Abstract
Background
Causal interpretation of associations between short interpregnancy interval (the duration from the preceeding birth to the conception of the next-born index child) and the offspring’s psychological and educational problems may be influenced by a failure to account for unmeasured confounding.
Methods
Using population-based Swedish data from 1973–2009, we estimated the association between interpregnancy interval and outcomes [autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), severe mental illness, suicide attempt, criminality, substance-use problem and failing grades] while controlling for measured covariates. We then used cousin comparisons, post-birth intervals (the interval between the second- and third-born siblings to predict second-born outcomes) and sibling comparisons to assess the influence of unmeasured confounding. We included an exploratory analysis of long interpregnancy interval.
Results
Interpregnancy intervals of 0–5 and 6–11 months were associated with higher odds of outcomes in cohort analyses. Magnitudes of association were attenuated following adjustment for measured covariates. Associations were eliminated for ADHD, severe mental illness and failing grades, but maintained magnitude for ASD, suicide attempt, criminality and substance-use problem in cousin comparisons. Post-birth interpregnancy interval and sibling comparisons suggested some familial confounding. Associations did not persist across models of long interpregnancy interval.
Conclusions
Attenuation of the association in cousin comparisons and comparable post-birth interval associations suggests that familial genetic or environmental confounding accounts for a majority of the association for ADHD, severe mental illness and failing grades. Modest associations appear independently of covariates for ASD, suicide attempt, criminality and substance-use problem. Post-birth analyses and sibling comparisons, however, show some confounding in these associations.
Keywords: pregnancy interval, birth spacing, psychopathology, case-comparison studies, interpregnancy interval
Key Messages
Previous research asserts a causal association between short interpregnancy interval and offspring psychopathology. However, several alternative hypotheses may better explain the associations between short interpregnancy interval and adverse offspring outcomes.
The current paper includes a series of traditional cohort analyses, cousin comparisons, post-birth interpregnancy interval negative control analyses and sibling comparisons to examine the pattern of associations.
For all studied outcomes, measured and unmeasured genetic or environmental confounding is present in the associations with short interpregnancy interval.
Associations between short interpregnancy interval and attention-deficit/hyperactivity disorder (ADHD), severe mental illness and failing grades are fully explained by familial genetic or environmental confounding, whereas associations with ASD, suicide attempt, criminality and substance-use problem show modest independent associations, though confounding is still present.
Introduction
Several recent studies have suggested that short interpregnancy interval, or the duration from the preceeding birth to the conception of the next-born index child, can lead to major long-term problems, including mental illness and low academic achievement.1–7 For example, an interpregnancy interval of less than 6 months is associated with a 300% elevated risk for offspring autism spectrum disorder (ASD)2 and a 150% elevated risk for schizophrenia.1,3 A causal relation between short interpregnancy interval and these burdensome outcomes is compelling because interpregnancy interval is a relatively modifiable risk factor.8 Further, there are plausible causal mechanisms whereby a short interpregnancy interval may not allow adequate restoration of the maternal nutritional foundation, especially of the fetal growth-relevant micronutrient folate.9 As such, negative effects of short interpregnancy interval could be reduced with a simple maternal folic acid supplement.10
More research is needed before resources are directed towards altering interpregnancy intervals for the targeted prevention of offspring psychological and educational outcomes, however.11 Traditional studies that compare outcomes across unrelated individuals may be confounded by genetic or environmental factors that influence both interpregnancy interval and the outcome.5 Potential confounding factors include maternal socio-economic variables, ethnicity, race, education, smoking status and maternal age.12–16 Additionally, within adolescent mothers, poor mental health, trauma history and behavioural aggression correlate with shorter interpregnancy intervals.13,17 Therefore, causal claims from traditionally designed studies5,6 should be made with caution. Previous studies have also been limited by skewed measurement of interpregnancy intervals (i.e. birth to birth rather than birth to conception),1,7 which confounds spacing with gestational age—a factor that influences both interpregnancy interval,18 as well as the likelihood of offspring psychopathology.19
Family-based designs are well equipped to explore alternative explanations to causal claims.20–22 Family-based designs that compare related individuals, such as cousins, have the ability to control for all factors that make those individuals similar. This is important when examining an exposure that may be highly influenced by genetic or environmental factors.23 Whereas sibling comparisons offer greater control of unmeasured genetic and environmental influences, they are problematic in relation to the nature of the exposure (i.e. interpregnancy interval); the first-born does not have an interpregnancy interval, there may be birth-order effects and the first-born’s outcomes may impact the interval prior to conception of the second-born. Therefore, cousin comparisons may be a better-suited study design24 to offer some control of unmeasured genetic factors (cousins share 12.5% of their genetic makeup on average) while avoiding the design issues of sibling comparisons.
Another way to explore alternative explanations (e.g. family culture contributing to both short interpregnancy intervals and increased likelihood of childhood behaviour disorders) is with a negative control design wherein the interpregnancy interval to the following (next-born) sibling is used to predict the outcome of the prior-born sibling. Since any association with this post-birth interval cannot be due to the pregnancy-related mechanisms through which interpregnancy interval theoretically functions, it may be taken to indicate a role of familial confounding, genetic or environmental.1 If, on the other hand, there is no association between post-birth interval and the prior-born’s outcomes whereas there is a robust association between the prior interpregnancy interval and the outcome, then there is support for an independent association. Two studies have published significant associations between post-birth interval and psychological outcomes. One study found that schizophrenia was predicted from both the offspring’s interpregnancy interval and the post-birth interval1 and another study showed that short post-birth intervals were associated with elevated risk of psychotic disorder,7 both suggestive of confounding.
