Why was the cohort set up?
By the late 1980s, it was clear that allergic diseases, including asthma, eczema, allergic rhinitis and food allergy, had been increasing in recent decades, but the extent of this increase and true prevalence in an unselected population had not been estimated. It was also known that allergic diseases may remit and relapse. So one disease may improve as the child grows, but another may take its place; this pattern was termed ‘atopic or allergic march’. However, the extent and the true nature of this transition were not clear. Additionally, little was known why allergic diseases are increasing, and even less about why allergic disease often remits as the child grows but sometimes relapses in adolescent or adult life. We therefore established the Isle of Wight Birth Cohort (IOWBC) 28 years ago, in January 1989.
The Isle of Wight is an island off the south coast of England, with a resident population of approximately 130 000. The environment is semirural with no heavy industry, and the local economy largely relies on tourism.
The focus of the IOWBC is to:
assess prevalence of allergic sensitization and clinical allergic manifestations in an unselected population during childhood and early adult life;
explore the natural history of allergic sensitization and clinical allergic manifestations from infancy to early adult life;
define the heterogeneity of asthma and allergic diseases across the life course;
identify predictive markers for asthma and allergy development that might guide future disease prevention or treatment measures;
develop novel therapeutic interventions;
identify environmental risk factors relevant to asthma and allergic diseases;
investigate genes, gene-environmental interactions and epigenetic mechanisms in the development of asthma and other allergic diseases.
The IOWBC was originally established with support from the local (Isle of Wight) Health Authority, which helped establish the cohort and the assessments at ages 1, 2 and 4 years. A grant from the charity ‘Asthma UK’ allowed assessment at age 10 years. Further funding from the National Institutes of Health (US) and the Medical Research Council (UK) has supported the cohort up to the most recent assessment at 26 years.
Who is in the cohort?
Recruitment
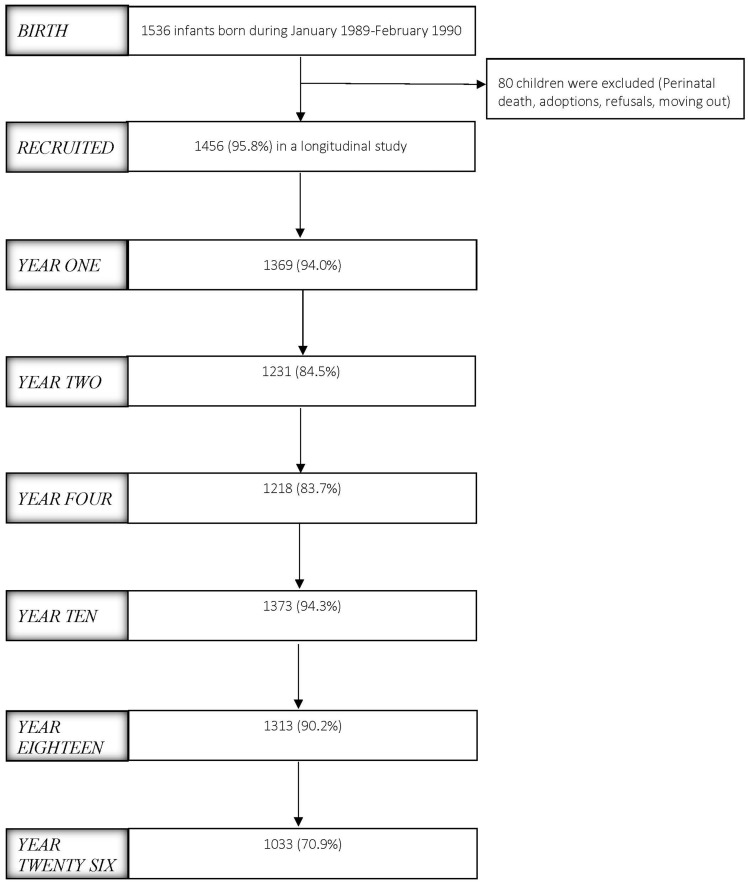
The IOWBC is a single-centre study designed to represent the community population. All children born on the Isle of Wight in a defined period were eligible for inclusion. Ethics approval was obtained from the local/national ethics committees at recruitment of the birth cohort between January 1989 and February 1990, and subsequently at each assessment. The cohort was recruited through the 1509 women who gave birth to 1536 children on the IOW during the recruitment period. All 1509 mothers were recruited and consented to complete questionnaires and provide samples soon after birth. Parental consent was obtained from 1456 of the 1536 children for inclusion into a longitudinal study of asthma and allergic disease (Figure 1).
Figure 1.
Flow diagram of study progress.
How often have they been followed up?
The children in the IOWBC have been seen on six occasions over the course of 26 years, at 1, 2, 4, 10, 18 and 26 years (Table 1). Extremely high retention rates have been obtained at all time points. Cohort particpants who attended or did not attend at various assessments were compared for information collected at birth, when parents of all 1536 infants responded and provided basic information on family history of allergy, birthweight, social status and exposures to pets and smoking (Table 2).
Table 1.
Information and samples collected from the Isle of Wight birth cohort
| Variable | Birth | Year 1 | Year 2 | Year 4 | Year 10 | Year 18 | Year 26 |
|---|---|---|---|---|---|---|---|
| Family history of asthma, eczema and rhinitis | x | x | x | x | x | x | x |
| Pregnancy complications and birth characteristics | x | – | – | – | – | – | – |
| Mode of feeding | – | x | x | – | – | – | – |
| Household pets | x | x | x | x | x | x | x |
| Socioeconomic status | x | x | x | x | x | x | x |
| Exposure to smoking | x | x | x | x | x | x | x |
| Height, weight, BMI | x | x | x | x | x | x | x |
| Housing characteristics | – | – | x | x | x | x | x |
| Wheeze and asthma | – | x | x | x | x | x | x |
| Nasal symptoms | – | x | x | x | x | x | x |
| Respiratory infections | – | x | x | x | x | x | x |
| Allergic symptoms | – | x | x | x | x | x | x |
| Eczema | x | x | x | x | x | x | |
| Food allergy | x | x | x | x | x | x | |
| Treatment and medications | – | x | x | x | x | x | x |
| Skin prick test | – | x | x | x | x | x | x |
| Total and specific IgEa | x | – | – | – | x | x | x |
| Spirometry and bronchodilator reversibility (FEV1, FVC, FEF25-75) | – | – | – | – | x | x | x |
| Bronchial provocation test (PC20 and methacholine and dose-response) | – | – | – | – | x | x | x |
| Exhaled nitric oxide | – | – | – | – | – | x | x |
| Urinary cotinine | – | – | – | – | x | x | x |
| Genome-wide genotyping | – | – | – | – | x | – | – |
| Genome-wide DNA-methylationb | x | – | – | – | x | x | – |
FVC, forced vital capacity; FEF, forced expiratory flow.
