophthalmol 2008;246:949–54. [DOI] [PubMed] [Google Scholar]
- 46.Hayreh SS, Jonas JB. Optic disk and retinal nerve fiber layer damage after transient central retinal artery occlusion: an experimental study in rhesus monkeys. Am J Ophthalmol 2000;129:786–95. [DOI] [PubMed] [Google Scholar]
- 47.Rudkin AK, Lee AW, Chen CS. Ocular neovascularization following central retinal artery occlusion: prevalence and timing of onset. Eur J Ophthalmol 2010;20:1042–6. [DOI] [PubMed] [Google Scholar]
- 48.Hayreh SS, Podhajsky P. Ocular neovascularization with retinal vascular occlusion. II. Occurrence in central and branch retinal artery occlusion. Arch Ophthalmol 1982;100:1585–96. [DOI] [PubMed] [Google Scholar]
- 49.Mason JO 3rd, Patel SA, Feist RM, et al. Ocular neovascularization in eyes with a central retinal artery occlusion or a branch retinal artery occlusion. Clin Ophthalmol 2015;9:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vu HT, Keeffe JE, McCarty CA, et al. Impact of unilateral and bilateral vision loss on quality of life. Br J Ophthalmol 2005;89:360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hankey GJ, Slattery JM, Warlow CP. Prognosis and prognostic factors of retinal infarction: a prospective cohort study. BMJ 1991;302:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helenius J, Arsava EM, Goldstein JN, et al. Concurrent acute brain infarcts in patients with monocular visual loss. Ann Neurol 2012;72:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park SJ, Choi NK, Yang BR, et al. Risk and Risk Periods for Stroke and Acute Myocardial Infarction in Patients with Central Retinal Artery Occlusion. Ophthalmology 2015;122:2336–43 e2. [DOI] [PubMed] [Google Scholar]
- 54.Rim TH, Han J, Choi YS, et al. Retinal Artery Occlusion and the Risk of Stroke Development: Twelve-Year Nationwide Cohort Study. Stroke 2016;47:376–82. [DOI] [PubMed] [Google Scholar]
- 55.Plant GT, Landau K. Thrombolysis for central retinal artery occlusion. J Neurol Neurosurg Psychiatry 2005;76:160–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein R, Klein BE, Jensen SC, et al. Retinal emboli and stroke: the Beaver Dam Eye Study. Arch Ophthalmol 1999;117:1063–8. [DOI] [PubMed] [Google Scholar]
- 57.Wang JJ, Cugati S, Knudtson MD, et al. Retinal arteriolar emboli and long-term mortality: pooled data analysis from two older populations. Stroke 2006;37:1833–6. [DOI] [PubMed] [Google Scholar]
- 58.Chang YS, Jan RL, Weng SF, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol 2012;154:645–652.e1. [DOI] [PubMed] [Google Scholar]
- 59.Biousse V Acute retinal arterial ischemia: an emergency often ignored. Am J Ophthalmol 2014;157:1119–21. [DOI] [PubMed] [Google Scholar]
- 60.Olsen TW, Pulido JS, Folk JC, et al. Retinal and Ophthalmic Artery Occlusions Preferred Practice Pattern(R). Ophthalmology 2017;124:P120–P43. [DOI] [PubMed] [Google Scholar]
- 61.Lee J, Kim SW, Lee SC, et al. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. Am J Ophthalmol 2014;157:1231–8. [DOI] [PubMed] [Google Scholar]
- 62.Biousse V, Trobe JD. Transient monocular visual loss. Am J Ophthalmol 2005;140:717–21. [DOI] [PubMed] [Google Scholar]
- 63.Lauda F, Neugebauer H, Reiber L, et al. Acute Silent Brain Infarction in Monocular Visual Loss of Ischemic Origin. Cerebrovasc Dis 2015;40:151–6. [DOI] [PubMed] [Google Scholar]
- 64.Golsari A, Bittersohl D, Cheng B, et al. Silent Brain Infarctions and Leukoaraiosis in Patients With Retinal Ischemia: A Prospective Single-Center Observational Study. Stroke 2017;48:1392–6. [DOI] [PubMed] [Google Scholar]
- 65.Greven CM, Slusher MM, Weaver RG. Retinal arterial occlusions in young adults. Am J Ophthalmol 1995;120:776–83. [DOI] [PubMed] [Google Scholar]
- 66.Abel AS, Suresh S, Hussein HM, et al. Practice Patterns After Acute Embolic Retinal Artery Occlusion. Asia Pac J Ophthalmol (Phila) 2017;6:37–9. [DOI] [PubMed] [Google Scholar]
- 67.Atkins EJ, Bruce BB, Newman NJ, et al. Translation of clinical studies to clinical practice: survey on the treatment of central retinal artery occlusion. Am J Ophthalmol 2009;148:172–3. [DOI] [PubMed] [Google Scholar]