The current study used one of the largest longitudinal, population-based databases in the world—the Swedish population registers—to examine the risk conferred by short interpregnancy interval on several offspring psychological and educational problems. Outcomes included ASD, attention-deficit/hyperactivity disorder (ADHD), severe mental illness, suicide attempt, criminality, substance-use problem and failing grades. To examine the influence of confounding, the current study utilized cousin comparisons and post-birth interpregnancy intervals. We further examined familial confounding by performing sibling comparisons in sensitivity analyses. In addition, we included an exploratory analysis of the impact of long interpregnancy interval on these outcomes, as very little work has been performed examining this factor.6
Methods
Study population
The institutional review board at Indiana University and the Regional Ethical Review Board in Stockholm approved this study. We first identified offspring and their mothers using the Swedish Medical Birth Register, which provided data on more than 96% of births in Sweden since 1973.25 After identifying fathers using the Multi-Generation Register,26 we then collected information on several parental characteristics and offspring outcomes from the following registers: (i) the National Crime Register provided information on criminal convictions since 1973,27 (ii) the National Patient Register provided diagnoses for psychopathological and substance-related inpatient hospital admissions since 1973,28 (iii) the National School Register provided information on offspring school grades beginning in 1983; (iv) the Education Register provided information on highest level of completed formal education through 2009; and (v) the Migration Register and the (vi) Cause of Death Register provided information important in determining the censoring information.
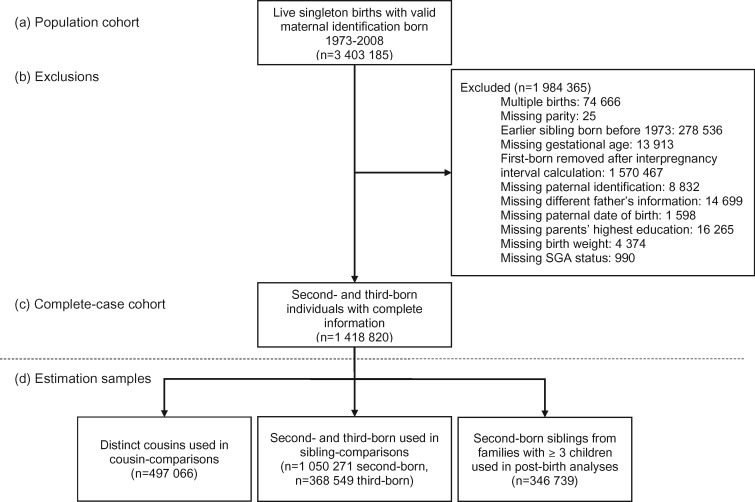
The initial population included live birth-related information for 3 403 185 individuals with valid maternal identifiers born between 1973 and 2008. The number of individuals removed for exclusionary reasons are listed in the data flow chart presented as Figure 1. We used a complete-case analysis given the mechanisms for missing data are not fully understood.29 The final cohort for main analyses consisted of 1 050 271 second-born and 368 549 third-born offspring. There were 784 640 distinct maternal-side grandmothers represented in the cohort used in cousin-comparison models and 497 066 distinct cousins. Post-birth interval analyses only included the 346 739 individuals who had third-born siblings and sibling-comparison analyses use all second- and third-born siblings. For childhood outcomes of ADHD and ASD, years of birth were limited to 1987 through 2007 in order to capture the highest-quality inpatient and outpatient diagnosis data (N = 973 391).
Figure 1.
Sample flow for (a) population cohort, (b) reasons for exclusions and the number of cases excluded, (c) the complete-case cohort, (d) estimation samples by type of individuals included in the specific model. Depending on outcome, year of birth restrictions to address age of diagnosis (at least 2, 12 or 15) or conviction were applied during analysis at this step. In particular, ASD and ADHD 1987–2007; severe mental illness, suicide attempt, substance-use problem, failing grades 1973–97; and criminality 1973–94. Follow-up for all outcomes was through 2009.
Measures
Interpregnancy interval
We defined interpregnancy interval as the number of completed months between the birth of the preceding (earlier-born) offspring and the date of conception of the index (next-born) offspring. Date of conception was obtained from information on gestational age at birth estimated from last menstrual period or ultrasound. We treated the second-born offspring as the index offspring. To allow prediction also by their post-birth interpregnancy interval, we used the subsample of second-born offspring that had a third sibling interpregnancy interval from the birth of the second-born to the conception of the third-born. We categorized interpregnancy intervals as 0–5 months, 6–11 months, 12–23 months and 24–35 months (referent).
Offspring outcomes
Outcomes were chosen because of previously indicated positive associations with the risk factor, biological relatedness to the previously studied outcomes, normative indicators of decreased functioning and resource costliness.1–7 All clinical diagnoses were according to the year-dependent ICD- 8, -9 and -10 codes as appropriate. ICD codes are presented in Supplementary Table 1A, available as Supplementary data at IJE online. Follow-up for each outcome was through 2009.
