Abstract
Background
Attention-deficit/hyperactivity disorder (ADHD) is increasing worldwide for reasons largely unknown and environmental chemicals with neurotoxic properties, such as persistent organic pollutants (POPs), have been proposed to play a role. We investigated the association between prenatal and postnatal exposure to polychlorinated biphenyl-153 (PCB-153), p-p´-dichlorodiphenyldichloroethylene (p-p’-DDE) and hexachlorobenzene (HCB) and ADHD in childhood.
Methods
We pooled seven European birth cohort studies encompassing 4437 mother–child pairs from the general population with concentrations of PCB-153, p-p´-DDE and HCB measured in cord blood, maternal blood or milk. We then calculated prenatal (birth) and postnatal (3, 6, 12 and 24 months) POP concentrations using a pharmacokinetic model. The operational definition of ADHD varied across cohorts and ranged from doctor diagnosis obtained from patient registries to maternal or teachers reports. We used multilevel (mixed) logistic regression models to estimate the associations between exposure to POPs at birth, 3, 6, 12 and 24 months and ADHD.
Results
The global prevalence of ADHD in our study was 6%. The mean age at assessment of ADHD was 5.8 years (range: 3.8–9.5 years). We found no association between exposure to PCB-153, p-p´-DDE and HCB at any age point between birth and 24 months and ADHD, in the pooled analyses (pooled odds ratios ranging from 1.00 to 1.01). A number of sensitivity analyses gave basically the same results.
Conclusions
In the largest study to date of 4437 children in seven European birth cohorts, we did not observe any association between either pre- or postnatal exposure (up to 24 months) to PCB-153, p-p´-DDE and HCB and the risk of ADHD before the age of 10 years.
Keywords: DDT, hexachlorobenzene, polychlorinated biphenyls, attention-deficit disorder with hyperactivity
Key Messages
In this pooled analysis of seven European population-based birth cohort studies including 4400 children, we examined whether prenatal and postnatal POPs exposure was associated with ADHD diagnosis in childhood.
A pharmacokinetic model was used to distinguish between prenatal and postnatal exposure, in order to reduce the risk of exposure misclassification.
We conclude that there are strong indications that POPs exposure during pregnancy or up to 24 months is not associated with ADHD diagnosis in children 3–10 years of age, at exposure levels typical of the general population in Europe.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is considered the most common neurobehavioral disorder in childhood, with a worldwide prevalence of about 5.3% in children and adolescents.1 Although there are major challenges tied to shifts in diagnostics and awareness of disease, it is agreed upon that there has been a substantial increase in the prevalence of ADHD during the last 20–25 years.2 Environmental factors may contribute to the increase of the ADHD prevalence during the last decades, since genes are stable over the period studied, yet the nature of such factors remains poorly understood.
A recent joint World Health Organization (WHO)–UN study on chemicals with endocrine-disrupting effects suggests that chemicals are, in part, responsible for the rise in neurodevelopmental disorders.3 Persistent organic pollutants (POPs) are of particular concern due to their biomagnifying and bioaccumulating properties, which lead to high concentrations in humans. They transfer to the fetus and infant through placenta and mother’s milk, the infant’s body burden increases during the length of breastfeeding to levels manifold those of the mother then, after cessation of breastfeeding, decreases in parallel with the continued infant growth in body mass (which dilutes the concentration in blood).4
Among these chemicals are the polychlorinated biphenyls (PCBs), p-p´-dichlorodiphenyltrichloroethane (p-p´-DDT) and its metabolite p-p´-dichlorodiphenyldichloroethylene (p-p´-DDE) and hexachlorobenzene (HCB), which were all used extensively in agriculture and/or industry from the 1930s until their ban (or, in the case of p-p´-DDT, restriction).5 Epidemiological and experimental evidence suggests that PCBs, p-p´-DDE and HCB are developmental neurotoxicants.6 However, few studies have specifically studied these toxicants in relation to ADHD. In a study of 800 Danish women with PCBs, p-p´-DDE and HCB measured during pregnancy, then followed for 20 years through national registries, no relation between the measured concentrations and ADHD was observed in their offspring.7 Some other studies also have failed to find an association between PCBs, p-p´-DDE or HCB and ADHD symptomatology.8–13 However, in a well-conducted study of 607 mother–child pairs residing near a PCB-contaminated harbour in New Bedford (Massachusetts), the authors found moderate associations between PCB and p-p´-DDE serum levels in the mothers and ADHD symptomatology in their children.14 Pre- and postnatal exposure was later estimated in the same study population using a pharmacokinetic model, concluding that larger effects were observed for prenatal than for postnatal exposure to PCB-153 and ADHD-related behaviours at 8 years of age.15 In another study, prenatal exposure to HCB was associated with an increase in ADHD symptoms in 475 children at 4 years of age in Spain.16
The inconsistency in results could be due to modest study samples, misclassification of exposure, varying exposure ranges and varying underlying unmeasured confounding patterns. To overcome these limitations, we pooled data from seven European birth cohorts encompassing in total 4437 mother–child pairs with concentrations of PCB-153, p-p´-DDE and HCB measured in cord blood, maternal blood or milk. We calculated pre- and postnatal exposure up to 2 years of life in all infants using a pharmacokinetic model,4 which was validated using data from one of the cohorts, and studied the associations with ADHD in childhood. We selected the 2-year postnatal time window, since some crucial processes, such as synaptogenesis, synaptic pruning and myelination, occur not only during pregnancy, but also during the first years of life.17
Methods
Description of cohorts
We selected birth cohort studies with available information on POPs and ADHD symptoms using two online inventories (www.enrieco.org and www.birthcohorts.net). Eighteen cohorts had relevant data according to the registries and seven of these cohorts agreed to participate: INUENDO (Greenland, Poland and Ukraine sub-cohorts),18 FLEHS I (Belgium),12,19 PELAGIE (France),20 PCB cohort (Slovakia),21 INMA (Gipuzkoa, Menorca, Sabadell and Valencia sub-cohorts) (Spain), HUMIS (Norway)22 and DUISBURG (Germany).23 The population sample was restricted to: live-born singleton births with data on concentrations of PCB-153 and/or p-p´-DDE and/or HCB, measured either in maternal serum/whole blood, cord serum/plasma or breast milk; and available information on ADHD symptoms. The INUENDO-Poland cohort was originally invited but then excluded before statistical analysis due to the low number of subjects with complete information on ADHD and POPs (n = 69). In total, the study sample encompassed 4437 mother–child pairs from seven European cohort studies. Ethical approval was obtained from the local authorized Institutional Review Boards.
Exposure assessment
POP concentrations were measured in cord serum/plasma in FLEHS I, PCB cohort, PELAGIE and INMA-Menorca; in maternal serum/whole blood in DUISBURG, INUENDO-Greenland, INUNEDO-Ukraine, INMA-Valencia, INMA-Sabadell and INMA-Gipuzkoa; and in breast milk in HUMIS. INMA-Gipuzkoa also collected cord blood in a half of participants, although these data were not used in the present study. In addition, we used POPs measured in child serum at 6 and 16 months in the Slovakian cohort to validate the pharmacokinetic calculations. All cohorts provided wet-weight and lipid-adjusted POP concentrations, plus information on lipid collection time (Supplementary Table 1, available as Supplementary data at IJE online). In our study, we used the PCB-153 congener as a marker of PCB exposure. We replaced POP concentrations below the limit of detection/quantification (LOD/LOQ) with a uniform randomly generated number between zero and the analysis-specific threshold reported from the laboratories. The percentage of values below LOD/LOQ for PCB-153 and p-p´-DDE was less than 4.0% for most cohorts, with some notable exceptions: FLEHS I had 17.8% and PELAGIE 14.6% below LOD/LOQ, for PCB-153 and p-p´-DDE, respectively (Tables 3 and 4). For HCB, percentage of values below LOD/LOQ varied: four cohorts had samples below LOD ranging from 3.6 % to 24.2% (FLEHS I) (Table 5).
Table 3.