Using maternal and cord blood.
Using Guthrie cards collected at day 7.
Table 2.
Comparison of participants who attended or did not attend at various assessments with regard to cohort characteristics and other information collected at birth
| 1 year |
2 years |
4 years |
10 years |
18 years |
26 years |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attended (n = 1369)a | Not attend (n = 167) | Attended (n = 1231) | Not attend (n = 305) | Attended (n = 1218) | Not attend (n = 318) | Attended (n = 1373) | Not attend (n = 163) | Attended (n = 1313) | Not attend (n = 213) | Attended (n = 1033) | Not attend (n = 503) | |
| Male gender | 697/1369 (50.9%) | 90/167 (53.9%) | 622/1231 (50.5%) | 165/305 (54.1%) | 621/1218 (51.0%) | 166/318 (52.2%) | 698/1373 (50.8%) | 89/163 (54.6%) | 654/1313 (49.8%)* | 133/223 (59.6%) | 473/1033 (45.8%)* | 314/503 (62.4%) |
| Low birthweight (< 2.5 kg) | 52/1347 (3.9%)* | 14/165 (8.5%) | 42/1210 (3.5%) | 24/302 (7.9%) | 45/1198 (3.8%)* | 21/314 (6.7%) | 53/1351 (3.9%)* | 13/161 (8.1%) | 48/1294 (3.7%)* | 18/218 (8.3%) | 40/1019 (3.9%) | 26/493 (5.3%) |
| Maternal asthma | 150/1362 (11.2%) | 15/155 (9.7%) | 129/1228 (10.5%) | 36/289 (12.5%) | 126/1211 (10.4%) | 39/306 (12.7%) | 143/1364 (10.5%) | 22/153 (14.4%) | 138/1304 (10.6%) | 27/213 (12.7%) | 112/1025 (10.9%) | 53/492 (10.8%) |
| Paternal asthma | 134/1354 (9.9%) | 15/150 (10.0%) | 115/1222 (9.4%) | 34/282 (12.1%) | 117/1208 (9.7%) | 32/296 (10.8%) | 131/1356 (9.7%) | 18/148 (12.2%) | 128/1296 (9.9%) | 21/208 (10.1%) | 98/1019 (9.6%) | 51/485 (10.5%) |
| Sibling asthma | 93/805 (11.6%) | 13/79 (16.5%) | 78/722 (10.8%)* | 28/162 (17.3%) | 73/712 (10.3%)* | 33/172 (19.2%) | 88/800 (11.0%)* | 18/84 (21.4%) | 88/756 (11.6%) | 18/128 (14.1%) | 70/602 (11.6%) | 36/282 (12.8%) |
| Maternal eczema | 166/1359 (12.2%) | 17/155 (11.0%) | 150/1225 (12.2%) | 33/289 (11.4%) | 145/1208 (12.0%) | 38/306 (12.4%) | 162/1361 (11.9%) | 21/153 (13.7%) | 161/1301 (12.4%) | 22/213 (10.3%) | 125/1023 (12.2%) | 58/491 (11.8%) |
| Paternal eczema | 86/1352 (6.4%) | 11/152 (7.2%) | 80/1220 (6.6%) | 17/284 (6.0%) | 78/1206 (6.5%) | 19/298 (6.4%) | 89/1355 (6.6%) | 8/149 (5.4%) | 90/1294 (7.0%)* | 7/210 (3.3%) | 74/1017 (7.3%) | 23/487 (4.7%) |
| Sibling eczema | 192/801 (24.0%) | 14/79 (17.7%) | 168/718 (23.4%) | 38/162 (23.5%) | 167/708 (23.6) | 39/172 (22.7%) | 195/796 (24.5%) | 11/84 (13.1%) | 181/752 (24.1%) | 25/128 (19.5%) | 136/599 (22.7%) | 70/281 (24.9%) |
| Maternal rhinitis | 278/1362 (20.4%) | 27/155 (17.4%) | 246/1228 (20.0%) | 59/289 (20.4%) | 257/1211 (21.2%)* | 48/306 (15.7%) | 270/1364 (19.8%) | 35/153 (22.9%) | 262/1304 (20.1%) | 43/213 (20.2%) | 210/1025 (20.5%) | 95/492 (19.3%) |
| Paternal rhinitis | 202/1353 (14.9%) | 18/152 (11.8%) | 179/1222 (14.6%) | 41/283 (14.5%) | 177/1207 (14.7%) | 43/298 (14.4%) | 201/1356 (14.8%) | 19/149 (12.8%) | 189/1296 (14.6%) | 31/209 (14.8%) | 151/1018 (14.8%) | 69/487 (14.2%) |
| Sibling rhinitis | 58/801 (7.2%) | 3/79 (3.8%) | 50/719 (7.0%) | 11/161 (6.8%) | 50/708 (7.1%) | 11/172 (6.4%) | 55/796 (6.9%) | 6/84 (7.1%) | 55/752 (7.3%) | 6/128 (4.7%) | 38/599 (6.3%) | 23/281 (8.2%) |
| Maternal smoking | 345/1354 (25.5%) | 48/155 (31.0%) | 295/1221 (24.2%)* | 98/288 (34.0%) | 257/1206 (21.3%)* | 136/303 (44.9%) | 325/1357 (23.9%)* | 68/152 (44.7%) | 311/1298 (24.0%)* | 82/211 (38.9%) | 233/1020 (22.8%)* | 160/489 (32.7%) |
| Paternal smoking | 539/1353 (39.8%) | 69/150 (46.0%) | 476/1221 (39.0%)* | 132/282 (46.8%) | 437/1207 (36.2%)* | 171/296 (57.8%) | 523/1355 (38.6%)* | 85/148 (57.4%) | 503/1296 (38.8%)* | 105/207 (50.7%) | 384/1018 (37.7%)* | 224/485 (46.2%) |
| Cat ownership | 452/1361 (33.2%) | 42/153 (27.5%) | 411/1227 (33.5%) | 83/287 (28.9%) | 403/1211 (33.3%) | 91/303 (30.0%) | 454/1364 (33.3%) | 40/150 (26.7%) | 434/1303 (33.3%) | 60/211 (28.4%) | 340/1023 (33.2%) | 154/491 (31.4%) |
| Dog ownership | 403/1361 (29.6%) | 38/153 (24.8%) | 363/1227 (29.6%) | 78/287 (27.2%) | 356/1211 (29.4%) | 85/303 (28.1%) | 396/1364 (29.0%) | 45/150 (30.0%) | 376/1303 (28.9%) | 65/211 (30.8%) | 293/1023 (28.6%) | 148/491 (30.1%) |
| Social class I–III | 441/799 (55.2%) | 45/91 (49.5%) | 319/715 (44.6%) | 85/175 (48.6%) | 347/721 (48.1%) | 57/169 (33.7%) | 376/811 (46.4%) | 28/79 (35.4%) | 357/779 (45.8%) | 47/111 (42.3%) | 292/601 (48.6%)* | 112/289 (38.8%) |
| Cord IgE > 0.5 | 129/1000 (12.9%) | 10/64 (15.6%) | 118/928 (12.7%) | 21/136 (15.4%) | 139/1064 (13.1%) | 0/152 (0%)* | 130/1016 (12.8%) | 9/48 (18.8%) | 122/973 (12.5%) | 17/91 (18.7%) | 96/756 (12.7%) | 43/308 (14.0%) |
Attended n = denotes maximum number of children who provided any information at a given assessment. There was some missing information depending on the specific question, with the details provided for each in the rows below.
