Abstract
The concept of objective nociceptive assessment and optimal pain management have gained increasing attention. Despite the known negative short- and long-term consequences of unresolved pain or excessive analgosedation, adequate nociceptive monitoring remains challenging in non-communicative, critically ill adults. In the intensive care unit (ICU), routine nociceptive evaluation is carried out by the attending nurse using the Behavior Pain Scale (BPS) in mechanically ventilated patients. This assessment is limited by medication use (e.g., neuromuscular blocking agents) and the inherent subjective character of nociceptive evaluation by third parties.
Here, we describe the use of two nociceptive reflex testing devices as tools for objective pain evaluation: the pupillary dilation reflex (PDR) and nociception flexion reflex (NFR). These measurement tools are non-invasive and well tolerated, providing clinicians and researchers with objective information regarding two different nociceptive processing pathways: (1) the pain-related autonomic reactivity and (2) the ascending component of the somatosensory system. The use of PDR and NFR measurements are currently limited to specialized pain clinics and research institutions because of impressions that these are technically demanding or time-consuming procedures, or even because of a lack of knowledge regarding their existence.
By focusing on the two abovementioned nociceptive reflex assessments, this study evaluated their feasibility as a physiological pain measurement method in daily practice. Pursuing novel technologies for evaluating the analgesia level in unconscious patients may further improve individual pharmacological treatment and patient related outcome measures. Therefore, future research must include large well-designed clinical trials in a real-life environment.
Keywords: Medicine, Issue 137, Pain Measurement, Reflex physiology, Pain physiopathology, Analgesia, Nociceptive assessment, Humans
Introduction
Many critically ill patients in the Intensive Care Unit (ICU) are prone to experience pain during daily care or during diagnostic or therapeutic procedures. Substandard nociceptive evaluation and consequent suboptimal pain management may increase stress and anxiety1. Persistent pain not only increases circulating catecholamines, compromises tissue perfusion and reduces oxygen delivery2 but also activates catabolic hypermetabolism, thus contributing to hyperglycemia, lipolysis and muscle loss. All of these elements impair the healing process and increase the risk of infections3,4,5,6.
As stated by the International Association for the Study of Pain (IASP), clinicians must use pain assessment tools that are valid for all patients, and self-reports remain the golden standard for pain evaluation. However, there are many situations in which patients are unable to communicate, especially because of critical illness or when they are mechanically ventilated (MV). The increased interest in ICU patient-related outcome measures has amplified the need for structured and reliable techniques for nociceptive assessment when a patient is unable to report pain and discomfort. Attempts to address this need have been hampered by the lack of specific, reproducible and feasible monitoring tools. In recent years, considerable effort has been directed toward providing physicians with more objective nociceptive parameters. However, many studies executed in the ICU have focused on the use of vital signs as possible surrogates for pain assessment and underlie not to use blood pressure or heart rate as a specific parameter for pain7,8.
As reported in previous research, untreated pain significantly compromises patient outcomes and should therefore always be assessed independently of vital signs, and assessments should not be influenced by a patient's inability to communicate7,8,9,10,11,12. This approach of objective nociceptive assessment has gained considerable support due to the known negative consequences of pain. Especially in ICU patients, physiological and psychological effects can be substantial and long-lasting and may significantly decrease health-related quality of life13,14.
Currently, no objective pain monitoring protocol exists that can readily be applied to a large group of critically ill patients. The implementation of objective assessment tools in ICU patients could optimize pain management and thus prevent the development of central sensitization syndromes. Moreover, opioid-induced hyperalgesia (OIH), chronification of pain, and long-lasting pain-related morbidity may decrease. Finally, the application of nociceptive reflex evaluation tools may provide a unique translational platform on which new pharmacological analgesic compounds can be tested.
The aim of the proposed methodology is to provide an overview of the technical requirements and provide a precise description of the protocols used to assess nociceptive reflexes in non-communicative ICU patients. Overall, we aim to provide a comprehensive guide for the use of objective pain measurement tools in the ICU and in other circumstances in which sedated or unconscious patients need to be assessed.
