Origins of the Mlomp Health and Demographic Surveillance System
The Mlomp Health and Demographic Surveillance System in Senegal was established in 1985 to study the demographic and health situation of a West African population with high mortality levels, to observe changes over time and to examine the factors involved.1 The rationale was the need for reliable demographic information concerning sub-Saharan Africa, in particular in rural areas for which very few data were available. When the Mlomp project started in 1985, Senegal already had two rural areas under long-term health and demographic surveillance: Niakhar, in the centre-west, where surveillance started in 1962,2 and Bandafassi, in the southeast, where surveillance started in 1970.3 The objective was to add a third HDSS in another rural area of Senegal, in a region whose historic, economic, and ethnic characteristics are very different from those of the two other sites, thus providing the opportunity to better cover the diversity of demographic and epidemiological situations in the country.
What does the Mlomp HDSS cover now?
The core Mlomp HDSS collects information on events—births, deaths, marriages and migrations. The objective is to obtain accurate estimates of demographic levels and trends in the community, and to study changes in marriage and fertility patterns, household, family and kinship.
Research on more specific topics is also conducted, making use of the data collected routinely by the Mlomp HDSS in combination with specific supplementary information on the study topic collected during one particular round. Examples of such research include studies of malaria, sexually transmitted diseases, contraceptive prevalence, breastfeeding, nutrition, etc.
The Mlomp HDSS is now a joint project of the Institut de Recherche pour le Développement (IRD) and the French Institute for Demographic Studies (INED), and is a member of the INDEPTH network [http://www.indepthnetwork.org].
Where is the Mlomp HDSS area?
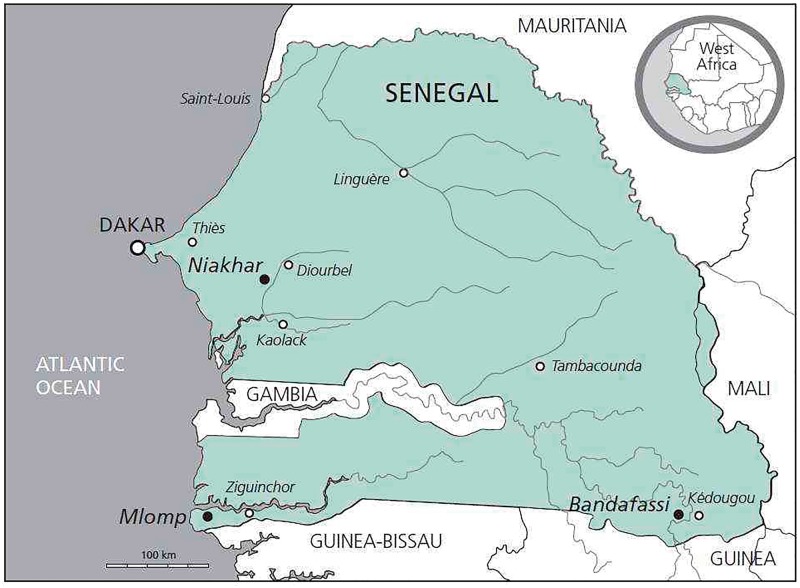
The Mlomp area is in the Ziguinchor Region, in the Département of Oussouye, in southwest Senegal, near the border between Senegal and Guinea-Bissau (Figure 1). It covers about half the Arrondissement of Loudia-Ouolof. The Mlomp HDSS site measures about 11 km × 7 km and has an area of 70 km2. Villages are grouped and surrounded by land that is flooded during the rainy season and is cultivated for rice.
Figure 1.
Location of Mlomp and the two other HDSS sites in Senegal.
The site is in a Guinea savannah and mangrove ecological zone. The area has a rainy season from June to October and a dry season from November to May, and had average annual rainfall of 1250 mm over the period 1985–2010.
The Mlomp HDSS site had a population of 8751 on 1 January 2015 and a population density of 125 inhabitants/km2. It has 11 villages. Most people belong to the Diola ethnic group and self-report as Christians or animists, with a small minority reporting that they are Muslims (3%). People speak Diola, and most also speak Oulof, the main national language in Senegal, and French, the teaching language at school. The area is rural, and rice cultivation is the main economic activity. The majority of the adult population engages in seasonal migrations. The residential unit is a household comprising a family of 6.3 persons on average in 2015, and polygyny is rare.
