Abstract
Background
Mothers’ smoking during pregnancy increases asthma risk in their offspring. There is some evidence that grandmothers’ smoking may have a similar effect, and biological plausibility that fathers’ smoking during adolescence may influence offspring’s health through transmittable epigenetic changes in sperm precursor cells. We evaluated the three-generation associations of tobacco smoking with asthma.
Methods
Between 2010 and 2013, at the European Community Respiratory Health Survey III clinical interview, 2233 mothers and 1964 fathers from 26 centres reported whether their offspring (aged ≤51 years) had ever had asthma and whether it had coexisted with nasal allergies or not. Mothers and fathers also provided information on their parents’ (grandparents) and their own asthma, education and smoking history. Multilevel mediation models within a multicentre three-generation framework were fitted separately within the maternal (4666 offspring) and paternal (4192 offspring) lines.
Results
Fathers’ smoking before they were 15 [relative risk ratio (RRR) = 1.43, 95% confidence interval (CI): 1.01–2.01] and mothers’ smoking during pregnancy (RRR = 1.27, 95% CI: 1.01–1.59) were associated with asthma without nasal allergies in their offspring. Grandmothers’ smoking during pregnancy was associated with asthma in their daughters [odds ratio (OR) = 1.55, 95% CI: 1.17–2.06] and with asthma with nasal allergies in their grandchildren within the maternal line (RRR = 1.25, 95% CI: 1.02–1.55).
Conclusions
Fathers’ smoking during early adolescence and grandmothers’ and mothers’ smoking during pregnancy may independently increase asthma risk in offspring. Thus, risk factors for asthma should be sought in both parents and before conception.
Funding
European Union (Horizon 2020, GA-633212).
Keywords: Asthma, mothers’, smoking during pregnancy, grandmothers’, smoking during pregnancy, fathers’, smoking during puberty, multilevel mediation model, Ageing Lungs in European Cohorts (ALEC) Study
Key Messages
Fathers’ smoking before the age of 15 was associated with an increased risk of asthma without nasal allergies in their offspring, suggesting an effect of paternal pre-adolescent environment on the next generation.
Grandmothers’ smoking during pregnancy was associated with an increased risk of asthma with nasal allergies in their grandchildren within the maternal line, suggesting a multi-generation effect of tobacco smoking.
A multi-generation perspective is needed to better understand major public health challenges, such as smoking and asthma, and to assess the value and feasibility of preventive interventions.
Introduction
Considerable resources are invested in smoking prevention, with substantial health benefits. Pregnant women are a target of such interventions, as consistent evidence has demonstrated the negative impact of prenatal exposures on offspring’s health. In particular, it is widely accepted that mothers’ smoking during pregnancy increases the risk of asthma and asthma-like symptoms in their offspring.1–4 Indeed, nicotine exposure during the pre- and perinatal periods appears to permanently affect the development of the lungs, with adverse effects on their final structure and function.5 These changes may increase the risk of asthma later in life and accelerate lung function decline with ageing.5–8
The enhanced understanding of the heritable effects of tobacco smoking through transmissible epigenetic phenomena opens a new paradigm,9,10 providing a biological basis for preventive interventions during pregnancy and even in young males. Animal studies support multi-generation effects of nicotine exposure during gestation and lactation on the lungs,11 but evidence in humans is scarce and controversial. There are reports that the risk of asthma increases for a child if the maternal grandmother had smoked when pregnant with the child’s mother, even if the child was not exposed to the mother’s smoking in utero.2,12,13 However, grandmothers’ smoking was not associated with their grandchildren’s respiratory outcomes through the maternal line in another population survey.14 Tobacco smoking may have heritable effects also within the paternal line, as fathers’ smoking during adolescence may cause epigenetic changes in sperm precursor cells that can be transmitted to later generations.15 Supporting evidence to the effect of fathers’ smoking during puberty on offspring’s health has been provided by the Respiratory Health in Northern Europe (RHINE) III study.16
The present study aims at investigating the pattern of associations between tobacco smoking and asthma across three generations [grandparents (F0), parents (F1), offspring (F2)], during different developmental stages within those generations (grandmothers/mothers’ pregnancies, fathers’ puberty). To fulfil this objective, we used data from the European Community Respiratory Health Survey (ECRHS).17–19
Methods
Study population
The ECRHS is an international, population-based, cohort study on respiratory health in subjects aged 20–44 at the time of recruitment (ECRHS I; 1991–93).17 At baseline, each participant was sent a brief screening questionnaire (stage 1) and, from those who responded, a 20% random sample was invited to undergo a more detailed clinical examination (stage 2). Follow-up of the participants in stage 2 took place in 1998–2002 (ECRHS II)18 and 2010–13 (ECRHS III).19 The participants underwent a standardized clinical interview, lung function tests and laboratory testing on all occasions. An additional sample of adults with asthma-like symptoms recruited at baseline was not included in the present analyses. Ethical approval was obtained for each centre from the appropriate ethics committee and written consent was obtained from each participant.