Critically ill unconscious adults admitted to the ICU were screened for study inclusion from October 2016 until December 2017. All were mechanically ventilated and received a strict analgosedation protocol containing propofol/remifentanil or propofol/sufentanil, which are the two most commonly used schemes in our hospital. A history of ophthalmologic surgery, known pupil reflex disorders, Horner or Adie's syndrome, previous eye trauma, cranial nerve lesions or acute intracranial hypertension caused by traumatic brain injury, tumor compression or bleeding, fulminant stroke, known (poly)neuropathy related to diabetes or other neurological conditions known to influence reflex activity, intra- or extracorporeal treatment (pacemaker, intra-aortic balloon pump, extracorporeal life support), chronic opioid use (>3 months), age <18 years, and the use of topical interfering eye drops (atropine, phenylephrine), α2 adrenergic agonists15, the use of other analgosedation protocols than described by the inclusion criteria or neuromuscular blocking agents were defined as exclusion criteria.
The demographic variables and medical data of the enrolled subjects, including the Simplified Acute Physiology Score II (SAPS II),16were extracted from the digital patient data management system (e.g., Metavision).
Pain Assessment
ICU patients were screened for study inclusion, which required a medical history and admission diagnosis to assess the inclusion and exclusion criteria mentioned above. Physiological reflexes were assessed in the ICU environment under real-life conditions: no specific modifications were made regarding temperature or noise control. Reflex assessment was executed during daytime working hours at the individual patient room of approximately 20 °C. All generated data (reflex characteristics) can be stored by each of the two devices when this function is enabled on the touch screen display.
Measurement of the Pupil Dilation Reflex
A pupillometry device was used for pupil dilation reflex (PDR) assessment using infrared video recording for quantitative pupil size evaluation. For the application of standardized nociceptive stimulation, two low-impedance Ag-AgCl electrodes were placed on the skin area innervated by the median nerve on the left arm after skin preparation (Figure 1). The current was fixed at 60 milliampères (mA) with a maximum acceptable resistance of 5 kOhms, defining a voltage limitation of 300 volts (V).
PDR assessment was performed using an inbuilt pupillary pain index (PPI) measurement protocol that generates an automatic electric stimulation pattern for dynamic pupil reflex evaluation. Standardized noxious stimulation was applied with increasing intensity (from 10 mA to 60 mA with incremental steps of 10 mA, a duration of 1 s, and a pulse width of 200 µs) until pupillary dilation greater than 13% ([maximal diameter - minimal diameter]/maximal diameter * 100) or maximal stimulation at 60 mA was achieved. When the defined criteria were reached, stimulation was automatically interrupted, and a PPI score was displayed (Table 1). Baseline pupil size (before standardized noxious stimulation), pupil reflex amplitude (PRA), stimulation intensity and the PPI score were recorded. The duration of PDR measurement was between 2 and 16 seconds depending on the number of required stimulations.
Several studies have suggested the use of pupillometry in non-communicative ICU adults. Paulus et al. demonstrated that PDR evaluation may predict analgesia requirements during endotracheal aspiration17. Moreover, this method may be able to reveal different levels of analgesia and could have discriminatory properties regarding different types of noxious procedures18,19. Recently, scientific interest has been directed toward the use of specific protocols for PDR assessment because of their low stimulation currents. The PPI protocol suggested in our approach has been previously investigated in anesthetized adults, revealing a significant correlation between PDR and opioid administration20. Furthermore, Sabourdin et al.21 demonstrated that PDR can be used to guide individual intraoperative remifentanil administration and therefore reduce intraoperative opioid consumption and postoperative rescue analgesia requirements.
Measurement of the Nociceptive Flexion Reflex
To assess the role of primary afferent fibers in the transmission of nociceptive signals from peripheral nociceptors to the sympathetic chain, the nociceptive flexion reflex (NFR) was evaluated. Reflex elicitation is mediated after A-delta fibers are activated by a complex interaction between neurons located in the dorsal horn of the spinal cord22. Rhudy and colleagues described the RIII reflex, a late response of the NFR with high-threshold nociceptive characteristics measured electromyographically (EMG) over the biceps femoris muscle after nociceptor activation.23
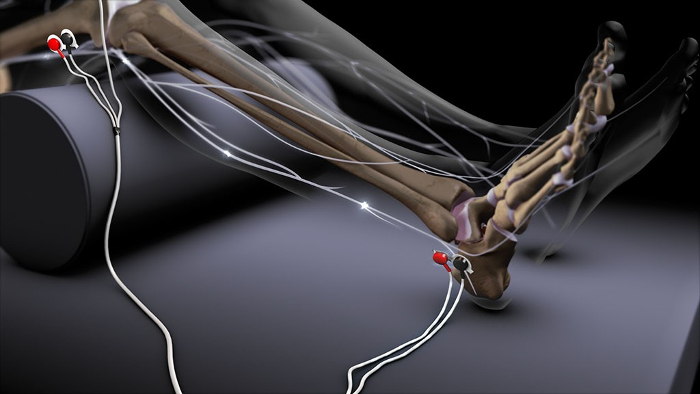
Increasing electrical stimulations are performed via cutaneous Ag-AgCl electrodes at the lateral malleolus, triggering the solely sensory sural nerve. The reflex response is evaluated in time and amplitude through EMG recording (Figure 2; Reprinted with permission of PH Dr med. Jan Baars, Managing Director, Dolosys GmbH.).
