Abstract
The nasal potential difference test has been used for almost three decades to assist in the diagnosis of cystic fibrosis (CF). It has proven to be helpful in cases of attenuated, oligo- or mono-symptomatic forms of CF usually diagnosed later in life, and of CF-related disorders such as congenital bilateral absence of vas deferens, idiopathic chronic pancreatitis, allergic bronchopulmonary aspergillosis, and bronchiectasis. In both clinical and preclinical settings, the test has been used as a biomarker to quantify responses to targeted therapeutic strategies for CF. Adapting the test to a mouse is challenging and can entail an associated mortality. This paper describes the adequate depth of anesthesia required to maintain a nasal catheter in situ for continuous perfusion. It lists measures to avoid broncho-aspiration of solutions perfused in the nose. It also describes the animal care at the end of the test, including administration of a combination of antidotes of the anesthetic drugs, leading to rapidly reversing the anesthesia with full recovery of the animals. Representative data obtained from a CF and a wild-type mouse show that the test discriminates between CF and non-CF. Altogether, the protocol described here allows reliable measurements of the functional status of trans-epithelial chloride and sodium transporters in spontaneously breathing mice, as well as multiple tests in the same animal while reducing test-related mortality.
Keywords: This Month in JoVE, Issue 137, Cystic fibrosis, CFTR, ENaC, nasal potential difference, ion transport, mouse models of disease, biomarker of therapeutic efficacy, diagnosis
Introduction
For almost three decades, electrical potential difference (PD) measurements have been used to evaluate the functional status of transmembrane ion transporters expressed at the nasal mucosa, as representative of the distal airways1. As a multistep dynamic test2,3, nasal PD allows functional dissection of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and epithelial sodium channel (ENaC) activity, both localized at the apical membranes of epithelial cells and exerting critical roles in airway surface hydration. The major clinical application of the nasal PD test is to assist in the diagnosis of CF, the most common fatal genetic disorder in Caucasian populations with an average incidence of 1 out of 2,500 live births in European countries. The test has long proved helpful in the diagnosis of attenuated, oligo- or mono-symptomatic forms of CF usually diagnosed later in life, and of CF-related disorders such as congenital bilateral absence of vas deferens, idiopathic chronic pancreatitis, allergic bronchopulmonary aspergillosis, and bronchiectasis4. More recently, clinometric evaluation of the therapeutic modulation of the basic CFTR defect5,6,7,8,9,10,11,12,13,14,15,16 has made use of the nasal PD in clinical trials of new CF therapies. In the preclinical setting, the test has been adapted to the mouse17 to allow investigation of the bioactivity of new CF target therapies18,19,20,21. In mice, the technique is delicate, based on species-related anatomical differences in size of the nasal region between rodents and humans, and mainly on the essential role of sensory inputs from the nasofacial region in rodents. It requires trained and skilled operators, dedicated equipment and supplies.
CF is a multi-systemic disorder of exocrine glands, in which chronic respiratory disease dominates the clinical picture. The disease is caused by mutations in the gene encoding the cyclic adenosine monophosphate (cAMP)-regulated CFTR chloride channel22. To date, more than 2,000 CFTR mutations have been identified23. The most common mutation24,25, found in almost 90% of CF alleles, corresponds to a deletion of the phenylalanine in position 508 of the polypeptide chain of the protein (F508del-CFTR). The CFTR protein is a purely ohmic small conductance chloride channel. There is also considerable evidence that CFTR regulates other transport mechanisms, in particular, ENaC26,27. Defective electrolyte transport, including reduced CFTR-dependent chloride conductance and increased ENaC-dependent sodium conductance, is a hallmark of CF epithelia. The former defect is reflected by a reduced or abolished repolarization in response to both an electrochemical gradient favoring chloride efflux and addition of isoprenaline (a β-adrenergic agonist that increases intracellular cAMP) or forskolin (an adenylate cyclase agonist, not approved for clinical use). The latter defect is reflected by a basal hyperpolarization of the nasal mucosa (a more negative PD) and an increased response to amiloride, a diuretic drug that blocks ENaC28.
CF mouse models have been frequently used in CF research and have been invaluable in dissecting CF pathology. Nowadays, at least fifteen models have been described29, three of which are homozygous for the most clinically relevant F508del mutation30,31,32. One of these three strains30, developed at Erasmus University in Rotterdam, has been used for nearly 20 years in the Université catholique de Louvain (UCL) laboratory. The Cftrtm1Eur model30 has proved to be very useful to study the multiorgan pathophysiology of CF disease and to test the efficacy of new therapeutic strategies18,19,20,21. Numerous problems may occur during or early after (<24 h) the nasal PD test in mice. In this paper, the adequate depth of anesthesia required for keeping a nasal catheter in situ for continuous perfusion, and measures to avoid broncho-aspiration of solutions perfused in the nose are described. The animal care at the end of the test is also described, including administration of a combination of antidotes of anesthetic drugs, leading to rapidly reversing the anesthesia with complete recovery of the animals. Altogether, these procedures allow reliable measurements in spontaneously breathing mice, reduced test-related mortality and repeating the test in the same animal. Representative data obtained from the nasal PD test in a CF and in a wild-type mouse are shown and discussed.
