Abstract
Background
Observational studies have shown that tobacco and alcohol use co-occur, but it is not clear whether this relationship is causal.
Methods
Using data from the Avon Longitudinal Study of Parents and Children (ALSPAC) and UK Biobank, we used observational methods to test the hypothesis that smoking heaviness increases alcohol consumption. Mendelian randomization (MR) analyses were then used to test the causal relationship between smoking heaviness and alcohol consumption using 55 967 smokers from four European studies [ALSPAC, The Nord-Trøndelag Health Study (HUNT), the Copenhagen General Population Study (CGPS) and UK Biobank]. MR analyses used rs1051730/rs16969968 as a genetic proxy for smoking heaviness.
Results
Observational results provided evidence of an association between cigarettes per day and weekly alcohol consumption (increase in units of alcohol per additional cigarette smoked per day = 0.10, 95% confidence interval (CI) 0.05 to 0.15, P ≤ 0.001 in ALSPAC; and 0.48, 95% CI 0.45 to 0.52, P ≤ 0.001 in UK Biobank). However, there was little evidence for an association between rs1051730/rs16969968 and units of alcohol consumed per week across ALSPAC, HUNT, CGPS and UK Biobank (standard deviation increase in units of alcohol per additional copy of the risk allele = –0.004, 95% CI –0.023 to 0.016, P=0.708, I2 = 51.9%). We had 99% and 88% power to detect a change of 0.03 and 0.02 standard deviation units of alcohol per additional copy of the risk allele, respectively.
Conclusions
Previously reported associations between smoking and alcohol are unlikely to be causal, and may be the result of confounding and/or reverse causation. This has implications for public health research and intervention research.
Keywords: Mendelian randomization, licit drugs, ALSPAC, HUNT, CGPS, UK Biobank
Key Messages
Observational studies have shown consistent strong evidence for an association between tobacco and alcohol use.
It has been suggested that reductions in tobacco use can be used as an intervention target for alcohol.
Our study suggests that heaviness of smoking does not causally influence level of alcohol consumption.
It is likely that previous findings were the subject of confounding, reverse causation or bias.
These findings could have implications for targeting smoking behaviour in interventions.
Introduction
Smoking and alcohol are among the most important preventable causes of morbidity and mortality.1–4 Many studies have examined the relationship between tobacco and alcohol use, with several of these focusing on smoking as a risk factor for later alcohol use in both adolescence5–11 and adulthood.12–16 However, current evidence is inconsistent and the observational data used in such studies are difficult to interpret due to the potential for unmeasured confounding and reverse causation. Determining whether there is a causal association between tobacco and alcohol use is important in the attempt to reduce the use of these drugs, as one could use prevention of tobacco use as a means of reducing alcohol misuse.
Mendelian randomization (MR) uses genetic variants known to be associated with an exposure of interest as a method of testing whether there is a causal association between exposure and disease.17 MR is based on three assumptions. First, the genetic instrument being used must be associated with the exposure of interest. Second, the genetic instrument must only influence the outcome through the exposure of interest. Third, the genetic instrument cannot be associated with any factors that confound the relationship between the exposure and the outcome.17 If the assumptions of MR hold, genetic variants associated with an exposure of interest should be independent of confounding factors.18 Furthermore, as genotype is determined at conception, it cannot be influenced by any stage of the disease process and therefore estimates cannot be the result of reverse causation.17,18
We hypothesized that a genetic determinant of smoking phenotypes would be associated with increased levels of alcohol use and would therefore provide evidence of a causal relationship (MR). To test this, we first assessed the relationship between heaviness of smoking and weekly alcohol consumption using observational methods. Data from the Avon Longitudinal Study of Parents and Children (ALSPAC) and UK Biobank were used. In MR analyses, we used two single-nucleotide polymorphisms (SNPs) in the nicotine acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4): rs1051730 and rs16969968, which are highly correlated and therefore can be used interchangeably.19 The rs16969968 SNP is a missense mutation that codes for a change in amino acid from aspartate to asparagine in the α5 nAChR subunit protein and is therefore of functional significance. Conversely, rs1051730 is a coding synonymous variant and is more likely to act as a proxy for a functional SNP.20 These variants have been shown to be robustly associated with the number of cigarettes consumed per day21–26 and have previously been used in MR studies.27–35 MR analyses were carried out using four European cohorts [ALSPAC, the Nord-Trøndelag Health Study (HUNT), the Copenhagen General Population Study (CGPS) and UK Biobank] with results from all studies being meta-analysed.