- 68.Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: Follow the Guidelines. Ophthalmology 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 69.Fraser SG, Adams W. Interventions for acute non-arteritic central retinal artery occlusion. Cochrane Database Syst Rev 2009;(1):CD001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Almeida DR, Mammo Z, Chin EK, et al. Surgical Embolectomy for Fovea-Threatening Acute Retinal Artery Occlusion. Retin Cases Brief Rep 2016;10:331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilbert AL, Choi C, Lessell S. Acute Management of Central Retinal Artery Occlusion. Int Ophthalmol Clin 2015;55:157–66. [DOI] [PubMed] [Google Scholar]
- 72.Pielen A, Pantenburg S, Schmoor C, et al. Predictors of prognosis and treatment outcome in central retinal artery occlusion: local intra-arterial fibrinolysis vs. conservative treatment. Neuroradiology 2015;57:1055–62. [DOI] [PubMed] [Google Scholar]
- 73.Augsburger JJ, Magargal LE. Visual prognosis following treatment of acute central retinal artery obstruction. Br J Ophthalmol 1980;64:913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reynard M, Hanscom TA. Neodymium:yttriumaluminum-garnet laser arteriotomy with embolectomy for central retinal artery occlusion. Am J Ophthalmol 2004;137:196–8. [DOI] [PubMed] [Google Scholar]
- 75.Opremcak E, Rehmar AJ, Ridenour CD, et al. Restoration of retinal blood flow via translumenal Nd:YAG embolysis/ embolectomy (TYL/E) for central and branch retinal artery occlusion. Retina 2008;28:226–35. [DOI] [PubMed] [Google Scholar]
- 76.Rudkin AK, Lee AW, Aldrich E, et al. Clinical characteristics and outcome of current standard management of central retinal artery occlusion. Clin Experiment Ophthalmol 2010;38:496–501. [DOI] [PubMed] [Google Scholar]
- 77.Rassam SM, Patel V, Kohner EM. The effect of acetazolamide on the retinal circulation. Eye (Lond) 1993;7:697–702. [DOI] [PubMed] [Google Scholar]
- 78.Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology 1995;102:2029–34; discussion 2034–5. [DOI] [PubMed] [Google Scholar]
- 79.Landa E, Rehany U, Rumelt S. Visual functions following recovery from non-arteritic central retinal artery occlusion. Ophthalmic Surg Lasers Imaging 2004;35:103–8. [PubMed] [Google Scholar]
- 80.Incandela L, Cesarone MR, Belcaro G, et al. Treatment of vascular retinal disease with pentoxifylline: a controlled, randomized trial. Angiology 2002;53 Suppl 1:S31–4. [PubMed] [Google Scholar]
- 81.Iwafune Y, Yoshimoto H. Clinical use of pentoxifylline in haemorrhagic disorders of the retina. Pharmatherapeutica 1980;2:429–38. [PubMed] [Google Scholar]
- 82.Arend O, Harris A, Martin BJ, et al. Retinal blood velocities during carbogen breathing using scanning laser ophthalmoscopy. Acta Ophthalmol (Copenh) 1994;72:332–6. [DOI] [PubMed] [Google Scholar]
- 83.Harino S, Grunwald JE, Petrig BJ, et al. Rebreathing into a bag increases human retinal macular blood velocity. Br J Ophthalmol 1995;79:380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deutsch TA, Read JS, Ernest JT, et al. Effects of oxygen and carbon dioxide on the retinal vasculature in humans. Arch Ophthalmol 1983;101:1278–80. [DOI] [PubMed] [Google Scholar]
- 85.Beiran I, Goldenberg I, Adir Y, et al. Early hyperbaric oxygen therapy for retinal artery occlusion. Eur J Ophthalmol 2001;11:345–50. [DOI] [PubMed] [Google Scholar]
- 86.Anderson B, Jr., Saltzman HA, Heyman A. The Effects of Hyperbaric Oxygenation on Retinal Arterial Occlusion. Arch Ophthalmol 1965;73:315–9. [DOI] [PubMed] [Google Scholar]
- 87.Cope A, Eggert JV, O’Brien E. Retinal artery occlusion: visual outcome after treatment with hyperbaric oxygen. Diving Hyperb Med 2011;41:135–8. [PubMed] [Google Scholar]
- 88.Menzel-Severing J, Siekmann U, Weinberger A, et al. Early hyperbaric oxygen treatment for nonarteritic central retinal artery obstruction. Am J Ophthalmol 2012;153:454–459.e2. [DOI] [PubMed] [Google Scholar]
- 89.Feltgen N, Neubauer A, Jurklies B, et al. Multicenter study of the European Assessment Group for Lysis in the Eye (EAGLE) for the treatment of central retinal artery occlusion: design issues and implications. EAGLE Study report no. 1 : EAGLE Study report no. 1. Graefes Arch Clin Exp Ophthalmol 2006;244:950–6. [DOI] [PubMed] [Google Scholar]
- 90.Page PS, Khattar NK, White AC, et al. Intra-Arterial Thrombolysis for Acute Central Retinal Artery Occlusion: A Systematic Review and Meta-Analysis. Front Neurol 2018;9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahn SJ, Kim JM, Hong JH, et al. Efficacy and safety of intra-arterial thrombolysis in central retinal artery occlusion. Invest Ophthalmol Vis Sci 2013;54:7746–55. [DOI] [PubMed] [Google Scholar]