The murine nasal PD test protocol is reported in three sessions: assessment and management before, during, and after the test. In the pre-test assessment and management, the protocol of preparation of the double lumen nasal catheter and of solutions used for continuous nasal perfusion is described in detail. During the assessment and management portions of the test, the experimental setup and the handling of the mouse is minutely dissected. Finally, management of the animal at the end of the test is described to improve full animal recovery.
Protocol
The studies and procedures were approved by the ethics committee for animal research of the UCL (2017/UCL/MD/015) and in agreement with the European Community regulations for animal use in research (CEE n° 86/609). The investigators are qualified for the animal experimentation following the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
1. Pre-test Assessment and Management
- Prepare the double lumen nasal catheter. NOTE: A nasal catheter is made as a double lumen capillary tube, one lumen being used for the continuous perfusion of solutions, and the other one as a measuring channel.
- Heat up the central section of a piece, about 20 cm long, of polyethylene tube (2.0 mm inner diameter; 3.0 mm outer diameter; Figure 1a) in the flame of a gas burner until it is soft enough for pulling (10 - 15 s).
- Pull the two ends apart to obtain a very thin capillary tube of appropriate length (~15 cm) and outer diameter (~0.1 mm) for the nasal probe (Figure 1b).
- Clean and degrease two such capillaries with pure ethanol, join them with tape (Figure 1c) and glue them together using cyanoacrylate glue.
- Cut away the excess length of the probes with a razor blade or a scalpel to obtain an optimal length of the double lumen catheter of about 8 cm (Figure 1d).
- Inject water through both lumens to verify that they are permeable.
- Apply a mark at a 5 mm distance from the tip. NOTE: The probe is inserted up to the mark into the nostril, so that all measurements are made at the same site inside the nasal cavity.
- Store the double lumen catheter in a dry box. NOTE: It can be used 6 - 10 times if appropriately cleaned immediately after the test.
- Prepare the cream mix.
- Gently mix electrolyte cream and saturated 3 M KCl solution in a 1:1 ratio (volume/volume), avoiding formation of tiny air bubbles. NOTE: The cream mix is used to build the electrode bridges for the measuring and the reference Ag/AgCl electrodes, as illustrated in Figure 1.
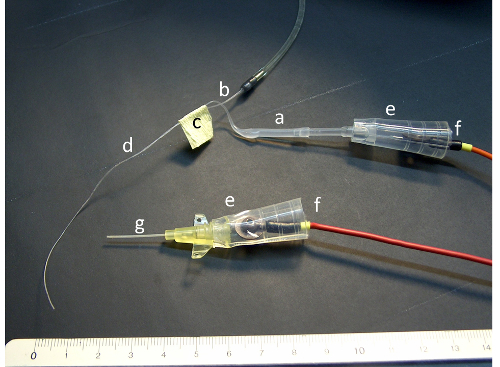
Figure 1: Probes with electrodes and bridges. The figure illustrates the polyethylene tube before (a) and after heating and pulling (b), the tape joining the two capillary parts (c) of the double lumen catheter (d), the silicone tube connectors (e) and the electrodes (f). For picture clarity, the connectors have not been filled with cream mix. Please click here to view a larger version of this figure.
| Solution Label | Solution description |
| A | Basal buffered salt solution |
| B | Chloride-free buffered salt solution |
| C | 10-2 M amiloride stock solution |
| D | 10-3 M forskolin stock solution |
Table 1: Stock solution for the nasal PD test in mice.
| Salt | mM | Molecular weight | g/L |
| Sodium chloride (NaCl) | 135 | 58.44 | 7,889 |
| Calcium chloride dihydrate (CaCl2.2H2O) | 2.25 | 147 | 0.331 |
| Magnesium chloride hexahydrate (MgCl2.6H2O) | 1.2 | 203.3 | 0.244 |
| Dipotassium phosphate (K2HPO4) | 2.4 | 174.2 | 0.418 |
| Monopotassium phosphate (KH2PO4) | 0.4 | 136.1 | 0.054 |
Table 2: Composition of basal buffered salt solution (stock solution A).
- Prepare the solutions.
- Prepare the four stock solutions required for the experimental protocol (Table 1).