Methods
Study populations
Four European cohort studies were utilized in this analysis (ALSPAC, HUNT, CGPS and UK Biobank). ALSPAC is a longitudinal birth cohort study situated in south-west England that recruited more than 14 000 pregnant women between 1991 and 1992.36,37 HUNT invited individuals in Nord-Trøndelag County in Norway who were aged 20 years or older between 1995 and 1997 to take part in the second wave of the study, and successfully recruited 65 215 individuals.38,39 CGPS is a study comprising randomly selected Copenhagen residents aged 20–100 years.27 UK Biobank recruited over 500 000 men and women (aged 37–73 years) between 2006 and 2010.40 Full description of each of the cohorts can be found in Supplementary Methods, available as Supplementary Data at IJE online.
Phenotypic measures
Smoking
Cigarettes per day in individuals who reported smoking was a continuous variable—a measure that has previously shown association with rs16969968/rs1051730.23,41,42 In ALSPAC, these data were collected during pregnancy (18 weeks’ gestation), but addressed regular smoking status pre-pregnancy. In UK Biobank, participants were asked about current and past smoking status during the baseline computer-administered questionnaire. Individuals were classed as current, former or never smokers. Individuals reporting regular use of pipes or cigars were excluded from analyses.
Alcohol
Alcohol consumption was a continuous measure of units of alcohol consumed per week, derived from the reporting of frequency of alcohol consumption. Different questions were asked in each of the four cohorts but all allowed the calculation of average weekly intake in units. Further information on the derivation of this variable is provided in Supplementary Table 1, available as Supplementary Data at IJE online. Non-drinkers were excluding from analyses.
Confounders
Potential confounders for the observational analysis (conducted in ALSPAC and UK Biobank; see ‘Statistical analysis’ below) included: sex (UK Biobank only), age in years, social class (0 ‘III manual skilled, IV and V unskilled manual or casual workers or those who rely on state for their income’ and 1 ‘I and II professional occupations and managerial and technical occupations and III non-manual skilled workers’43 (ALSPAC only); highest level of education (0 ‘college degree or higher’ and 1 ‘A level equivalent or lower’) (UK Biobank only); partner’s smoking (reported by the mother) (0 ‘no’ and 1 ‘yes’) (ALSPAC only); partner’s drinking (reported by the mother) (0 ‘never/very occasionally’, 1 ‘occasionally’ and 2 ‘daily’) (ALSPAC only).
Genetic measures
Genotyping information for ALSPAC, HUNT, CGPS and UK Biobank is provided in Supplementary Methods, available as Supplementary Data at IJE online. The rs1051730/rs16969968 variants were used as a proxy for heaviness of smoking (measured here as cigarettes per day) and were coded 0, 1 and 2 for genotypes CC, CT and TT, respectively. The minor allele frequency (MAF) was 0.33 for both rs1051730 and rs16969968.
Statistical analysis
Linear regression was used to assess the association between cigarettes per day and units of alcohol per week [adjusted for age (both cohorts), social class, partner’s smoking and drinking (ALSPAC only), sex and education (UK Biobank only)] in ALSPAC and UK Biobank only.
Using data from ALSPAC and UK Biobank, we tested the association between rs1051730/rs16969968 and cigarettes per day as a test of the first assumption of MR (genotype must be associated with the exposure). We examined associations between rs1051730/rs16969968 and confounders to test the third assumption of MR (that the genetic instrument cannot be associated with any factors that confound the relationship between the exposure and the outcome). Both CGPS27 and HUNT28 have previously published the association between rs16969968/rs1051730 and units of alcohol per week. The aim of these previous analyses was not to assess the relationship between smoking heaviness and alcohol consumption; however, both tested the association between rs1051730 and alcohol consumption as a test for genetic pleiotropy (i.e. considering alcohol as a potential confounder in their analysis). Additionally, these previous analysis have assessed the association between rs1051730 and smoking behaviour and a range of relevant confounders.27,28
Deviation from Hardy-Weinberg equilibrium was assessed as a test of missingness that might arise from genotyping errors, clinical ascertainment or by chance.44
In each of the four cohorts (ALSPAC, HUNT, CGPS and UK Biobank), units of alcohol per week (excluding non-drinkers) (Supplementary Table 1, available as Supplementary Data at IJE online) was standardized (i.e. converted to a Z-score) for consistency between datasets. We then tested for association between rs1051730/rs16969968 and standardized units per week stratified by smoking status (current smokers/former smokers/never smokers). Analysis in never and former smokers tests the pleiotropy assumption of MR (i.e. the genotype cannot be associated with the outcome through any other phenotype). Additional sensitivity analysis was conducted stratifying the sample in ever smokers and never smokers.