Following Willer et al., using the described reflex registration setup, the required stimulation intensity to elicit the NFR (threshold tracking) can be used as an objective nociceptive assessment correlating with subjective pain scores24,25,26,27,28. Subsequently, numerous studies have been conducted to identify reflex characteristics (mainly reflex threshold and amplitude) and their correlation with pain intensity sensation in conscious adults. These studies revealed that the reflex threshold and response amplitude is closely related to pain intensity27,29,30.Furthermore, standardized NFR scoring criteria, such as the reflex peak and the mean reflex EMG activity, can be used as reliable criteria for defining this NFR23,31,32. According to recent research, the defined reflex characteristics contributing to the NFR, despite their empirically derived origin, showed good test-retest reliabilities33,34. The duration of NFR recording, taking into account the (variable) step size range (0.5 mA - 2 mA), interstimulus interval of 8 seconds with an interval randomization of 20% to avoid possible habituation and reflex range between 90 - 180 ms after stimulation35, was between 5 and 15 minutes depending on the necessary stimulation intensity to elicit the NFR and therefore the number of required stimulations (maximum of 100 mA).
Protocol
This single-center cohort study was performed in accordance with the ethical standards of ICH-GCP and the Declaration of Helsinki after it was approved by the institutional review board and ethics committee of the Antwerp University Hospital, Belgium (study identifier: 16/33/334). The study was registered at Clinicaltrials.gov (NCT02916004) before its initiation.
All included patients were sedated in accordance with the standard hospital sedation protocol before study enrollment. The patients were titrated to a Richmond Agitation-Sedation Scale (RASS) set by the ICU physician. Patients were sedated to a RASS - 4 prior to study inclusion. All patients were routinely titrated to a Behavior Pain Scale (BPS) of 3 by the ICU analgosedation protocol.
Note: Determining therapeutic measures solely on the basis of the excitability of the recorded pain reflexes is not recommended. When interpreting the measurements, possible effects on the efferent branch of the reflex arc must be considered. Patients who are sedated or anesthetized have a higher pain reflex threshold than non-sedated patients. For reflex assessment, higher currents may be required. Monitoring of physiological parameters (heart rate, blood pressure, breathing rate) is recommended.
1. Safety Precautions
Verify potential confounders for noise control (other devices, alternating mattress).
Verify if the ambient temperature is in the normal range.
2. Positioning of the Subject
Position the patient in the bed to maintain angles of 120° of flexion of the hip and 130 - 160° at the knee.
Place the palmar side of the wrist upwards.
Ensure that the non-measured eye is closed during reflex recording.
3. Preparation of the Skin for Electrode Application
Note: This will reduce electrode impedance.
Clip or shave hair at the application sites.
Check the application sites, they must be clean and dry. If necessary, remove any body lotion by cleaning the skin with soap and water and rub the skin gently with a dry wash cloth or gauze.
Abrade the application sites with available abrasive material. Use the skin preparation paper over a large area rather than only a single swipe.
Apply each electrode immediately after skin preparation.
4. Placement of the Electrodes for Pupil Dilation Reflex (PDR) Assessment
Note: Please see the Figures for an overview of electrodes application. Magnetic and electrical fields may appear as background noise or other artefacts in the measurement trace. Maximal acceptable noise level using the followings protocol is set from values higher than 10 µV. High noise level is defined when the maximum amplitude in the area before the stimulation (‘noise area’, i.e., -130 ms up to -10 ms before stimulation) exceeds this adjustable threshold (‘maximum acceptable noise level’). Noise values are not used to calculate the threshold and the stimulation is repeated with the current intensity until an EMG signal with no noise is determined. To limit the occurrence of artefacts, verify the device has been updated to the latest version. Artefacts can be reduced by optimal electrode placement and skin preparation.
Use Ag-AgCl electrodes with highly conductive wet gel to ensure an optimal signal during reflex recording.
Maintain an inter-electrode distance of 30 mm (center-to-center).
Place two stimulation electrodes for PDR recording, at the wrist on the skin area innervated by the median nerve, keeping the palmar side of the wrist facing upwards.