Where differences were found to be statistically significant between those who attended and did not attend defined as P < 0.05.
What has been assessed and/or measured?
Hospital records were used to gather information on maternal height and weight at week 14 (± 4) of gestation, pregnancy characteristics and complications and birth characteristics. Samples of maternal blood and cord blood at birth, and the child’s blood from a heel prick at 7 days of age, were collected on Guthrie cards.
Questionnaires, both study-specific and standardized questionnaires (International Study of Asthma and Allergic Diseases in Childhood from 10 years onwards when these became available) seeking information on asthma and allergy status and common environmental exposures, were completed by most participants or their parents at various assessments throughout childhood and early adult life (Table 3). Mothers completed a questionnaire soon after the birth of their children (Table 4); and at 1 and 2 years, children were seen by a doctor, nurse or health visitor and a questionnaire completed. If parents reported any allergy-related symptoms in their child, they were asked to attend the clinic for a visit when examination and allergy skin prick tests (SPT) were carried out. Physical examination at all assessments included height, weight and signs of allergic diseases such as wheeze and eczema. At 4, 10, 18 and 26 years, all participants were invited to attend the research centre for an assessment, which included questionnaire, physical examination and skin prick test. At 10, 18 and 26 years, spirometry and bronchial provocation tests were carried out and blood and urine samples were collected. Exhaled nitric oxide was measured at 18 and 26 years. A subgroup of children were invited for sputum induction at 10 and 18 years. At all ages, those who could not attend the centre for a personal visit were asked to complete a telephone or postal questionnaire. At 26 years online questionnaires were first introduced, in addition to telephone and postal questionnaires, to achieve optimal participation. Where possible, participants were asked to give permission for access to their medical records which provided more accurate data regarding physician diagnosis and treatments received.
Table 3.
Procedures conducted in the Isle of Wight cohort (n)
| Variable | Birth | Year 1 | Year 2 | Year 4 | Year 10 | Year 18 | Year 26 |
|---|---|---|---|---|---|---|---|
| Questionnaires | 1536 | 1369 | 1231 | 1218 | 1373 | 1313 | 1033 |
| Physical examination | – | 323 | 410 | 977 | 1036 | 864 | 544 |
| Height/weight | 1501 | 1090 | 399 | 1053 | 1043 | 964 | 681 |
| Skin prick tests | – | 323 | 410 | 977 | 1036 | 851 | 556 |
| Spirometry | – | – | – | – | 980 | 839 | 544 |
| Bronchial provocation tests | – | – | – | – | 784 | 586 | Ongoing |
| Sputum induction | – | – | – | – | 25 | 100 | – |
| Exhaled nitric oxide | – | – | – | – | – | 822 | 542 |
| Blood samples |
|
– | – | – | 950 | 550 | 503 |
| Urine | – | – | – | – | 970 | 650 | 528 |
| Saliva | – | – | – | – | 850 | 500 | 22 |
Table 4.
Demographic characteristics of the parents of the Isle of Wight Birth Cohort particiants (recorded at recruitment)
| Mothers | Fathers | |
|---|---|---|
| Mean age (years) | 27.25 | – |
| Smoking (n) | 393 | 608 |
| Cat ownership (n) | 494 | – |
| Dog ownership (n) | 442 | – |
| Asthma (n) | 165 | 149 |
| Hay fever (n) | 305 | 220 |
| Eczema (n) | 183 | 97 |
| Food allergy (n) | 71 | 38 |
| Any allergic disease (n) | 528 | 388 |
Serum total immunoglobulin E (IgE) was measured at birth (in the cord serum), and again at 10 and 18 years. Specific IgE screens for aero and food allergens were carried out at 10 and 18 years. Serum leptin and urinary cotinine were measured at 10 and 18 years. In a subgroup, a panel of cytokines was measured at 10 and 18 years. Genome-wide genotyping is being carried out currently and data will be available shortly. Genome-wide epigenotyping with DNA methylation was carried out in a subgroup of participants in whole-blood-derived DNA collected at 18 years and is now being extended to all participants with blood samples available at birth (using Guthrie cards) and at 10 and 18 years.
What has it found?
Nearly 100 original articles have been published, describing prevalence, natural history and genetic and environmental risk factors for asthma and allergic diseases up to 18 years of age, and data collected at the age of 26 years are currently being analysed. A list of publications arising from the IOWBC can be found at [http://www.allergyresearch.org.uk].