We opted to use linear regression over two-stage least-squares regression. This is because the second assumption of MR (that the SNP should only be associated with the outcome through the exposure of interest) is likely to be violated when the phenotype (e.g. cigarettes per day) does not adequately capture the exposure through which the genetic variant operates. This has been described in detail elsewhere.45,46 In brief, results from two-stage least-squares regression may be biased when this assumption is violated. In this situation, the genetic variant is still a valid instrument to provide evidence of causality, but is not a valid instrument for quantifying the effect of the measured phenotype on the outcome. We assume a constant effect of smoking on alcohol consumption and, as a result, we identify the average effect of smoking heaviness on alcohol consumption in the sample.
The effect sizes between rs16969968/rs1051730 and standardized units per week stratified by smoking status for each study were pooled in a meta-analysis. We used DerSimonian and Laird random-effects meta-analysis47 using the metan command in Stata 13.48,49
Quanto50 was used to calculate the sample size required to obtain different effects in the MR analyses. A continuous trait design was specified with a gene-only hypothesis, using a desired power of 0.80, a significance level of 0.05 (two-sided) and a log additive mode of inheritance.
All analysis was carried out following STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (Supplementary Table 2, available as Supplementary Data at IJE online). The Instrumental Variable Checklist51 (Supplementary Table 3, available as Supplementary Data at IJE online) was used for MR analysis and the instrumental variable flow chart considered throughout analyses and reporting.52
Results
Observational results
A total of 8030 individuals had genetic information collected in ALSPAC and provided information on their smoking status. Of these, 2198 (27.4%) were smokers and provided information on alcohol consumption. The median number of cigarettes per day and units of alcohol per week were 15 (IQR = 15) and 4 (IQR = 3.5), respectively. Following inclusion of covariate data, the sample size for the complete case analysis was 1359 (Supplementary Table 4, available as Supplementary Data at IJE online). In UK Biobank, 335 921 individuals had genetic information and provided data on their smoking status. Of these, 30 241 (9.0%) were smokers and provided information on alcohol consumption. The median number of cigarettes per day and units of alcohol per week were 15 (IQR = 10) and 8 (IQR=14), respectively. Following inclusion of covariate data, the sample size for the complete case analysis was 15 323 (Supplementary Table 5, available as Supplementary Data at IJE online).
There was strong evidence for an association between cigarettes consumed per day and units of alcohol per week [change in units per week for each additional cigarette per day smoked = 0.09, 95% confidence interval (CI) 0.04 to 0.15, P ≤ 0.001 in ALSPAC; and 0.65, 95% CI 0.61 to 0.69, P ≤ 0.001 in UK Biobank], which remained after adjustment for confounders (change in units per week for each additional cigarette smoked = 0.10, 95% CI 0.05 to 0.15, P ≤ 0.001 in ALSPAC; and 0.48, 95% CI 0.45 to 0.52, P ≤ 0.001 in UK Biobank) (Table 1).
Table 1.
Unadjusted and adjusted effect sizes for observational analysis examining the association between cigarettes per day and units of alcohol per week in ALSPAC and UK Biobank
| Adjustment | N | Coef* | 95% CI | LR(χ2) | LR test P-value |
|---|---|---|---|---|---|
| ALSPAC | |||||
| Unadjusted (all available data) | 2198 | 0.11 | 0.07–0.16 | 23.02 | ≤0.001 |
| Unadjusted (complete case analysis) | 1359 | 0.09 | 0.04–0.15 | 12.01 | ≤0.001 |
| Socio-economic position | 1359 | 0.10 | 0.04–0.15 | 12.94 | ≤0.001 |
| Age | 1359 | 0.09 | 0.04–0.15 | 12.39 | ≤0.001 |
| Partner’s smoking | 1359 | 0.09 | 0.04–0.15 | 12.10 | ≤0.001 |
| Partner’s drinking | 1359 | 0.10 | 0.05–0.15 | 13.67 | ≤0.001 |
| Fully Adjusted | 1359 | 0.10 | 0.05–0.15 | 14.66 | ≤0.001 |
| UK Biobank | |||||
| Unadjusted (all available data) | 15 462 | 0.65 | 0.61–0.69 | 1090.35 | ≤0.001 |
| Unadjusted (complete case analysis) | 15 323 | 0.65 | 0.61–0.69 | 1067.82 | ≤0.001 |
| Education | 15 323 | 0.65 | 0.61–0.69 | 1037.70 | ≤0.001 |
| Age | 15 323 | 0.65 | 0.61–0.69 | 1047.03 | ≤0.001 |
| Sex | 15 323 | 0.48 | 0.44–0.51 | 611.50 | ≤0.001 |
| Fully adjusted | 15 323 | 0.48 | 0.45–0.52 | 624.42 | ≤0.001 |
Coefficients describe the increase in units of alcohol per week for each additional cigarette smoked per day. LR, likelihood ratio.