Infant blood concentrations for PCB-153 pre- and postnatal exposure (3, 6, 12 and 24 months), estimated through pharmacokinetic modelling for lipophilic compounds (ng/g lipid)
| Measured concentrations |
Estimated PCB-153 (ng/g lipid) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCB-153 (ng/g lipid) |
Birth |
3 months |
6 months |
12 months |
24 months |
||||||||||||||||
| Cohort | Matrix | n | P25 | Median | P75 | %<LOD | P25 | Median | P75 | P25 | Median | P75 | P25 | Median | P75 | P25 | Median | P75 | P25 | Median | P75 |
| INUENDO-Greenland | MB | 518 | 56.0 | 106.2 | 207.2 | 1.4% | 48.9 | 93.0 | 189.1 | 91.4 | 172.5 | 343.9 | 97.0 | 195.7 | 385.5 | 98.6 | 217.2 | 440.8 | 87.6 | 191.6 | 390.0 |
| INUENDO-Ukraine | MB | 490 | 17.2 | 26.3 | 37.5 | 3.7% | 14.5 | 22.3 | 32.3 | 25.1 | 38.2 | 55.9 | 26.3 | 42.4 | 63.5 | 23.9 | 40.5 | 65.8 | 21.2 | 35.8 | 58.5 |
| FLEHS I | CB | 256 | 16.9 | 33.8 | 57.1 | 17.8% | 16.9 | 33.8 | 57.2 | 10.1 | 29.9 | 68.1 | 7.8 | 25.9 | 69.7 | 6.4 | 21.9 | 58.2 | 5.7 | 18.9 | 52.5 |
| PELAGIE | CB | 188 | 23.6 | 35.6 | 55.6 | 0% | 23.6 | 35.6 | 55.6 | 11.0 | 28.8 | 61.8 | 7.8 | 22.6 | 58.0 | 6.4 | 18.5 | 49.8 | 5.9 | 16.5 | 45.7 |
| PCB cohort | CB | 455 | 87.6 | 132.9 | 207.4 | 0% | 87.6 | 132.9 | 207.4 | 83.6 | 191.7 | 317.0 | 77.5 | 183.8 | 342.7 | 70.2 | 168.0 | 341.6 | 63.8 | 154.3 | 311.0 |
| INMA-Menorca | CB | 304 | 57.0 | 74.6 | 105.0 | 1.2% | 57.0 | 74.6 | 105.0 | 36.9 | 113.8 | 168.5 | 26.3 | 105.7 | 176.4 | 20.9 | 90.1 | 148.0 | 18.7 | 79.5 | 128.1 |
| INMA-Valencia | MB | 347 | 36.8 | 52.3 | 68.3 | 2.4% | 31.8 | 45.3 | 60.0 | 29.0 | 62.7 | 94.7 | 22.2 | 66.8 | 112.3 | 18.1 | 59.8 | 111.3 | 16.2 | 51.6 | 99.0 |
| INMA-Sabadell | MB | 442 | 23.8 | 33.6 | 49.5 | 7% | 20.3 | 29.7 | 42.7 | 26.6 | 44.9 | 68.9 | 22.9 | 46.2 | 78.2 | 20.0 | 39.9 | 76.4 | 17.6 | 35.3 | 67.5 |
| INMA-Gipuzkoa | MB | 276 | 38.9 | 52.1 | 72.5 | 2.3% | 33.1 | 44.7 | 62.4 | 48.1 | 71.8 | 103.3 | 41.9 | 82.1 | 129.2 | 33.5 | 78.4 | 130.7 | 29.3 | 69.1 | 116.8 |
| HUMIS | M | 1015 | 24.9 | 32.2 | 42.9 | 0% | 27.5 | 36.0 | 47.4 | 47.1 | 62.0 | 81.8 | 61.5 | 82.8 | 110.0 | 68.4 | 94.6 | 129.7 | 70.2 | 103.5 | 144.1 |
| DUISBURG | MB | 112 | 43.0 | 63.0 | 87.0 | 0% | 39.9 | 56.8 | 79.4 | 61.5 | 96.5 | 138.1 | 72.6 | 121.4 | 175.1 | 78.8 | 148.0 | 221.3 | 70.2 | 139.6 | 203.3 |
MB: maternal blood; CB: cord blood; M: milk; LOD: limit of detection; P25: 25th percentile; P75: 75th percentile.
Table 4.
Infant blood concentrations for p-p´-DDE pre- and postnatal exposure (3, 6, 12 and 24 months), estimated through pharmacokinetic modelling for lipophilic compounds (ng/g lipid)
| Measured concentrations |
Estimated p-p’-DDE (ng/g lipid) |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
p-p’-DDE (ng/g lipid) |
Birth |
3 months |
6 months |
12 months |
24 months |
||||||||||||||||||||
| Cohort | Matrix | n | P25 | Median | P75 | %<LOD | P25 | Median | P75 | P25 | Median | P75 | P25 | Median | P75 | P25 | Median | P75 | P25 | Median | P75 | ||||
| INUENDO-Greenland | MB | 518 | 159.0 | 299.5 | 537.4 | 1.9% | 136.5 | 262.3 | 492.7 | 247.3 | 475.1 | 902.7 | 266.4 | 556.1 | 1030.8 | 266.8 | 585.0 | 1170.4 | 236.1 | 515.1 | 1031.2 | ||||
| INUENDO-Ukraine | MB | 490 | 427.3 | 634.3 | 909.7 | 0% | 361.3 | 541.0 | 782.5 | 627.6 | 925.6 | 1363.2 | 661.6 | 1017.8 | 1593.3 | 595.0 | 980.3 | 1612.1 | 528.6 | 874.7 | 1433.3 | ||||
| FLEHS I | CB | 256 | 67.8 | 125.2 | 218.9 | 0.4% | 67.8 | 125.2 | 218.9 | 58.5 | 134.6 | 272.4 | 44.0 | 122.9 | 268.3 | 35.9 | 98.9 | 246.2 | 30.9 | 86.7 | 218.9 | ||||
| PELAGIE | CB | 188 | 30.3 | 59.8 | 101.0 | 14.6% | 30.3 | 59.8 | 101.0 | 16.2 | 39.1 | 109.5 | 12.5 | 32.4 | 94.2 | 10.0 | 26.4 | 81.8 | 9.1 | 25.4 | 75.8 | ||||
| PCB cohort | CB | 455 | 313.0 | 507.6 | 817.0 | 0.2% | 313.0 | 507.6 | 817.0 | 283.8 | 690.7 | 1193.8 | 262.6 | 665.1 | 1277.1 | 223.2 | 615.5 | 1314.0 | 200.8 | 564.4 | 1203.0 | ||||
| INMA-Menorca | CB | 304 | 587.3 | 1062.0 | 1804.2 | 0% | 238.7 | 431.7 | 733.4 | 219.2 | 530.2 | 1063.4 | 197.3 | 551.4 | 1009.4 | 154.8 | 443.0 | 908.6 | 136.0 | 389.0 | 786.2 | ||||
| INMA-Valencia | MB | 347 | 110.3 | 179.4 | 288.3 | 0.5% | 96.7 | 153.5 | 248.2 | 102.7 | 201.9 | 396.9 | 88.4 | 225.8 | 468.1 | 72.8 | 201.6 | 453.2 | 63.7 | 179.1 | 396.6 | ||||
| INMA-Sabadell | MB | 442 | 71.9 | 116.1 | 181.9 | 0% | 63.2 | 103.0 | 157.7 | 91.1 | 150.9 | 256.9 | 88.6 | 153.6 | 285.1 | 72.8 | 143.6 | 281.4 | 64.1 | 126.1 | 248.6 | ||||
| INMA-Gipuzkoa | MB | 276 | 60.5 | 92.5 | 142.7 | 2.3% | 52.6 | 82.2 | 123.8 | 73.2 | 129.1 | 210.7 | 73.9 | 145.7 | 238.1 | 63.3 | 141.3 | 251.5 | 54.6 | 124.5 | 221.1 | ||||
| HUMIS | BM | 1015 | 32.3 | 46.6 | 71.3 | 0% | 35.7 | 51.6 | 79.1 | 60.3 | 88.9 | 136.8 | 75.8 | 116.8 | 181.7 | 85.7 | 134.2 | 209.6 | 91.1 | 145.4 | 236.6 | ||||
| DUISBURG | MB | 112 | 67.0 | 98.5 | 140.0 | 0% | 58.8 | 86.5 | 126.4 | 96.7 | 141.2 | 206.5 | 112.1 | 177.7 | 278.8 | 119.2 | 212.7 | 346.6 | 108.5 | 187.1 | 312.9 | ||||
MB, maternal blood; CB, cord blood; BM, breast milk; LOD, Limit of Detection; P25, 25th percentile; P75, 75th percentile.
Table 5.