Prevalence
We described the population prevalence of allergic disorders at various ages in this unselected birth cohort. The prevalence was generally described as period prevalence, i.e. in the past 12 months, at each assessment. We used study-specific questionnaires in the first three assessments, and later repeated these questionnaires also using standardized International Study of Asthma and Allergy in Childhood (ISAAC) questionnaires1 which had become available between the 4- and 10-year follow-ups. The overall prevalence of one or more allergic diseases varied from ∼25% in the first 2 years to 40% at 4 and 50% by the age of 18 years.2–6
Asthma
Reported asthma at 1, 2 and 4 years, defined as recurrent wheezing, increased from 8.7% at 1 year to 14.9% at 4 years.2,4,7 At ages 10, 18 and 26 years, we characterized cohort children extensively for asthma and allergic diseases using standardized questionnaires, lung function, bronchial provocation tests, and sputum induction.5,6,8–11 We also described wheezing phenotypes during the first 10 years of life, identifying that more severe disease had an early onset and could be distinguished from more transient disease using risk scoring systems.8,9,12,13 We investigated early life risk factors for the development of asthma and bronchial hyper-responsiveness (BHR) during later childhood10,13 and how these factors influence symptom expression in those with BHR10 and induce earlier onset of disease.14
Nearly 5% of adolescents reported wheezing in the absence of diagnosed asthma and showed few pathophysiological hallmarks of asthma. This ‘undiagnosed wheeze’ phenotype was associated with smoking and paracetamol use.15 Applying cluster analysis methods on IOWBC data, we have defined wheeze and rhinitis clusters to explore phenotypes of these conditions using adolescents.16,17 By 18 years, severe asthma clusters with evidence of impaired lung function, high morbidity and higher smoking prevalence were identifiable.
Allergic rhinitis
Rhinitis was defined as nasal and/or eye symptoms of sneezing, rhinorrhoea, nasal blockage and streaming/itchy eyes when not having a ‘cold’ or respiratory infection. At 1 and 2 years, the prevalence was low (∼3%) but gradually increased so that by 26 years it had reached 42%.2–4,7,16,18–20
Atopic dermatitis
Atopic dermatitis, using modified Hannifin and Rajka definition, was approximately 10% during early childhood.2–4,7,21
Peanut allergy
We were among the first to describe the prevalence of peanut allergy in 4-year-old children in the IOWBC.22 More recently we described the natural history of peanut allergy over the first 18 years of life.23 Subsequently, the prevalence rates in the IOWBC were compared with another cohort of children of the same age, born a few years later on the IOW and assessed for peanut allergy. This showed that sensitization increased 3-fold and clinical allergy to peanut doubled during the 1990s.24 In early 2000, we recruited another birth cohort on the IOW (Food Allergy and Intolerance Research cohort) and assessed children for food allergy during early childhood. Therefore, we were able to compare prevalence in these three sequential cohorts of children, all aged 3–4 years but born 5–6 years apart. We found that after the initial rise in 1990s, the peanut prevalence in the UK stabilized during the past decade.25
Allergic sensitization
The relationship of allergic sensitization, asthma and allergic disease was investigated from the ages of 4 to 26 years.4,12,26–28 Atopy (SPT positive to any allergen) was 29% at 4 years.4 A strong relationship was found between allergic diseases such as asthma with house dust mite, allergic rhinitis with grass pollen, and eczema with egg.7 Various childhood atopic phenotypes were described and their relationships with wheeze and asthma were defined. The population-attributable risk of atopy for asthma was 44%, for rhinitis it was 46% and for eczema, 32%.2 We recently showed that fractional exhaled nitric oxide (FeNO) is associated with atopy and atopic asthma, but not with non-atopic asthma. This has implications for the use of FeNO for the diagnosis and management of asthma.27
Natural history
Overall, the prevalence of wheeze and asthma has continued to rise from early childhood to early adult life. However, there was fluidity such that a proportion of children who had wheeze at one follow-up were not wheezing at the next, but other non-wheezing children had acquired wheeze, so that the trend of period prevalence remained upwards. The remission, relapse and new onset (in those who were previously disease free) was also seen in other allergic manifestations including eczema, rhinitis, food allergy and allergic sensitization.20,23,29–32 However, the net trend for asthma and rhinitis was generally upwards, whereas for eczema and food allergy there was a relatively high prevalence in early childhood followed by overall stable figures of around 10-15% for eczema and 1–3% for food allergy.
We were among the first to report that children with egg allergy in infancy have a 5–6-fold increased risk of acquiring aeroallergen sensitization and respiratory symptoms by age 4,33 thus shifting the allergic phenotype from food allergy to aeroallergen sensitization with associated asthma and rhinitis.
Risk factors for allergic diseases
Sex
Boys suffered from asthma, eczema, rhinitis and atopy (allergic sensitization) more than girls throughout childhood and early adult life (up to age 26 years). For asthma, a gender reversal occurs during adolescence; thus at age 18 girls had more asthma than boys.2,4,6–8,20,21,31,34
Parental allergy
As expected, parental asthma and allergy had a consistent effect on childhood asthma and eczema over the entire childhood and adolescent period,2,3,6,7,18,21,34–36 with some disease specificity such that parental asthma increased the risk of asthma more than of eczema or rhinitis.4 We also showed that the risk is sex specific, such that boys had higher risk of asthma when their fathers were affected by asthma and girls had a higher risk when mothers were diagnosed with asthma.35
Breastfeeding and asthma
The effect of breastfeeding on asthma remains controversial. We showed that breastfeeding for at least 3 months protects against early childhood wheezing, 3,7 possibly as a result of attenuating the adverse effect of respiratory infections and maternal smoking on asthma.37–39 We also showed that breastfeeding is associated with better lung function at 10 and 18 years of age.38,40 However, the effect on allergic diseases in later childhood and adolescence was less clear.41 Our data suggest that some of the conflicting results on method of feeding and allergy may be due to reverse causation.42
Low birthweight
Low birthweight was shown to be a risk factor for asthma, atopy and lung function.34,36,39,43–45
Exposure to smoking
We demonstrated the adverse effects of maternal smoking exposure on the developing fetus, with increased risk of wheeze and nasal symptoms during infancy,2,7,18,34,46 and of active smoking on lung health during adolescence.6,15 Maternal smoking also had an effect on eczema at age 4 years.46 The genetic susceptibility and epigenetic mechanisms mediating this susceptibility have been studied (see below). These findings have paved the way to identifying susceptible smokers at risk of future chronic obstructive pulmonary disease (COPD).
Lower socioeconomic group
Children among the lower socioeconomic group had a higher level of infant wheezing, even after adjusting for confounding factors such as maternal smoking and lack of breastfeeding.2,3,7
Presence of pets
We have not found an effect of exposure to furry pets on asthma or other allergic diseases at any age.2,3,18,21,34,47 A similar conclusion was reached in a meta-analysis of data from various European birth cohorts including the Isle of Wight.48
Season of birth
We have found effect of season of birth, with a higher level of asthma and rhinitis during the summer.2,3,7,49 Autumn births were associated with rhinitis, and autumn and winter combined had more eczema.