MR results
In both ALSPAC and UK Biobank, there was evidence for an association between rs1051730/rs16969968 and cigarettes per day in those who smoked (change in cigarettes smoked per day for each additional copy of the risk allele = 0.91, 95% CI 0.41 to 1.40, P ≤ 0.001 in ALSPAC; and 0.95, 95% CI 0.79 to 1.12, P ≤ 0.001 in UK Biobank), which is consistent with previous evidence that each additional copy of the risk allele is responsible for an approximately one-cigarette-per-day increase in smoking heaviness.25,26 There was no evidence for association between rs1051730/rs16969968 and potential confounding factors in ALSPAC or UK Biobank (Supplementary Tables 4 and 5, available as Supplementary Data at IJE online) or a departure from Hardy-Weinberg equilibrium (ALSPAC χ2P-value = 0.34; HUNT χ2P-value = 0.12; CGPS χ2P-value = 0.32; UK Biobank χ2P-value = 0.89).
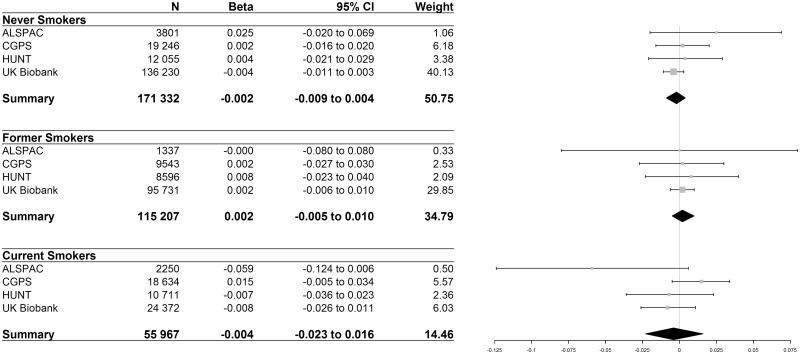
In total, 55 967 smokers were included when pooling results from the ALSPAC, HUNT, CGPS and UK Biobank studies. There was little evidence for an association between rs1051730/rs16969968 and units of alcohol per week (where β coefficients represent the standard deviation change for each additional copy of the risk allele) in never (β = –0.002, 95% CI –0.009 to 0.004, P = 0.49), former (β = 0.002, 95% CI –0.005 to 0.010, P = 0.55) or current smokers (β = –0.004, 95% CI –0.023 to 0.016, P = 0.71) (Figure 1). Additionally, there was little evidence for an association between rs16969968/rs1051730 and units of alcohol per week in ever smokers (β = 0.003, 95% CI –0.004to 0.009, P = 0.45) (Supplementary Figure 1, available as Supplementary Data at IJE online). In ALSPAC and UK Biobank, MR results using all available data were consistent with those using complete case data from the observational analysis (Supplementary Table 6, available as Supplementary Data at IJE online).
Figure 1.
Effect sizes represent the standard deviation increase in units of alcohol per week for each additional copy of the minor (risk) allele. P values for association in: never smokers = 0.496, former smokers = 0.549, current smokers = 0.708. Test of heterogeneity: never smokers I2 = 0.0%, p = 0.558; former smokers I2=0.0%, p = 0.986; current smokers I2 = 51.9%, p= 0.101. Note: weights are from random effects meta-analysis.