We defined ASD as including pervasive developmental disorder, disintegrative psychosis, Heller’s syndrome and schizophrenic syndrome of childhood.30 We defined ADHD according to hyperkinetic and attention-deficit hyperactivity disorder diagnoses.31 For both childhood outcomes, offspring had to have been at least 2 years old at the time of diagnosis. Data for ASD and ADHD included inpatient psychiatric diagnosis beginning in 1987 and outpatient specialist diagnosis beginning in 200130,32 through 2007. We defined ‘severe mental illness’ as measured by the age at the first inpatient hospitalization for bipolar disorder, broadly defined schizophrenia or other non-organic psychotic disorders33; ‘suicide attempt’ as indicated by the age at first attempt recorded in inpatient care records as the primary or secondary reason for care34; criminality according to the age at first occurrence of any criminal conviction under the Swedish Penal code, beginning at age 15, the Swedish age of legal responsibility35; and ‘substance-use problem’ according to first inpatient hospitalization involving a primary or secondary diagnosis of alcohol or any other non-nicotine substance-use disorder.36 We also defined ‘failing grades’ according to summary grades in grade nine.37 Summary grade scores were calculated by summing the numeric value of grades across 16 different subjects (pass = 10, pass with distinction = 15 and pass with honors = 20) for a maximum summary grade of 320. A score of 160 indicated that the mean across the 16 grades was only at ‘pass’; any score below this was used as an indicator for failing grades.37 The minimum age for all adult psychopathology outcomes was 12 years old, except for criminality. Those born from 1973 to 1997 were informative for all adult outcomes except for criminality, in which those born in 1973–94 were informative. Outcome-dependent sample information is presented in Table 1.
Table 1.
Kaplan–Meier estimates and sample details by outcome and interpregnancy interval category
| Interpregnancy interval (months) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–5 |
6–11 |
12–23 |
24–35 |
||||||||
| Outcome | Birth year | n cases | Total N | n | KME (n %) | n | KME (n %) | n | KME (n %) | n | KME (n %) |
| ASD | 1987–2007 | 6112 | 973 391 | 260 | 1.80 | 895 | 1.38 | 1744 | 1.07 | 943 | 1.08 |
| ADHD | 1987–2007 | 11 945 | 973 391 | 449 | 3.42 | 1 539 | 2.60 | 3295 | 2.16 | 1945 | 2.27 |
| Severe mental illness | 1973–97 | 6162 | 957 099 | 234 | 1.99 | 756 | 1.87 | 1712 | 1.49 | 1206 | 1.36 |
| Suicide attempt | 1973–97 | 11 247 | 957 099 | 485 | 3.22 | 1430 | 2.53 | 3136 | 2.01 | 2311 | 1.97 |
| Criminality | 1973–94 | 99 452 | 824 200 | 4299 | 23.81 | 12 658 | 18.81 | 29 662 | 16.13 | 21 029 | 15.56 |
| Substance-use problem | 1973–97 | 17 512 | 957 099 | 745 | 4.70 | 2282 | 3.95 | 5093 | 3.13 | 3398 | 2.85 |
| Failing grades | 1973–97 | 99 917 | 957 099 | 4333 | (21.08) | 12 026 | (14.94) | 28 414 | (13.23) | 20 637 | (13.53) |
KME, Kaplan–Meier product-limit survival estimate percentage at 20 (ASD, ADHD) or 30 years (all other outcomes). KMEs were not calculated for failing grades because it is a logistic outcome. Numbers across interpregnancy interval categories do not add to n cases because data for long interpregnancy interval categories (i.e. 36–71 and 72+ months) are not included in this presentation.
Measured covariates
We included various measured covariates depending on the statistical model. We chose covariates based on their correlations with interpregnancy interval and psychopathological outcomes.19 See Supplementary Table 2A, available as Supplementary data at IJE online, for associations between interpregnancy interval and measured covariates in our sample. Measured covariates included maternal and paternal age at the index birth, highest education level, nationality and whether the earlier-born offspring had a different biological father. Some adjusted models also included measured lifetime parental psychopathology. In particular, we included parental criminality as indexed by any criminal conviction under the Swedish Penal code beginning at age 15, the Swedish age of legal responsibility,35,38 substance-use problem defined as an inpatient hospitalization involving a primary or secondary diagnosis of alcohol or any other non-nicotine substance-use disorder,39 suicide attempt as indicated by an attempt recorded in inpatient care records as the a primary or secondary reason for care34 and severe mental illness as measured by an inpatient hospitalization for bipolar disorder, broadly defined as schizophrenia or other non-organic psychotic disorders.33 Except for criminality, the minimum age for all parental mental health outcomes was 12 years old. All clinical diagnoses were according to ICD versions 8, 9 and 10, and are presented in Supplementary Table 1A, available as Supplementary data at IJE online.
Statistical analyses
We used Cox survival analyses for right-censored outcomes (e.g. attempted suicide) and logistic regression analyses for dichotomous outcomes (i.e. failing grades) when predicting the second-born, index offspring’s outcomes. For the survival analyses, if the offspring had not received a diagnosis within the study period, they contributed person-time at risk until death, emigration or the end date of follow-up (31 December 2009), whichever came first.
A series of models were performed for each outcome. Model 1 was a baseline model that only adjusted for offspring sex and year of birth. Model 2 adjusted for offspring sex, year of birth and maternal and paternal age, highest education, nationality and whether the fathers were different. Model 3 additionally adjusted for parental psychopathology variables including maternal and paternal criminality, attempted suicide, substance misuse and severe mental illness.