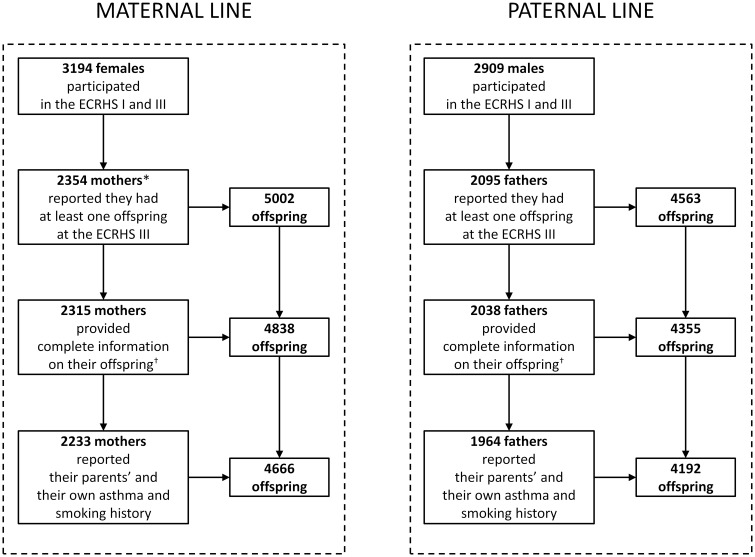
The 4449 subjects (from 26 centres in Europe and Australia; Supplementary Table 1, available as Supplementary data at IJE online) who had participated in both the ECRHS I and the ECRHS III, and who had reported at least one offspring at the ECRHS III clinical interview, were eligible for the present analyses (Figure 1). Among these individuals, 2233 mothers and 1964 fathers provided complete information on gender, birth year, asthma and nasal allergies (including hay fever) of their 4666 and 4192 offspring, respectively, as well as information on their parents’ (grandparents) and their own asthma and smoking history.
Figure 1.
Study population of parents and offspring, according to the parental line. *Six mothers were excluded because their age at their child’s birth was <13 years. †Complete information on offspring’s gender, birth year, asthma and nasal allergies (including hay fever).
Definitions
Offspring’s asthma was classified as: ‘ever asthma with nasal allergies’; ‘ever asthma without nasal allergies’; or ‘never asthma’. Grandparental and parental ever asthma (‘present’ vs ‘absent’) was reported by parents at baseline or at the ECRHS II and III (5.6% of grandparents and 8.7% of parents).
The parents provided detailed information on their own smoking history (including when they had started and quitted smoking) at each clinical interview. Mothers’ smoking was classified according to the birth year of each offspring: ‘smoking when the offspring was in utero’ (mothers smoked during their child’s birth year and/or during the previous year; these mothers also smoked during other periods); ‘smoking during other periods’ [mothers stopped smoking at least 2 years prior to their child's birth year (at least 3 months before conception) and/or started or restarted smoking after their child's birth year]; or ‘not smoking’. Fathers’ smoking was classified as: ‘smoking initiation before 15 years of age’ (before the mean age of completed puberty in boys);20 ‘smoking initiation at 15 years of age or older’; or ‘not smoking’. At ECRHS I, the parents provided information on their mother’s smoking during the period around their birth. Consequently, grandmothers’ smoking was categorized as: ‘smoking when the parent was in utero’; ‘smoking during other periods (or unknown smoking period)’; or ‘not smoking’.
Grandparents’ education level was parent-reported and considered low if both grandparents had only studied up to the minimum school-leaving age. Mothers’ and fathers’ education levels were self-reported and considered low if less than or equal to the minimum school-leaving age in their country before the start of the ECRHS.21 An ‘unknown’ category was used when no information on education was available.
Statistical analyses
Mediation models22 within a hierarchical framework were used to investigate the multi-generation pattern of associations between tobacco smoking and asthma within the maternal and paternal lines. Our data have a hierarchical structure (see the Supplementary Appendix, available as Supplementary data at IJE online) because we evaluated multiple offspring (level 1 units) from the same parent (i.e. the participants in the ECRHS III; level 2 units) and because many parents had been sampled from each of the different centres (level 3 units).
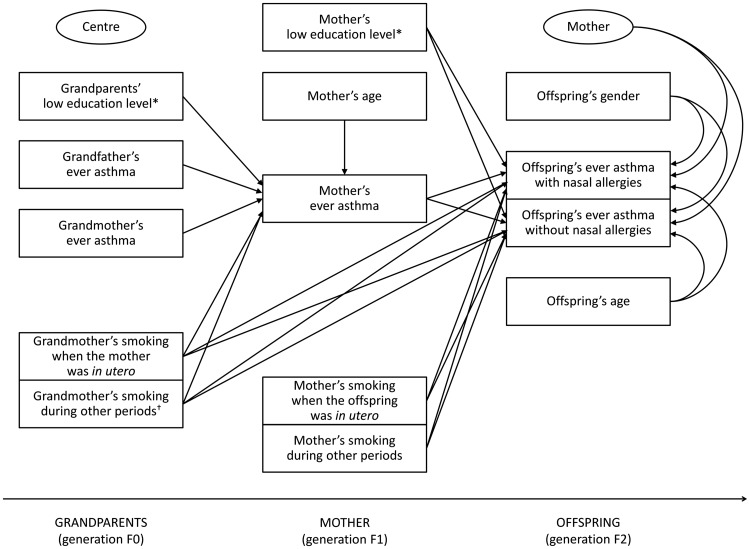
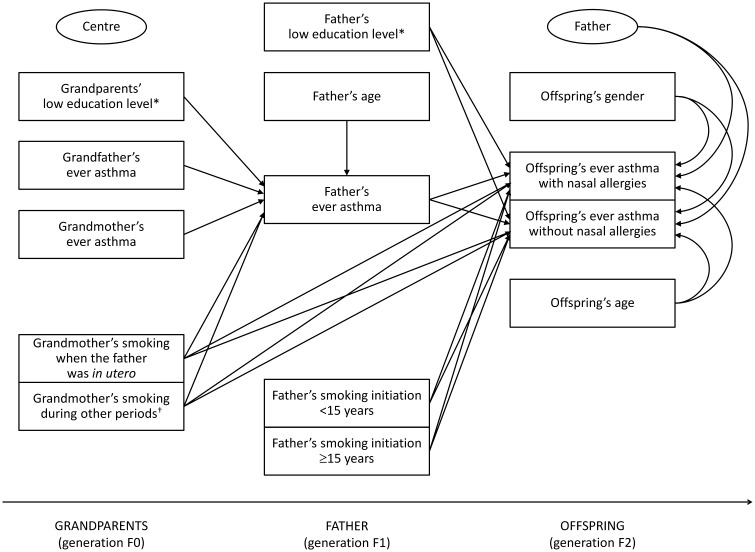
The following variables were included in the mediation models (the paths investigated in the analyses are represented in Figures 2 and 3):
Figure 2.