We calculated that our sample size of 55 967 current smokers had at least 99% and 88% power to detect a change of 0.03 and 0.02 standard deviations of units of alcohol per additional copy of the minor allele, respectively.
Discussion
We corroborate the strong association between tobacco and alcohol consumption in observational data sets using ALSPAC and UK Biobank cohorts. However, we found no clear evidence of a causal effect when using a genetic marker of smoking heaviness in an MR framework using current smokers from four European cohorts. Our analysis suggests that the reported associations between smoking and alcohol could be strongly influenced by confounding factors and/or reverse causality, and that smoking heaviness is not causally associated with alcohol consumption.
Two studies have assessed the relationship between tobacco and alcohol using MR. The results reported in this manuscript are in agreement with Vrieze and colleagues, who did not report an association.53 However, as the previous analysis uses an adolescent sample, the results may not be comparable with an adult sample, such as those we report here. Vink and colleagues reported that both cigarettes per day and smoking cessation were associated with glasses of alcohol per week when using polygenic risk scores as proxies for smoking phenotypes.54 This analysis used weak P-value thresholds to generate their polygenic risk scores and therefore provide evidence for shared aggregated genetic risk factors between tobacco and alcohol use, rather than testing for a causal relationship. Following meta-analysis of data from four studies, the sample size of the analysis we report here increased to 55 967 and is therefore much larger than that reported by Vink and colleagues, providing more robust evidence for no causal association between heaviness of smoking and alcohol consumption.
As alcohol consumption does not remain stable over time, smoking may have a causal effect on levels of alcohol consumption regardless of when people start drinking. Here, we have examined the dose—response relationship between heaviness of smoking among current smokers with levels of alcohol consumption, not the effect of smoking initiation on subsequent alcohol use. By examining this using MR, our results cannot be influenced by reverse causation. One study conducted by Irons and colleagues (2007) examined the causal effect of alcohol use on tobacco use using MR finding little evidence for an effect.55 This study, in conjunction with the results reported here, provides evidence that previously reported associations between tobacco and alcohol use are not the result of reverse causation and that confounding factors are responsible for the co-occurrence of these substances.
A number of limitations need to be considered when interpreting these results. First, when excluding women with missing data on all genetic, outcome, exposure and covariate measures, there is a large amount of attrition from the original ALSPAC dataset. Second, both smoking and alcohol consumption in this study were assessed using self-report. The potential effect of this limitation is reduced by the use of a genetic variant as a determinant of smoking, as it is unlikely to vary with regard to reporting bias. Nevertheless, biological assessment at least for tobacco use (based on cotinine data for smoking) would be advantageous in any further studies.56 However, alcohol biomarkers for chronic consumption are unreliable.57 Third, it is likely that MR analysis is underpowered to rule out any association between tobacco and alcohol. We would not expect to see the same effect size between the non-genetic observational analysis and the MR analysis (since the genetic variants only explain a small proportion of the variance in heaviness of smoking). However, as the observed effects are very close to the null, the conclusion can be made that the MR result is consistent with a null result. Furthermore, the meta-analysis of results with those from the HUNT and CGPS cohorts provide further evidence that is consistent with the null hypothesis. Fourth, the design of MR and meta-analysis are susceptible to population stratification. There is potential for population stratification between each of the datasets used. However, the effect in each of these studies has been examined separately and no difference was observed, suggesting it is reasonable to conclude that population stratification is not affecting these results. We did observe heterogeneity between the observational results in ALSPAC and UK Biobank, but the direction of the association was consistent. The differences in the magnitude of the association could be explained by differences in age and gender between the two populations, the fact that the data were collected ∼15 years apart or that the ALSPAC sample comprised individuals who might have been trying to get pregnant at the time. Finally, we cannot completely rule out the possibility that collider bias may affect these results. As the genetic variant influences likelihood of smoking cessation,58 stratifying analyses into former and current smokers could induce collider bias. However, there is little evidence to suggest that the variant influences smoking initiation, so stratification of results into ever and never smokers should be less problematic. Furthermore, collider bias could also arise if selection into the study samples is related to both alcohol consumption and rs16969968/rs1051730 (if heavier smoking makes individuals less likely to participate). This is most likely to be an issue in UK Biobank, which has very low participation rates and is not likely to be very representative of individuals of the target age group living in the UK.59 When excluding UK Biobank from the meta-analysis, results remained the same (Supplementary Figure 2, available as Supplementary Data at IJE online).