In Model 4, we limited the sample to maternal cousins with different interpregnancy interval categories and used fixed-effects modelling or conditional logistic regression. In other words, a risk for outcome in second-born offspring was compared with their second-born maternal cousin with a differing interpregnancy interval category. Model 4 additionally included all covariates in Model 3.
Model 5 used post-birth interpregnancy interval (the interval between the second- and third-born offspring within a family) to predict second-born outcomes in the subsample that had a third sibling.1 This model also adjusted for the same measured covariates as in Models 3 and 4. Again, if a positive association is identified using post-birth interval, familial confounding is implicated. If there is no association between post-birth interval and the prior-born’s outcomes, then an independent and unique association with interpregnancy interval may be warranted, given other results.
Sensitivity analysis
We performed a sibling comparison in an effort to use a family-based design with different assumptions from the cousin-comparison design while continuing to control for unmeasured environmental and genetic factors that may influence the relation. For this analysis, the interpregnancy interval was calculated between the first- and second-born (for the second-born) as well as between the second- and third-born offspring (for the third-born); second- and third-born offspring outcomes were compared in a model that also adjusted for measured covariates that may have varied between siblings (i.e. offspring sex, birth year, parity and different father).
We also examined associations following long interpregnancy intervals. In particular, as compared with a reference category of 24–35 months, we examined outcomes associated with interpregnancy intervals of 36–71 months and 72+ months across all models used in the main analyses (i.e. baseline and adjusted, cousin comparisons and post-birth analyses).
Results
Table 2 presents demographic information for the second-born offspring.
Table 2.
Descriptive characteristics and covariates for n = 1 050 271 (74%) index, second-born offspring in the sample of n = 1 418 820
| Variable | n (%) |
|---|---|
| Second-born offspring | 1 050 271 |
| Interpregnancy interval (months) | |
| 0–5 | 27 888 (2.7) |
| 6–11 | 128 096 (12.2) |
| 12–23 | 369 173 (35.2) |
| 24–35a | 233 983 (22.3) |
| 36–71 | 216 631 (20.6) |
| 72+ | 74 500 (7.1) |
| Maternal age (years) | |
| <20 | 6618 (0.6) |
| 20–29a | 596 101 (56.8) |
| 30–39 | 433 634 (41.3) |
| ≥40 | 13 918 (1.3) |
| Paternal age (years) | |
| <20 | 1299 (0.1) |
| 20–29a | 390 057 (37.1) |
| 30–39 | 576 730 (54.9) |
| ≥40 | 82 185 (7.8) |
| Highest maternal education | |
| ≤9 years primary and lower secondarya | 17 979 (1.7) |
| 9 years primary and lower secondary | 88 677 (8.4) |
| 3 years upper secondary | 514 757 (49.0) |
| Post-secondary/and or post-graduate | 428 858 (40.8) |
| Highest paternal education | |
| ≤9 years primary and lower secondarya | 43 960 (4.2) |
| 9 years primary and lower secondary | 131 470 (12.5) |
| 3 years upper secondary | 528 773 (50.4) |
| Post-secondary/and or post-graduate | 346 068 (33.0) |
| Mother of Swedish nationality | 933 506 (88.9) |
| Father of Swedish nationality | 926 035 (88.2) |
| First- and second-born to different fathers | 93 379 (8.9) |
| Maternal psychopathology | |
| Criminality | 109 882 (10.5) |
| Attempted suicide | 23 099 (2.2) |
| Substance-use problem | 17 300 (1.7) |
| Severe mental illness | 8378 (0.8) |
| Paternal psychopathology | |
| Criminality | 393 941 (37.5) |
| Attempted suicide | 16 520 (1.6) |
| Substance-use problem | 33 079 (3.2) |
| Severe mental illness | 6140 (0.6) |
denotes reference category for model estimation.
Odds and hazard ratios predicting all studied outcomes can be seen in Table 3. In the baseline models, an interpregnancy interval of 0–5 months was associated with higher odds of every studied outcome; the baseline associations were moderate and varied from HR = 1.38 [95% confidence interval (CI) = 1.22–1.56] for ADHD and HR = 1.70 (95% CI = 1.44–2.00) for ASD when compared with interpregnancy interval of 24–35 months. Across outcomes, magnitudes of association were minimally attenuated following adjustment for measured covariates in Model 2. The associations were moderately to greatly attenuated for the shortest interpregnancy interval in Model 3, where parental psychopathology measures were included as measured covariates.
Table 3.