- Prepare the basal buffered solution (stock solution A; Table 2) and the chloride-free buffered solution (stock solution B; Table 3) by mixing the salts in pure water at room temperature. Adjust pH to 7.4 (range 7.0 - 7.6) using 1 N NaOH to raise pH, 1 N HCl to lower pH. Check osmolarity of the solution (275 mOsm/L).
- Store in labeled glass bottles at 4 °C for up to 3 months or frozen in plastic bottles for up to 6 months.
- Prepare the 10-2 M amiloride solution (stock solution C) by adding 26.6 mg of amiloride hydrochloride to 10 mL of pure water. Heat the mixture for 5 to 10 minutes at 70 °C. As amiloride is sensitive to light, keep solution C in a dark container. Label the container and store at 4 °C for up to 3 months.
- Prepare the 10-3 M forskolin solution (stock solution D) by adding 10 mg of forskolin to 24.36 mL of pure water. Label 0.1 mL aliquots of the forskolin stock solution and store at -20 °C for up to 6 months.
- Prepare fresh solutions A1, B1 and B2 (Table 4).
- Prepare fresh solution A1 (basal buffered salt solution plus 10-4 M amiloride) by obtaining 10 mL of basal salt solution (stock solution A) and adding 0.1 mL of 10-2 M amiloride solution (stock solution C). Label the container and use within 24 h of preparation.
- Prepare fresh solution B1 (chloride buffered solution plus 10-4 M amiloride) by obtaining 10 mL of chloride-free buffered salt solution (stock solution B) and adding 0.1 mL of 10-2 M amiloride solution (stock solution C). Label the container and use within 24 hours of preparation.
- Prepare fresh solution B2 (chloride-free buffered salt solution plus 10-4 M amiloride and 10-5 M forskolin) by obtaining 10 mL of chloride-free buffered salt solution (stock solution B) and adding 0.1 mL of 10-2 M amiloride solution (stock solution C) and 0.1 mL of 10-3 M forskolin solution (stock solution D). Label the container and use within 2 hours of preparation.
| Salt | mM | Molecular weight | g/L |
| Sodium gluconate (monosodium salt) | 135 | 218.1 | 29,444 |
| Calcium gluconate (anhydrous powder) | 2.2 | 430.4 | 0.947 |
| Magnesium sulfate heptahydrate (MgCl2.6H2O) | 1.2 | 246.5 | 0.296 |
| Dipotassium phosphate (K2HPO4) | 2.4 | 174.2 | 0.418 |
| Monopotassium phosphate (KH2PO4) | 0.4 | 136.1 | 0.054 |
Table 3: Composition of chloride-free buffered solution (stock solution B).
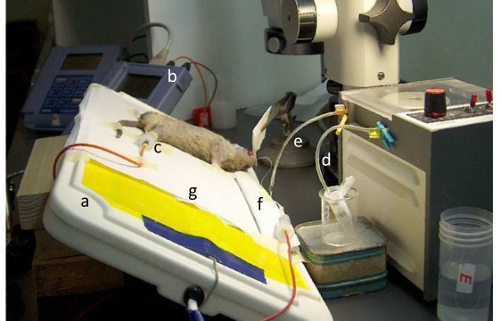
Figure 2: Position of the mouse during perfusion of the nasal mucosa. The figure illustrates the heating pad (a), the voltmeter (b), the reference electrode inserted in the subcutaneous space in a hind limb (c), the proximal (d) and the distal (e) outlets of the peristaltic pump, the pillow (f), and the bed sheet (g). Please click here to view a larger version of this figure.
2. Assessment and Management During the Test
- Prepare the experimental setup.
- To prevent hypothermia during and after the test, use two heating pads maintained at physiological temperatures, one for the measuring setup (18.8 x 37.5 cm; Figure 2a), and the other one (15.5 x 15.5 cm) for the box in which the animal will be placed for recovery at the end of the test.
- Switch on the computer loaded with the software for data capture connected to the data memory high input impedance (>1012Ω) and high resolution (0.1 mV) voltmeter (Figure 2b).
- Connect the electrodes to the voltmeter. Connect the positive measuring electrode to the nasal catheter and the negative reference electrode to the catheter inserted in the subcutaneous space in a hind limb, as shown in Figure 2c.
- Dip the tips of the electrodes together in the cream mix. Check the initial electrode offset value. Reject the electrodes if the value is larger than (±) 2 mV (ideally 0 mV).
- Switch on the peristaltic pump and insert the pump tube into position, as illustrated in Figure 2.
- Fill one lumen of the nasal catheter with electrolyte cream mix through a 10 µL pipette tip. Adapt the catheter to a silicone tube connector (Figure 1e), fill the connector with cream mix and insert the positive electrode in the connector (Figure 1f). Check the measuring electrode bridge offset value. Reject the bridges if the value is larger than (±) 2 mV (ideally 0 mV).