MR techniques are particularly pertinent to behavioural exposures—such as tobacco smoking, alcohol consumption, cannabis and other drug use—which cannot be randomized, cluster with other risk behaviours and confounders, and lack effective interventions that could be used in trials that randomize the removal of the exposure. We find no clear evidence for the prevailing assumption that tobacco causally affects alcohol consumption, which has great implications for public health and intervention research. Interventions that target reductions in tobacco consumption may not necessarily also lead to any change in alcohol consumption, and interventions that seek to target both smoking and alcohol will need to incorporate active ingredients for each substance.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in ALSPAC, the midwives for their help with recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK MRC and the Wellcome Trust (grant ref. 102215/2/12/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors who will serve as guarantors for the contents of this paper. This research has been conducted using the UK Biobank Resource. The HUNT study is a collaborative effort of the HUNT Research Centre (Faculty of Medicine, NTNU, Norwegian University of Science and Technology), Nord-Trøndelag County Council, Central Norway Health Authority and the Norwegian Institute of Public Health. HUNT Research Centre provided the data.
Conflict of interest: A.T. is in receipt of a GRAND award from Pfizer for research that is unrelated to this manuscript.
Funding
The CGPS was financially supported by the Copenhagen County Foundation and Herlev Hospital, Copenhagen University Hospital. This research was specifically supported by a Wellcome Trust PhD Studentship awarded to M.T. (grant ref. 097088/Z/11/Z). M.R.M. and A.E.T. are members of the United Kingdom Centre for Tobacco and Alcohol Studies, a UKCRC Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council (MRC) and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. M.T., M.M., and A.E.T. work in the MRC Integrative Epidemiology Unit at the University of Bristol, which is supported by the MRC and the University of Bristol (grant ref. MC_UU_12013/6).
References
- 1. Chaloupka F, Warner K.. The economics of smoking In: Culter A, Newhouse JP (eds). Handbook of Health Economics. 1 Amsterdam: Elsevier Science, 2000, pp. 1539–627. [Google Scholar]
- 2. World Health Organisation. Global Status Report on Alcohol and Health. Geneva: World Health Organization, 2011, p. xxi. [Google Scholar]
- 3. Hanna EZ, Yi HY, Dufour MC, Whitmore CC.. The relationship of early-onset regular smoking to alcohol use, depression, illicit drug use, and other risky behaviors during early adolescence: results from the youth supplement to the Third National Health and Nutrition Examination Survey. J Subst Abuse 2001;13:265–82. [DOI] [PubMed] [Google Scholar]
- 4. Miller TR, Lestina DC, Smith GS.. Injury risk among medically identified alcohol and drug abusers. Alcoholism Clin Exp Res 2001;25:54–59. [PubMed] [Google Scholar]
- 5. Chen XG, Unger JB, Palmer P. et al. Prior cigarette smoking initiation predicting current alcohol use: evidence for a gateway drug effect among California adolescents from eleven ethnic groups. Addict Behav 2002;27:799–817. [DOI] [PubMed] [Google Scholar]
- 6. Wetzels JJ, Kremers SP, Vitoria PD, de Vries H.. The alcohol-tobacco relationship: a prospective study among adolescents in six European countries. Addiction 2003;98:1755–63. [DOI] [PubMed] [Google Scholar]
- 7. Sutherland I, Willner P.. Patterns of alcohol, cigarette and illicit drug use in English adolescents. Addiction 1998;93:1199–208. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki K, Kimura M, Takeda A, Matsushita S.. Is adolescent tobacco use a gateway drug to adult alcohol abuse? A Japanese longitudinal prospective study on adolescent drinking. Nihon Arukoru Yakubutsu Igakkai zasshi 2008;43:44–53. [PubMed] [Google Scholar]
- 9. Clark DB, Kirisci L, Moss HB.. Early adolescent gateway drug use in sons of fathers with substance use disorders. Addict Behav 1998;23:561–66. [DOI] [PubMed] [Google Scholar]
- 10. van Leeuwen AP, Verhulst FC, Reijneveld SA, Vollebergh WA, Ormel J, Huizink AC.. Can the gateway hypothesis, the common liability model and/or, the route of administration model predict initiation of cannabis use during adolescence? A survival analysis—the TRAILS study. J Adolesc Health 2011;48:73–78. [DOI] [PubMed] [Google Scholar]