Hazard or odds ratios predicting child and adult psychopathology across interpregnancy interval and model
| Interpregnancy interval (months) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–5 |
6–11 |
12–23 |
24–35 | |||||||
| Outcome and model | HR/OR | 95% CI | HR/OR | 95% CI | HR/OR | 95% CI | ||||
| ASD | ||||||||||
| Model 1 | 1.70 | 1.44 | 2.00 | 1.35 | 1.22 | 1.50 | 0.99 | 0.90 | 1.08 | Ref |
| Model 2 | 1.63 | 1.38 | 1.93 | 1.38 | 1.25 | 1.54 | 1.03 | 0.94 | 1.12 | Ref |
| Model 3 | 1.59 | 1.34 | 1.87 | 1.37 | 1.23 | 1.52 | 1.03 | 0.94 | 1.12 | Ref |
| Model 4/cousin comparison | 1.63 | 1.04 | 2.55 | 1.38 | 1.05 | 1.80 | 1.06 | 0.83 | 1.35 | Ref |
| Model 5/post-birth | 1.50 | 1.15 | 1.94 | 1.29 | 1.08 | 1.54 | 1.12 | 0.96 | 1.31 | Ref |
| ADHD | ||||||||||
| Model 1 | 1.38 | 1.22 | 1.56 | 1.05 | 0.97 | 1.14 | 0.90 | 0.84 | 1.14 | Ref |
| Model 2 | 1.29 | 1.14 | 1.47 | 1.09 | 1.01 | 1.18 | 0.95 | 0.89 | 1.01 | Ref |
| Model 3 | 1.18 | 1.04 | 1.34 | 1.06 | 1.98 | 1.14 | 0.95 | 0.89 | 1.01 | Ref |
| Model 4/cousin comparison | 1.16 | 0.82 | 1.63 | 0.95 | 0.77 | 1.18 | 0.84 | 0.70 | 1.00 | Ref |
| Model 5/post-birth | 1.27 | 1.05 | 1.54 | 0.99 | 0.87 | 1.14 | 1.00 | 0.89 | 1.13 | Ref |
| Severe mental illness | ||||||||||
| Model 1 | 1.61 | 1.37 | 1.88 | 1.45 | 1.31 | 1.60 | 1.18 | 1.09 | 1.27 | Ref |
| Model 2 | 1.51 | 1.29 | 1.77 | 1.43 | 1.29 | 1.58 | 1.18 | 1.09 | 1.28 | Ref |
| Model 3 | 1.32 | 1.13 | 1.55 | 1.34 | 1.22 | 1.49 | 1.17 | 1.08 | 1.27 | Ref |
| Model 4/cousin comparison | 1.04 | 0.64 | 1.69 | 1.05 | 0.81 | 1.36 | 0.88 | 0.72 | 1.08 | Ref |
| Model 5/post-birth | 1.46 | 1.12 | 1.90 | 1.32 | 1.11 | 1.57 | 1.12 | 0.97 | 1.30 | Ref |
| Suicide attempt | ||||||||||
| Model 1 | 1.67 | 1.49 | 1.87 | 1.30 | 1.21 | 1.40 | 1.00 | 0.94 | 1.06 | Ref |
| Model 2 | 1.48 | 1.32 | 1.65 | 1.28 | 1.19 | 1.34 | 1.01 | 0.95 | 1.07 | Ref |
| Model 3 | 1.26 | 1.13 | 1.42 | 1.19 | 1.11 | 1.29 | 0.99 | 0.93 | 1.05 | Ref |
| Model 4/cousin comparison | 1.34 | 1.01 | 1.79 | 1.20 | 1.01 | 1.42 | 1.03 | 0.89 | 1.18 | Ref |
| Model 5/post-birth | 1.19 | 0.98 | 1.44 | 1.14 | 1.00 | 1.30 | 0.99 | 0.89 | 1.10 | Ref |
| Criminality | ||||||||||
| Model 1 | 1.62 | 1.56 | 1.69 | 1.24 | 1.21 | 1.27 | 1.04 | 1.02 | 1.06 | Ref |
| Model 2 | 1.40 | 1.34 | 1.45 | 1.21 | 1.18 | 1.24 | 1.05 | 1.03 | 1.07 | Ref |
| Model 3 | 1.25 | 1.20 | 1.30 | 1.15 | 1.12 | 1.18 | 1.03 | 1.01 | 1.05 | Ref |
| Model 4/cousin comparison | 1.18 | 1.08 | 1.28 | 1.04 | 0.99 | 1.10 | 1.01 | 0.97 | 1.06 | Ref |
| Model 5/post-birth | 1.20 | 1.12 | 1.29 | 1.11 | 1.06 | 1.16 | 1.03 | 0.99 | 1.07 | Ref |
| Substance-use problem | ||||||||||
| Model 1 | 1.65 | 1.50 | 1.81 | 1.34 | 1.26 | 1.42 | 1.11 | 1.06 | 1.17 | Ref |
| Model 2 | 1.46 | 1.33 | 1.60 | 1.32 | 1.24 | 1.40 | 1.12 | 1.07 | 1.18 | Ref |
| Model 3 | 1.25 | 1.14 | 1.37 | 1.23 | 1.16 | 1.31 | 1.11 | 1.05 | 1.16 | Ref |
| Model 4/cousin comparison | 1.20 | 0.98 | 1.45 | 0.93 | 0.82 | 1.05 | 1.07 | 0.97 | 1.19 | Ref |
| Model 5/post-birth | 1.08 | 0.91 | 1.27 | 1.15 | 1.03 | 1.28 | 1.02 | .93 | 1.11 | Ref |
| Failing grades | ||||||||||
| Model 1 | 1.69 | 1.62 | 1.77 | 1.10 | 1.07 | 1.13 | 0.97 | 0.95 | 0.99 | Ref |
| Model 2 | 1.36 | 1.30 | 1.42 | 1.10 | 1.06 | 1.13 | 1.00 | 0.98 | 1.02 | Ref |
| Model 3 | 1.19 | 1.14 | 1.25 | 1.03 | 1.00 | 1.06 | 0.98 | 0.96 | 1.01 | Ref |
| Model 4/cousin comparison | 1.12 | 0.97 | 1.30 | 0.97 | 0.86 | 1.09 | 0.98 | 0.88 | 1.08 | Ref |
| Model 5/post-birth | 1.23 | 1.14 | 1.33 | 1.15 | 1.09 | 1.21 | 1.08 | 1.03 | 1.12 | Ref |
Number of differentially exposed cousin pairs included n = 338 604; post-IPI sample included n = 346 739; models were adjusted for the following measured covariates: Model 1: offspring sex, year of birth; Model 2: offspring sex, year of birth, maternal and paternal age, highest education, nationality, different fathers; Model 3: offspring sex, year of birth, maternal and paternal age, highest education, nationality, different fathers, maternal and paternal criminality, attempted suicide, substance misuse and severe mental illness; Model 4/cousin comparison: offspring sex, year of birth, maternal and paternal age, highest education, nationality, different fathers, maternal and paternal criminality, attempted suicide, substance misuse and severe mental illness; Model 5/post-birth interpregnancy interval: offspring sex, year of birth, maternal and paternal age, highest education, nationality, different fathers, maternal and paternal criminality, attempted suicide, substance misuse and severe mental illness, first-born preterm birth, low birth weight and small for gestational age; Model 5 predicts the second-born’s outcomes from the post-birth interpregnancy interval (interval between second-born and third-born) in a subsample of families with three children. Follow-up through 2009.