Two-level mediation model within the maternal line. The two ellipses represent: (i) the level 2 unit (mother; the presence of arrows indicates the random intercept terms at level 2); and (ii) the cluster variable (centre; the absence of arrows indicates that cluster-robust standard errors were computed in order to take the correlation among mothers within centres into account). *The ‘unknown’ category is not shown. †Smoking during other periods (or unknown smoking period).
Figure 3.
Two-level mediation model within the paternal line. The two ellipses represent: (i) the level 2 unit (father; the presence of arrows indicates the random intercept terms at level 2); and (ii) the cluster variable (centre; the absence of arrows indicates that cluster-robust standard errors were computed in order to take the correlation among fathers within centres into account). *The ‘unknown’ category is not shown. †Smoking during other periods (or unknown smoking period).
offspring’s ever asthma with or without nasal allergies as the multinomial-distributed outcome;
maternal/paternal ever asthma as the Bernoulli-distributed mediator;
grandmother’s and grandfather’s ever asthma, grandmother’s smoking, grandparents’ education level and maternal/paternal age as the potential predictors of the mediator;
grandmother’s smoking, maternal/paternal smoking and education level and offspring’s gender and age as the potential predictors of the outcome.
Both mediation models had a complex two-level structure in which the predictors of the mediator and the mediator were measured at level 2 (parent), whereas the outcome was measured at level 1 (offspring). This type of mediation model has been labelled ‘2→2→1’ in the literature.23 Random intercept terms at level 2 were included in the models. Cluster-robust standard errors were computed in order to take the correlation among parents within each of the different centres (cluster variable) into account.
Due to the complex mediation pattern (see the Supplementary Appendix, available as Supplementary data at IJE online), only controlled direct effects24 (i.e. the effects of exposures on the outcome that would be observed if the mediator were controlled uniformly at a fixed value) were calculated. In particular, the direct effects on the Bernoulli-distributed mediators and the direct effects on the multinomial-distributed outcome were summarized as odds ratios (ORs) and relative risk ratios (RRRs), respectively. The interactions of the offspring’s gender with maternal/paternal smoking and asthma were evaluated by testing the significance of the extra parameters in the models. The statistical analyses were carried out using STATA 14.2 (StataCorp, College Station, TX) and Mplus 8 (Muthén & Muthén, Los Angeles, CA).
Sensitivity analyses
Sensitivity analyses (see the Supplementary Appendix, available as Supplementary data at IJE online) were performed in order to check whether:
the covariates included in the models represent the ‘minimal sufficient adjustment set’ (i.e. the group of measured covariates that needs to be included in order to eliminate confounding) through a directed acyclic graph (DAG; Supplementary Figure 1, available as Supplementary data at IJE online),25 using DAGitty (http://dagitty.net);
the inclusion of one unmeasured confounder in the models26 changes the estimate of the direct effects of grandmothers’ smoking on offspring’s asthma, using the Umediation package [https://github.com/SharonLutz/Umediation] in R3.4.1.
Results
Main characteristics of the subjects
The 2233 mothers and 1964 fathers included in the present analyses were of similar age, and their parents had similar education levels (Table 1). Mothers, compared with fathers, were more likely to have ever had asthma (18.3 vs 12.7%), to report that their mothers (11.0 vs 7.6%) and fathers (9.2 vs 7.4%) had ever had asthma, and to report that their mothers had smoked during their pregnancy (10.5 vs 6.7%).
Table 1.
Main characteristics of the parents and grandparents, according to the parental line
| Maternal line |
Paternal line |
||
|---|---|---|---|
| N parents | n = 2233 | n = 1964 | P-valuea |
| Grandmother’s ever asthma, % | 11.0 | 7.6 | <0.001 |
| Grandfather’s ever asthma, % | 9.2 | 7.4 | 0.04 |
| Grandparents’ education level, % | 0.56 | ||
| low | 45.7 | 47.0 | |
| high | 52.1 | 51.1 | |
| unknown | 2.2 | 1.9 | |
| Grandmother’s smoking, % | <0.001 | ||
| when the parent was in utero | 10.5 | 6.7 | |
| during other periods (or unknown smoking period) | 13.5 | 15.3 | |
| not smoking | 76.0 | 78.0 | |
| Parent’s ages (years), median (range) | 55 (40–67) | 55 (40–67) | 0.08 |
| Parent’s ever asthma, % | 18.3 | 12.7 | <0.001 |
| Parent’s education level, % | 0.35 | ||
| low | 14.1 | 12.6 | |
| high | 82.2 | 83.4 | |
| unknown | 3.8 | 4.0 | |
| Father’s smoking initiation, % | – | ||
| <15 years of age | – | 12.2 | |
| ≥15 years of age | – | 51.3 | |
| not smoking | – | 36.6 |
Obtained by using Pearson chi-square and Wilcoxon-Mann-Whitney tests.