Infant blood concentrations for HCB pre- and postnatal exposure (3, 6, 12 and 24 months), estimated through pharmacokinetic modelling for lipophilic compounds (ng/g lipid)
| Measured concentrations |
Estimated HCB (ng/g lipid) |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCB (ng/g lipid) |
Birth |
3 months |
6 months |
12 months |
24 months |
||||||||||||||||||||
| Cohort | Matrix | n | P25 | Median | P75 | %<LOD | P25 | Median | P75 | P25 | Median | P75 | P25 | Median | P75 | P25 | Median | P75 | P25 | Median | P75 | ||||
| INUENDO-Greenland | MB | 189 | 34.1 | 60.5 | 105.9 | 0% | 30.4 | 56.3 | 94.8 | 55.0 | 102.0 | 170.0 | 57.4 | 113.8 | 191.5 | 62.0 | 111.1 | 232.8 | 54.8 | 97.9 | 203.9 | ||||
| INUENDO-Ukraine | MB | 161 | 77.8 | 100.9 | 145.0 | 0% | 65.5 | 86.3 | 127.7 | 113.4 | 145.2 | 223.4 | 112.4 | 169.0 | 277.8 | 108.1 | 188.1 | 273.6 | 96.1 | 166.7 | 240.6 | ||||
| FLEHS I | CB | 248 | 10.2 | 22.3 | 36.5 | 24.20% | 10.1 | 22.3 | 36.5 | 6.1 | 21.2 | 43.3 | 5.0 | 17.3 | 46.8 | 3.9 | 14.2 | 40.3 | 3.5 | 12.5 | 35.5 | ||||
| PELAGIE | CB | 188 | 5.4 | 11.1 | 17.3 | 21.2% | 5.4 | 11.1 | 17.3 | 3.1 | 7.3 | 19.1 | 2.2 | 6.0 | 17.0 | 1.8 | 5.1 | 15.0 | 1.7 | 4.6 | 13.5 | ||||
| PCB cohort | CB | 452 | 41.2 | 75.6 | 133.1 | 3.6% | 41.2 | 75.6 | 133.1 | 34.9 | 103.4 | 199.8 | 27.4 | 98.7 | 215.1 | 22.2 | 88.5 | 204.9 | 20.3 | 79.4 | 191.6 | ||||
| INMA-Menorca | CB | 251 | 234.1 | 310.0 | 457.5 | 11.6% | 234.1 | 310.0 | 457.5 | 136.5 | 447.3 | 678.0 | 95.7 | 451.3 | 692.2 | 73.6 | 364.1 | 588.6 | 64.6 | 316.0 | 514.9 | ||||
| INMA-Valencia | MB | 347 | 40.5 | 74.6 | 118.2 | 4.3% | 34.0 | 64.1 | 104.4 | 32.4 | 82.3 | 151.7 | 30.0 | 81.7 | 171.4 | 24.8 | 71.2 | 160.5 | 21.7 | 62.0 | 140.4 | ||||
| INMA-Sabadell | MB | 442 | 22.8 | 40.3 | 65.2 | 8.1% | 19.5 | 35.3 | 58.3 | 28.2 | 53.0 | 86.3 | 27.5 | 54.8 | 93.2 | 22.4 | 49.4 | 89.5 | 19.6 | 43.6 | 78.4 | ||||
| INMA-Gipuzkoa | MB | 276 | 22.1 | 35.2 | 57.3 | 8.4% | 18.3 | 30.8 | 49.3 | 27.8 | 45.2 | 84.1 | 23.3 | 52.8 | 89.8 | 19.3 | 50.5 | 94.4 | 16.7 | 44.7 | 83.3 | ||||
| HUMIS | BM | 1015 | 8.7 | 10.7 | 13.1 | 0% | 9.5 | 11.8 | 14.6 | 16.3 | 20.5 | 25.3 | 20.9 | 27.5 | 34.5 | 23.0 | 31.7 | 40.4 | 23.4 | 34.2 | 46.0 | ||||
| DUISBURG | MB | 112 | 21.0 | 27.0 | 39.0 | 0% | 18.1 | 23.9 | 34.8 | 28.9 | 39.0 | 58.0 | 34.2 | 48.6 | 76.6 | 34.4 | 65.2 | 94.0 | 30.4 | 61.0 | 91.7 | ||||
MB: maternal blood; CB: cord blood; BM: breast milk; LOD: Limit of Detection; P25: 25th percentile; P75: 75th percentile.
The pharmacokinetic model: input variables
We calculated individual child PCB-153, p-p´-DDE and HCB concentrations at birth, 3, 6, 12, 24 months based on a pharmacokinetic model.4
The model’s equations use a number of input variables: child’s and mother’s weight at birth, 3, 6, 12 and 24 months and proportion of breastfeeding at each month, summed up to a cumulative proportion of breastfeeding at 3, 6, 12 and 24 months.
Child’s weight data at birth and at multiple and varying age points during first years of life were measured: by nurses or doctors (PCB cohort); recorded during paediatric examinations in children’s health cards and reported by parents (HUMIS); obtained by study staff and from paediatric records (INMA); or parent-reported (FLEHS I, DUISBURG, PELAGIE and INUENDO). We used the obtained data to estimate child’s weight at 3, 6, 12 and 24 months, using a cohort-specific, sex-specific, multilevel (mixed) linear model fitted with cubic polynomials, adjusted for child’s height at birth, birth weight, gestational age, parity, maternal height, maternal weight and age at delivery and with random effects for infant. We used estimates that incorporated the estimated random effect for each individual. For DUISBURG and INUENDO, we used standard WHO growth curves24 as they had information on child’s weight on fewer than two data points after birth.
Maternal weight and height were based on self-reports in all cohorts. For all cohorts except for HUMIS, only pre-pregnancy weight was available. In the HUMIS cohort, maternal weight at the end of pregnancy, after delivery, at milk-sampling time and at 6, 12, and 24 months was also collected. We used the HUMIS maternal weight data to fit a predictive model of maternal weight development postpartum, using an ordinary linear regression model with a cubic polynomial in child’s age plus mother’s height, age, pre-pregnancy weight, duration of total and exclusive breastfeeding and parity, and used this model to estimate individual maternal weights at delivery, 3, 6, 12 and 24 months for all cohorts (see Supplementary Methods 2, available as Supplementary data at IJE online).
Duration of exclusive and partial breastfeeding was reported in all cohorts. During partial breastfeeding, the proportion of human milk to other food steadily declines and this decline was mapped in HUMIS where 2600 mothers had been asked to report the proportions of milk to other food at each month during the first 12 months. These proportions were assumed to apply for children with the same duration pattern of exclusive and partial breastfeeding in other cohorts, and used to estimate cumulative monthly proportion of breast milk to other food. Finally, the proportion of breastfeeding at each month was summed up to a cumulative proportion of breastfeeding at 3, 6, 12 and 24 months.
Pharmacokinetic model
For cohorts with POPs measured in cord blood, this was taken to represent exposure at birth. We then calculated postnatal exposure using three equations for the transfer of POPs from mother to child4 (see also Supplementary Methods 3, available as Supplementary data at IJE online).
The first equation estimates the transfer of milk fat to the infant during the breastfeeding period. We first used published equations25 to estimate the infant’s total need for milk, taking the infant’s age, sex and birth weight into consideration. Then we multiplied the total need by the cumulative milk proportions, and used a standard milk fat percentage of 3.4% to calculate the amount of milk fat ingested by the infant.4
The second equation calculates temporal concentrations of POPs in breast milk, taking into account the decrease in concentration due to transfer of POPs from the mother to the child, as well as any changes in concentration due to maternal weight changes.
The third and final equation calculates the concentration in the infant, based on the amount of milk fat consumed by the infant up to the given age, the temporal concentration of POPs in the milk fat and the dilution in concentration due to infant body growth in the same period.
The equations allow both forward and backward calculations and, depending on the matrix wherein the concentrations were measured (maternal pregnancy serum, cord blood, breast milk), different applications of the model were used to encompass appropriate calculations and derive the needed estimates. Thus, the same calculations were used to back-calculate human milk concentrations to estimated concentrations in maternal blood at time of delivery. Model equations were performed using STATA 13 (Stata Corporation, College Station).
Validation of the pharmacokinetic model
We evaluated the exposure estimates obtained by using our pharmacokinetic model with concentrations measured in serum at age 6 and 16 months in 455 children from the Slovakian PCB cohort. We calculated the Spearman correlation between estimated and measured blood concentrations for PCB-153, p-p´-DDE and HCB due to skewed distributions with long tails towards high values in both measured and predicted values. The Spearman correlations (with errors excluded as described in Supplementary Methods 3, available as Supplementary data at IJE online) were 0.75 (PCB-153, N = 311), 0.76 (p-p´-DDE, N = 311) and 0.77 (HCB, N = 278) at 6 months and were 0.79 (PCB-153, N = 308), 0.82 (p-p´-DDE, N = 309) and 0.79 (HCB, N = 278) at 16 months. The explained variances from linear regressions for PCB-153, p-p´-DDE and HCB on the logged data were 0.61, 0.56 and 0.62 at 6 months, respectively; at 16 months, they were 0.67, 0.66 and 0.66, respectively. Results from this evaluation are presented in Supplementary Methods 4 and Supplementary Figures 5 and 6, available as Supplementary data at IJE online.
ADHD assessment
We used patient registries and three different questionnaire instruments to assess ADHD symptoms.