In Model 4, in which second-born cousins with varying interpregnancy intervals were compared, associations were fully attenuated for ADHD (HR = 1.16, 95% CI = 0.82–1.63), severe mental illness (HR = 1.04, 95% CI = 0.64–1.69) and failing grades (HR = 1.12, 95% CI = 0.97–1.30). For ASD (HR = 1.63, 95% CI = 1.04–2.55), suicide attempt (HR = 1.34, 95% CI = 1.01–1.79) and criminality (HR = 1.18, 95% CI = 1.08–1.28), moderate associations persisted. The magnitude of association remained elevated for substance-use problem (HR = 1.20, 95% CI = 0.98–1.45), though confidence intervals were large.
In Model 5, post-birth intervals (i.e. the interval between the second- and third-born offspring) were associated with higher odds of all outcomes but substance-use problem, suggesting the presence of familial confounding. In other words, the length of interval after the birth of the second-born offspring to the next sibling’s conception significantly predicted the outcomes of the second-born offspring.
Sensitivity and exploratory analyses
Sibling comparison
Outcome rates of the second- and third-born siblings were compared if their interpregnancy intervals differed; the subset included 569 802 differentially exposed sibling pairs. Results are presented in Supplementary Table 3A, available as Supplementary data at IJE online. In summary, after controlling for all factors that siblings share, there were no statistically significant associations or notably high magnitudes of association between short interpregnancy interval and the studied outcomes.
Long interpregnancy interval
We also explored the relation between long interpregnancy intervals and the studied outcomes. This was approached as an exploratory analysis, as little research has been done on this exposure, which is correlated with parental age. We examined 36–71 months and 72+ months as compared with the reference range of 24–35 months. Supplementary Table 4A, available as Supplementary data at IJE online, presents the results. For the longest interpregnancy interval period, odds of all studied outcomes were higher compared with the reference interval period (24–35 months) in the baseline model. These associations, however, were dramatically attenuated following covariate adjustment and none remained in the cousin comparisons. There were also weak associations between post-birth interpregnancy intervals and ADHD, suicide attempt, criminality, substance-use problem and failing grades, supporting the interpretation that associations with these outcomes may largely be due to familial confounding.
Discussion
Using a Swedish population cohort, we explored the relation between interpregnancy interval and offspring psychological and educational problems, particularly problems associated with substantial morbidity and previously shown to be associated with short interpregnancy interval. Our findings suggest that much, if not all, of the association between short interpregnancy interval and elevated risk for offspring child and adult psychological and educational outcomes identified in general samples are due to genetic or shared environmental confounding. Whereas our baseline and adjusted findings are in agreement with previous research reporting associations between short interpregnancy interval and ASD, severe mental illness and academic performance,1–7 the more rigorous examination in the current study including within-family and post-birth analyses does not support causal interpretations. However, the pattern of association is outcome-specific. For ADHD, severe mental illness and failing grades, the association was fully attenuated in cousin and sibling comparisons and post-birth analyses supported familial confounding. For ASD, suicide attempt, criminality and substance-use problem, elevated magnitudes of association were maintained through cousin comparisons, with wider confidence intervals than in previous models. This supports a modest independent effect even when controlling for all factors that make cousins similar to each other. Post-birth and sibling-comparison analyses, however, suggested familial confounding was present even for these maintained associations.
Others have applied a sibling ‘case-comparison’ approach to study these associations and our results differ from their conclusions. This may be because the previous projects used the first- and second-born offspring, as compared with our use of cousins and the second- and third-born offspring in sibling comparisons.2,5 We used cousins to remove birth-order bias and bias introduced from prodromal symptoms emerging in the older-born sibling prior to the birth or diagnosis of the second-born.2,5 In our sibling comparisons, we did not include first-borns because they do not have a prior interpregnancy interval. An additional design difference is that previous studies examined rates of ASD in the second sibling across different interpregnancy interval categories, given that the first sibling had not been diagnosed with ASD.2,5 Future research might include direct comparison of these approaches.