- Fill the second lumen of the nasal catheter with the fresh solution A1. Dip the tip of the nasal catheter in the vial containing solution A. Reverse the direction of the peristaltic pump to fill a length of the nasal catheter corresponding to 10 s of perfusion. NOTE: This allows recording basal PD values for 10 s before applying amiloride. Dip the tip of the nasal catheter in the cream mix inside the connector of the reference electrode (Figure 1).
- Begin handling the mouse.
- Record the mouse weight to calculate the exact dose of anesthetics to be injected by the intraperitoneal (ip) route17.
- Using a 26G (0.45 x 10 mm) needle, inject premedication intraperitoneally to promote the induction of anesthesia: a fixed dose of 50 µL of 5 mg/mL midazolam. Wait for 5 min.
- Prepare the anesthetic mix (fentanyl, medetomidine, droperidol at final concentrations of 0.05, 0.40, and 20 mg/kg body weight respectively, and clonidine at the fixed dose of 0.375 µg) allowing optimal and stable depth of anesthesia (stage III, plane 2)17. Inject the volume of anesthetic mix intraperitoneally, and then inject the volume of clonidine. NOTE: After 15 min, the mouse should be asleep and the anesthesia can last for at least 30 min.
- Fill the catheter and the connector with cream mix and insert the negative electrode in the connector (Figure 1f).
- Fix the catheter and the electrode with tape as required to prevent any displacement. After the placement of the reference electrode and bridges, verify the measuring electrode bridge offset value again by dipping the tip of the nasal catheter in the electrode cream mix inside the connector of the IV catheter. Record the stable final offset value (ideally less than (±) 2 mV).
- To absorb fluid from the oral cavity, move the tongue sideways (Figure 3a) and insert a pointed wick of filter paper (the 'pipe'; Figure 3b) about 1 cm into the mouth. To absorb fluid running out of the perfused nostril, place a second piece of filter paper (the 'handkerchief'; Figure 3c) held at the tip of the nose.
- Check a positive control value of the epithelial potential by placing the tip of the nasal catheter in contact with the inside of the mouth. NOTE: The reading should be stable and range between -10 mV and -20 mV.
- 5 min after injection of the anesthetic drugs, gently tilt the heating pad by about 30° with the animal head downwards. Monitor the maximal basal PD value.
- When it is stable over a period of about 30 s, start perfusing the nasal mucosa, at a constant rate of 10 µL/ min, with the 4 buffered solutions (A, A1, B1, B2) in succession. Perfuse the first solution (step 1.3.1.1) for 10 s and each following solution (step 1.4) for 5 min or until a stable value has been reached. Stop perfusing forskolin (B2) when the transient forskolin response starts going down.
- Select a 1 s interval for data recording. Start recording data at the time of starting perfusion. Display data as a function of time on the computer screen.
- Stop data capture at the end of perfusion with solution B2. Save data as a spreadsheet file.
- Correct data by subtracting the final offset value.
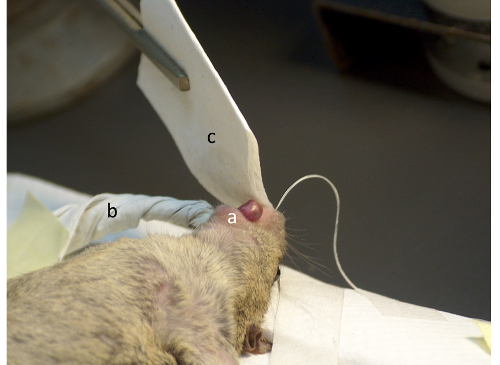
Figure 3: Position of the mouse on the heating pad with the nasal catheter and the filter papers in place. The figure illustrates the tongue put sideways (a), the pipe (b), and the handkerchief (c). Please click here to view a larger version of this figure.
3. Post-test Assessment and Management
Release the mouse from the heating pad.
Wipe the mouse nose with the 'bed sheet' (Figure 2g).
Inject intraperitoneally the anesthetic antagonist mix composed of a fixed dose (4 µg) of naloxone, a competitive morphine antagonist, and atipamezole, a medetomidine specific antidote (2 mg/kg body weight).
Lay the mouse on the smaller heating pad in the recovery box until full recovery, that is usually observed after 1 to 2 h. NOTE: In the UCL laboratory, about 10% mortality, mainly related to bronchoaspiration of solutions perfused in the nasal cavity during the test, is observed irrespective of the gender or genotype.
Representative Results
In order to illustrate the characteristic ion transport abnormalities in CF, nasal PD measurements were performed following the protocol described above in an F508del-CF mouse and in a wild-type control of the FVB/129 genetic background from the Brussels colony of Cftrtm1Eur mice30. This clinically relevant model, harboring the most common and one of the most severe F508del-CFTR mutation23,24,25, is the best currently available CF mouse model30,31,32.