The first school opened in 1949, two other primary schools in 1960 and 1972 and a secondary school in 1985. That year 20% of women aged 15–49 had been to school for at least 1 year. In 2000 the proportion was 55%, and 79% of 15–19 year-olds had been to school for at least 1 year.
Most dwellings have mud walls and roofs made of corrugated iron (65% in 2004) or thatch (35%). In 2004 half of them had pit latrines and the other half no toilet facilities. Water is from wells. No one had electricity in 2004. The area is 10 km from the small town of Oussouye and 50 km from the regional capital, Ziguinchor, the closest locality with a hospital where caesarian sections can be performed.
A non-governmental health centre, run by French Roman Catholic nurses, was opened in 1961 in the centre of the area. It is well supplied with medicines and equipped to perform simple laboratory tests. The village also runs a maternity clinic, opened in 1968, close to the health centre. The proportion of deliveries in a maternity facility increased from 50% in 1961 to >95% in 1970.4 Most of the children (99%) are correctly vaccinated against measles, yellow fever, BCG (bacillus Calmette-Guérin) and diphtheria–pertussis–tetanus–polio (as recommended by the expanded immunization programme).1
Who is covered by the Mlomp HDSS and how often have they been followed up?
The initial census
The initial census was organized in late 1984 and early 1985. It counted a population of 6218. The extent of the area under surveillance has not changed, but the population has increased to 8751 by the beginning of 2015.
Criteria used for in-migration and out-migration
At the census, a person was considered a member of the compound if the head of the compound declared him/her to be so. This definition was broad and resulted in a de jure population under study. Thereafter, a criterion was used to decide whether and when a person was to be excluded or included in the population.
A person was considered to exit the study population through either death or emigration. Part of the population of Mlomp engages in seasonal migration, with seasonal migrants sometimes remaining 1 or 2 years outside the area before returning. A person who is absent for two successive yearly rounds, without returning in between, is regarded as having emigrated and is no longer a member of the study population at the date of the second round. A new person enters the study population either as an infant born to a woman in the study population or through immigration.
Update rounds
Once each year, usually in February, all households are visited by investigators who check the list of people present in each one the previous year, and who record all births, marriages, migrations and deaths (including their causes) since the preceding visit (Box 1).
Box 1.
Information collected or checked at each annual round of the Mlomp HDSS
| Household | Name and identification number (ID) of head of household |
| Residents | Update of residency status (for all) |
| (still resident, died, out-migrated ?) | |
| Update of marital status (for adults aged 13 years or more) (formed a new union ? still in union with X ?) | |
| Update of maternities (for women aged 13-55 years) (delivered a liveborn baby since the last visit ? a stillbirth ?) | |
| Update of pregnancy status (for women aged 13-55 years) (currently pregnant ?) | |
| Birth | Date and place of birth |
| Names and sex of child | |
| Mother’s and father’s names and identification numbers (ID) (links) | |
| Death | Date and place of death |
| Cause of death (verbal autopsy) | |
| Out-migration | Date of out-migration |
| New residence (village and household if within the Bandafassi HDSS) | |
| Reason for out-migration | |
| In-migration | Date of in-migration |
| Previous residence (village and household if within the Bandafassi HDSS) | |
| Names, sex, date of birth and ID (if yet registered in the Bandafassi HDSS) | |
| Reason for out-migration | |
| Names, survival status, residence and ID of father and mother | |
| Union and birth histories with ID of spouses and children (for adults) | |
| Union formation | Date of cohabitation |
| Names, mother’s and father’s names, previous residence and ID of new spouse | |
| Union disruption | Date of decohabitation |
| Cause of disruption |
For each death identified, information on its cause is obtained from a close relative of the dead person, usually the mother in the case of a child’s death, using a verbal autopsy questionnaire. Verbal autopsies collect detailed data on symptoms and signs during the terminal illness, so that the cause of death can be determined by a physician or a computer algorithm. Verbal autopsies have been performed for all deaths since the beginning of the study.
What has been measured and how have the Mlomp HDSS databases been constructed?
The core data collected throughout the Mlomp HDSS include individual and household identifying information, parent identification and spousal relationships at each point in time. Sufficient data are collected to link individuals of all ages to both parents, facilitating household structure and kinship studies. Other data are collected at various times in the context of specific surveys often on population samples, such as serological, parasitological or resistance surveys for studies of sexually transmitted disease and malaria;5–13 and contraceptive prevalence, breastfeeding, and nutritional surveys.