Cord and maternal IgE
The presence of cord IgE was associated with maternal IgE36 and increased the risk of allergic sensitization at 4 years.3 We also showed that IgE at birth (cord IgE) decreases with increasing birth order.50 This provides an alternative explanation to the hygiene hypothesis for the lower incidence of allergic disease observed in younger siblings, as it seems they are born with lower cord IgE. We subsequently showed that this effect on children may be transmitted from mothers, as their IgE also decreases with the increasing number of children they have delivered.51 We demonstrated that the birth order effect is dependent on genetic susceptibility. An interaction between an IL13 gene single nucleotide polymorphism (SNP; rs20541) and birth order was found, whereby the effect of this SNP on skin test (ages 4 to 18), total IgE (age 10), and inhalant screen (age 10) was restricted to first-born children.52
Predictors
Using longitudinal and repeated assessments of allergic disease in our birth cohort and available information on risk factors and biomarkers, we attempted to identify predictive markers for asthma and allergy.13,33,53,54 We initially focused on cord blood IgE, and found that an elevated cord IgE increases the risk of allergic sensitization during childhood.53,55 Although it did not increase the risk of respiratory symptoms in early childhood, it did increase the risk of asthma at age 10.55 However, the sensitivity of cord IgE was too low to be used as a predictive marker for allergic disease.53,54 Another important issue in paediatric allergy is the outcome of infant wheeze and its relationship with later childhood asthma. We developed predictive scores, based on a set of four risk factors (maternal asthma, allergic sensitization, recurrent chest infections and absence of nasal symptoms). Among children with a risk score of 4, 83% persisted with their wheeze, whereas of those with a risk score of zero, 80% went into remission.13 Egg allergy combined with eczema during infancy had a high (> 80%) positive predictive value for allergic sensitization and respiratory symptoms.33
Genetics
We identified a novel gene (ATPAF1) association with childhood asthma, using a genome-wide approach on pooled DNA.56 Cohort data were also used to identify a novel gene regulating neutrophil function, which is responsible for severity in cystic fibrosis.57 Using a candidate gene approach, we investigated the association of IL13 with cord IgE and atopic eczema.58,59 We have also demonstrated that filaggrin loss-of-function mutations contribute to allergic comorbidity including food allergy.60–63 Gene-gene interaction between GATA3 and STAT6 with IL13 on rhinitis and eczema, respectively, was demonstrated.64,65
Exposure to tobacco smoke increases the risk of wheeze and lung function deficit. We have shown the interaction of pre- and postnatal smoking exposure with genetic polymorphisms in genes encoding IL-13, IL-1R antagonist and GSTM2-5, on development of asthma and lung function.66–68 Exposures related to birth order modified the effect of IL13 polymorphism on allergic sensitization, whereas filaggrin loss-of-function mutations modified the effect of breastfeeding on eczema.52,69
Epigenetics
We have explored epigenetic mechanisms using genome-wide DNA methylation.49,70–82 Common environmental exposures such as smoking alter epigenetic profile, which in turn is shown to be associated with the risk of allergic diseases (Table 5). Interestingly, tetanus vaccination between 10 and 18 years was related to differential methylation, which in turn reduced the risk of asthma at 18 years.79 Using a two-stage model we showed that genetic variants in combination with living conditions, for instance use of oral contraceptives in girls, may change the DNA methylation, which in turn modifies the genetic associations related to asthma.82 The interaction of DNA methylation with genetic variants was demonstrated for a number of allergic markers and diseases.70–73,77
Table 5.
Epigenetic associations identified in the Isle of Wight Birth Cohort participants
| Main CpG site/s | Genes | Exposure | Outcomes | Reference |
|---|---|---|---|---|
|
|
– | On asthma risk and temporal asthma transition | Zhang et al. Clin Epigenet 2014;6:8 |
| cg07548383 | FLG | – | Eczema | Ziyab. J Eur Acad Dermatol Venereol 2012;7:e32721 |
| cg09791102 | IL-4R | – | Asthma at age 18 years | Soto-Ramirez et al. Clin Epigenet 2013;5:1 |
| cg00666422 | LEP | – | Lung function and asthma | Mukherjee. Int J Mol Epidemiol Genet 2016;7:1–17 |
|
|
– | Eczema | Quraishi et al. Clin Epigenet 2015;7:68 |
|
|
– | Atopy and high serum IgE | Everson. Genome Med 2015;7:89 |
|
LEPR/LEPROT | Smoking | Serum pleptin/BMI | Yousefi et al. Int J Mol Epidemiol Genet 2013;4:86–100 |
| cg13566430 | IL-13 | Smoking | Asthma | Patil et al. Clin Epigenet 2013;5:22 |
|
|
Season of birth | Allergic disease | Lockett. Allergy 2016. Mar 12. doi: 10.1111/all.12882 |
| cg05575921 | AHRR | Maternal smoking | – | Joubert. Am J Hum Genet 2016;98:680–96 |
|
KIAA1549L PSMG3, TFAMP1 | Tetanus vaccination | Asthma | Janjanam et al. Vaccine 2016;34:6493–501 |
| CpG islands | NHP2L1, WRB and PPIEL | – | – | Docherty et al. J Med Genet 2014;51:229–38 |
What are the main strengths and weaknesses?
Strengths
The IOWBC is an unselected whole-population cohort that truly represents the community from which the cohort is drawn. There has been a high retention rate of over 70% throughout, with availability of information and samples from the parental generation, comprehensive assessment that covered not only asthma but all chronic allergic conditions, prospective and extensive phenotyping, and genome-wide (epi)genotyping.
Weaknesses
Despite reletively high retention, some self-selection was observed in chidren who attended at various assessments (Table 2). For instance at 18 and 26 years, girls attended more than boys and children who were assessed tended to have a lower proportion of parental smoking and low (< 2.5 kg) birthweight. However, as the follow-up rates were consistently high (80-90%) and imbalances were few, this reletively modest selection bias does not affect the validity or generalizability of the findings. The Isle of Wight is a relatively small island (∼20 miles across) and therefore there is a lack of diversity, both in terms of environment (no industrial exposure) and race (> 90% Caucasian), hence raising potential questions regarding generalizability of findings. The population is, however, not genetically inbred and there is frequent movement of people from mainland England.
Can I get hold of the data? Where can I find out more?