For our exploratory analysis of long interpregnancy intervals, the population-wide associations were fully attenuated by adjustment (measured covariates and cousin comparisons). Post-birth analyses showed modest positive association between the longest interpregnancy intervals and suicide attempt, criminality, substance-use problem and failing grades. Given the attenuation of the associations throughout the traditional and cousin-comparison analyses, this elevation in post-birth analyses may indicate familial confounding. As stated, previous work on long interpregnancy interval is limited.6 Most previous work has grouped any interpregnancy interval over 373 or 45 months1 together and/or treated the group as the reference category,2,5 thereby limiting the conclusions that could be drawn. Our results should also be interpreted with caution, as a long interpregnancy interval is correlated with older parental age and may be associated with infertility, maternal infection and breastfeeding (and therefore a longer period of maternal nutrient depletion).1,8,10,36,40 In addition, the long interpregnancy interval may be due to the older-born offspring’s early-manifesting psychological outcomes. More research on the ramifications of a long interpregnancy interval is needed.
We were able to draw our conclusions by utilizing several designs that account for unmeasured confounding with differing assumptions and limitations in a large sample. The family-based designs of cousin comparison and sibling comparison in sensitivity analyses enabled us to account for unmeasured environmental and genetic factors shared by cousins or siblings that may influence the associations. The cousin-comparison approach removed the problems associated with comparing outcomes across siblings where the first-born did not experience an interpregnancy interval and three-child families are required. We also performed a post-birth interval analysis to examine alternative hypotheses. Despite these strengths, important limitations must be considered. First, due to the relative ethnic homogeneity of the Swedish population, future analyses across ethnic and racial groups are needed because interpregnancy intervals vary across these groups.12,14,15 Similarly, prenatal care is advanced and comprehensive in Sweden and may have influenced interpregnancy interval length.41 Second, we cannot rule out the possibility that ‘stoppage’, or the decision to not have a second child due to diagnosis (e.g. ASD) in the first child, may have influenced family structure. If these families were not included in the sample because they did not have a second child, our estimates may be biased because of the non-inclusion of a subgroup with high familial risk for certain disorders. Third, every design has inherent limitations and assumptions, such as shared characteristics across cousins,21 and family-based designs are not randomized–controlled studies; therefore, we cannot rule out all possible confounding factors. Further, confidence intervals became wider as the sample was more limited by relatedness. By combining multiple designs, however, we hope to triangulate on the ‘real’ association. Finally, there may be some interpregnancy interval-length category exposure misclassification because of conception date error. In addition, we were unable to identify spontaneous or induced abortions,42 which may have influenced interpregnancy interval length.
Our findings suggest that associations of short interpregnancy interval and elevated offspring psychological and educational problems are modest and outcome-dependent. Familial factors, either shared genetic or environmental, appear to play a role in these associations.11–17
Funding
This work was supported by grants from the National Institute of Child Health and Development (HD061817) to B.M.D., the National Institute of Mental Health (T32MH094011 T32MH103213) to Q.A.C. and A.S., respectively, the Indiana University Mabel LaDuke Lauder Fund to Q.A.C., the National Science Foundation Graduate Research Fellowship (1342962) to A.S., the Swedish Council for Working Life and Social Research to P.L. and the Swedish Initiative for Research on Microdata in the Social and Medical Sciences (SIMSAM) framework grant (340-2013-5867) to C.A. and International Postdoc Grant (350-2012-340) to A.S.O.
Conflict of interest: H.L. has served as a speaker for Eli-Lilly and Shire and has received research grants from Shire; all outside the current work. All other authors report no competing interests.
Supplementary Material
References
- 1. Smits L, Pedersen C, Mortensen PB, van Os J.. Association between short birth intervals and Schizophrenia in the offspring. Schizophr Res 2004;70:49–56. [DOI] [PubMed] [Google Scholar]
- 2. Cheslack-Postava K, Liu K, Bearman PS.. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics 2011;127:246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gunawardana L, Smith GD, Zammit S, Whitley E, Gunnell D, Lewis S.. Pre-conception interpregnancy interval and risk of schizophrenia. Br J Psychiatry 2011;199:338–39. [DOI] [PubMed] [Google Scholar]
- 4. Zerbo O, Yoshida C, Gunderson EP, Dorward K, Croen LA.. Interpregnancy interval and risk of autism spectrum disorders. Pediatrics 2015;136:651–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunnes N, Suren P, Bresnahan M, Hornig M, Lie KK, Lipkin WI.. Interpregnancy interval and risk of autistic disorder. Epidemiology 2013;24:906–12. [DOI] [PubMed] [Google Scholar]
- 6. Durkin MS, DuBois LA, Maenner MJ.. Inter-pregnancy intervals and the risk of autism spectrum disorder: results of a population-based study. J Autism Dev Disord 2015;45:2056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riordan DV, Morris C, Hattie J, Stark C.. Interbirth spacing and offspring mental health outcomes. Psychol Med 2012;42:2511–21. [DOI] [PubMed] [Google Scholar]
- 8. Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol 2012;72:1272–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winkvist A, Rasmussen KM, Habicht JP.. A new definition of maternal depletion syndrome. Am J Public Health 1992;82:691–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med 2011;17:389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klebanoff MA. Interpregnancy interval and pregnancy outcomes: causal or not? Obstet Gynecol 2017;129:405–07. [DOI] [PubMed] [Google Scholar]