Information collected during the baseline and follow-up surveys is coded and stored in databases designed in 1985 and subsequently modernized. The data are held in multiple event tables in the database, with a single view table describing all Mlomp HDSS events. Each person who has been member of the resident population at one time, or has been mentioned as spouse or relative of a resident member, is described with an identity number enabling linkage between the different tables (a total of about 50 000 identity numbers have been attributed). The information collected during each annual survey is coded and entered with Access software and, after verification, added to the database using PostgreSQL software.
Key findings and publications
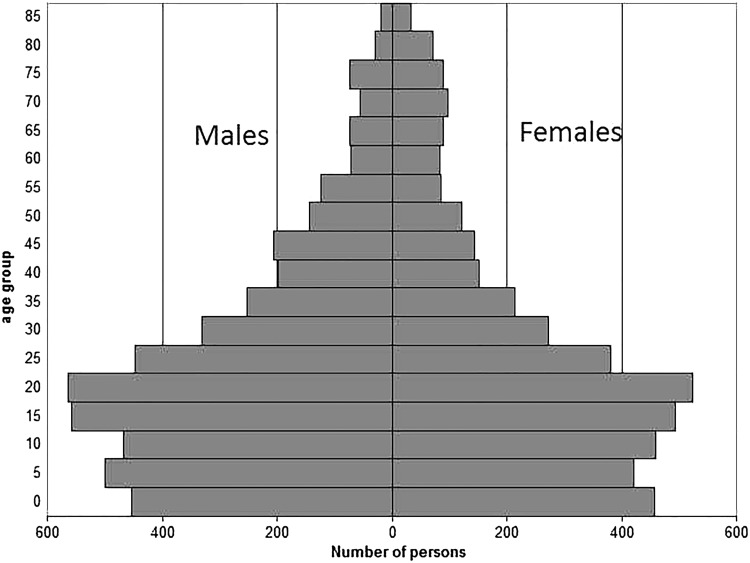
Basic demographic indices for the period 1985–2014 are summarized in Table 1. Mortality and fertility indicators for the two other Senegalese HDSS, Bandafassi and Niakhar, are also provided in Table 1 for comparison. The Mlomp population pyramid on 1 January 2015 is shown in Figure 2.
Table 1.
Demographic characteristics of the Mlomp HDSS
| Period |
||||||
|---|---|---|---|---|---|---|
| 1985–89 | 1990–94 | 1995–99 | 2000–04 | 2005–09 | 2010–14 | |
| Total population under surveillancea | 6951 | 7563 | 7643 | 8001 | 8662 | 8751 |
| Crude birth rate/1000 | 24.3 | 22.9 | 22.2 | 22 | 22.2 | 22.3 |
| Crude death rate/1000 | 8.8 | 11.1 | 11.5 | 11.3 | 8.1 | 7.7 |
| Net migration rate/1000 | 6.6 | 4.7 | −8.6 | −2.0 | 2.2 | −12.8 |
| Population growth rate/1000b | 22.1 | 16.5 | 2.1 | 8.7 | 16.3 | 1.8 |
| Neonatal mortality/1000 livebirths | 39 | 22 | 32 | 21 | 27 | 22 |
| Infant mortality/1000 livebirths | 53 | 63 | 52 | 40 | 42 | 31 |
| Under-five mortality /per 1000 livebirths | 89 | 135 | 108 | 88 | 60 | 39 |
| For comparison: | ||||||
| Bandafassi HDSS under-five mortality | 274 | 253 | 232 | 192 | 151 | 100 |
| Niakhar HDSS under-five mortality | 260 | 190 | 220 | 141 | 74 | 50 |
| Life expectancy at birth (males) (years) | 58 | 56 | 67 | 55 | 64 | 66 |
| Life expectancy at birth (females) (years) | 70 | 62 | 64 | 63 | 72 | 74 |
| For comparison: | ||||||
| Bandafassi HDSS life expectancy (males) | 42 | 46 | 47 | 54 | 57 | 61 |
| Bandafassi HDSS life expectancy (females) | 46 | 49 | 50 | 52 | 58 | 63 |
| Niakhar HDSS life expectancy (males) | 47 | 53 | 48 | 57 | 62 | 67 |
| Niakhar HDSS life expectancy (females) | 49 | 56 | 54 | 61 | 66 | 71 |
| Total fertility rate (children per woman) | 5.3 | 4.4 | 3.6 | 3.2 | 3.4 | 3.3 |
| For comparison: | ||||||
| Bandafassi HDSS total fertility rate | 6.2 | 6.5 | 6.4 | 6.8 | 6.3 | 6.0 |
| Niakhar HDSS total fertility rate | 8.0 | 7.2 | 6.8 | 6.8 | 6.4 | 6.1 |
Figure 2.