- 11. Maldonado-Molina MM, Lanza ST.. A framework to examine gateway relations in drug use: an application of latent transition analysis. J Drug Issues 2010;40:901–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Faeh D, Viswanathan B, Chiolero A, Warren W, Bovet P.. Clustering of smoking, alcohol drinking and cannabis use in adolescents in a rapidly developing country. BMC Public Health 2006;6:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. John U, Meyer C, Rumpf HJ, Schumann A, Thyrian JR, Hapke U.. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol 2003;38:606–12. [DOI] [PubMed] [Google Scholar]
- 14. Picone GA, Sloan F, Trogdon JG.. The effect of the tobacco settlement and smoking bans on alcohol consumption. Health Econ 2004;13:1063–80. [DOI] [PubMed] [Google Scholar]
- 15. Hyland A, Hassan LM, Higbee C. et al. The impact of smokefree legislation in Scotland: results from the Scottish ITC: Scotland/UK longitudinal surveys. Eur J Public Health 2009;19:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foster JH, Ferguson CS.. Home drinking in the UK: trends and causes. Alcohol Alcohol 2012;47:355–58. [DOI] [PubMed] [Google Scholar]
- 17. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G.. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008;27:1133–63. [DOI] [PubMed] [Google Scholar]
- 18. Davey Smith G, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S.. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med 2007;4:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ware JJ. Exploring Associations between the Nicotinic Acetylcholine Receptor Gene Cluster CHRNA5-A3-B4 and Smoking-Related Behaviours. Cardiff, UK: Cardiff University, 2012. [Google Scholar]
- 20. Laura Jean Bierut M, Stitzel JA, Wang JC. et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 2008;165:1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caporaso N, Gu F, Chatterjee N. et al. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One 2009;4:e4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thorgeirsson TE, Gudbjartsson DF, Surakka I. et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet 2010;42:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breitling LP, Dahmen N, Mittelstrass K. et al. Smoking cessation and variations in nicotinic acetylcholine receptor subunits alpha-5, alpha-3, and beta-4 genes. Biol Psychiatry 2009;65:691–95. [DOI] [PubMed] [Google Scholar]
- 24. Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 2010;42:441–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thorgeirsson T, Geller F, Sulem P. et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 2008;452:638-U9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ware J, van den Bree M, Munafo M.. Association of the CHRNA5-A3-B4 gene cluster with heaviness of smoking: a meta-analysis. Nicotine Tob Res 2011;13:1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rode L, Bojesen SE, Weischer M, Nordestgaard BG.. High tobacco consumption is causally associated with increased all-cause mortality in a general population sample of 55,568 individuals, but not with short telomeres: a Mendelian randomization study. Int J Epidemiol 2014;43:1473–83. [DOI] [PubMed] [Google Scholar]
- 28. Asvold BO, Bjorngaard JH, Carslake D. et al. Causal associations of tobacco smoking with cardiovascular risk factors: a Mendelian randomization analysis of the HUNT Study in Norway. Int J Epidemiol 2014;43:1458–70. [DOI] [PubMed] [Google Scholar]
- 29. Taylor AE, Fluharty ME, Bjørngaard JH. et al. Investigating the possible causal association of smoking with depression and anxiety using Mendelian randomisation meta-analysis: the CARTA consortium. BMJ Open 2014;4:e006141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morris RW, Taylor AE, Fluharty ME. et al. Heavier smoking may lead to a relative increase in waist circumference: evidence for causal relationship from a Mendelian randomisation meta-analysis: the CARTA consortium. BMJ Open 2015;5:e008808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freathy RM, Kazeem GR, Morris RW. et al. Genetic variation at CHRNA5-CHRNA3-CHRNB4 interacts with smoking status to influence body mass index. Int J Epidemiol 2011;40:1617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis SJ, Araya R, Davey Smith G. et al. Smoking is associated with, but does not cause, depressed mood in pregnancy—a Mendelian randomization study. PloS One 2011;6:e21689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Linneberg A, Jacobsen RK, Skaaby T. et al. Effect of smoking on blood pressure and resting heart rate: a Mendelian randomization meta-analysis in the CARTA consortium. Circ Cardiovasc Genet 2015;8:832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taylor AE, Morris RW, Fluharty ME. et al. Stratification by smoking status reveals an association of CHRNA5-A3-B4 genotype with body mass index in never smokers. PLoS Genet 2014;10:e1004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Skaaby T, Taylor AE, Jacobsen RK. et al. Investigating the causal effect of smoking on hay fever and asthma: a Mendelian randomization meta-analysis in the CARTA consortium. Sci Rep-UK 2017;7:2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Golding J, Pembrey M, Jones R, Team AS.. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 2001;15:74–87. [DOI] [PubMed] [Google Scholar]