In the HUMIS cohort, information on ADHD was obtained from the Norwegian Patient Registry,26 which held updated diagnosis of ADHD up to August 2014, at a time when the children’s mean and median age was 10 years (range 6–12 years). In the rest of the cohorts, three different questionnaires were used: the Attention syndrome scale of the Child Behavior Checklist for Toddlers (CBCL-ADHD)27 was used in the PCB cohort (4 years); the Hyperactivity/Inattention problems subscale of the Strengths and Difficulties Questionnaire (SDQ-Hyperactivity/Inattention)28 was used in INUENDO (7–8 years), FLEHS I (8 years) and PELAGIE (6 years); and, finally, the ADHD Criteria of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (ADHD-DSM-IV) list,29 was used in the four INMA regions (4–5 years) and DUISBURG (9 years) (Table 1). All questionnaires were completed by parents except in the INMA cohorts, where they were completed by teachers. For all tests, higher scores indicated more ADHD symptomatology. We used established cut-offs reported in the literature to correspond to clinical ADHD symptomatology. The CBCL-ADHD is based on the sum of six items (range 0–12). The raw scores have been standardized within each individual in the PCB cohort (using a T-score distribution) and then a T-score > 65 was used as the cut-off score to classify children with ADHD.27 The SDQ-hyperactivity/Inattention is composed of five items (total scores obtainable ranges from 0 to 10). A score ≥7 points has been used to classify children with ADHD.28 Finally, ADHD-DSM-IV consists of 18 symptoms categorized into two separate groups: inattention (9 symptoms) and hyperactivity/impulsivity (9 symptoms). Each ADHD symptom is rated on a four-point scale (0 = never or rarely, 1 = sometimes, 2 = often or 3 = very often). Then, in the binary scoring method, the first two response options of the original responses (i.e. options 0 and 1) are recoded as the symptom being absent (recoded as 0), whereas the next two response options (i.e. options 2 and 3) are recoded as the symptom being present (recoded as 1). A score of ≥6 symptoms of inattention or hyperactivity was used as the cut-off for an ADHD case.29 Our operational definition of ADHD was either symptoms above the aforementioned thresholds or a doctor diagnosis registered in a patient registry.
Table 1.
Description of the outcome
| Cohort | Country | Test | Evaluator | Age (years) mean (SD) | N | N with ADHD | (%) |
|---|---|---|---|---|---|---|---|
| INUENDO-Greenland | Greenland | SDQ | Parents | 7.9 (0.6) | 518 | 31 | 6.0 |
| INUENDO-Ukraine | Ukraine | SDQ | Parents | 7.0 (0.4) | 490 | 26 | 5.3 |
| FLEHS I | Belgium | SDQ | Parents | 8.3 (0.3) | 256 | 39 | 15.2 |
| PELAGIE | France | SDQ | Parents | 6.0 (0.2) | 188 | 13 | 6.9 |
| PCB cohort | Slovakia | CBCL-ADHD | Parents | 3.8 (0.1) | 455 | 29 | 6.4 |
| INMA-Menorca | Spain | ADHD-DSM-IV | Teachers | 4.6 (0.3) | 304 | 49 | 16.1 |
| INMA-Valencia | Spain | ADHD-DSM-IV | Teachers | 5.9 (0.4) | 347 | 25 | 7.2 |
| INMA-Sabadell | Spain | ADHD-DSM-IV | Teachers | 4.4 (0.3) | 442 | 23 | 5.2 |
| INMA-Gipuzkoa | Spain | ADHD-DSM-IV | Teachers | 4.4 (0.2) | 310 | 8 | 2.6 |
| HUMIS | Norway | NPR | Medical doctor | 7.0 (0.1) | 1015 | 25 | 2.5 |
| DUISBURG | Germany | ADHD-DSM-IV | Parents | 9.5 (0.4) | 112 | 7 | 6.3 |
NPR, Norwegian Patient Registry; CBCL-ADHD, Attention-Deficit and Hyperactivity problems subscale of the Child Behavior Checklist for Toddlers; SDQ, Hyperactivity/Inattention problems subscale of the Strengths and Difficulties Questionnaire; ADHD-DSM-IV, ADHD Criteria of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (ADHD-DSM-IV).
From here on, we will refer to the main outcome of the study as ADHD.
Other variables
We identified eight potential confounders a priori using directed acyclic graphs (DAGs) (Supplementary Figure 7, available as Supplementary data at IJE online): maternal pre-pregnancy body mass index (BMI, continuous), maternal age (years, continuous), maternal education (low, medium, high), maternal smoking during pregnancy (yes/no), maternal parity (nulliparous yes/no), duration of total breastfeeding (months, continuous) and child’s sex (male/female). Categories for primary and secondary education varied, so we combined categories to create relative low, medium and high per cohort. Models testing the association up to birth were not adjusted by duration of total breastfeeding.
Statistical analyses
We imputed missing data on covariates, separately by cohort, using multiple imputation by chained equations assuming data is missing at random30,31 and, for each imputation set (n = 5), we ran the equations that estimate the postnatal concentrations of POPs. We combined exposure, outcome and covariate data from individual cohorts into one pooled data set and then used a multilevel (mixed) logistic regression model to estimate associations between exposure to POPs at birth, 3, 6, 12 and 24 months and ADHD. Models were fitted via maximum likelihood, using the STATA 13.0 ‘mi estimate’ function to pool five imputation results. The predictors used in the fixed-effects part of the model were the exposure and the potential confounders, and cohort was included as an independent random-effect part of the model. For each compound and time-point, we tested for heterogeneity of effects over cohorts by adding both a random intercept and a random slope with independent variances for the intercept and the slope term and the covariance set to zero. We assessed assumptions of linearity (on the log-odds scale) using cubic splines [comparing Akaike information criterion (AIC) and Bayesian information criterion (BIC) statistics for linear term model and spline model] and diagnostic plots, and calculated delta-betas (from corresponding logistic models without random terms) to find possible influential observations. We assessed assumptions of normality of residuals and assessed the combination of high leverage and residuals in order to fit regression models with and without influential observations. We also tested possible effect modification by child’s sex, maternal education and parity using a p-value < 0.10 to indicate statistical significance. As a sensitivity analysis, we repeated the main analysis leaving out one cohort at a time to determine the influence of a particular cohort and leaving out cohorts with the highest and lowest prevalence of ADHD. We repeated the models stratifying by matrix type: maternal blood cohorts, cord blood cohorts and breast milk cohorts. We also repeated the models but excluding the three cohorts (INUENDO-Greenland, INUENDO-Ukraine and DUISBURG) with fewer than two measurements on children’s weight after birth. Moreover, we re-ran the analysis for the five cohorts where SDQ had been applied and where we thus had the opportunity to separate inattentive and hyperactive symptomatology; SDQ-inattention (SDQ-items 15 and 25) ranged from 0 to 4, whereas SDQ-hyperactivity (SDQ-items 2, 10 and 21) ranged from 0 to 6. We then used a multilevel (mixed) negative binomial regression model to estimate associations between exposure to POPs at birth, 3, 6, 12 and 24 months and SDQ-inattention and SDQ-hyperactivity. Models were fitted via maximum likelihood, using the STATA 13.0 ‘mi estimate’ function to pool the five imputation datasets. The predictors used in the fixed-effects part of the model were the exposure and the potential confounders, and cohort was included as the independent random-effect part of the model.
Results
The global prevalence of ADHD in our study was 6.0% (range: 2.5–16.1) and Table 1 shows the outcome distribution in all participating cohorts. The mean age of assessment of ADHD was 5.8 years (range: 3.8–9.5 years). Percentage of highly educated mothers and duration of breastfeeding varied markedly across cohorts (Table 2). Tables 3–5 show the measured POPs concentrations as well as the estimated pre- and postnatal concentrations. In general, the highest prenatal and postnatal concentrations for the three POPs were observed in INUENDO-Greenland, INUENDO-Ukraine (except for PCB-153), PCB cohort and INMA-Menorca. The lowest prenatal and postnatal concentrations were observed in PELAGIE, HUMIS and FLEHS I (except for p-p´-DDE). We observed moderate to high correlations between prenatal and postnatal POP concentration (Supplementary Table 8, available as Supplementary data at IJE online). These correlations tended to decrease over the child’s age, the highest correlations observed between the prenatal and 3-month concentrations. Overall correlations for PCB-153 between pre- and postnatal ranged from 0.82 (prenatal vs 3 months) to 0.64 (prenatal vs 24 months); for p-p´-DDE, correlations ranged between 0.91 (prenatal vs 3 months) and 0.76 (prenatal vs 24 months); and for HCB, correlations ranged between 0.89 (prenatal vs 3 months) and 0.72 (prenatal vs 24 months).
Table 2.