Population pyramid of Mlomp HDSS in Senegal on 1 January 2015.
The population pyramid has a trough at the base of the pyramid related to the recent fertility decline, which has been rapid in the Mlomp community (see below).
Seasonal migration is important in Mlomp, with the majority of the young adult population being absent for more than half of the year (7 months on average).15–17 A share of the women aged 20–34 years (53% in 2015), most of them unmarried, are employed as domestic servants in the main cities of Senegal and The Gambia; once married, women usually stay at home all year round. A large proportion of the men aged 20–40 (55%) migrate to harvest palm wine in other villages both within and outside the region, or near the main cities of Senegal or The Gambia. They also go away to fish, practise a trade or do odd jobs in other regions. Some of these men continue to migrate after marriage and until an advanced age, although the proportion of migrants diminishes after age 35.
The population increased at a rate of 1.1% per annum on average over the period 1985–2014, with natural increase (1.3% per annum) being slightly counterbalanced by a net migration rate of –0.2% (Table 1).
The Mlomp HDSS has produced estimates of mortality by sex, age and period. Life expectancy at birth has increased for males from 58 years in 1985–89 to 66 years in 2010–14, and for females, from 70 to 74 years (Table 1). However the increase has not been regular due to the irregular pace of child mortality decline.
Malaria mortality: a temporary increase in the 1990s
The data collected in the Mlomp HDSS show that the risk for a newborn child of dying before age five was 89 per thousand over the period 1985–89, a relatively low level at that time compared with the national level, and levels in other rural areas of the country: in Bandafassi and Niakhar HDSS, for example, child mortality was three times higher (Table 1).18 Its low level in Mlomp was linked to high child vaccination coverage, which resulted in a relatively low mortality from vaccine-preventable infectious diseases, and also to successful antimalaria campaigns organized by the health centre in the 1970s and 1980s (almost all children received chloroquine prophylaxis during the malaria season and all fever cases treated at the dispensary received malaria presumptive treatment). Malaria mortality was reduced as a consequence.1
Malaria has been studied in the Mlomp HDSS since the beginning of surveillance in 1985, via entomological and parasitological surveys. Parasitaemia among children has been measured at different seasons, and the proportion of malaria strains resistant to different chemicals has been measured repeatedly.19,20 Malaria mortality can be retraced with great accuracy because most of the children who die following bouts of fever have been examined at the local health centre, where biological tests (using thick blood films with parasite density measurement) are performed to verify the malaria diagnosis.21
Malaria-related mortality increased 7-fold in Mlomp between 1990 and 1993, and remained at high levels until the end of the 1990s. This rise was attributable to the appearance and spread in the early 1990s of malaria strains that were resistant to chloroquine, the antimalarial widely used in Senegal both preventively and curatively up to 2003, and which dramatically reduced malaria mortality in Mlomp from 1975 until the emergence of chloroquine resistance.21 This unfavourable trend in malaria deaths is one of the reasons for the renewed upturn in overall child mortality in Mlomp in the 1990s. The Mlomp area was used to test successfully new malaria treatments (amodiaquine + sulphadoxine/pyrimetamine (AQ + SP) and artesunate + amodiaquine (ACT),22–26 and to examine antimalarial drug safety.27–29 A dramatic decline in mortality attributable to malaria took place in Mlomp from 2000 onwards. The investment in antimalaria programmes at the national level probably had a substantial effect thanks to the introduction of new treatments (AQ + SP replaced chloroquine for the first-line treatment of malaria in 2003, ACT in 2006), the introduction in 2007 of rapid diagnostic tests for use in health facilities and the mass distribution of insecticide-treated nets (ITN) from 2008 onwards.21,30–33 Similar patterns of increase and decrease in overall and malaria child mortality occurred over the same periods in the two other Senegalese HDSS (Bandafassi and Niakhar), suggesting that these changes were taking place across the entire country.