- 37. Fraser A, Macdonald-Wallis C, Tilling K. et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holmen J, Midthjell K, Krüger Ø. et al. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): objectives, contents, methods and participation. Norsk Epidemiologi 2003;13:19–32. [Google Scholar]
- 39. Krokstad S, Langhammer A, Hveem K. et al. Cohort profile: the HUNT study, Norway. Int J Epidemiol 2013;42:968–77. [DOI] [PubMed] [Google Scholar]
- 40. Collins R. What makes UK Biobank special? Lancet 2012;379:1173–74. [DOI] [PubMed] [Google Scholar]
- 41. Thorgeirsson TE, Gudbjartsson DF, Surakka I. et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet 2010;42:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caporaso N, Gu F, Chatterjee N. et al. Genome-wide and candidate gene association study of cigarette smoking behaviors. PloS One 2009;4:e4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Galobardes B, Shaw M, Lawlor DA, Lynch JW.. Indicators of socioeconomic position (part 2). J Epidemiol Community Health 2006;60:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodriguez S, Gaunt TR, Day IN.. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol 2009;169:505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taylor AE, Davies NM, Ware JJ, Vanderweele T, Smith GD, Munafo MR.. Mendelian randomization in health research: using appropriate genetic variants and avoiding biased estimates. Econ Human Biol 2013;13:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Munafò MR, Timofeeva MN, Morris RW. et al. Association between genetic variants on chromosome 15q25 locus and objective measures of tobacco exposure. J Natl Cancer Inst 2012;104:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deeks JJ, Altman DG, Bradburn MJ.. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis In: Egger M, Davey Smith G, and Altman DG (eds), Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd edn. New Jersey: John Wiley & Sons, 2008, pp. 285–312. [Google Scholar]
- 48. StataCorp. Stata Statistical Software: Release 13, 2013.
- 49. Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JAC.. Metan: fixed- and random-effects meta-analysis. Stata J 2008;8:3–28. [Google Scholar]
- 50. Gauderman WJ. Sample size requirements for matched case-control studies of gene-environment interaction. Stat Med 2002;21:35–50. [DOI] [PubMed] [Google Scholar]
- 51. Davies NM, Smith GD, Windmeijer F, Martin RM.. Issues in the reporting and conduct of instrumental variable studies: a systematic review. Epidemiology 2013;24:363–69. [DOI] [PubMed] [Google Scholar]
- 52. Swanson SA, Hernán MA.. Commentary: how to report instrumental variable analyses (suggestions welcome). Epidemiology 2013;24:370–74. [DOI] [PubMed] [Google Scholar]
- 53. Vrieze SI, McGue M, Iacono WG.. The interplay of genes and adolescent development in substance use disorders: leveraging findings from GWAS meta-analyses to test developmental hypotheses about nicotine consumption. Hum Genet 2012;131:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vink JM, Hottenga JJ, de Geus EJ. et al. Polygenic risk scores for smoking: predictors for alcohol and cannabis use? Addiction 2014;109:1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Irons DE, McGue M, Iacono WG, Oetting WS.. Mendelian randomization: a novel test of the gateway hypothesis and models of gene-environment interplay. Dev Psychopathol 2007;19:1181–95. [DOI] [PubMed] [Google Scholar]
- 56. Vartiainen E, Seppala T, Lillsunde P, Puska P.. Validation of self reported smoking by serum cotinine measurement in a community-based study. J Epidemiol Community Health 2002;56:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lees R, Kingston R, Williams TM, Henderson G, Lingford-Hughes A, Hickman M.. Comparison of ethyl glucuronide in hair with self-reported alcohol consumption. Alcohol Alcohol 2012;47:267–72. [DOI] [PubMed] [Google Scholar]
- 58. Taylor AE, Munafò MR.. Commentary: Does mortality from smoking have implications for future Mendelian randomization studies? Int J Epidemiol 2014;43:1483–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Munafò MR, Tilling K, Taylor AE, Evans DM, Davey Smith G.. Collider scope: when selection bias can substantially influence observed associations. Int J Epidemiol 2018;47:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.