Characteristics of the cohorts [mean (SD) or n (%)]
| INUENDO |
INMA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Greenland (n = 518) | Ukraine (n = 490) | FLEHSI (n = 256) | PELAGIE (n = 188) | PCB cohort (n = 455) | Menorca (n = 304) | Valencia (n = 347) | Sabadell (n = 442) | Gipuzkoa (n = 310) | HUMIS (n = 1015) | DUISBURG (n = 112) | |
| Low birth weight (<2500 g) | 22 (4.2) | 12 (2.5) | 6 (2.3) | 3 (1.6) | 18 (3.9) | 11 (3.6) | 22 (6.3) | 18 (4.1) | 11 (3.6) | 75 (7.4) | 6 (5.4) |
| Gestational age (weeks) | 39.6 (1.7) | 39.1 (1.2) | 39.2 (1.3) | 39.5 (1.2) | 39.7 (1.0) | 39.3 (1.5) | 39.5 (1.9) | 39.7 (1.3) | 39.8 (1.4) | 39.6 (2.5) | 39.7 (1.25) |
| Missings (%) | 0 | 2 (0.4) | 0 | 0 | 5 (1) | 0 | 0 | 0 | 0 | 3 (0.3) | 0 |
| Determination of GA | |||||||||||
| 1st day last menstruation | 518 (100) | 490 (100) | – | – | – | – | 347 | 442 | 310 | – | 112 (100) |
| Ultrasound | – | – | – | – | 456 (100) | – | – | – | – | – | – |
| Combination 1&2 | – | – | – | – | – | – | – | – | – | – | – |
| Other | – | – | 256 (100) | 188 (100) | – | 304 (100) | – | – | – | 1015 | – |
| Missings (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Term | |||||||||||
| Preterm (<37 weeks) | 23 (4.4) | 9 (1.8) | 12 (4.7) | 2 (1.1) | 5 (1.1) | 13 (4.3) | 23 (6.6) | 13 (2.9) | 11 (3.6) | 114 (11.2) | 5 (4.5) |
| Term (37–42 weeks) | 487 (94.0) | 479 (97.8) | 244 (95.3) | 186 (98.9) | 446 (97.8) | 291 (95.7) | 317 (91.4) | 426 (96.4) | 293 (94.5) | 806 (79.4) | 107 (95.5) |
| Over term (>42 weeks) | 8 (1.6) | – | – | – | – | – | 7 (2.0) | 3 (0.7) | 6 (1.9) | 92 (9.1) | – |
| Missings (%) | 0 | 2 (0.4) | 0 | 0 | 5 (1.1) | 0 | 0 | 0 | 0 | 3 (0.3) | 0 |
| Child’s sex | |||||||||||
| Boy | 277 (53.5) | 259 (52.9) | 122 (47.7) | 89 (47.3) | 226 (49.6) | 146 (48.7) | 180 (51.9) | 227 (51.4) | 155 (50.0) | 555 (54.7) | 63 (56.3) |
| Girl | 241 (46.5) | 229 (46.7) | 134 (52.3) | 99 (52.7) | 229 (50.2) | 156 (51.3) | 167 (48.1) | 215 (48.6) | 155 (50.0) | 458 (45.1) | 49 (43.7) |
| Missings (%) | 0 | 2 (0.4) | 0 | 0 | 1 (0.2) | 0 | 0 | 0 | 0 | 2 (0.2) | 0 |
| Maternal age at delivery | |||||||||||
| <25 years | 119 (23.0) | 258 (52.6) | 17 (6.6) | 13 (6.9) | 189 (41.5) | 37 (12.2) | 11 (3.2) | 18 (4.1) | 4 (1.3) | 155 (15.3) | 4 (3.6) |
| 25–29 years | 56 (10.8) | 135 (27.6) | 113 (44.1) | 72 (38.3) | 159 (34.9) | 120 (39.5) | 93 (26.8) | 128 (29.0) | 51 (16.5) | 350 (34.5) | 20 (17.9) |
| 30–34 years | 40 (7.7) | 55 (11.2) | 101 (39.5) | 74 (39.4) | 72 (15.8) | 107 (35.2) | 172 (49.6) | 191 (43.2) | 171 (55.2) | 343 (33.8) | 51 (45.5) |
| ≥35 years | 43 (8.3) | 18 (3.7) | 24 (9.4) | 29 (15.4) | 26 (5.7) | 40 (13.1) | 68 (19.6) | 104 (23.5) | 84 (27.1) | 167 (16.4) | 37 (33.0) |
| Missings (%) | 260 (50.2) | 24 (4.9) | 1 (0.4) | 0 | 9 (2.0) | 0 | 3 (0.86) | 1 (0.2) | 0 | 0 | 0 |
| Maternal pre-pregnancy BMI | |||||||||||
| <18.5 kg/m2 | 16 (3.1) | 72 (14.7) | 12 (4.7) | 10 (5.3) | 67 (14.7) | 0 | 11 (3.2) | 25 (5.7) | 10 (3.2) | 39 (3.8) | 0 |
| 18.5–24.9 kg/m2 | 316 (61.0) | 347 (70.8) | 183 (71.5) | 143 (76.1) | 264 (57.9) | 145 (47.7) | 234 (67.4) | 292 (66.1) | 242 (78.1) | 609 (60.0) | 47 (42.0) |
| 25–29.9 kg/m2 | 129 (24.9) | 52 (10.6) | 39 (15.2) | 26 (13.8) | 65 (14.3) | 119 (39.1) | 67 (19.3) | 84 (19.0) | 44 (14.2) | 234 (23.1) | 49 (43.8) |
| ≥30 kg/m2 | 53 (10.2) | 11 (2.2) | 16 (6.3) | 8 (4.3) | 20 (4.4) | 28 (9.2) | 35 (10.1) | 41 (9.3) | 14 (4.5) | 105 (10.3) | 16 (14.2) |
| Missings (%) | 4 (0.8) | 8 (1.6) | 6 (2.3) | 1 (0.5) | 39 (8.6) | 12 (3.95) | 0 | 0 | 0 | 28 (2.8) | 0 |
| Maternal height (cm) | 162 (6) | 165 (5) | 166 (6) | 164 (5) | 164 (6) | 160 (5.8) | 162 (6.3) | 162 (5.9) | 163 (5.9) | 167 (6.3) | 168 (6) |
| Missings (%) | 3 (0.6) | 5 (1.0) | 3 (1.2) | 1 (0.5) | 15 (3.2) | 12 (4.0) | 0 | 0 | 0 | 15 (1.5) | 0 |
| Parity | |||||||||||
| 0 | 165 (31.8) | 392 (80.0) | 145 (56.6) | 79 (42.0) | 178 (39.0) | 127 (41.8) | 198 (57.1) | 253 (57.2) | 166 (53.6) | 421 (41.5) | 60 (53.6) |
| ≥1 | 353 (68.2) | 98 (20.0) | 111 (43.4) | 109 (58.0) | 277 (60.8) | 177 (58.2) | 149 (42.9) | 187 (42.3) | 144 (46.4) | 593 (58.4) | 52 (46.4) |
| Missings (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (0.5) | 0 | 1 (0.1) | 0 |
| Maternal education | |||||||||||
| Low | 260 (50.2) | 262 (53.5) | 13 (5.1) | 28 (14.9) | 207 (45.4) | 175 (57.6) | 91 (26.2) | 105 (23.8) | 34 (11.0) | 86 (8.5) | 13 (11.6) |
| Medium | 191 (36.9) | 168 (34.3) | 188 (73.4) | 33 (17.6) | 219 (48.0) | 73 (24.0) | 152 (43.8) | 189 (42.8) | 115 (37.1) | 136 (13.4) | 39 (34.8) |
| High | 7 (1.3) | 0 | 53 (20.7) | 126 (67.0) | 27 (5.9) | 45 (14.8) | 104 (30.0) | 145 (32.8) | 160 (51.6) | 779 (76.8) | 60 (53.6) |
| Missings (%) | 60 (11.6) | 60 (12.2) | 2 (0.8) | 1 (0.5) | 2 (0.4) | 11 (3.6) | 0 | 3 (0.7) | 1 (0.3) | 14 (1.4) | 0 |
| Duration of total breastfeeding (exclusive and partial) (months) | 9.27 (9.3) | 7.7 (3.9) | 4.44 (5.6) | 3.1 (3.8) | 8.7 (8.8) | 4.81 (4.2) | 5.31 (4.3) | 6.40 (4.6) | 6.71 (4.6) | 12.2 (5.3) | 9.2 (5.3) |
| Missings (%) | 69 (13.3) | 44 (8.9) | 14 (5.1) | 2 (1.1) | 5 (1.1) | 0 | 0 | 1 (0.2) | 13 (4.2) | 7 (0.7) | 13 (11.0) |
| Duration of exclusive breastfeeding (months) | 4.02 (1.6) | 4.51 (1.7) | 2.13 (2.1) | – | 3.07 (1.8) | 2.40 (2.1) | 2.83 (2.2) | 2.32 (2.0) | 3.0 (2.2) | 4.6 (2.0) | 5.12 (2.4) |
| Missings (%) | 146 (28.2) | 46 (9.4) | 7 (2.5) | – | 3 (0.7) | 0 | 0 | 4 (0.9) | 23 (7.4) | 7 (0.7) | 14 (11.8) |
| Maternal smoking during pregnancy | |||||||||||
| Non-smoking | 54 (10.4) | 248 (50.6) | 234 (91.4) | 166 (88.3) | 368 (80.7) | 197 (64.8) | 213 (61.4) | 317 (71.7) | 238 (76.8) | 750 (73.9) | 94 (83.9) |
| Yes | 376 (72.6) | 112 (22.9) | 21 (8.2) | 20 (10.6) | 71 (15.6) | 107 (35.2) | 134 (38.6) | 119 (26.9) | 62 (20.0) | 67 (6.6) | 18 (16.1) |
| Missings (%) | 88 (17.0) | 130 (26.5) | 1 (0.4) | 2 (1.1) | 16 (3.5) | 0 | 0 | 6 (1.4) | 10 (3.2) | 198 (19.5) | 0 |
| Type of sample | |||||||||||
| Blood mother | 518 (100) | 490 (100) | – | – | – | – | 347 | 442 | 310 | – | 112 (100) |
| Cord blood | – | – | 256 (100) | 188 (100) | 455 (100) | 304 (100) | – | – | – | – | – |
| Milk | – | – | – | – | – | – | – | – | – | 1015 | – |
| Missings (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
BMI, body mass index; GA, gestational age.