Half of the parents had two offspring, and 24.0% of the mothers and 22.5% of the fathers had only one child (Supplementary Table 2, available as Supplementary data at IJE online). The 4666 offspring in the maternal line (females: 50.3%; age range: 1–51 years) were more likely to have ever had asthma with or without nasal allergies (6.8 vs 6.0% and 8.2 vs 4.8%, respectively) than the 4192 offspring in the paternal line (females: 49.1%; age range: 0–48 years; Table 2). Of all the offspring, 12.5% were born to the 239 fathers (12.2%; Table 1) who had started smoking before they were 15, and 29.2% had been exposed to their mother’s smoking during pregnancy (Table 2).
Table 2.
Main characteristics of the offspring, according to the parental line
| Maternal line |
Paternal line |
||
|---|---|---|---|
| N offspring | n = 4666 | n = 4192 | P-value |
| Offspring’s gender (female), % | 50.3 | 49.1 | – a |
| Offspring’s age (years), median (range) | 26 (1–51) | 24 (0–48) | – a |
| Offspring’s ever asthma, % | <0.001b | ||
| with nasal allergies | 6.8 | 6.0 | |
| without nasal allergies | 8.2 | 4.8 | |
| never asthma | 85.0 | 89.2 | |
| Mother’s smoking, % | – | ||
| when the offspring was in utero | 29.2 | – | |
| during other periods | 26.2 | – | |
| not smoking | 44.6 | – |
Not computed because of the hierarchical data structure (offspring nested within parents).
Obtained by using the likelihood ratio test for the comparison of the goodness-of-fit of the following nested models: (i) two-level multinomial regression model (parent = level 2 unit) with the offspring’s ever asthma as the outcome and the parental line as the covariate; and (ii) the previous model with no covariates.
Recurrence of asthma across three generations
The risk of mothers’ asthma (generation F1) was higher if their parents (generation F0) had ever had asthma (grandmothers’ asthma: OR = 2.24; grandfathers’ asthma: OR = 2.60; Table 3). The risk of asthma with or without nasal allergies in offspring (generation F2) was higher if the offspring’s mother had ever had asthma (RRR = 2.50 and 1.69, respectively). Similar results were found within the paternal line (Table 4). Whether the offspring was a boy or a girl did not modify the association of parents’ asthma with the offspring’s asthma (tests for interaction: P-value >0.9). These estimates did not change when grandparental/parental smoking and education levels were excluded from the models (Supplementary Tables 3 and 4, available as Supplementary data at IJE online).
Table 3.
Controlled direct effects24 within the maternal line
| F1 |
F2 |
|||
|---|---|---|---|---|
| Mother’s ever asthma | Offspring's ever asthma with nasal allergies | Offspring's ever asthma without nasal allergies | ||
| Generation | OR (95% CI) | RRR (95% CI) | RRR (95% CI) | |
| F0 | Grandmother’s ever asthma (present vs absent) | 2.24 (1.58–3.17) | – | – |
| Grandfather’s ever asthma (present vs absent) | 2.60 (1.98–3.42) | – | – | |
| Grandparents’ education levela (low vs high) | 0.71 (0.58–0.87) | – | – | |
| Grandmother’s smoking (vs not smoking) | ||||
| when the mother was in utero | 1.55 (1.17–2.06) | 1.25 (1.02–1.55) | 1.31 (0.86–1.98) | |
| during other periods (or unknown smoking period) | 1.12 (0.83–1.52) | 1.20 (0.88–1.63) | 1.12 (0.85–1.48) | |
| F1 | Mother’s age (1-year increase) | 1.00 (0.99–1.02) | – | – |
| Mother’s ever asthma (present vs absent) | – | 2.50 (1.95–3.22) | 1.69 (1.25–2.28) | |
| Mother’s education levela (low vs high) | – | 1.31 (0.93–1.83) | 1.79 (1.26–2.55) | |
| Mother’s smoking (vs not smoking) | ||||
| when the offspring was in utero | – | 1.06 (0.76–1.49) | 1.27 (1.01–1.59) | |
| during other periods | – | 0.87 (0.61–1.24) | 0.96 (0.71–1.28) | |
| F2 | Offspring’s gender (female vs male) | – | 0.80 (0.66–0.97) | 0.89 (0.71–1.12) |
| Offspring’s age (1-year increase) | – | 0.98 (0.97–1.00) | 0.96 (0.95–0.98) | |
Comparison of the goodness-of-fit between the present mediation model and the cluster-robust (centre = cluster variable) two-level (mother = level 2 unit) multinomial regression model (outcome: offspring’s ever asthma with or without nasal allergies; covariates: grandmother’s smoking, mother’s ever asthma, education level and smoking, offspring’s gender and age): P-value (Satorra-Bentler scaled chi-square test for nested models27 with 8 degrees of freedom) <0.0001.
aThe estimates for the ‘unknown’ category are not shown.
Table 4.