Representative nasal PD tracings, obtained in a 4-month old female mouse homozygous for the F508del-CF mutation and in an age- and sex-matched wild-type littermate, are shown in Figure 4. During the first two phases of the test, the functional status of ENaC was studied by perfusing solutions A and A1, the latter containing amiloride. The functional status of CFTR (and of alternative chloride transporters in the absence of forskolin) was assessed during the last two phases of the test, when the contribution of ENaC remained blocked by amiloride.
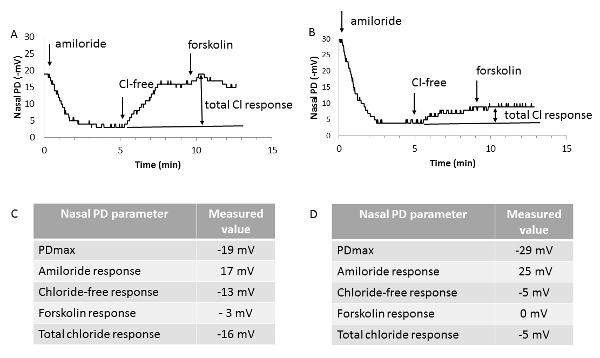
In the F508del-CF mouse, a hyperpolarized baseline value (a more negative PDmax compared to the wild-type mouse value) together with an increased amiloride response were observed; both findings reflect the CFTR-associated ENaC overactivity. More consistently, a drastically reduced repolarization in response to both an electrochemical gradient favorable to chloride efflux and addition of forskolin, named here as total chloride response, was observed. Even though the magnitude of the forskolin response in wild-type mice is small (-3 mV), in CF, the response is usually blunted, consistent with the CFTR loss-of-function.
Figure 4 - Representative nasal PD tracings. Representative nasal PD tracings from a homozygous normal mouse (A) and a mouse homozygous for the F508del-CFTR mutation (B), together with the individual values obtained for the nasal PD parameters (C and D). PDmax: maximal baseline stable value. Amiloride response: difference between the values of nasal PD at the end and at the beginning of perfusion of the nasal mucosa with basal buffered salt solution containing amiloride (solution A1). Chloride-free response: difference between the values of nasal PD at the end and at the beginning of perfusion of the nasal mucosa with chloride-free buffered salt solution plus amiloride (solution B1). Forskolin response: difference between the values of nasal PD at the end and at the beginning of perfusion of the nasal mucosa with chloride-free buffered salt solution plus forskolin and amiloride (solution B2). Total chloride response: sum of the last two parameters obtained under zero-chloride perfusion. Arrows indicate changes of solutions perfused into the nostril. Please click here to view a larger version of this figure.
Antidotes of the anesthetics were applied at the end of the tests, thus reducing the duration of the anesthesia, which can be up to 45 min beyond test completion. As recovery of the animals occurred without after-effects, they were tested again after an interval of seven days, when the same protocol was applied. The same nostril was explored during both tests. Examples of the second test and paired differences of each individual nasal PD parameter between the two tests are shown in Figure 5. As previously reported35, the between-test differences were close to nil, in particular for total chloride response, reflecting the functional status of CFTR-dependent chloride transport, defective in CF.
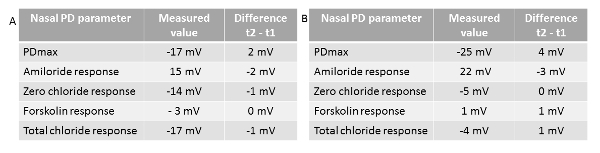
Figure 5 - Individual values of nasal PD parameters. Values were obtained in a second test (t2) performed in a homozygous normal mouse (A) and a mouse homozygous of the F508del-CFTR mutation (B), together with the paired differences between the second and the first test (t1) for each corresponding parameter. PDmax: maximal baseline stable value. Amiloride response: difference between the values of nasal PD at the end and at the beginning of perfusion of the nasal mucosa with the basal buffered salt solution containing amiloride (solution A1). Chloride-free response: difference between the values of nasal PD at the end and at the beginning of perfusion of the nasal mucosa with the chloride-free buffered salt solution plus amiloride (solution B1). Forskolin response: difference of the values of nasal PD at the end and at the beginning of perfusion of the nasal mucosa with the chloride-free buffered salt solution plus forskolin and amiloride (solution B2). Total chloride response: sum of the last two parameters obtained under zero-chloride perfusion. Please click here to view a larger version of this figure.
| Solution Label | Solution description |
| A1 | Basal buffered salt solution (A) plus 10-4 M amiloride |
| B1 | Chloride-free buffered salt solution (B) plus 10-4 M amiloride |
| B2 | Chloride-free buffered salt solution (B) plus 10-4 M amiloride plus 10-5 M forskolin |
Table 4: List of the fresh solutions for the nasal PD test in mice.