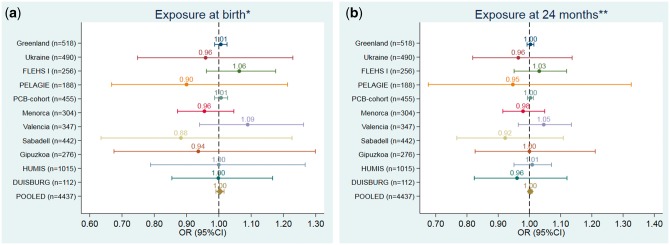
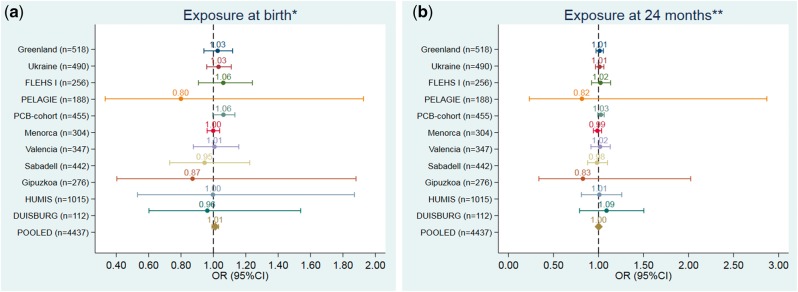
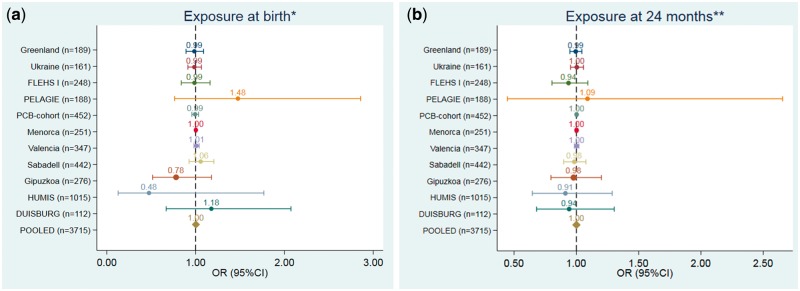
Figures 1–3 and Supplementary Tables 9–11, available as Supplementary data at IJE online, show the adjusted association between POPs exposure at different time-points and ADHD. The pooled data showed no associations between PCB-153, p-p´-DDE and HCB exposure at any of the time-points and ADHD [pooled odds ratios (ORs) ranging from 1.00 to 1.01]. These null OR point estimates were precise, with 95% confidence intervals (CIs) ranging from 0.99 to 1.01 for PCB-153, from 0.99 to 1.03 for p-p´-DDE and from 1.00 to 1.01 for HCB. None of the individual cohort analyses showed any clear trend association between POPs exposure and ADHD. The only exception was for p-p´DDE, were a borderline increased OR was observed for the PCB cohort at birth and 24 months, although, in both cases, the lower confidence limit comprised the null value. There was no heterogeneity between cohorts in any of the analysis for any exposure (Supplementary Table 12, available as Supplementary data at IJE online). There were no deviations from linearity (from AIC and BIC statistics comparing linear term model vs spline models) between POPs and ADHD (Supplementary Table 13, available as Supplementary data at IJE online). There was no significant difference in estimates from regression models fitted with and without influential observations (data not shown). We observed no effect modification by child’s sex, maternal education or parity in the pooled analysis (data not shown).
Figure 1.
Associations between pre- and postnatal (at 24 months) exposure to PCB-153 (ng/g) and the risk of ADHD (based on 10 ng/g lipid PCB-153 increase). (a) Exposure at birth. (b) Exposure at 24 months. *Models were adjusted for: maternal pre-pregnancy body mass index (kg/m2, continuous), maternal age (years, continuous), maternal education (low, medium, high), maternal smoking during pregnancy (yes/no), maternal parity (nulliparous, yes/no) and child’s sex (male/female). **Models were also adjusted for duration of total breastfeeding (months, continuous).
Figure 2.
Associations between pre- and postnatal (at 24 months) exposure to p-p´-DDE (ng/g) and the risk of ADHD (based on 100 ng/g lipid p-p´-DDE increase). (a) Exposure at birth. (b) Exposure at 24 months. *Models were adjusted for: maternal pre-pregnancy body mass index (kg/m2, continuous), maternal age (years, continuous), maternal education (low, medium, high), maternal smoking during pregnancy (yes/no), maternal parity (nulliparous, yes/no) and child’s sex (male/female). **Models were also adjusted for duration of total breastfeeding (months, continuous).
Figure 3.
Associations between pre- and postnatal (at 24 months) exposure to HCB (ng/g) and the risk of ADHD (based on 100 ng/g lipid HCB increase). (a) Exposure at birth. (b) Exposure at 24 months. *Models were adjusted for: maternal pre-pregnancy body mass index (kg/m2, continuous), maternal age (years, continuous), maternal education (low, medium, high), maternal smoking during pregnancy (yes/no), maternal parity (nulliparous, yes/no) and child’s sex (male/female). **Models were also adjusted for duration of total breastfeeding (months, continuous).
When we removed one cohort at a time, pooled estimates did not change (data not shown). When we repeated the analyses restricting to the type of matrix (maternal blood, cord blood and milk), results were similar (data not shown). Also, we did observe the same results when the three cohorts with fewer than two measurements on children’s weight after birth (INUENDO-Greenland, INUENDO-Ukraine and DUISBURG) were excluded from the analyses (data not shown). When we separated SDQ scores into primarily inattentive or primarily hyperactive symptomatology and used these scores continuously, also no association was observed between POPs exposure and any outcome (Supplementary Table 14, available as Supplementary data at IJE online). Excluding cohorts with high (FLEHS I and INMA-Menorca) or low (HUMIS) prevalence of ADHD did not change the results (data not shown).
Discussion
To our knowledge, this is the largest study to investigate the association between prenatal and postnatal exposure to POPs and ADHD in the general population. In a sample of 4437 children with an estimated prevalence of ADHD about 6%, we did not observe any association between either pre- or postnatal exposure (up to 24 months) to PCB-153, p-p´-DDE and HCB and the risk of ADHD before the age of 10 years.
This study has several strengths: the large sample size (almost 4500 children in seven European cohort studies), prenatal and postnatal exposure assessment using a model to estimate postnatal exposure to lipophilic environmental toxicants, a wide range of exposure concentrations and centralized statistical analysis allowing a standardized protocol to increase comparability of the data. Furthermore, we were able to adjust for all known potential confounders. Additionally, the study design by itself controls for unmeasured confounding to some degree, since the underlying confounding patterns are likely to vary across countries.
However, there are some limitations. A weakness of the present study is related to the outcome measurement. Different questionnaires (SDQ, CBCL-ADHD and ADHD-DSM-IV) with different evaluators (parents and teachers) were used, and only one cohort had information on specialist diagnosis from medical registries. These questionnaires include different numbers of questions and assign different weight to the main ADHD symptoms (inattention, hyperactivity or impulsivity), although they all likely to tap into overlapping aspects associated with ADHD. Furthermore, the diagnosis of ADHD is uncertain in preschool children, since it has been shown that only ∼50% of the children diagnosed before 4 years of age will still fulfil the diagnostic criteria at 6 years of age.32 In our study, the age at assessment of ADHD ranged from 3.8 to 9.5 years, with approximately 10% between 3.8 and 4 years of age. However, we did obtain an overall prevalence of 6% ADHD in our study, which is not far from the worldwide prevalence of 5.3%.1 In addition, we consistently found no associations between prenatal and postnatal exposure to PCB-153, p-p´-DDE and HCB and ADHD, robust in a number of sensitivity analyses, and also when restricting to the HUMIS cohort that had objective diagnosis from its national registry. The use of ADHD diagnosis instead of continuum of ADHD symptoms might reduce our ability to capture subclinical effects of POPs on neuropsychological development that may be important for population health. However, in cohorts that had assessed ADHD using the SDQ questionnaire (INUENDO-Greenland, INUENDO-Ukraine, FLEHS I, PELAGIE and PCB cohort), we did not detect an association between POPs exposure and ADHD symptoms as a continuous outcome (data not shown).