5. Placement of the Electrodes for Nociceptive Flexion Reflex (NFR) Assessment
Note: Please see the Figures for an overview of electrodes application.
Use two stimulation electrodes at the ankle and place the electrodes distal to the lateral malleolus, stimulating the sural nerve area.
Use two registration electrodes for EMG recording at the biceps femoris muscle. Place the electrodes four finger breadths above the popliteal fossa, posterior to the iliotibial band on the ipsilateral leg.
Use one reference electrode, placed at the quadriceps tendon.
6. Safety Check
Identify the materials: battery status (PDR tool), accessibility of a plug connection nearby (NFR evaluation monitor), lead wires, and connections to the labeled device sockets.
Identify the patient: patient number, medical history, current medications, behavior pain scale, and sedation depth.
7. Pupillary Dilation Reflex Assessment: Getting Started
Attach the lead wire to the stimulation electrodes at the wrist. Verify that the black-labeled part is attached to the most distal electrode.
Turn the infrared camera on.
Select the measurement protocol: ‘pupillary pain index’ (PPI) through menu selection on the touch screen display. Perform an impedance control indicated by the colored symbols, if necessary repeat the preparation procedure.
Clean the camera and eye cab with water and disinfect them.
8. Pupillary Dilation Reflex Assessment: Installation
- Open the eyelid and place the camera in an optimal position.
- Let the rubber eyecup rest on the orbit, enclosing the whole eye.
- Verify whether pupil detection has been set correctly and adjust the camera if necessary. The operator may have to raise the eyelid more.
- Center the pupil in the middle of the screen and verify the position by pursuing a pupil completely colored green.
Close the contralateral eye, decreasing the consensual light response.
Wait for a least 5 seconds to start the measurement, ensuring a stabilization period necessary for pupil accommodation (dark measurement environment).
9. Pupillary Dilation Reflex Assessment: Measurement
- Start the test by pushing the trigger button. Hold the button until the pupil assessment is complete (a few seconds). Ensure that the entire measurement cycle is executed by 2 audible signals (first at the start, second when the test is finished)
- Do not move the camera during measurement; a countdown is shown on the screen when stimulation intensity is increasing automatically from 10 mA up to maximum 60 mA.
Identify the results automatically displayed after 15 seconds on the screen Baseline pupil size (mm) before noxious stimulation (yellow horizontal line). Maximal pupil size (mm) after noxious stimulation (white horizontal line). Different levels of noxious stimulations by colored bands and values. Maximal pupil variation (% and mm). PPI score
Save the measurement results by pressing the icon after pupil assessment.
10. Nociception Flexion Reflex Assessment: Getting Started
Attach the lead wires for stimulation, recording and reference. Verify whether the black-labeled parts are attached to the most distal electrodes; white is for reference value recording at the knee.
Turn the device on when connected to a power supply. Identify the USB flash drive if data storage is desired.
11. Nociceptive Flexion Reflex Assessment: Installation
- Press the settings button to go to the configuration menu to verify the stimulation settings and the threshold determination procedure for reflex measurement in unconscious sedated patients.
- Verify Measurement technique is on threshold tracking.
- Verify the Stimulus type is determined as RIII reflex.
- Select off when asked for NRS input.
- Choose Peak Z Score as Evaluation criterion.
- Use >100 number of stimuli.
- Initiate stimulation at 1 mA intensity, with minimum and maximum step size of 0.5 mA.
- Verify the Interstimulus interval is defined as 8 s with a reflex range of 90 – 180 ms.
12. Nociceptive Flexion Reflex Assessment: Measurement
Start the measurement, i.e., automatic reflex threshold tracking.
Reduce impedances when ‘High noise level’ appears by repeating the skin preparation protocol.
- Identify reflex features.
- Identify the currents applied to the patient and number of stimulations.
- Identify the raw EMG displayed 200 ms before to 300 ms after stimulation via the EMG electrode on the thigh.
- Identify the reflex range and the reflex threshold value. The parameter is shown numerically (value in mA).
Representative Results
We used both reflex assessments in a total of 40 critically ill ventilated subjects (38% females) at the ICU department using the previously described protocol. Patients with various indications for analgo-sedation were included: 58% for primary respiratory insufficiency, 23% due to multiple organ failure, 10% of the patients had a septic shock, and 9% were defined as being sedated for other reasons (e.g., cardiogenic reasons). All measurements were performed by the same investigator. Sedative agent dosing was never adjusted during the assessment. The pupil characteristics and EMG responses are shown in Table 2.