The cohort profile is available on [www.allergyresearch.org.uk]. We encourage collaboration to maximize the use of data and samples. We are in the process of finalizing details of how the data can be accessed and the process of submitting an application to access the data. Please contact Mr Stephen Potter [stephen.potter@iow.nhs.uk].
Profile in a nutshell
IOWBC is a whole-population prospective, observational study investigating prevalence, natural history and risk and protective factors for the development of asthma and allergic diseases.
All children (n = 1536) born on the Isle of Wight between 1 January 1989 and 28 February 1990 were enrolled, with 1456 consenting for long-term follow-up.
Participants have been assessed six times since birth, with a high (> 70%) retention of the cohort participants.
A wide range of phenotypic and environmental information has been collected using questionnaires and hospital medical records, study procedures and genetic and epigenetic assessments; and over 10 000 biological samples have been collected.
Funding
Recruitment and initial assessment for the first 4 years of age was supported by the Isle of Wight Health Authority. The 10-year follow-up of this study was funded by the National Asthma Campaign, UK (Grant No 364) and the 18-year follow-up by NIH/NHLBI R01 HL082925-01. The 21 year assessment was funded by Medical Research Council, UK (G23369). The 26 years assessment was supported by The David Hide Asthma & Allergy Research Trust.
Conflict of interest: None declared.
References
- 1. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 1998;351:1225–32. [PubMed] [Google Scholar]
- 2. Arshad SH, Stevens M, Hide DW.. The effect of genetic and environmental factors on the prevalence of allergic disorders at the age of two years. Clin Exp Allergy 1993;23:504–11. [DOI] [PubMed] [Google Scholar]
- 3. Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW.. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. J Allergy Clin Immunol 1998;101:587–93. [DOI] [PubMed] [Google Scholar]
- 4. Arshad SH, Tariq SM, Matthews S, Hakim E.. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics 2001;108:E33. [DOI] [PubMed] [Google Scholar]
- 5. Kurukulaaratchy RJ, Fenn M, Twiselton R, Matthews S, Arshad SH.. The prevalence of asthma and wheezing illnesses amongst 10-year-old schoolchildren. Respir Med 2002;96:163–69. [DOI] [PubMed] [Google Scholar]
- 6. Kurukulaaratchy RJ, Raza A, Scott M. et al. Characterisation of asthma that develops during adolescence; findings from the Isle of Wight Birth Cohort. Respir Med 2012;106:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arshad SH, Hide DW.. Effect of environmental factors on the development of allergic disorders in infancy. J Allergy Clin Immunol 1992;90:235–41. [DOI] [PubMed] [Google Scholar]
- 8. Kurukulaaratchy RJ, Fenn MH, Waterhouse LM, Matthews SM, Holgate ST, Arshad SH.. Characterization of wheezing phenotypes in the first 10 years of life. Clin Exp Allergy 2003;33:573–78. [DOI] [PubMed] [Google Scholar]
- 9. Kurukulaaratchy RJ, Fenn M, Matthews S, Arshad SH.. Characterisation of atopic and non-atopic wheeze in 10 year old children. Thorax 2004;59:563–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurukulaaratchy RJ, Matthews S, Waterhouse L, Arshad SH.. Factors influencing symptom expression in children with bronchial hyperresponsiveness at 10 years of age. J Allergy Clin Immunol 2003;112:311–16. [DOI] [PubMed] [Google Scholar]
- 11. Arshad SH, Raza A, Lau L. et al. Pathophysiological characterization of asthma transitions across adolescence. Respir Res 2014;15:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurukulaaratchy RJ, Matthews S, Arshad SH.. Relationship between childhood atopy and wheeze: what mediates wheezing in atopic phenotypes? Ann Allergy Asthma Immunol 2006;97:84–91. [DOI] [PubMed] [Google Scholar]
- 13. Kurukulaaratchy RJ, Matthews S, Holgate ST, Arshad SH.. Predicting persistent disease among children who wheeze during early life. Eur Respir J 2003;22:767–71. [DOI] [PubMed] [Google Scholar]
- 14. Kurukulaaratchy RJ, Matthews S, Arshad SH.. Does environment mediate earlier onset of the persistent childhood asthma phenotype? Pediatrics 2004;113:345–50. [DOI] [PubMed] [Google Scholar]
- 15. Raza A, Kurukulaaratchy RJ, Grundy JD. et al. What does adolescent undiagnosed wheeze represent? Findings from the Isle of Wight Cohort. Eur Respir J 2012;40:580–88. [DOI] [PubMed] [Google Scholar]
- 16. Kurukulaaratchy RJ, Zhang H, Patil V. et al. Identifying the heterogeneity of young adult rhinitis through cluster analysis in the Isle of Wight birth cohort. J Allergy Clin Immunol 2015;135:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurukulaaratchy RJ, Zhang H, Raza A. et al. The diversity of young adult wheeze: a cluster analysis in a longitudinal birth cohort. Clin Exp Allergy 2014;44:724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arshad SH, Kurukulaaratchy RJ, Fenn M, Waterhouse L, Matthews S.. Rhinitis in 10-year-old children and early life risk factors for its development. Acta Paediatr 2002;91:1334–38. [DOI] [PubMed] [Google Scholar]
- 19. Patil VK, Kurukulaaratchy RJ, Venter C. et al. Changing prevalence of wheeze, rhinitis and allergic sensitization in late childhood: findings from 2 Isle of Wight birth cohorts 12 years apart. Clin Exp Allergy 2015;45:1430–38. [DOI] [PubMed] [Google Scholar]
- 20. Kurukulaaratchy RJ, Karmaus W, Raza A, Matthews S, Roberts G, Arshad SH.. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life. Clin Exp Allergy 2011;41:851–59. [DOI] [PubMed] [Google Scholar]
- 21. Kurukulaaratchy R, Fenn M, Matthews S, Hasan Arshad S.. The prevalence, characteristics of and early life risk factors for eczema in 10-year-old children. Pediatr Allergy Immunol 2003;14:178–83. [DOI] [PubMed] [Google Scholar]
- 22. Tariq SM, Stevens M, Matthews S, Ridout S, Twiselton R, Hide DW.. Cohort study of peanut and tree nut sensitization by age of 4 years. BMJ 1996;313:514–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arshad SH, Venter C, Roberts G, Dean T, Kurukulaaratchy R.. The natural history of peanut sensitization and allergy in a birth cohort. J Allergy Clin Immunol 2014;134:1462–63.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grundy J, Matthews S, Bateman B, Dean T, Arshad SH.. Rising prevalence of allergy to peanut in children: Data from 2 sequential cohorts. J Allergy Clin Immunol 2002;110:784–89. [DOI] [PubMed] [Google Scholar]