Another potential limitation of the present study could be the heterogeneity in the models. As shown in Supplementary Table 12, available as Supplementary data at IJE online, the random-effects parameters (the standard deviation of the random intercept), which represent the baseline level of the outcome (ADHD), between the cohorts are different from zero, meaning that the cohorts have different prevalence of ADHD. However, the standard deviation of the random-slope term, which represents the difference in the effect of the exposures on ADHD over the cohorts, is close to zero, which means that, in this study, there is no difference in the effect of the toxicants on ADHD over the cohorts. Thus, the different levels of ADHD across cohorts cannot be explained by differential population sensitivity to the toxicants studied, but are likely due to other risk factors not studied in this paper.
Another major limitation is that POPs were measured in three different matrices, and therefore at three different time-windows: maternal blood during pregnancy, cord blood and milk. Although, these toxicants are stable in adults due to their long half-lives (PCB-153 about 14 years,33p-p´-DDE about 13 years34 and HCB about 9 years),35 their concentrations vary substantially in the perinatal period. Thus, we modelled postnatal exposure.4 Although there are uncertainties tied to such models (amongst others, they presuppose equilibrium between the fat compartments in the mother and her fetus, of which we lack exact knowledge), their use is necessary to avoid drawing multiple blood samples from the infants. This exposure model was selected in view of its efficiency and simplicity compared with the previous postnatal models. Moreover, we evaluated our chosen exposure model using data from the PCB cohort where actual concentrations had been measured in child blood at 6 and at 16 months. We observed satisfactory results by showing a moderate to high correlation (Spearman Rho > 0.75) between estimated and measured POP concentrations. Moreover, the explained variances from linear regressions on the logged data were 0.61, 0.56 and 0.62 at 6 months, respectively. At 16 months, values were similar (0.67 for PCB-153), 0.66 for p-p´-DDE and 0.66 for HCB). These values are similar to or better than those obtained by a previously published pharmacokinetic method:36 0.59 (N = 216), 0.49 (N = 216) and 0.40 (N = 210).
Further, it seems likely that misclassification will increase with the time between measured concentration and the age at estimated concentration, and this could lead to less power to detect an effect of exposure with later age. However, the effect estimates in our study are consistent over time. There is also misclassification based on the way we calculate fat percentage based on measured BMI. This may lead to a differential bias, as BMIs in the normal range are less accurate in estimating the fat percentage than BMIs in the higher range, and ADHD is associated with BMI.37 However, we observed no interaction with obesity in our study. We show that, when applying our model, the predicted values tend to be systematically higher than the measured values in the low end of the concentrations. This leads to an overestimation of the estimated exposure effects (Supplementary Figures 5 and 6, available as Supplementary data at IJE online). However, importantly for this study, the overestimation goes to zero when the true exposure effect goes to zero.
Child’s weight after birth at a number of different ages was available in most cohorts and we used this to estimate child’s weight at the selected time-points. However, we did not have information about change in child weight in three cohorts and instead used general growth curves from the WHO, which may have reduced the precision of the estimated postnatal values for those three specific cohorts. Maternal weight change after delivery was also estimated at the selected time-points based on measured change in maternal weight after delivery in HUMIS and pre-pregnancy BMI in the other cohorts. Nutrition data were not available and therefore no information was added about the environmental chemicals exposure through food or water. These calculations may have decreased model precision and could lead to exposure misclassification, decreasing our chance of observing an effect. However, breastfeeding is the major source of body burden of PCB, DDE and HCB in young children,36,38 and we had more accurate information on breastfeeding in some cohorts, where mothers were asked about not only total breastfeeding, but the proportion of breastfeeding during the partial-breastfeeding period. We did a number of sensitivity analyses, amongst others excluding cohorts with fewer than two measurements on children’s weight, and the results remained the same, indicating that no major bias has occurred. In accordance with other studies,39,40 we chose lipid-adjusted POP concentrations and did not apply conversion factors due to the considerable uncertainty associated with these. Study-specific conversion factors are difficult to apply to other studies with differing distributions of underlying co-factors.
The null effects of PCB-153, p-p´-DDE and HCB exposure on ADHD suggested in the present study are in accordance with some of the previous studies, which overall report consistent findings.8–13 In one of these previous studies that included the FLEHS I cohort,12 increased odds of SDQ-hyperactivity subscale at 7–8 years were reported for PCB and p-p´-DDE exposure, although the estimates reported were rather imprecise. Only in the New Bedford cohort study, the authors report increased ADHD at 8 years, obtained either through a teacher’s rating scale or by using neuropsychological measures of inattentive and impulsive behaviours, in association with prenatal exposure to PCBs and p-p´-DDE.14,15,41 The different results from the two studies conducted in the New Bedford cohort and the present pooled analysis may be explained by different PCB exposure profile and ADHD assessment. In the New Bedford cohort, children were exposed via the mother to specific PCB mixtures due to the proximity to an industry and the de-chlorination processes that occurred.42 Most child population exposure to PCBs is through the food chain, which gives rise to a different PCB mixture. In our study, we used the PCB-153 congener as a marker of PCB exposure and this should be comparable across child cohorts with background exposures. Furthermore, it should also be noted that, in the New Bedford cohort, the authors assessed ADHD by the Conners’ Rating Scale for Teachers, which includes 59 items and separates the different ADHD symptomatologies. They also treated the outcome as a continuous score. Finally, ADHD was measured at older ages in New Bedford (8 years) compared with most of the cohorts participating in the present study. Together, these factors could have made the New Bedford study more sensitive to detect small effects. We also included in our analysis a subsample of the Spanish study (INMA-Menorca cohort) that previously reported a negative association between prenatal HCB exposure and ADHD.16 Differences in the final population included in the present study, exposure assessment modelling, covariate adjustment and analytical strategy explain the different results.
Animal studies have shown that postnatal exposure to PCB-153 (or PCB-126) through mothers’ milk is related to increased motor activity and attention deficits in male rats.43 In experimental studies, a mixture of PCB and polybrominated diphenyl ether (PBDE) co-exposure has been associated with hyperactivity and deficits in inhibitory control,44 disruption in endocrine activity45 and with an increase in dopamine concentrations in the prefrontal cortex, which may be the link between PCB and altered behaviour in rats.46 Monkeys exposed to PCBs exhibit some of the characteristics found in children with attention-deficit disorder.47 In mice, exposure to a low dose of DDT during neonatal nervous system development caused behavioural and neurochemical changes in adulthood.48 HCB exposure increased the activity level in a study in rats.49 However, not all animal studies report increased risk of ADHD symptoms in association with POPs.50 Effects of PCB-153 on ADHD behaviour may depend on the exposure level, whereby high doses may aggravate ADHD symptoms in genetically vulnerable individuals that could possibly explain findings in one study and not another. An animal model of ADHD using rats concluded that, in normal controls, postnatal exposure to PCB-153 may not constitute an environmental risk factor for developing the full range of ADHD symptoms.51 It is unclear to what the extent the findings from animal studies can be generalized to the human population; however, it appears overall that the literature supports that, in children from the general population, background exposure to POPs in subjects with no genetic susceptibility does not increase the risk of ADHD.
The aetiology of ADHD is not yet completely understood. The heritability of ADHD is estimated to be about 77%.52 However, our recent understanding is that ADHD results from complex interactions of genetic, environmental and social factors.53 Identified factors include pregnancy and delivery complications leading to hypoxia, low birth weight, maternal smoking, fetal exposure to alcohol, environmental toxins and dietary factors.54 Psychosocial adversities in the home environment (e.g. low social class, low income, large family size, family dysfunction and single-parent families), maternal mental disorders, violence and stress during pregnancy also appear to play a role in the aetiology of this disorder.52,55 Our inability to take into account the complex gene–environment interactions and the individual psychosocial vulnerabilities to chemical exposures might have affected our results.
In summary, we found no increased risk of ADHD in association with prenatal and early postnatal exposure to PCB-153, p-p´-DDE and HCB in a sample of 4437 children from the general population of seven European birth cohort studies. Future pooled studies require psychosocial, genetic and epigenetic information together with environmental contaminants in order to ascertain whether vulnerability to environmental toxicant exposure may contribute to ADHD prevalence.
Funding
Funding was provided as follows: this research was primarily supported by a grant from the European Community’s Seventh Framework Program [FP7/2007–2013] under grant agreement DENAMIC no.282957.
HUMIS: This research was funded by a grant from the Norwegian Research Council, under the NEVRINOR programme grant agreement no. 226402. The study was approved by the Regional Ethics Committee for Medical Research in Norway (ref. S-02122) and the Norwegian Data Inspectorate (refs 2002/1398), and participation did not occur until after informed consent was obtained.
PELAGIE: The PELAGIE study received funding for this research from the French National Research Agency (ANR-2010-PRSP-007).