Controlled direct effects24 within the paternal line
| F1 |
F2 |
|||
|---|---|---|---|---|
| Father’s ever asthma | Offspring's ever asthma with nasal allergies | Offspring's ever asthma without nasal allergies | ||
| Generation | OR (95% CI) | RRR (95% CI) | RRR (95% CI) | |
| F0 | Grandmother’s ever asthma (present vs absent) | 3.08 (1.96–4.85) | – | – |
| Grandfather’s ever asthma (present vs absent) | 2.38 (1.51–3.75) | – | – | |
| Grandparents’ education levela (low vs high) | 0.96 (0.71–1.30) | – | – | |
| Grandmother’s smoking (vs not smoking) | ||||
| when the father was in utero | 0.82 (0.47–1.44) | 1.60 (0.95–2.68) | 1.08 (0.55–2.13) | |
| during other periods (or unknown smoking period) | 1.02 (0.62–1.67) | 1.24 (0.81–1.91) | 1.35 (0.87–2.09) | |
| F1 | Father’s age (1-year increase) | 0.99 (0.96–1.02) | – | – |
| Father’s ever asthma (present vs absent) | – | 2.37 (1.63–3.43) | 1.70 (1.14–2.53) | |
| Father’s education levela (low vs high) | – | 0.47 (0.27–0.83) | 0.87 (0.49–1.53) | |
| Father’s smoking initiation (vs not smoking) | ||||
| <15 years of age | – | 1.19 (0.74–1.90) | 1.43 (1.01–2.01) | |
| ≥15 years of age | – | 0.98 (0.71–1.36) | 0.88 (0.70–1.11) | |
| F2 | Offspring’s gender (female vs male) | – | 0.71 (0.59–0.84) | 0.83 (0.70–0.98) |
| Offspring’s age (1-year increase) | – | 1.00 (0.98–1.02) | 0.96 (0.94–0.99) | |
Comparison of the goodness-of-fit between the present mediation model and the cluster-robust (centre = cluster variable) two-level (father = level 2 unit) multinomial regression model (outcome: offspring’s ever asthma with or without nasal allergies; covariates: grandmother’s smoking, father’s ever asthma, education level and smoking initiation, offspring’s gender and age): P-value (Satorra-Bentler scaled chi-square test for nested models27 with 8 degrees of freedom) <0.0001. aThe estimates for the ‘unknown’ category are not shown.
Associations of tobacco smoking with asthma across three generations
Grandmothers’ smoking when mothers were in utero (generation F0) was significantly associated with maternal asthma (generation F1; OR = 1.55; Table 3). In turn, mothers’ smoking when the offspring was in utero (generation F1) was significantly associated with asthma without nasal allergies in their offspring (generation F2; RRR = 1.27). Within the paternal line, we did not find any association between grandmothers’ smoking during pregnancy and fathers’ asthma (Table 4). However, if fathers had started smoking before they were 15, the risk of asthma without nasal allergies in their offspring was higher (RRR = 1.43). The associations of parental smoking with asthma without nasal allergies in their offspring were not significantly different whether the offspring was a boy or a girl (tests for interaction: P-value >0.2).
Grandmothers’ smoking when mothers were in utero (generation F0) was positively associated with asthma with nasal allergies in their grandchildren (generation F2; RRR = 1.25; Table 3). This association did not reach statistical significance when fathers were in utero.
Sensitivity analyses
The DAG analysis supported the assumption that the measured covariates included in the models represent the ‘minimal sufficient adjustment set’ (see the Supplementary Appendix, available as Supplementary data at IJE online). In addition, the simulation analyses showed that the inclusion of one unmeasured confounder in the models had a limited impact on the estimate of the direct effects of grandmothers’ smoking on offspring’s asthma (Supplementary Figure 2, available as Supplementary data at IJE online).
Discussion
We have shown that fathers’ smoking during early puberty is associated with a higher risk of asthma without nasal allergies in their offspring, suggesting an effect of paternal pre-adolescent environment on the next generation. We have also shown that grandmothers’ smoking when mothers were in utero is a possible risk factor for asthma with nasal allergies in their grandchildren, suggesting a multi-generation effect of tobacco smoking. Finally, we have confirmed the higher risk of asthma in the offspring of mothers who smoked during their pregnancy and the recurrence of asthma across generations. Our findings have considerable public health implications with regard to the environment of male adolescents and to forecast the health of future generations.
Recurrence of asthma across three generations
We have found that asthma susceptibility recurred from grandparents to grandchildren, irrespective of the parent/offspring’s gender. These results support the well-established evidence that the offspring of asthmatic parents are at a higher risk of asthma.28 Although some case-control and cross-sectional surveys on asthma recurrence have shown that this was more marked for mothers,29 a longitudinal study has found a comparable risk in the parental lines,30 in agreement with our findings.
The association of mothers’ asthma with their offspring’s asthma can be explained through a combination of genetic and non-genetic factors in utero (e.g. genetic imprinting, the trans-placental passage of Th2 cytokines and immunological cells31), maternally dependent postnatal exposures such as breastfeeding,32 and hormonal factors.33 Asthma phenotypes, which mainly depend on the effect of paternal asthma, are likely mediated either by hormonal mechanisms or through imprinting.33
Associations of tobacco smoking with asthma across three generations
Tobacco smoking has adverse effects on human fertility, reproduction, and early development.34,35 The most consistent association with offspring’s asthma has been found for maternal smoking during pregnancy,1–4 which may permanently affect the lungs.5 Animal studies have shown that nicotine can penetrate the placental barriers and disturb alveolar development,36 expression of nicotinic receptors,37 and lung function.38 In agreement with this knowledge, we have found that grandmothers’ smoking during pregnancy was associated with asthma in their sons and daughters and, in turn, maternal smoking during pregnancy was associated with asthma in their offspring, irrespective of the offspring’s gender.