Vital signs remained unchanged during measurements, even with high (>60 mA) nociceptive stimulation. Therefore, no nociceptive reflex assessment had to be terminated early due to an increase in blood pressure, heart rate or change in ventilatory parameters. Identification of the PDR was possible in all subjects using the described protocol. Nevertheless, the NFR was identified in only 72% of the patients. Moreover, NFR threshold tracking was not possible in 13% of the patients despite optimal measurement conditions, suggesting a deep analgosedation level. However, excessive nociceptive stimulation (i.e., stimulation currents above 100 mA) was not used.
Figure 1: Schematic presentation of electrode application for standardized nociceptive stimulation used to elicit the PDR. Application of two stimulation electrodes at the skin area innervated by the median nerve.
Figure 2: Schematic presentation of electrode application for NFR assessment. Application of two stimulation electrodes at the skin area innervated by the sural nerve, two recording electrodes placed at the ipsilateral biceps femoris, and 1 reference electrode. Please note that the black lead wire is attached to the most distally placed electrode, and the red lead wire is attached to the proximal electrode (courtesy of Dolosys GmbH, PD Dr med. Jan Baars, Managing Director). Please click here to view a larger version of this figure.
| Maximum stimulation intensity (mA) | Pupil reactivity | Generated PPI score |
| 10 | Pupil dilation is greater than 13% during 10-mA stimulation | 9 |
| 20 | Pupil dilation is greater than 13% during 20-mA stimulation | 8 |
| 30 | Pupil dilation is greater than 13% during 30-mA stimulation | 7 |
| 40 | Pupil dilation is greater than 13% during 40-mA stimulation | 6 |
| 50 | Pupil dilation is greater than 13% during 50-mA stimulation | 5 |
| 60 | Pupil dilation is greater than 13% during 60-mA stimulation | 4 |
| 60 | Pupil dilation is greater than 13% during the second 60-mA stimulation | 3 |
| 60 (5% < dilation < 13%) | Pupil dilation is greater than 13% during the third 60-mA stimulation | 2 |
| 60 (dilation ≤ 5%) | Pupil dilation is greater than 13% during the last 60-mA stimulation | 1 |
| Note: if the pupil dilation is over 20% during stimulation, the PPI score is increased with one point |
Table 1: PPI scoring algorithm.
| Analgesia Protocol | Overall | Remifentanil | Sufentanil | No opioid |
| Number of subjects | 40 | 32 | 5 | 3 |
| PDR elicitable | 100% | 100% | 100% | 100% |
| PDR stimulation intensity (mean ± SD, mA) | 49.75 ± 12.91 | 49.69 ± 2.31 | 54.00 ± 6.00 | 43.33 ± 6.67 |
| PDR PPI score (mean ± SD) | 4.55 ± 0.39 | 5.09 ± 0.50 | 4.00 ± 1.73 | 6.33 ± 0.88 |
| NFR elicitable | 72% | 69% | 60% | 0% |
| NFR measurement error (no reflex assessed) | 15% | 19% | 20% | - |
| NFR threshold (mean ± SD) | 44.93 ± 4.93 | 39.93 ± 4.65 | 48.22 ± 16.84 | 53.33 ± 8.37 |
Table 2: Pupil characteristics and EMG responses after nociceptive reflex assessment. 'Error' measurements are defined as high impedance or noise problems during measurements. This can be explained by skin moisturing problems or skin pathology resulting in sub-optimal measurements or wall-outlet dysfunction.
Discussion
This paper describes the application of two nociceptive reflex devices for objective (patient-independent) pain assessment in adult ICU patients. Moreover, the evaluation of the PDR and the NFR characteristics are described.
Pain and delirium are common in hospitalized patients, often in combination, and may adversely affect outcome parameters. In the ICU, opioids are frequently administered, sometimes in combination with other sedative agents, to protect patients against stressful stimuli such as nursing care or various diagnostic or therapeutic procedures and to improve mechanical ventilation therapy, or they may be necessary due to critical illness. However, extensive evidence indicates that (unnecessary) prolonged administration of analgosedation to ICU patients negatively affects morbidity and mortality. Furthermore, the implementation of reliable evidence-based analgosedation protocols could further improve patient outcomes36,37,38.