Discussion
The purpose of this paper is to describe an adequate protocol for measuring nasal PD under continuous perfusion of solutions in spontaneously breathing mice for a length of time required for testing the integrity of ion transporters, mainly CFTR and ENaC. All steps of the protocol have been carefully optimized to ensure full animal recovery and good quality and reproducible data. In particular, critical steps are anesthesia assessment and management, and adequate animal position and care during and after the test.
Previous studies showed that plane 2 stage III of anesthetic exposure, which can be achieved by applying the cocktail mixture used here17, is associated with regular breathing and with absence of negative inotropic effect and of blink, pupillary and pedal withdrawal reflexes. At this level of anesthetic depth, the nasal catheter can be kept in situ for continuous perfusion of the nasal cavity and for use as a bridge of the measuring electrode with good tolerance. Also, insertion of the catheter in the subcutaneous space, serving as the bridge of the reference electrode, was not followed by any painful reaction or sign of harmful effect. In rodents, the essential role of sensory input from the nasofacial region in the control of behavior vis-a-vis an external situation, including a threat, makes adequate depth of anesthesia particularly challenging when operating in the nasal cavity. Nebulization instead of continuous nasal perfusion has been applied to perform nasal PD test in some mouse studies33,34. However, this method leads to non-reliable results, owing to repeated removals and reinsertions of the nasal probe. As a matter of fact, owing to non-homogenous distribution of cell types in the mouse nasal mucosa20, repositioning the tip of the probe at the same location in the nostril is critical. Moreover, responses to changes of solutions, in particular chloride-free solutions, rapidly vanish when perfusion is interrupted.
Broncho-aspiration of solutions leading to respiratory arrest is a critical limitation of the procedure and is the major cause of mortality of the test. Several essential measures aim at preventing it, including a dorsal decubitus position of the animal, lightly tilting it with its head downwards, and absorbing excess fluid from the oral and nasal cavities17. A rapid and reversible level of anesthesia with complete recovery of animals is ensured by applying antidotes of anesthetic drugs at the end of the test. The protocol presented here allows reliable measurements in spontaneously breathing mice and repeating the test in the same animal. It impacts on the number of mice required to get statistical significance35 and on complying to the 3R (replace, refine and reduce) rules for animal use in experimental procedures36. In humans as in mice, the lowest between-test variability has been found for total chloride response, suggesting that it as the most reliable nasal PD parameter to detect efficacy of new CF therapeutic strategies. In CF mice, the measurement error of the total chloride response was shown to be less than ±1.7 mV35. In other words, when evaluating the bioactivity of a CFTR-correcting drug in F508del-CF mice, a difference between total chloride response in the absence and in the presence of treatment larger than 2 mV indicates 95% chance of a drug-related improving effect.
Data interpretation of representative tracings illustrates the ability of the nasal PD test to discriminate between CF and wild-type mice18,19,20,21,35 and shows that the F508del-CF Erasmus mouse model30 mimics the human nasal mucosa with respect to the typical clinical ion transport abnormalities. However, in the animal model, a residual chloride conductance is detectable, resulting either from a residual F508del-CFTR function or from a contribution of alternative non-CFTR-dependent chloride channels. Translating results from CF research from preclinical into clinical settings implies dealing with several major differences between the two settings. The mouse CF phenotype displays an attenuated respiratory syndrome. The absence of multiple therapies together with the fact that the mouse model is housed in privileged conditions with hygienic barriers also contribute to the differences37. The protocol described here shows a very low variability35 and it has been adapted to the pig38,39 and the ferret models40. In a previous study, the experimental protocol was modified by including perfusion of the mouse nasal mucosa with an inhibitor of alternative chloride transporters, to explore the possible contribution of non-CFTR-dependent calcium-activated chloride channels18. The test has also been used to study sodium transport in the β-ENaC overexpressing mouse model41, engineered to mimic CF-lung disease42. In the future, further applications of the test could be considered to study other transporters, such as the ATP12A, a CFTR-independent H+-pump protein expressed in human and in pig but absent in mouse airways43. Altogether, the protocol described here allows reliable measurements of the functional status of transepithelial chloride and sodium transporters in spontaneously breathing mice, reduced test-related mortality and multiple tests in the same animal.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors thank Prof. J. Lebacq for critically editing the manuscript. Cftrtm1Eur (homozygous F508del-CFTR (FVB/129) mice were developed by the Erasmus MC, Rotterdam, The Netherlands, with the support of European Economic Community European Coordination Action for Research in Cystic Fibrosis EU FP6 LHHM-CT-2005-018932.