Adult mortality: a large gender gap
The Mlomp HDSS data show that the level of adult mortality has not changed much over the surveillance period. The large gender gap, which is particular to Mlomp and is not observed in the two other Senegalese HDSS, is partly explained by the relatively moderate level of maternal mortality and the high mortality from external causes for men.34–36
Maternal mortality is around 270 maternal deaths per 100 000 live births over the entire surveillance period, a relatively low level for a rural area of Senegal. Since well before the beginning of the surveillance period, nearly all women have given birth in maternity clinics (>95%). This is related to a tradition in the population to deliver outside the village, in huts specially built for this purpose and called ‘kalambas’.4 When a maternity clinic opened in Mlomp in 1968, located in the centre of the study area, women were encouraged successfully by missionaries and health workers to abandon the kalambas and use this modern facility.
Mortality due to violence and accidents is four times higher for males than for females (the standardized annual rate of deaths from external causes is 160.7 per 100 000 inhabitants over the period 1985–2004 for males, and 39.5 for females). The high excess mortality of males from external causes is related to some specific causes of death such as falls from trees (men climb up palm trees to collect palm wine), drowning (fishing is also an important male activity) and war deaths (due to the conflict to gain independence from Senegal).35
HIV infection: a higher risk for seasonal migrants
The Mlomp HDSS has been used to check the presence of HIV 1 and HIV2 viruses in the south of Senegal at the end of the 1980s, at a time when very little was known about the viruses responsible for HIV and their geographical distribution in Africa. Serological surveys conducted in 1990 and 1995 showed an HIV seroprevalence close to 1% among individuals ≥20 years old, and an annual incidence rate of 1 per 1000 each year during the period 1990–95.5,37,38 Interviews on sexuality, risk factors for HIV and other sexually transmitted disease (STD) infections, as well as perceptions of AIDS and its prevention, showed that seasonal migration was a risk factor for HIV infection.17
Marriage, sexuality, fertility: are the Mlomp people forerunners of new ways of life in rural Africa?
Surveys on sexuality and unions have shown that first marriage has been increasingly delayed in Mlomp since the middle of the 20th century, with women’s mean age at first marriage increasing from about 20 years around 1950 to 32 years at the beginning of the 21st century, and for men, from about 24 years to 38 years.39
Conversely, mean age at first sexual intercourse has decreased, in particular for men: for those born in the 1930s and 1940s, sexual debut occurred at around age 24 years on average, and for those born in the 1960, at around 18 years. There has been little change, on the contrary, for women, with sexual debut occurring at around age 18 or 19 on average, with only a slight decrease of 0.6 years over a 25-year period.39
Fertility has declined considerably, from 5.3 children per woman on average in 1985–89 to 3.2 in 2000–04, and has since remained at this level. No such rapid decline has been observed in the two other HDSS, where fertility has remained at six children per woman or higher in the past three decades, with only a slow decrease, as has also been the case also in most rural areas across the country. The low level in Mlomp results from a combination of factors. First, marriage is increasingly late. Second, the unions which almost always precede women’s first marriage are not very fertile: the first birth is late (mean age at first birth, which was already high at the beginning of the surveillance period at 21.2 years in 1985–89, has increased further since then to reach 23.3 years in 2010–14) and the subsequent births are spaced. Sexual intercourse is infrequent on average, widely spaced and partly protected (among unmarried women below 30 living in union with a partner, only one-third were using contraception at the beginning of the 2000s, condoms in most cases). The majority of young women who give birth often wait a long time before they have sex again. Third, within marriage, sexual intercourse is more regular, but births remain spaced because of the long duration of breastfeeding and temporary separations of spouses during seasonal migrations; only a minority of married women use contraception (20% in the early 2000s).39
Mlomp HDSS: an ideal site for methodological studies
The Mlomp HDSS is regularly used for methodological studies to evaluate the quality of data collected by censuses and demographic surveys in countries with limited vital registration, and to test new ‘unconventional’ techniques to improve data collection. In collaboration with the Senegalese statistical office in charge of the census (‘Agence nationale de la statistique et de la démographie’), an evaluation of the data collected by the two most recent national censuses of Senegal (those of 2002 and 2013) is currently ongoing by matching, at the individual level, the census data collected for the Mlomp villages with the data of the Mlomp HDSS.40 The objectives are to measure the exhaustiveness of the census, and also study errors and bias in reporting of age, survival of parents, household deaths in the past 12 months and reporting of causes of death, especially maternal deaths and deaths from external causes. As this evaluation is also performed in the two other Senegalese HDSS (Bandafassi and Niakhar), it allows examination of the variations in data quality across regions within the country.