- 25. Venter C, Hasan Arshad S, Grundy J. et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy 2010;65:103–08. [DOI] [PubMed] [Google Scholar]
- 26. Kurukulaaratchy RJ, Matthews S, Arshad SH.. Defining childhood atopic phenotypes to investigate the association of atopic sensitization with allergic disease. Allergy 2005;60:1280–86. [DOI] [PubMed] [Google Scholar]
- 27. Scott M, Raza A, Karmaus W. et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax 2010;65:258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang H, Karmaus W, Gan J. et al. Adjusting wheal size measures to correct atopy misclassification. Int J Gen Med 2011;4:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziyab AH, Raza A, Karmaus W. et al. Trends in eczema in the first 18 years of life: results from the Isle of Wight 1989 birth cohort study. Clin Exp Allergy 2010;40:1776–84. [DOI] [PubMed] [Google Scholar]
- 30. Venter C, Maslin K, Patil V. et al. The prevalence, natural history and time trends of peanut allergy over the first 10 years of life in two cohorts born in the same geographical location 12 years apart. Pediatr Allergy Immunol 2016;27:804–11. [DOI] [PubMed] [Google Scholar]
- 31. Roberts G, Zhang H, Karmaus W. et al. Trends in cutaneous sensitization in the first 18 years of life: results from the 1989 Isle of Wight birth cohort study. Clin Exp Allergy 2012;42:1501–09. [DOI] [PubMed] [Google Scholar]
- 32. Soto-Ramirez N, Ziyab AH, Karmaus W. et al. Epidemiologic methods of assessing asthma and wheezing episodes in longitudinal studies: measures of change and stability. J Epidemiol 2013;23:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tariq SM, Matthews SM, Hakim EA, Arshad SH.. Egg allergy in infancy predicts respiratory allergic disease by 4 years of age. Pediatr Allergy Immunol 2000;11:162–67. [DOI] [PubMed] [Google Scholar]
- 34. Arshad SH, Kurukulaaratchy RJ, Fenn M, Matthews S.. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest 2005;127:502–08. [DOI] [PubMed] [Google Scholar]
- 35. Arshad SH, Karmaus W, Raza A. et al. The effect of parental allergy on childhood allergic diseases depends on the sex of the child. J Allergy Clin Immunol 2012;130:427–34.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arshad SH, Twiselton R, Smith J, Hide DW.. Influence of genetic and environmental factors on the level of IgE at birth. Pediatr Allergy Immmunol 1992;3:79–83. [Google Scholar]
- 37. Karmaus W, Dobai AL, Ogbuanu I, Arshard SH, Matthews S, Ewart S.. Long-term effects of breastfeeding, maternal smoking during pregnancy, and recurrent lower respiratory tract infections on asthma in children. J Asthma 2008;45:688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ogbuanu IU, Karmaus W, Arshad SH, Kurukulaaratchy RJ, Ewart S.. Effect of breastfeeding duration on lung function at age 10 years: a prospective birth cohort study. Thorax 2009;64:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Balte P, Karmaus W, Roberts G, Kurukulaaratchy R, Mitchell F, Arshad H.. Relationship between birth weight, maternal smoking during pregnancy and childhood and adolescent lung function: A path analysis. Respir Med 2016;121:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soto-Ramirez N, Alexander M, Karmaus W. et al. Breastfeeding is associated with increased lung function at 18 years of age: a cohort study. Eur Respir J 2012;39:985–91. [DOI] [PubMed] [Google Scholar]
- 41. Bion V, Lockett GA, Soto-Ramirez N. et al. Evaluating the efficacy of breastfeeding guidelines on long-term outcomes for allergic disease. Allergy 2016;71:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fussman C, Todem D, Forster J, Arshad H, Urbanek R, Karmaus W.. Cow’s milk exposure and asthma in a newborn cohort: repeated ascertainment indicates reverse causation. J Asthma 2007;44:99–105. [DOI] [PubMed] [Google Scholar]
- 43. Sonnenschein-van der Voort AM, Arends LR, de Jongste JC. et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol 2014;133:1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. den Dekker HT, Sonnenschein-van der Voort AM, de Jongste JC. et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J Allergy Clin Immunol 2016;137:1026–35. [DOI] [PubMed] [Google Scholar]
- 45. Kurukulaaratchy RJ, Waterhouse L, Matthews SM, Arshad SH.. Are influences during pregnancy associated with wheezing phenotypes during the first decade of life? Acta Paediatr 2005;94:553–58. [DOI] [PubMed] [Google Scholar]
- 46. Tariq SM, Hakim EA, Matthews SM, Arshad SH.. Influence of smoking on asthmatic symptoms and allergen sensitization in early childhood. Postgrad Med J 2000;76:694–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arshad SH. Pets and atopic disorders in infancy. Br J Clin Pract 1991;45:88–89. [PubMed] [Google Scholar]
- 48. Lodrup Carlsen KC, Roll S, Carlsen KH. et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PloS One 2012;7:e43214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lockett GA, Soto-Ramirez N, Ray MA. et al. Association of season of birth with DNA methylation and allergic disease. Allergy 2016;71:1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karmaus W, Arshad H, Mattes J.. Does the sibling effect have its origin in utero? Investigating birth order, cord blood immunoglobulin E concentration, and allergic sensitization at age 4 years. Am J Epidemiol 2001;154:909–15. [DOI] [PubMed] [Google Scholar]
- 51. Karmaus W, Arshad SH, Sadeghnejad A, Twiselton R.. Does maternal immunoglobulin E decrease with increasing order of live offspring? Investigation into maternal immune tolerance. Clin Exp Allergy 2004;34:853–59. [DOI] [PubMed] [Google Scholar]
- 52. Ogbuanu IU, Karmaus WJ, Zhang H. et al. Birth order modifies the effect of IL13 gene polymorphisms on serum IgE at age 10 and skin prick test at ages 4, 10 and 18: a prospective birth cohort study. Allergy Asthma Clin Immunol 2010;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tariq SM, Arshad SH, Matthews SM, Hakim EA.. Elevated cord serum IgE increases the risk of aeroallergen sensitization without increasing respiratory allergic symptoms in early childhood. Clin Exp Allergy 1999;29:1042–48. [DOI] [PubMed] [Google Scholar]
- 54. Hide DW, Arshad SH, Twiselton R, Stevens M.. Cord serum IgE: an insensitive method for prediction of atopy. Clin Exp Allergy 1991;21:739–43. [DOI] [PubMed] [Google Scholar]