- 12. Schelar E, Franzetta K, Manlove J.. Repeat Teen Childbearing: Differences across States and by Race and Ethnicity. Child Trends Research Brief Washington, DC: Child Trends, 2007. [Google Scholar]
- 13. Crittenden CP, Boris NW, Rice JC, Taylor CA, Olds DL.. The role of maternal health factors, behavioral factors, and past experiences in the prediction of rapid repeat pregnancy in adolescence. J Adolesc Health 2009;44:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khoshnood B, Lee K, Wall S, Hsieh H, Mittendorf R.. Short interpregnancy intervals and the risk of adverse birth outcomes among five racial/ethnic groups in the United States. Am J Epidemiol 1998;148:798–805. [DOI] [PubMed] [Google Scholar]
- 15. Rawlings JS, Rawlings VB, Read JA.. Prevalence of low birth weight and preterm delivery in relation to the interval between pregnancies among white and black women. N Engl J Med 1995;332:69–74. [DOI] [PubMed] [Google Scholar]
- 16. Grant BF, Goldstein RB, Chou SP. et al. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Mol Psychiatry 2009;14:1051–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patchen L, Caruso D, Lanzi RG.. Poor maternal mental health and trauma as risk factors for short interpregnancy interval among adolescent mothers. J Psychiatr Ment Health Nurs 2009;16:401–03. [DOI] [PubMed] [Google Scholar]
- 18. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta A.. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA 2006;295:1809–23. [DOI] [PubMed] [Google Scholar]
- 19. D’Onofrio BM, Class QA, Rickert ME, Larsson H, Långström N, Lichtenstein P.. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry 2013;70:1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rutter M. Proceeding from observed correlation to causal inference: the use of natural experiments. Perspect Psychol Sci 2007;2:377–95. [DOI] [PubMed] [Google Scholar]
- 21. D’Onofrio BM, Lahey B, Turkheimer E, Lichtenstein P.. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health 2013;103:S46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lahey BB, D’Onofrio BM.. All in the family: comparing siblings to test causal hypotheses regarding environmental influences on behavior. Curr Dir Psychol Sci 2010;19:319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Downs JM, Jonas S.. Short inter-pregnancy interval and schizophrenia: overestimating the risk. Br J Psychiatry 2012;200:160.. [DOI] [PubMed] [Google Scholar]
- 24. D’Onofrio BM, Class QA, Rickert ME. et al. Traditional epidemiologic approaches to understanding the consequences of early-life exposures. Behav Genet 2016;46:315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centre for Epidemiology. The Swedish Medical Birth Register: A Summary of Content and Quality. Stockholm: Socialstyrelsen, 2003. [Google Scholar]
- 26. Statistics Sweden. Multi-Generation Register 2005: A Description of Contents and Quality. Orebro: Statistics Sweden, 2006. [Google Scholar]
- 27. Fazel S, Grann M.. The population impact of severe mental illness on violent crime. Am J Psychiatry 2006;163:1397–403. [DOI] [PubMed] [Google Scholar]
- 28. Centre for Epidemiology. The Swedish Hospital Discharge Register http://www.socialstyrelsen.se/publikationer2011/externalreviewandvalidationoftheswedishnationalinpatientregister (12 October 2017, date last accessed).
- 29. Bartlett JW, Seaman SR, White IR, Carpenter JR, for the Alzheimer’s Disease Neuroimaging Initiative. Multiple imputation of covariates by fully conditional specification: accommodating the substantive model. Stat Methods Med Res 2015;24:462–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Indring S, Rai D, Dal H, Dalman C, Sturm H, Zander E.. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PLoS One 2012;7:e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Larsson H, Chang Z, D’Onofrio BM, Lichtenstein P.. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol Med 2014;44:2223–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larsson H, Rydén E, Boman M, Långström N, Lichtenstein P, Landén M.. Does attention deficit hyperactivity disorder share etiologic factors with bipolar disorder and schizophrenia? Br J Psychiatry 2013;203:103–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lichtenstein P, Yip BH, Bjork C. et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 2009;373:234–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tidemalm D, Langstrom N, Lichtenstein P, Runeson B.. Risk of suicide after suicide attempt according to coexisting psychiatric disorder: Swedish cohort study with long term follow-up. BMJ 2008;337:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fazel S, Grann M, Carlstrom E, Lichtenstein P, Langstrom N.. Risk factors for violent crime in schizophrenia: a national cohort study of 13, 806 patients. J Clin Psychiatry 2009;70:362–69. [DOI] [PubMed] [Google Scholar]
- 36. Smits L, Essed GGM.. Short interpregnancy intervals and unfavorable pregnancy outcome: role of folate depletion. Lancet 2001;358:2074–77. [DOI] [PubMed] [Google Scholar]
- 37. Lambe M, Hultman C, Torrang A, MacCabe J, Cnattingius S.. Maternal smoking during pregnancy and school performance at age 15. Epidemiology 2006;17:524–30. [DOI] [PubMed] [Google Scholar]
- 38. D’Onofrio BM, Singh AL, Iliadou AN, Lambe M, Hultman CM, Grann M.. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in Sweden. Arch Gen Psychiatry 2010;67:529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. D’Onofrio BM, Rickert ME, Langstrom N. et al. Familial confounding of the association between maternal smoking during pregnancy and offspring substance use problems. Arch Gen Psychiatry 2012;69:1140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atladottir HO, Thorsen P, Ostergaard L. et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010;40:1423–30. [DOI] [PubMed] [Google Scholar]
- 41. Teitler JO, Das D, Kruse L, Reichman NE.. Prenatal care and subsequent birth intervals. Perspect Sex Health Reprod Health 2012;44:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buckles KS, Munnich EL.. Birth spacing and sibling outcomes. J Hum Resour 2012;47:613–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.