- 92.Pettersen JA, Hill MD, Demchuk AM, et al. Intra-arterial thrombolysis for retinal artery occlusion: the Calgary experience. Can J Neurol Sci 2005;32:507–11. [PubMed] [Google Scholar]
- 93.Agarwal N, Gala NB, Karimi RJ, et al. Current endovascular treatment options for central retinal arterial occlusion: a review. Neurosurg Focus 2014;36:E7. [DOI] [PubMed] [Google Scholar]
- 94.Noble J, Weizblit N, Baerlocher MO, et al. Intra-arterial thrombolysis for central retinal artery occlusion: a systematic review. Br J Ophthalmol 2008;92:588–93. [DOI] [PubMed] [Google Scholar]
- 95.Aldrich EM, Lee AW, Chen CS, et al. Local intraarterial fibrinolysis administered in aliquots for the treatment of central retinal artery occlusion: the Johns Hopkins Hospital experience. Stroke 2008;39:1746–50. [DOI] [PubMed] [Google Scholar]
- 96.Hattenbach LO, Kuhli-Hattenbach C, Scharrer I, et al. Intravenous thrombolysis with low-dose recombinant tissue plasminogen activator in central retinal artery occlusion. Am J Ophthalmol 2008;146:700–6. [DOI] [PubMed] [Google Scholar]
- 97.Nowak RJ, Amin H, Robeson K, et al. Acute central retinal artery occlusion treated with intravenous recombinant tissue plasminogen activator. J Stroke Cerebrovasc Dis 2012;21:913e5–8. [DOI] [PubMed] [Google Scholar]
- 98.Arnold M, Koerner U, Remonda L, et al. Comparison of intra-arterial thrombolysis with conventional treatment in patients with acute central retinal artery occlusion. J Neurol Neurosurg Psychiatry 2005;76:196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hwang G, Woo SJ, Jung C, et al. Intra-arterial thrombolysis for central retinal artery occlusion: two cases report. J Korean Med Sci 2010;25:974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Biousse V, Calvetti O, Bruce BB, et al. Thrombolysis for central retinal artery occlusion. J Neuroophthalmol 2007;27:215–30. [DOI] [PubMed] [Google Scholar]
- 101.Mercier J, Kastler A, Jean B, et al. Interest of local intra-arterial fibrinolysis in acute central retinal artery occlusion: Clinical experience in 16 patients. J Neuroradiol 2015;42:229–35. [DOI] [PubMed] [Google Scholar]
- 102.Biousse V Thrombolysis for acute central retinal artery occlusion: is it time? Am J Ophthalmol 2008;146:631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Egan RA, Van Stavern R. Should Patients With Acute Central Retinal Artery Occlusion Be Treated With Intraarterial t-PA? J Neuroophthalmol 2015;35:205–9. [DOI] [PubMed] [Google Scholar]
- 104.Chen CS, Lee AW, Campbell B, et al. Efficacy of intravenous tissue-type plasminogen activator in central retinal artery occlusion: report from a randomized, controlled trial. Stroke 2011;42:2229–34. [DOI] [PubMed] [Google Scholar]
- 105.Dumitrascu OM, Shen JF, Kurli M, et al. Is Intravenous Thrombolysis Safe and Effective in Central Retinal Artery Occlusion? A Critically Appraised Topic. Neurologist 2017;22:153–6. [DOI] [PubMed] [Google Scholar]
- 106.Page PS, Cambon AC, James RF. Visual Improvement after Intra-Arterial Thrombolysis for Central Retinal Artery Occlusion Does Not Correlate with Time to Treatment. Interv Neurol 2016;5:131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schumacher M, Schmidt D, Jurklies B, et al. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology 2010;117:1367–75 e1. [DOI] [PubMed] [Google Scholar]
- 108.Preterre C, Godeneche G, Vandamme X, et al. Management of acute central retinal artery occlusion: Intravenous thrombolysis is feasible and safe. Int J Stroke 2017:1747493016687578. [DOI] [PubMed] [Google Scholar]
- 109.Streifler JY, Eliasziw M, Benavente OR, et al. The risk of stroke in patients with first-ever retinal vs hemispheric transient ischemic attacks and high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Arch Neurol 1995;52:246–9. [DOI] [PubMed] [Google Scholar]
- 110.Kvickstrom P, Lindblom B, Bergstrom G, et al. Amaurosis fugax - delay between symptoms and surgery by specialty. Clin Ophthalmol 2016;10:2291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Naylor AR, Robinson TG, Eveson D, et al. An audit of management practices in patients with suspected temporary monocular blindness. Br J Ophthalmol 2014;98:730–3. [DOI] [PubMed] [Google Scholar]