- 55. Sadeghnejad A, Karmaus W, Davis S, Kurukulaaratchy RJ, Matthews S, Arshad SH.. Raised cord serum immunoglobulin E increases the risk of allergic sensitization at ages 4 and 10 and asthma at age 10. Thorax 2004;59:936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schauberger EM, Ewart SL, Arshad SH. et al. Identification of ATPAF1 as a novel candidate gene for asthma in children. J Allergy Clin Immunol 2011;128:753–60.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gu Y, Harley IT, Henderson LB. et al. Identification of IFRD1 as a modifier gene for cystic fibrosis lung disease. Nature 2009;458:1039–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sadeghnejad A, Karmaus W, Hasan Arshad S, Ewart S.. IL13 gene polymorphism association with cord serum immunoglobulin E. Pediatr Allergy Immunol 2007;18:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arshad SH, Karmaus W, Kurukulaaratchy R, Sadeghnejad A, Huebner M, Ewart S.. Polymorphisms in the interleukin 13 and GATA binding protein 3 genes and the development of eczema during childhood. Br J Dermatol 2008;158:1315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ziyab AH, Karmaus W, Yousefi M. et al. Interplay of filaggrin loss-of-function variants, allergic sensitization, and eczema in a longitudinal study covering infancy to 18 years of age. PloS One 2012;7:e32721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ziyab AH, Karmaus W, Zhang H. et al. Allergic sensitization and filaggrin variants predispose to the comorbidity of eczema, asthma, and rhinitis: results from the Isle of Wight birth cohort. Clin Exp Allergy 2014;44:1170–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Venkataraman D, Soto-Ramirez N, Kurukulaaratchy RJ. et al. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J Allergy Clin Immunol 2014;134:876–82.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ziyab AH, Karmaus W, Zhang H. et al. Association of filaggrin variants with asthma and rhinitis: is eczema or allergic sensitization status an effect modifier? Int Arch Allergy Immunol 2014;164:308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huebner M, Kim DY, Ewart S, Karmaus W, Sadeghnejad A, Arshad SH.. Patterns of GATA3 and IL13 gene polymorphisms associated with childhood rhinitis and atopy in a birth cohort. J Allergy Clin Immunoly 2008;121:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ziyab AH, Davies GA, Ewart S. et al. Interactive effect of STAT6 and IL13 gene polymorphisms on eczema status: results from a longitudinal and a cross-sectional study. BMC Med Genet 2013;14:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ramadas RA, Sadeghnejad A, Karmaus W. et al. Interleukin-1R antagonist gene and pre-natal smoke exposure are associated with childhood asthma. Eur Respir J 2007;29:502–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sadeghnejad A, Karmaus W, Arshad SH, Kurukulaaratchy R, Huebner M, Ewart S.. IL13 gene polymorphisms modify the effect of exposure to tobacco smoke on persistent wheeze and asthma in childhood, a longitudinal study. Respir Res 2008;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alexander M, Karmaus W, Holloway JW. et al. Effect of GSTM2-5 polymorphisms in relation to tobacco smoke exposures on lung function growth: a birth cohort study. BMC Pulm Med 2013;13:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ziyab AH, Mukherjee N, Ewart S. et al. Filaggrin gene loss-of-function variants modify the effect of breast-feeding on eczema risk in early childhood. Allergy 2016;71:1371–73. [DOI] [PubMed] [Google Scholar]
- 70. Zhang H, Tong X, Holloway JW. et al. The interplay of DNA methylation over time with Th2 pathway genetic variants on asthma risk and temporal asthma transition. Clin Epigenet 2014;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ziyab AH, Karmaus W, Holloway JW, Zhang H, Ewart S, Arshad SH.. DNA methylation of the filaggrin gene adds to the risk of eczema associated with loss-of-function variants. J Eur Acad Dermatol Venereol 2013;27:e420–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Soto-Ramirez N, Arshad SH, Holloway JW. et al. The interaction of genetic variants and DNA methylation of the interleukin-4 receptor gene increase the risk of asthma at age 18 years. Clin Epigenet 2013;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mukherjee N, Lockett GA, Merid SK. et al. DNA methylation and genetic polymorphisms of the Leptin gene interact to influence lung function outcomes and asthma at 18 years of age. Int J Mol Epidemiol Genet 2016;7:1–17. [PMC free article] [PubMed] [Google Scholar]
- 74. Quraishi BM, Zhang H, Everson TM. et al. Identifying CpG sites associated with eczema via random forest screening of epigenome-scale DNA methylation. Clin Epigenet 2015;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Everson TM, Lyons G, Zhang H. et al. DNA methylation loci associated with atopy and high serum IgE: a genome-wide application of recursive Random Forest feature selection. Genome Med 2015;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yousefi M, Karmaus W, Zhang H, Ewart S, Arshad H, Holloway JW.. The methylation of the LEPR/LEPROT genotype at the promoter and body regions influence concentrations of leptin in girls and BMI at age 18 years if their mother smoked during pregnancy. Int J Mol Epidemiol Genet 2013;4:86–100. [PMC free article] [PubMed] [Google Scholar]
- 77. Patil VK, Holloway JW, Zhang H. et al. Interaction of prenatal maternal smoking, interleukin 13 genetic variants and DNA methylation influencing airflow and airway reactivity. Clin Epigenet 2013;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Joubert BR, Felix JF, Yousefi P. et al. DNA methylation in newborns and maternal smoking in pregnancy: Genome-wide Consortium meta-analysis. Am J Hum Genet 2016;98:680–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Janjanam VD, Mukherjee N, Lockett GA. et al. Tetanus vaccination is associated with differential DNA-methylation: Reduces the risk of asthma in adolescence. Vaccine 2016;34:6493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Terry W, Zhang H, Maity A, Arshad H, Karmaus W.. Unified variable selection in semi-parametric models. Stat Methods Med Res 2017;26:2821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang H, Maity A, Arshad H, Holloway J, Karmaus W.. Variable selection in semi-parametric models. Stat Methods Med Res 2016;25:1736–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guthikonda K, Zhang H, Nolan VG. et al. Oral contraceptives modify the effect of GATA3 polymorphisms on the risk of asthma at the age of 18 years via DNA methylation. Clin Epigenet 2014;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]