A key finding is the association of grandmothers’ smoking when the mother was in utero with asthma with nasal allergies in their grandchildren, irrespective of maternal asthma and smoking status during pregnancy. This is consistent with previous studies on humans.2,12,13 Epigenetic changes may be a potential explanation for this association (see the Supplementary Appendix, available as Supplementary data at IJE online).39,40 In fact, tobacco smoking may cause heritable modifications of the epigenome, particularly in the prenatal period and shortly after birth.41 Animal data have shown that these epigenetic changes may be inherited by second-generation offspring, and affect lung function.42 One study on humans has highlighted a link between prenatal smoke exposure, DNA methylation changes and asthma-related lung function.43 An alternative explanation is that the association between grandmothers’ smoking and grandchildren’s asthma might be due to confounding effects of other lifestyle and environmental factors. However, we controlled for education level, which may act as a proxy for some of these factors. The results pertaining to the education levels of parents/grandparents are discussed in the Supplementary Appendix, available as Supplementary data at IJE online.
A ground-breaking finding of our study is that paternal smoking before 15 years of age was associated with asthma without nasal allergies in their offspring, irrespective of gender. This is of particular concern, as smoking in 11-15-year-old boys has increased in Europe over recent decades (Alessandro Marcon, data presented at the European Respiratory Society International Congress 2016). At present, public health strategies do not focus on the environment of male adolescents with regard to the health of their future offspring, and to do so would represent a paradigm shift in preventive policies. Our results are consistent with findings from the RHINE study,16 a questionnaire-based postal follow-up of the ECRHS subjects from the seven Nordic centres listed in Supplementary Table 1, available as Supplementary data at IJE online. A minority of the parents evaluated in RHINE (11.5%) also underwent clinical examinations as part of the ECRHS and are included in this report. The present work is based on clinical interview data from these Nordic centres and 19 additional centres (located in other parts of Europe and Australia). One report from the Avon Longitudinal Study of Parents and Children (ALSPAC), showing that body fat increases in the sons of fathers who had started smoking in early puberty, also supports the hypothesis that paternal lifestyle and exposures well before conception may influence the health of their offspring.44 The heritable effect of smoking in young males seems biologically plausible. Male adolescence represents a critical period for the germ line development15 and for the susceptibility to tobacco-related DNA damage. Reproductive cells in male adolescents are characterized by an increased number of cell divisions, and they have a 6-fold higher risk of DNA mutations than female oocytes.45 Smokers have altered spermatozoal mRNA profiles compared with non-smokers.46 Tobacco smoking could also induce changes in the miRNA profiles of spermatozoa, leading to harmful phenotypes that are hypothesized to be transmitted to future generations through the male germ line.47 Altered miRNA is involved in perturbation of cell death and apoptosis pathways.47 Spermatozoal miRNA could be transferred to the oocyte at fertilization48 and target epigenetic compounds, which are important in DNA methylation and histone modification, and it could mediate gene expression during embryogenesis and alter phenotypes in future progeny.
Curiously, in our study, grandmothers’ smoking during pregnancy was associated with asthma with nasal allergies in their grandchildren, whereas maternal smoking during pregnancy and paternal smoking during puberty were associated with asthma without nasal allergies in their offspring. We speculate that parental smoking may have a detrimental effect on lung growth and function during fetal development, whereas grandmothers’ smoking could give rise to epigenome changes that alter the expression of inflammatory genes or regulate immune development.
Strengths and weaknesses
The information in the present study was available from three generations of subjects. The parents were selected from the general population in different countries and they were interviewed in clinical settings following a highly standardized protocol. Moreover, the analyses were carried out using appropriate statistical methods for evaluating the complex pattern of associations among variables in different generations.
There are very few epidemiological studies with detailed information on respiratory health across generations and, in the ECRHS centres involved in the Ageing Lungs in European Cohorts (ALEC) study [www.alecstudy.org], this work is being extended to include health assessment of children and registry-based collection of grandparents’ health status. However, the ECRHS is not a family-based study. It recruited a representative sample of men (fathers) and women (mothers), but their partners (co-parent of the offspring) did not participate in the study. Moreover, the information regarding grandparents and offspring was parent-reported, rather than directly assessed. This could have generated an information bias across generations and between the parental lines (see the Supplementary Appendix, available as Supplementary data at IJE online).
It is also possible that important confounders [e.g. parental socioeconomic status (SES) and offspring’s smoking history] were not included in the models. However, as we believe that an early start of smoking is one of the major SES-related exposures responsible for the influence of SES on health, we might expect that adjusting for this would attenuate our smoking-related associations. Moreover, in our simulations, unmeasured confounding had a limited impact on the estimated associations of grandmothers’ smoking with offspring’s asthma.
Finally, we investigated the associations of grand-maternal/parental smoking with offspring’s atopic/non-atopic asthma, rather than conditioning on offspring’s nasal allergies. Indeed, the inclusion of offspring’s asthma as the outcome and offspring’s nasal allergies as a mediator in the models would induce spurious exposure-outcome associations (collider bias), if we assume:49 (i) an effect of grand-maternal/parental smoking on offspring’s nasal allergies; (ii) the possibility of unmeasured confounders associated with offspring’s asthma and nasal allergies (but not with grand-maternal/parental smoking); and (iii) a probable link between nasal allergies and asthma in offspring.
Conclusions
The present analyses suggest that smoking during pregnancy and male puberty may increase the risk of asthma in the next generation, and that the effect of smoking during pregnancy may continue into a further generation within the maternal line. Our results provide further evidence on asthma recurrence across multiple generations. Therefore, risk factors for asthma should be sought before conception, in men and in women, to improve the health of future generations.