Profile in a nutshell
The Mlomp Health and Demographic Surveillance System (HDSS) is located in southwest Senegal, near the border with Guinea-Bissau. The area is 500 km from the national capital, Dakar. The population under surveillance is rural and in 2015 comprised 8751 inhabitants living in 11 villages.
Data have been collected through annual rounds since the surveillance started in 1985. On each visit, investigators review the composition of all households, check the lists of people who were present in each household the previous year and gather information about births, marriages, migrations and deaths (including their causes) since then. Data are partly open access and may be shared under a collaboration agreement.
Total fertility in Mlomp declined in the 1980s and 1990s down to slightly more than three children per woman on average at the beginning of the 2000s, and has since remained at this relatively low level for a rural area of Western Africa.
Malaria-related mortality increased 7-fold in Mlomp in the early 1990s following the appearance and spread of malaria strains that were resistant to chloroquine; this rise was followed by a dramatic decline from 2000 onwards due to new antimalaria strategy developed at the national level.
In collaboration with ‘Agence nationale de la statistique et de la démographie’ (National statistical office of Senegal), the Mlomp HDSS is used to validate national censuses and to test new techniques for improving data collection.
Future analysis projects
We plan to continue providing data on demographic trends: mortality (child, adult); fertility (intensity, timing, non-marital, fostered children); and marriage (marital patterns, divorce, new ways of life). We are further exploring changes in household, family and kinship size and composition in relation to the increase in life expectancy and population ageing. Evaluation of the data collected by the national censuses will continue, and we plan to test new ‘unconventional’ techniques to improve data collection.
Strengths and weaknesses
A major strength of the Mlomp HDSS is the long duration of the follow-up which provides continuous time-series over 32 years, as the data collection method has remained practically unchanged, and a unique historical perspective on demographic changes in the study area. The main weakness of Mlomp HDSS is its size, which is often too small for trials. However, when combined with the information collected in the two other HDSS in rural Senegal (Niakhar2 and Bandafassi3), its data can be used to perform multisite studies. The three sites are well suited for comparative analysis: they are located in very different regions and environments in a country with contrasting population densities and health services, and the data collection methods are very similar.
Data sharing and collaboration
Part of the data are open access at [www.indepth-ishare.org], and other data may be shared within the framework of a collaboration agreement. Collaborative research projects are encouraged. Enquiries and queries can be submitted to the first or the last author.
Funding
The following institutions have provided financial support to the Mlomp HDSS: Institut national d’études démographiques, Institut français de recherche pour le développement, Muséum national d’histoire naturelle, Agence nationale de recherches sur le SIDA, Agence nationale de la recherche, European Union, World Health Organization, Institut national de la santé et de la recherche médicale and Centre national de la recherche scientifique.
Conflict of interest: None declared.
References
- 1. Pison G, Trape J-F, Lefebvre M, Enel C.. Rapid decline in child mortality in a rural area of Senegal. Int J Epidemiol 1993;22:72–80. [DOI] [PubMed] [Google Scholar]
- 2. Delaunay V, Douillot L, Diallo A. et al. Profile: The Niakhar Health and Demographic Surveillance System. Int J Epidemiol 2013;42:1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pison G, Douillot L, Kante AM. et al. Health and Demographic Surveillance System Profile: Bandafassi Health and Demographic Surveillance System (Bandafassi HDSS), Senegal. Int J Epidemiol 2014;43:739–48. [DOI] [PubMed] [Google Scholar]