The described reflex evaluation techniques can be considered quality indicators in healthcare and are closely associated with the use of opioids; further implementation could result in shorter ICU stays and improved short- and long-term outcomes. Furthermore, measuring nociceptive reflex thresholds through nociceptive assessments could result in targeted and patient-specific opioid administration. Therefore, evaluation and validation of the available objective pain assessment tools in critically-ill patients are urgently needed. Infrared pupillometry for PDR assessment has shown promising results39,40. Consistent with previous studies, this study demonstrated that pupillometry in unconscious patients in a very technological environment is feasible, fast, and straightforward41,42. Moreover, using the derived PPI score, the clinician is provided with an indication of the level of analgesia. Our study has clearly demonstrated that NFR can be routinely evaluated in ICU patients. However, it raises some significant points. First, NFR assessment may not be measurable due to persistent high electrode impedance despite maximal skin preparation. Secondly, we identified patients in whom NFR was not present, even with the maximal stimulation intensity. Despite the fact that NFR measurement is more challenging to perform, NFR threshold evaluation has shown promising results in patients under propofol-remifentanil sedation43.
Improving reflex assessment skills, the authors advise the performer to take some key steps into account. It is imperative to pursue a low electrode impedance for generating high quality output. As such, cleaning the skin with isopropyl alcohol should be limited to patients in whom electrode adhesion may be problematic (lotion-covered skin) since it may dehydrate the skin and therefore increase impedance. Abrading the skin at the electrode application site with intended material will optimize measurement variables. However, care should be taken not to injure the skin of the patient. Before reflex assessment, the user can easily perform an impedance control in a similar way for both devices, looking to the colored electrode symbol on the main screen. A green symbol indicates an optimal electrode impedance, a yellow symbol implies a 'good' impedance. When the symbol is red colored, the impedance is too high for measurement and the skin preparation procedure should be repeated. In addition, the use of (very) small stimulation electrodes is recommended (i.e., 45 mm × 30 mm) to avoid electrode overlap which may lead to incorrect reflex recording. Finally, explore the device settings before starting reflex measurement as default settings or stimulation characteristics can change between different patient populations. The issue of obvious concern is that of high unnecessary currents application in mainly awake, conscious patients.
Despite the growing interest in physiological pain assessment in unconscious patients2,16,17,18, there are some limitations that need to be acknowledged for both devices. Most notably, the pupillometer uses an inbuilt measurement model called 'pupillary pain index' containing stepwise increasing tetanic stimulations. The measurement protocol is stopped when the pupil dilates more than 13% from its baseline size, a fixed cut-off criteria. By using this inbuilt limit, the occurrence of tachycardia and hypertension in response to nociceptive stimulations is assumed. Although pupillometry stimulation models are more frequently used, data confirming this hypothesis is lacking. Furthermore, the true challenge of this model lies in the practical implementation of these tests in routine clinical practice. Although more objective and patient-independent nociceptive reflex measurements may offer new perspectives for analgesic management, preparation and measurements require approximately 15 minutes (especially for NFR assessment), which remains challenging in a fast-paced work environment. Moreover, no normative data are currently available for 'normal reflex ranges' in critically ill patients. Optimizing the skills and expertise of health care workers with respect to the use of these highly innovative tools may generate extraordinary results that can further classify analgesia levels, improve pain detection, prevent chronic pain disorders and enable (re)evaluation of pain management. Moreover, opportunities for economic valorization may arise, and the use of objective pain assessment tools may offer a unique translational platform for the testing of new pharmacological, analgesic compounds.
Measurements of more objective nociceptive reflexes, such as the PDR and NFR, may help clinicians evaluate patients' specific analgesic needs, especially in those who are not able to report pain levels themselves. Whether these two assessment tools can be applied on a wide scale in daily practice remains to be determined. The ability of both innovative devices to predict nociceptive status and their ability to guide clinicians in optimizing analgesic treatment in non-communicative critically ill patients warrants further investigation.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by departmental grants from the Multidisciplinary Pain Center (PCT), Anesthesiology and Critical Care Medicine departments of the Antwerp University Hospital (UZA), Belgium. In addition, an educational grant (Dehousse mandaat) was received from the University of Antwerp (UA). The authors want to thank Dr. Tom Schepens for his expert help during revision of this article.