Supplementary Material
Acknowledgements
The ALEC project leader is Deborah Jarvis. The manuscript was done with ALEC Workpackage 2 led by Cecilie Svanes. Other Workpackage leaders in ALEC are John Henderson (University of Bristol, Bristol, UK), Judith Garcia-Aymerich [ISGlobal, Centre for Research in Environmental Epidemiology (CREAL), Barcelona, Spain], Nicole Probst-Hensch (Swiss Tropical and Public Health Institute, University of Basel, Basel, Switzerland) and Cosetta Minelli (Imperial College London, London, UK). The principal investigators and team members of the ECRHS are reported in the Supplementary Appendix, available as Supplementary data at IJE online. The authors commemorate the late Professor Roberto de Marco, a passionate scientist and enlightened man who led the Unit of Epidemiology and Medical Statistics (University of Verona) until October 2015. He contributed to the ALEC study with extraordinary commitment and inspiration.
Funding
The present analyses are part of the Ageing Lungs in European Cohorts (ALEC) Study [www.alecstudy.org], which has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 633212. The coordination of the European Community Respiratory Health Survey (ECRHS) was supported by the European Commission (phases 1 and 2) and the Medical Research Council (phase 3). Local funding agencies for the ECRHS are reported in the Supplementary Appendix, available as Supplementary data at IJE online.
Conflict of interest: J.W.H. reports grants from the European Union’s Horizon 2020 programme (633212), the Medical Research Council UK (MC_PC_15078) and the National Institutes of Health USA (R01 AI091905, R01 AI121226) during the conduct of the study. R.J. reports grants from the Estonian Research Council (personal grant No. 562) during the conduct of the study, grants/grants pending from the Estonian Research Council (personal research grant No. 562), personal fees for consulting and lecturing from GlaxoSmithKline, Boehringer and Novartis and travel/accommodation/meeting expenses paid by GlaxoSmithKline and Boehringer, outside the submitted work. C.R. reports personal fees for consulting and lecturing from ALK, Astra Zeneca, GSK, Boheringer and Novartis, outside the submitted work. A.G.C. reports grants from Chiesi Farmaceutici and GlaxoSmithKline Italy, during the conduct of the study. P.D. reports personal fees for consulting and lecturing from ALK and Stallergenes Greer and personal fees for consulting from Circassia, Chiesi Farmaceutici, ThermofisherScientific and Menarini, outside the submitted work. D.J. reports grants from the Medical Research Council and the European Union’s Horizon 2020 programme, during the conduct of the study. All other authors declare no competing interests.
References
- 1. Jaakkola JJ, Gissler M.. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health 2004;94:136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li YF, Langholz B, Salam MT, Gilliland FD.. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest 2005;127:1232–41. [DOI] [PubMed] [Google Scholar]
- 3. Skorge TD, Eagan TM, Eide GE, Gulsvik A, Bakke PS.. The adult incidence of asthma and respiratory symptoms by passive smoking in utero or in childhood. Am J Respir Crit Care Med 2005;172:61–66. [DOI] [PubMed] [Google Scholar]
- 4. Fuentes-Leonarte V, Estarlich M, Ballester F. et al. Pre- and postnatal exposure to tobacco smoke and respiratory outcomes during the first year. Indoor Air 2015;25:4–12. [DOI] [PubMed] [Google Scholar]
- 5. Maritz GS, Harding R.. Life-long programming implications of exposure to tobacco smoking and nicotine before and soon after birth: evidence for altered lung development. Int J Environ Res Public Health 2011;8:875–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Svanes C, Omenaas E, Jarvis D, Chinn S, Gulsvik A, Burney P.. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax 2004;59:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maritz GS. Perinatal exposure to nicotine and implications for subsequent obstructive lung disease. Paediatr Resp Rev 2013;14:3–8. [DOI] [PubMed] [Google Scholar]
- 8. Dratva J, Zemp E, Dharmage SC. et al. Early life origins of lung ageing: early life exposures and lung function decline in adulthood in two European cohorts aged 28-73 years. PLoS One 2016;11:e0145127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pembrey M, Saffery R, Bygren LO; Network in Epigenetic Epidemiology. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J Med Genet 2014;51:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeVries A, Vercelli D.. Epigenetic mechanisms in asthma. Ann Am Thorac Soc 2016;13(Suppl 1):S48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maritz GS, Mutemwa M.. The effect of grand maternal nicotine exposure during gestation and lactation on lung integrity of the F2 generation. Pediatr Pulmonol 2014;49:67–75. [DOI] [PubMed] [Google Scholar]
- 12. Magnus MC, Håberg SE, Karlstad Ø, Nafstad P, London SJ, Nystad W.. Grandmother’s smoking when pregnant with the mother and asthma in the grandchild: the Norwegian Mother and Child Cohort Study. Thorax 2015;70:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lodge CJ, Bråbäck L, Lowe AJ, Dharmage SC, Olsson D, Forsberg B.. Grandmaternal smoking increases asthma risk in grandchildren: a nationwide Swedish cohort. Clin Exp Allergy 2018;48:167–74. [DOI] [PubMed] [Google Scholar]
- 14. Miller LL, Henderson J, Northstone K, Pembrey M, Golding J.. Do grandmaternal smoking patterns influence the etiology of childhood asthma? Chest 2014;145:1213–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soubry A, Hoyo C, Jirtle RL, Murphy SK.. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. Bioassays 2014;36:359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Svanes C, Koplin J, Skulstad SM. et al. Father's environment before conception and asthma risk in his children: a multi-generation analysis of the Respiratory Health in Northern Europe study. Int J Epidemiol 2017;46:235–45. [DOI] [PubMed] [Google Scholar]