- 4. Enel C, Pison G, Lefebvre M. [ From traditional childbirth to modern childbirth in Senegal.] De l'accouchement traditionnel à l'accouchement moderne au Sénégal. Cahiers Santé 1993;3:441–46. [Google Scholar]
- 5. Diop O, Pison G, Diouf I, Enel C, Lagarde E.. Incidence of HIV-1 and HIV-2 infections in a rural community in southern Senegal. AIDS 2000;14:1671–72. [DOI] [PubMed] [Google Scholar]
- 6. Enel C, Lagarde E, Pison G.. The evaluation of surveys of sexual behaviour: a study of couples in rural Senegal. Health Transit Rev 1994;4:111–24. [PubMed] [Google Scholar]
- 7. Enel C, Pison G.. Veuvage et lévirat: une étude de cas à Mlomp (Sénégal). In: Locoh T. (ed). Genre et sociétés en Afrique. Paris: Presses universitaires de France (P.U.F; ), 2007. [Google Scholar]
- 8. Enel C, Pison G.. Pour mieux comprendre l’exode rural des jeunes femmes au Sénégal: la méthode des sœurs In: Vallin J. (ed). Du genre et de l'Afrique Hommage à Thérèse Locoh. Paris: INED/Presses universitaires de France (PUF; ), 2009. [Google Scholar]
- 9. Lagarde E, Enel C, Pison G.. Reliability of reports of sexual behaviour: a study of married couples in rural West Africa. Am J Epidemiol 1995;141:1194–200. [DOI] [PubMed] [Google Scholar]
- 10. Lagarde E, Pison G, Enel C.. A study of sexual behavioural change in rural Senegal. J Acqu Immune Defic Syndr 1996;11:282–87. [DOI] [PubMed] [Google Scholar]
- 11. Lagarde E, Pison G, Enel C.. Knowledge, attitudes and perception of AIDS in rural Senegal: relationship to sexual behaviour and behaviour change. AIDS 1996;10:327–34. [DOI] [PubMed] [Google Scholar]
- 12. Lagarde E, Pison G, Enel C.. Improvement in AIDS knowledge, perceptions and behaviours over a short period in a rural community of Senegal. Int J STD AIDS 1997;8:681–87. [DOI] [PubMed] [Google Scholar]
- 13. Lagarde E, Pison G, Enel C, Delaunay V, Gabadinho A.. Résultats d'une étude préliminaire sur les facteurs de variation de l'infection par le VIH et les maladies sexuellement en zone rurale d'Afrique de l'Ouest. Rev Epidemiol Sante Publique 1997;45:271–78. [PubMed] [Google Scholar]
- 14. INDEPT -Network. INDEPTHStats 2018. http://wwwindepth-networkorg/data-stats/indepthstats.
- 15. Enel C, Pison G.. Sexual relations in the rural area of Mlomp (Casamance, Senegal) In: Dyson T. (ed). Sexual Behaviour and Networking: Anthropological and Socio-Cultural Studies on the Transmission of HIV. Liège, Belgium: Derouaux Ordina Editions, 1992. [Google Scholar]
- 16. Enel C, Pison G, Lefebvre M.. Migrations and nuptiality changes. A case study in rural Senegal In: Bledsoe C, Pison G (eds). Nuptiality in Sub-Saharan Africa: Contemporary Anthropological and Demographic Perspectives. Oxford, UK: Clarendon Press, 1994. [Google Scholar]
- 17. Pison G, Le Guenno B, Lagarde E, Enel C, Seck C.. Seasonal migration: a risk factor for HIV infection in rural Senegal. J Acquir Immune Defic Syndr 1993;6:196–200. [PubMed] [Google Scholar]
- 18. Masquelier B, Reniers G, Pison G.. Divergences in trends in child and adult mortality in sub-Saharan Africa: survey evidence on the survival of children and siblings. Popul Stud 2014;68:161–77. [DOI] [PubMed] [Google Scholar]
- 19. Sokhna C, Molez J-F, Ndiaye P, Sane B, Trape J-F.. Tests in vivo de chimiosensibilité de Plasmodium falciparum à la chloroquine au Sénégal: évolution de la résistance et estimation de l'efficacité thérapeutique. Bull Soc Pathol Exot 1997;90:83–89. [PubMed] [Google Scholar]
- 20. Agnamey P, Brasseur P, De Pecoulas PE, Vaillant M, Olliaro P.. Plasmodium falciparum in vitro susceptibility to antimalarial drugs in Casamance (southwestern Senegal) during the first 5 years of routine use of artesunate-amodiaquine. Antimicrob Agents Chemother 2006;50:1531–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trape J-F, Pison G, Preziosi M-P. et al. Impact of chloroquine resistance on malaria mortality. Comptes rendus de l'Académie des sciences, Sciences de la vie, Paris 1998;321:689–97. [DOI] [PubMed] [Google Scholar]