References
- Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. New England Journal of Medicine. 1981;305(25):1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Middleton PG, Geddes DM, Alton EFW. Protocols for in vivo measurement of the ion transport defects in cystic fibrosis nasal epithelium. European Respiratory Journal. 1994;7(11):2050–2056. [PubMed] [Google Scholar]
- Knowles MR, Paradiso AM, Boucher RC. In vivo nasal potential difference: techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Human Gene Therapy. 1995;6(4):445–455. doi: 10.1089/hum.1995.6.4-445. [DOI] [PubMed] [Google Scholar]
- Paranjape SM, Zeitlin PL. Atypical cystic fibrosis and CFTR-related disorders. Clinical Reviews in Allergy & Immunology. 2008;35(3):116–123. doi: 10.1007/s12016-008-8083-0. [DOI] [PubMed] [Google Scholar]
- Wilschanski M, et al. A pilot study of the effect of gentamicin on nasal potential difference measurements in CF patients carrying stop mutations. American Journal of Respiratory and Critical Care Medicine. 2000;161(3):860–865. doi: 10.1164/ajrccm.161.3.9904116. Pt 1. [DOI] [PubMed] [Google Scholar]
- Clancy JP, et al. Evidence that systemic gentamicin suppresses premature stop mutations in patients with CF. American Journal of Respiratory and Critical Care Medicine. 2001;163(7):1683–1692. doi: 10.1164/ajrccm.163.7.2004001. [DOI] [PubMed] [Google Scholar]
- Wilschanski M, et al. Gentamicin-induced correction of CFTR function in patients with CF and CFTR stop mutations. New England Journal of Medicine. 2003;349(15):1433–1441. doi: 10.1056/NEJMoa022170. [DOI] [PubMed] [Google Scholar]
- Sermet-Gaudelus I, et al. In vitro prediction of stop-codon suppression by intravenous gentamicin in patients with CF: a pilot study. BMC Medicine. 2007;5:5. doi: 10.1186/1741-7015-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy JP, et al. No detectable improvements in CF transmembrane conductance regulator by nasal aminoglycosides in patients with CF with stop mutations. American Journal of Respiratory and Cell Molecular Biology. 2007;37(1):57–66. doi: 10.1165/rcmb.2006-0173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem E, et al. Effectiveness of PTC124 treatment of CF caused by nonsensemutations: a prospective phase II trial. Lancet. 2008;372(9640):719–727. doi: 10.1016/S0140-6736(08)61168-X. [DOI] [PubMed] [Google Scholar]
- Sermet-Gaudelus I, et al. Ataluren (PTC124) induces CF transmembrane conductance regulator protein expression and activity in children with nonsense mutation CF. American Journal of Respiratory and Critical Care Medicine. 2010;182(10):1262–1272. doi: 10.1164/rccm.201001-0137OC. [DOI] [PubMed] [Google Scholar]
- Wilschanski M, et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. European Respiratory Journal. 2011;38(1):59–69. doi: 10.1183/09031936.00120910. [DOI] [PubMed] [Google Scholar]
- Accurso FJ, et al. Effect of VX-770 in persons with CF and the G551D-CFTR mutation. New England Journal of Medicine. 2010;363(21):1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy JP, et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2012;67(1):12–18. doi: 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard A, Lebecque P, Dingemanse J, Leal T. A randomized placebo-controlled trial of miglustat in cystic fibrosis based on nasal potential difference. Journal of Cystic Fibrosis. 2012;11(3):231–236. doi: 10.1016/j.jcf.2011.12.004. [DOI] [PubMed] [Google Scholar]
- De Boeck K, et al. CFTR biomarkers: time for promotion to surrogate end-point. European Respiratory Journal. 2013;41:203–216. doi: 10.1183/09031936.00057512. [DOI] [PubMed] [Google Scholar]
- Leal T, et al. Successful protocol of anaesthesia for measuring transepithelial nasal potential difference in spontaneously breathing mice. Laboratory Animals. 2006;40(1):43–52. doi: 10.1258/002367706775404480. [DOI] [PubMed] [Google Scholar]
- Lubamba B, et al. Preclinical evidence that sildenafil and vardenafil activate chloride transport in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2008;177(5):506–515. doi: 10.1164/rccm.200703-344OC. [DOI] [PubMed] [Google Scholar]
- Lubamba B, et al. Airway delivery of low-dose miglustat normalizes nasal potential difference in F508del cystic fibrosis mice. American Journal of Respiratory and Critical Care Medicine. 2009;179(11):1022–1028. doi: 10.1164/rccm.200901-0049OC. [DOI] [PubMed] [Google Scholar]
- Lubamba B, et al. Inhaled PDE5 inhibitors restore chloride transport in cystic fibrosis mice. European Respiratory Journal. 2011;37(1):72–78. doi: 10.1183/09031936.00013510. [DOI] [PubMed] [Google Scholar]