References
- Chamorro C, Romera MA. [Pain and fear in the ICU] Medicina Intensiva. 2015;39(7):442–444. doi: 10.1016/j.medin.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Lusk B, Lash AA. The stress response, psychoneuroimmunology, and stress among ICU patients. Dimensions of Critical Care Nursing. 2005;24(1):25–31. doi: 10.1097/00003465-200501000-00004. [DOI] [PubMed] [Google Scholar]
- Bernardini R, et al. Plasma beta-endorphin levels and natural-killer cells in two cases of congenital indifference to pain. Child's Nervous System. 1992;8(2):83–85. doi: 10.1007/BF00298446. [DOI] [PubMed] [Google Scholar]
- Greisen J, et al. Acute pain induces an instant increase in natural killer cell cytotoxicity in humans and this response is abolished by local anaesthesia. British Journal of Anaesthesia. 1999;83(2):235–240. doi: 10.1093/bja/83.2.235. [DOI] [PubMed] [Google Scholar]
- Koga C, et al. Anxiety and pain suppress the natural killer cell activity in oral surgery outpatients. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2001;91(6):654–658. doi: 10.1067/moe.2001.115465. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, et al. The effects of epidural block on the distribution of lymphocyte subsets and natural-killer cell activity in patients with and without pain. Anesthesia & Analgesia. 2001;92(2):463–469. doi: 10.1097/00000539-200102000-00035. [DOI] [PubMed] [Google Scholar]
- Arbour C, Gelinas C. Are vital signs valid indicators for the assessment of pain in postoperative cardiac surgery ICU adults? Intensive and Critical Care Nursing. 2010;26(2):83–90. doi: 10.1016/j.iccn.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Rose L, et al. Critical care nurses' pain assessment and management practices: a survey in Canada. American Journal of Critical Care. 2012;21(4):251–259. doi: 10.4037/ajcc2012611. [DOI] [PubMed] [Google Scholar]
- Arroyo-Novoa CM, et al. Pain related to tracheal suctioning in awake acutely and critically ill adults: a descriptive study. Intensive and Critical Care Nursing. 2008;24(1):20–27. doi: 10.1016/j.iccn.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Stotts NA, et al. Wound care pain in hospitalized adult patients. Heart & Lung. 2004;33(5):321–332. doi: 10.1016/j.hrtlng.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Puntillo KA, et al. Challenge of assessing symptoms in seriously ill intensive care unit patients: can proxy reporters help? Critical Care Medicine. 2012;40(10):2760–2767. doi: 10.1097/CCM.0b013e31825b94d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DP, Anger KE, Szumita PM. Pathophysiology, assessment, and management of pain in critically ill adults. American Journal of Health-System Pharmacy. 2015;72(18):1531–1543. doi: 10.2146/ajhp140541. [DOI] [PubMed] [Google Scholar]
- Granja C, Amaro A, Dias C, Costa-Pereira A. Outcome of ICU survivors: a comprehensive review. The role of patient-reported outcome studies. Acta Anaesthesiologica Scandinavica. 2012;56(9):1092–1103. doi: 10.1111/j.1399-6576.2012.02686.x. [DOI] [PubMed] [Google Scholar]
- Schelling G, Kapfhammer HP. Surviving the ICU does not mean that the war is over. Chest. 2013;144(1):1–3. doi: 10.1378/chest.12-3091. [DOI] [PubMed] [Google Scholar]
- Larson MD. Effect of dexmedetomidine, an a2-adrenoceptor agonist, on human pupillary reflexes during general anaesthesia. British Journal of Clinical Pharmacology. 2001;51:27–33. doi: 10.1046/j.1365-2125.2001.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. The Journal of the American Medical Association. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- Paulus J, et al. Pupillary reflex measurement predicts insufficient analgesia before endotracheal suctioning in critically ill patients. Critical Care. 2013;17(4):R161. doi: 10.1186/cc12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant I, et al. Reflex pupillary dilatation in response to skin incision and alfentanil in children anaesthetized with sevoflurane: a more sensitive measure of noxious stimulation than the commonly used variables. British Journal of Anaesthesia. 2006;96(5):614–619. doi: 10.1093/bja/ael073. [DOI] [PubMed] [Google Scholar]
- Li D, Miaskowski C, Burkhardt D, Puntillo K. Evaluations of physiologic reactivity and reflexive behaviors during noxious procedures in sedated critically ill patients. Journal of Critical Care. 2009;24(3):e479–e413. doi: 10.1016/j.jcrc.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Wildemeersch D, Baeten M, Peeters N, Saldien V, Vercauteren M, Hans G. Pupillary dilation reflex and pupillary pain index evaluation during general anaesthesia: a pilot study. RJACC. 2018. [DOI] [PMC free article] [PubMed]
- Sabourdin N, et al. Pupillometry-guided Intraoperative Remifentanil Administration versus Standard Practice Influences Opioid Use: A Randomized Study. Anesthesiology. 2017;127(2):284–292. doi: 10.1097/ALN.0000000000001705. [DOI] [PubMed] [Google Scholar]
- Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans -- review article. Pain. 2002;96(1-2):3–8. doi: 10.1016/s0304-3959(02)00018-0. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: a comparison of different scoring criteria. Pain. 2007;128(3):244–253. doi: 10.1016/j.pain.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer JC, Bathien N. Pharmacological modulations on the nociceptive flexion reflex in. Pain. 1977;3(2):111–119. doi: 10.1016/0304-3959(77)90074-4. [DOI] [PubMed] [Google Scholar]
- Willer JC. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain. 1977;3(1):69–80. doi: 10.1016/0304-3959(77)90036-7. [DOI] [PubMed] [Google Scholar]
- Willer JC, Boureau F, Berny J. Nociceptive flexion reflexes elicited by noxious laser radiant heat in man. Pain. 1979;7(1):15–20. doi: 10.1016/0304-3959(79)90103-9. [DOI] [PubMed] [Google Scholar]
- Chan CW, Dallaire M. Subjective pain sensation is linearly correlated with the flexion reflex in man. Brain Research. 1989;479(1):145–150. doi: 10.1016/0006-8993(89)91344-9. [DOI] [PubMed] [Google Scholar]
- Guieu R, Blin O, Pouget J, Serratrice G. Analgesic effect of indomethacin shown using the nociceptive flexion reflex in humans. Annals of the Rheumatic Diseases. 1992;51(3):391–393. doi: 10.1136/ard.51.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhudy JL, Williams AE, McCabe KM, Nguyen MA, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42(5):579–587. doi: 10.1111/j.1469-8986.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- Willer JC, Boureau F, Albe-Fessard D. Supraspinal influences on nociceptive flexion reflex and pain sensation in man. Brain Research. 1979;179(1):61–68. doi: 10.1016/0006-8993(79)90489-x. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, France CR. Reliability and validity of a brief method to assess nociceptive flexion reflex (NFR) threshold. Journal of Pain. 2011;12(7):782–791. doi: 10.1016/j.jpain.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France CR, Rhudy JL, McGlone S. Using normalized EMG to define the nociceptive flexion reflex (NFR) threshold: further evaluation of standardized NFR scoring criteria. Pain. 2009;145(1-2):211–218. doi: 10.1016/j.pain.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Jurth C, Rehberg B, von Dincklage F. Reliability of subjective pain ratings and nociceptive flexion reflex responses as measures of conditioned pain modulation. Pain Research and Management. 2014;19(2):93–96. doi: 10.1155/2014/698246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GN, Rice DA, Jourdain K, McNair PJ. Influence of stimulation location and posture on the reliability and comfort of the nociceptive flexion reflex. Pain Research and Management. 2012;17(2):110–114. doi: 10.1155/2012/619124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini G, et al. The lower limb flexion reflex in humans. Neurobiology. 2005;77:353–395. doi: 10.1016/j.pneurobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Chanques G, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Critical Care Medicine. 2006;34(6):1691–1699. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- Robinson BR, et al. An analgesia-delirium-sedation protocol for critically ill trauma patients reduces ventilator days and hospital length of stay. Journal of Trauma. 2008;65(3):517–526. doi: 10.1097/TA.0b013e318181b8f6. [DOI] [PubMed] [Google Scholar]
- Payen JF, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Critical Care Medicine. 2001;29(12):2258–2263. doi: 10.1097/00003246-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Sabourdin N, et al. Pupillometry-guided Intraoperative Remifentanil Administration versus Standard Practice Influences Opioid Use: A Randomized Study. Anesthesiology. 2017;127(2):284–292. doi: 10.1097/ALN.0000000000001705. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz AC, et al. The relevance of pupillometry for evaluation of analgesia before noxious procedures in the intensive care unit. Anesthesia & Analgesia. 2015;120(6):1297–1300. doi: 10.1213/ANE.0000000000000609. [DOI] [PubMed] [Google Scholar]
- Wildemeersch D, et al. Pain assessment by pupil dilation reflex in response to noxious stimulation in anaesthetized adults. Acta Anaesthesiologica Scandinavica. 2018. [DOI] [PMC free article] [PubMed]
- Larson MD, et al. Portable infrared pupillometry in critical care. Critical Care. 2016;20(1):161. doi: 10.1186/s13054-016-1349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Dincklage F, et al. Monitoring of the responsiveness to noxious stimuli during anaesthesia with propofol and remifentanil by using RIII reflex threshold and bispectral index. British Journal of Anaesthesia. 2010;104(2):201–208. doi: 10.1093/bja/aep357. [DOI] [PubMed] [Google Scholar]