- 22. Adjuik M, Agnamey P, Babiker A. et al. Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomised, multicentre trial. Lancet 2002;359:1365–72. [DOI] [PubMed] [Google Scholar]
- 23. Brasseur P, Guiguemde R, Diallo S. et al. Amodiaquine remains effective for treating uncomplicated malaria in west and central Africa. Trans R Soc Trop Med Hyg 1999;93:645–50. [DOI] [PubMed] [Google Scholar]
- 24. Ndiaye JL, Randrianarivelojosia M, Sagara I. et al. Randomized, multicentre assessment of the efficacy and safety of ASAQ – a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar J 2009;8:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olliaro P, Nevill C, LeBras J. et al. Systematic review of amodiaquine treatment in uncomplicated malaria. Lancet 1996;348:1196–201. [DOI] [PubMed] [Google Scholar]
- 26. Zwang J, Olliaro P, Barennes H. et al. Efficacy of artesunate-amodiaquine for treating uncomplicated falciparum malaria in sub-Saharan Africa: a multi-centre analysis. Malar J 2009;8:203.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brasseur P, Agnamey P, Gaye O, Vaillant M, Taylor WR, Olliaro PL.. Efficacy and safety of artesunate plus amodiaquine in routine use for the treatment of uncomplicated malaria in Casamance, southern Senegal. Malar J 2007;6:150.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brasseur P, Vaillant MT, Olliaro PL.. Antimalarial drug safety information obtained through routine monitoring in a rural district of South-Western Senegal. Malar J 2012;11:402.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Winkler WE. The State of Record Linkage and Current Research Problems. Suitland, MD: Statistical Research Division, US Census Bureau, 1999. [Google Scholar]
- 30. Pison G, Douillot L, Duthé G, Kanté AM, Sokhna C, Trape J-F.. Successes and Failures in the Fight Against Child Mortality in Sub-Saharan Africa: Lessons From Senegal, a Country With Low AIDS Prevalence. Paris: French Institute for Demographic Studies, 2013. [Google Scholar]
- 31. Brasseur P, Badiane M, Cisse M, Agnamey P, Vaillant MT, Olliaro PL.. Changing patterns of malaria during 1996-2010 in an area of moderate transmission in Southern Senegal. Malar J 2011;10:203.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brasseur P, Gaye O, Badiane M. et al. Changes in malaria in southern Senegal with the introduction of artesunate plus amodiaquine and parasitological diagnosis. Am J Trop Med Hyg 2010;83:1318. [Google Scholar]
- 33. Duthé G. Malaria resurgence in Senegal: measuring malaria mortality in Mlomp. Population 2008;63:443–67. [Google Scholar]
- 34. Duthé G, Pison G.. Adult mortality in a rural area of Senegal: non-communicable diseases have a large impact in Mlomp. Demogr Res 2008;19:1419–34. [Google Scholar]
- 35. Guyavarch E, Pison G, Duthé G, Marra A, Chippaux J-P.. Mortality due to external causes in three rural areas of Senegal [La mortalité violente dans trois régions rurales du Sénégal]. Eur J Popul 2010;26:483–505. [Google Scholar]
- 36. Duthé G, Pison G, Delaunay V, Douillot L.. L’effet à long terme de la vie reproductive sur la mortalité des femmes en milieu rural sénégalais. Afr Popul Stud 2016;30. [Google Scholar]
- 37. Le Guenno B, Pison G, Enel C, Lagarde E, Seck C.. HIV2 seroprevalence in three rural regions of Senegal: low levels and heterogeneous distribution. Trans R Soc Trop Med Hyg 1992;86:301–02. [DOI] [PubMed] [Google Scholar]
- 38. Le Guenno B, Pison G, Enel C, Lagarde E, Seck C.. Clinical and immunological impact of HIV2 infection in a rural community of Senegal. J Med Virol 1992;38:67–80. [DOI] [PubMed] [Google Scholar]
- 39. Pison G, Gabadinho A, Enel C.. Mlomp (Senegal): demographic levels and trends [Mlomp (Sénégal) : niveaux et tendances démographiques]. Dossiers et recherches de l'INED 2001;103:1–182. [Google Scholar]
- 40. Masquelier B, Ndiaye CT, Pison G. et al. Evaluation of indirect mortality estimates in three population surveillance sites in Senegal [Evaluation des estimations indirectes de mortalité dans trois observatoires de population au Sénégal]. Afr Popul Stud 2016;30:2227–41. [Google Scholar]