- Vidovic D, et al. rAAV-CFTRΔR Rescues the Cystic Fibrosis Phenotype in Human Intestinal Organoids and Cystic Fibrosis Mice. American Journal of Respiratory and Critical Care Medicine. 2016;193(3):288–298. doi: 10.1164/rccm.201505-0914OC. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269(5225):847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- Lubamba B, Dhooghe B, Noel S, Leal T. Cystic fibrosis: insight into CFTR pathophysiology and pharmacotherapy. Clinical Biochemistry. 2012;45(15):1132–1144. doi: 10.1016/j.clinbiochem.2012.05.034. [DOI] [PubMed] [Google Scholar]
- Kerem B, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Riordan JR, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4925):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. Journal of Biological Chemistry. 1997;272(22):14037–14040. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- Ismailov II, et al. Regulation of epithelial sodium channels by the cystic fibrosis transmembrane conductance regulator. Biological Chemistry. 1996;271(9):4725–4732. doi: 10.1074/jbc.271.9.4725. [DOI] [PubMed] [Google Scholar]
- Althaus M. ENaC inhibitors and airway re-hydration in cystic fibrosis: state of the art. Current Molecular Pharmacology. 2013;6(1):3–12. doi: 10.2174/18744672112059990025. [DOI] [PubMed] [Google Scholar]
- Wilke M, et al. Mouse models of cystic fibrosis: phenotypic analysis and research applications. Journal of Cystic Fibrosis. 2011;10:152–171. doi: 10.1016/S1569-1993(11)60020-9. Suppl 2. [DOI] [PubMed] [Google Scholar]
- Van Doorninck JH, et al. A mouse model for the cystic fibrosis delta F508 mutation. The EMBO Journal. 1995;14(18):4403–4411. doi: 10.1002/j.1460-2075.1995.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge WH, et al. Generation and characterization of a delta F508 cystic fibrosis mouse model. Nature Genetics. 1995;10(4):445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- Zeiher BG, et al. A mouse model for the delta F508 allele of cystic fibrosis. Journal of Clinical Investigation. 1995;96(4):2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Taylor CJ, McGray J. Modification of the nasal membrane potential difference with inhaled amiloride and loperamide in the cystic fibrosis (CF) mouse. Thorax. 1996;51(12):1229–1232. doi: 10.1136/thx.51.12.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Taylor CJ, Colledge WH, Ratcliff R, Evans MJ. Sodium channel blockers and uridine triphosphate: effects on nasal potential difference in cystic fibrosis mice. European Respiratory Journal. 2000;15(1):146–150. doi: 10.1183/09031936.00.15114600. [DOI] [PubMed] [Google Scholar]
- Leonard A, et al. Comparative Variability of Nasal Potential Difference Measurements in Human and Mice. Open Journal of Respiratory Disease. 2012;2:43–56. [Google Scholar]
- Tannenbaum J, Bennett BT. Russell and Burch's 3Rs then and now: the need for clarity in definition and purpose. Journal of the American Association for Laboratory Animal Science. 2015;54(2):120–132. [PMC free article] [PubMed] [Google Scholar]
- Pritchett-Corning KR, et al. AALAS/FELASA Working Group on Health Monitoring of rodents for animal transfer. Journal of the American Association for Laboratory Animal Science. 2014;53(6):633–640. [PMC free article] [PubMed] [Google Scholar]
- Salinas DB, et al. CFTR involvement in nasal potential differences in mice and pigs studied using a thiazolidinone CFTR inhibitor. American Journal of Physiology. Lung Cell Molecular Physiology. 2004;287(5):936–943. doi: 10.1152/ajplung.00354.2003. [DOI] [PubMed] [Google Scholar]
- Fisher JT, et al. Comparative processing and function of human and ferret cystic fibrosis transmembrane conductance regulator. Journal of Biological Chemistry. 2012;287(26):21673–21685. doi: 10.1074/jbc.M111.336537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaza N, et al. Use of ferrets for electrophysiologic monitoring of ion transport. PLoS One. 2017;12(10):0186984. doi: 10.1371/journal.pone.0186984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal T, Beka M, Panin N, Mall MA, Noel S. Nasal potential difference in βENaC-overexpressing mouse reveals pH-sensitive channel hyperactivity and shift of subunits stoichiometry. Journal of Cystic Fibrosis. 2017;16(S1):72. [Google Scholar]
- Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nature Medicine. 2004;10(5):487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- Shah VS, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351(6272):503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]