- 17. Burney PG, Luczynska C, Chinn S, Jarvis D.. The European Community Respiratory Health Survey. Eur Respir J 1994;7:954–60. [DOI] [PubMed] [Google Scholar]
- 18. European Community Respiratory Health Survey II Steering Committee. The European Community Respiratory Health Survey II. Eur Respir J 2002;20:1071–79. [DOI] [PubMed] [Google Scholar]
- 19. Amaral AFS, Newson RB, Abramson MJ. et al. Changes in IgE sensitization and total IgE levels over 20 years of follow-up. J Allergy Clin Immunol 2016;137:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Håkonsen LB, Olsen J, Støvring H. et al. Maternal cigarette smoking during pregnancy and pubertal development in sons. A follow-up study of a birth cohort. Andrology 2013;1:348–55. [DOI] [PubMed] [Google Scholar]
- 21. Hill M. Social Policy: A Comparative Analysis. London: Prentice-Hall/Harvester Wheatsheaf, 1996. [Google Scholar]
- 22. Muthén BO, Muthén LK, Asparouhov T.. Regression and Mediation Analysis Using Mplus. Los Angeles, CA: Muthén & Muthén, 2016. [Google Scholar]
- 23. Krull JL, MacKinnon DP.. Multilevel modeling of individual and group level mediated effects. Multivariate Behav Res 2001;36:249–77. [DOI] [PubMed] [Google Scholar]
- 24. Pearl J. Direct and indirect effects. In: Proceedings of the Seventeenth Conference on Uncertainty in Artificial Intelligence, 2-5 August 2001 San Francisco, CA: Morgan Kaufmann Publishers Inc., 2001.
- 25. Greenland S, Pearl J, Robins JM.. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- 26. Lutz SM, Thwing A, Schmiege S. et al. Examining the role of unmeasured confounding in mediation analysis with genetic and genomic applications. BMC Bioinformatics 2017;18:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Satorra A, Bentler PM.. Ensuring positiveness of the scaled difference chi-square test statistic. Psychometrika 2010;75:243–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomsen SF. Genetics of asthma: an introduction for the clinician. Eur Clin Respir J 2015;2:24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim RH, Kobzik L, Dahl M.. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One 2010;5:e10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paaso EM, Jaakkola MS, Lajunen TK, Hugg TT, Jaakkola JJ.. The importance of family history in asthma during the first 27 years of life. Am J Respir Crit Care Med 2013;188:624–26. [DOI] [PubMed] [Google Scholar]
- 31. Lim RH, Kobzik L.. Maternal transmission of asthma risk. Am J Reprod Immunol 2009;61:1–10. [DOI] [PubMed] [Google Scholar]
- 32. Guilbert TW, Stern DA, Morgan WJ, Martinez FD, Wright AL.. Effect of breastfeeding on lung function in childhood and modulation by maternal asthma and atopy. Am J Respir Crit Care Med 2007;176:843–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raby BA, Van Steen K, Celedón JC, Litonjua AA, Lange C, Weiss ST.. Paternal history of asthma and airway responsiveness in children with asthma. Am J Respir Crit Care Med 2005;172:552–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rogers JM. Tobacco and pregnancy. Reprod Toxicol 2009;28:152–60. [DOI] [PubMed] [Google Scholar]
- 35. Bisht S, Faiq M, Tolahunase M, Dada R.. Oxidative stress and male infertility. Nat Rev Urol 2017;14:470–85. [DOI] [PubMed] [Google Scholar]
- 36. Maritz GS, Dennis H.. Maternal nicotine exposure during gestation and lactation interferes with alveolar development in the neonatal lung. Reprod Fertil Dev 1998;10:255–61. [DOI] [PubMed] [Google Scholar]
- 37. Sekhon HS, Jia Y, Raab R. et al. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest 1999;103:637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sekhon HS, Keller JA, Benowitz NL, Spindel ER.. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med 2001;164:989–94. [DOI] [PubMed] [Google Scholar]
- 39. Skinner MK. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol Cell Endocrinol 2014;398:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arshad SH, Karmaus W, Zhang H, Holloway JW.. Multigenerational cohorts in patients with asthma and allergy. J Allergy Clin Immunol 2017;139:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perera F, Herbstman J.. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol 2011;31:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rehan VK, Liu J, Naeem E. et al. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med 2012;10:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patil VK, Holloway JW, Zhang H. et al. Interaction of prenatal maternal smoking, interleukin 13 genetic variants and DNA methylation influencing airflow and airway reactivity. Clin Epigenetics 2013;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pembrey ME, Bygren LO, Kaati G. et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet 2006;14:159–66. [DOI] [PubMed] [Google Scholar]
- 45. Forster P, Hohoff C, Dunkelmann B, et al. Elevated germline mutation rate in teenage fathers. Proc Biol Sci 2015;282:20142898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Linschooten JO, Van Schooten FJ, Baumgartner A. et al. Use of spermatozoal mRNA profiles to study gene-environment interactions in human germ cells. Mutat Res 2009;667:70–76. [DOI] [PubMed] [Google Scholar]
- 47. Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH.. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics 2012;7:432–39. [DOI] [PubMed] [Google Scholar]
- 48. Amanai M, Brahmajosyula M, Perry AC.. A restricted role for sperm-borne microRNAs in mammalian fertilization. Biol Reprod 2006;75:877–84. [DOI] [PubMed] [Google Scholar]
- 49. Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology 2003;14:300–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.