INMA:This study was funded by grants from the EU (European Union): NEWGENERIS FP6–2003-Food-3-A-016320, FP7-ENV-2011 cod 282957, HEALTH.2010.2.4.5–1; and by grants from Spain: grants from Instituto de Salud Carlos III (Red INMA G03/176 and CB06/02/0041, FIS-FEDER: PI 03/1615, PI04/1509, PI04/1112, PI04/1436, PI04/1931, PI/04/2018, PI05/1079, PI05/1052, PI06/0867, PI06/1213, PI07/0314, PI/08/1151, PI09/02647, FIS-PI041436, FIS-PI081151, FISS-PI042018, FISS-PI09/02311, FISPI06/0867 FIS-PS09/00090, FIS-PI07/0252, PS09/00090, PI11/01007, PI11/02591, PI11/02038, PI13/1944, PI13/2032, PI14/00891, PI14/01687, PI17/00663 and Miguel Servet-FEDER: CP11/00178 and MS13/00054 and MSII16/00051), Generalitat de Catalunya-CIRIT 1999SGR 00241, La Fundació La Marató de TV3 (090430), Conselleria de Sanitat Generalitat Valenciana, Department of Health of the Basque Government (2005111093 and 2009111069), Provincial Government of Gipuzkoa (DFG06/004 and DFG08/001), Obra Social Cajastur, Universidad de Oviedo, Consejería de Salud de la Junta de Andalucía (grant number 183/07), EU Commission (QLK4–1999-01422, QLK4–2002-00603, and CONTAMED FP7-ENV-212502), and Fundación Roger Torné. ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. A full roster of the INMA Project Investigators can be found at http://www.proyectoinma.org/presentacion-inma/listadoinvestigadores/en_listado-investigadores.html
INUENDO: The INUENDO study was funded by European Commission’s 7th and 5th Framework Programmes (FP7-ENV-2008–1-226217 and QLK4-CT-2001–00202).
PCB cohort: The PCB cohort was funded by National Institutes of Health grant R01 CA096525, Slovak Research and Development Agency grants APVT-21–016804, APVV-0571–12, APVV-0444–11 and by the ITMS project no. 26240120033 based on the supporting operational Research and development programme from the European Regional Development Fund.
FLEHS I:The studies of the Flemish Center of Expertise on Environment and Health were commissioned, financed and steered by the Ministry of the Flemish Community (Department of Economics, Science and Innovation; Flemish Agency for Care and Health; and Department of Environment, Nature and Energy).
DUISBURG:The Duisburg Birth Cohort Study was initiated and financially supported by the North Rhine-Westphalia State Environment Agency. Follow-up was funded by the German Federal Environment Agency (registry no. 3708 61 201 3).
Supplementary Material
Acknowledgements
We thank all participants for their generous collaboration.
Conflict of interest: none declared.
References
- 1. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA.. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007;164:942–48. [DOI] [PubMed] [Google Scholar]
- 2. Surén P, Bakken IJ, Lie KK. et al. Differences across counties in the registered prevalence of autism, ADHD, epilepsy and cerebral palsy in Norway. Tidsskr Nor Laegeforen 2013;133:1929–34. [DOI] [PubMed] [Google Scholar]
- 3. WHO/UNEP. An Assessment of the State of the Science of Endocrine Disruptors Prepared by a Group of Experts for the United Nations Environment Programme (UNEP) and WHO [Internet] 2012. http://www.who.int/ceh/publications/endocrine/en/ (14 March 2017, date last accessed).
- 4. Stigum H, Iszatt N, Polder A, Mandal S, Eggesbø M.. A novel model to characterize postnatal exposure to lipophilic environmental toxicants and application in the study of hexachlorobenzene and infant growth. Environ Int 2015;85:156–62. [DOI] [PubMed] [Google Scholar]
- 5. Stockholm Convention on Persistent Organic Pollutants [Internet]. http://chm.pops.int/TheConvention/Overview/tabid/3351/Default.aspx (14 March 2017, date last accessed).
- 6. Grandjean P, Landrigan PJ.. Neurobehavioural effects of developmental toxicity. Lancet Neurol 2014;13:330–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strøm M, Hansen S, Olsen SF. et al. Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes—a prospective study with long-term follow-up. Environ Int 2014;68:41–48. [DOI] [PubMed] [Google Scholar]
- 8. Boucher O, Jacobson SW, Plusquellec P. et al. Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Québec. Environ Health Perspect 2012;120:1456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee D-H, Jacobs DR, Porta M.. Association of serum concentrations of persistent organic pollutants with the prevalence of learning disability and attention deficit disorder. J Epidemiol Community Health 2007;61:591–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neugebauer J, Wittsiepe J, Kasper-Sonnenberg M, Schöneck N, Schölmerich A, Wilhelm M.. The influence of low level pre- and perinatal exposure to PCDD/Fs, PCBs, and lead on attention performance and attention-related behavior among German school-aged children: results from the Duisburg Birth Cohort Study. Int J Hyg Environ Health 2015;218:153–62. [DOI] [PubMed] [Google Scholar]
- 11. Newman J, Behforooz B, Khuzwayo AG, Gallo MV, Schell LM.. PCBs and ADHD in Mohawk adolescents. Neurotoxicol Teratol 2014;42:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sioen I, Den Hond E, Nelen V. et al. Prenatal exposure to environmental contaminants and behavioural problems at age 7-8years. Environ Int 2013;59:225–31. [DOI] [PubMed] [Google Scholar]
- 13. Kyriklaki A, Vafeiadi M, Kampouri M. et al. Prenatal exposure to persistent organic pollutants in association with offspring neuropsychological development at 4years of age: the Rhea mother-child cohort, Crete, Greece. Environ Int 2016;97:204–11. [DOI] [PubMed] [Google Scholar]
- 14. Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA.. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol 2010;171:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verner M-A, Hart JE, Sagiv SK, Bellinger DC, Altshul LM, Korrick SA.. Measured prenatal and estimated postnatal levels of polychlorinated biphenyls (PCBs) and ADHD-related behaviors in 8-year-old children. Environ Health Perspect 2015;123:888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ribas-Fitó N, Torrent M, Carrizo D, Júlvez J, Grimalt JO, Sunyer J.. Exposure to hexachlorobenzene during pregnancy and children’s social behavior at 4 years of age. Environ Health Perspect 2007;115:447–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casey BJ, Tottenham N, Liston C, Durston S.. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci 2005;9:104–10. [DOI] [PubMed] [Google Scholar]
- 18. Toft G, Axmon A, Giwercman A. et al. Fertility in four regions spanning large contrasts in serum levels of widespread persistent organochlorines: a cross-sectional study. Environ Health 2005;4:26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koppen G, Den Hond E, Nelen V. et al. Organochlorine and heavy metals in newborns: results from the Flemish Environment and Health Survey (FLEHS 2002-2006). Environ Int 2009;35:1015–22. [DOI] [PubMed] [Google Scholar]
- 20. Cartier C, Warembourg C, Le Maner-Idrissi G. et al. Organophosphate insecticide metabolites in prenatal and childhood urine samples and intelligence scores at 6 years of age: results from the Mother-Child PELAGIE Cohort (France). Environ Health Perspect 2016;124:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hertz-Picciotto I, Trnovec T, Kočan A. et al. PCBs and early childhood development in Slovakia: study design and background. Fresenius Environ Bull 2003;12:208–14. [Google Scholar]
- 22. Eggesbø M, Stigum H, Longnecker MP. et al. Levels of hexachlorobenzene (HCB) in breast milk in relation to birth weight in a Norwegian cohort. Environ Res 2009;109:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wittsiepe J, Schrey P, Lemm F, Eberwein G, Wilhelm M.. Polychlorinated dibenzo-p-dioxins/polychlorinated dibenzofurans (PCDD/Fs), polychlorinated biphenyls (PCBs), and organochlorine pesticides in human blood of pregnant women from Germany. J Toxicol Environ Health Part A 2008;71:703–09. [DOI] [PubMed] [Google Scholar]
- 24. WHO. The WHO Child Growth Standards [Internet]. http://www.who.int/childgrowth/standards/en/ (26 November 2015, date last accessed).
- 25. Hattis D, Ginsberg G, Sonawane B. et al. Differences in pharmacokinetics between children and adults–ii. Children's variability in drug elimination half-lives and in some parameters needed for physiologically-based pharmacokinetic modeling. Risk Anal 2003;23:117–42. [DOI] [PubMed] [Google Scholar]
- 26. Surén P, Bakken IJ, Aase H. et al. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. Pediatrics 2012;130:e152–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Achenbach T, Rescorla L.. Manual for the ASEBA Preschool Forms and Profiles Burlington, VT: Research Center for Children, Youth, & Families, 2000.
- 28. Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiat 1997;38:581–86. [DOI] [PubMed] [Google Scholar]
- 29. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (Manual diagnóstico y estadístico de los trastornos mentales DSM-IV), 4th edn. Barcelona: Masson, 2002.
- 30. Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: Wiley, 1987. [Google Scholar]
- 31. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. [DOI] [PubMed] [Google Scholar]
- 32. Bufferd SJ, Dougherty LR, Carlson GA, Rose S, Klein DN.. Psychiatric disorders in preschoolers: continuity from ages 3 to 6. Am J Psychiatry 2012;169:1157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ritter R, Scheringer M, MacLeod M, Moeckel C, Jones KC, Hungerbühler K.. Intrinsic human elimination half-lives of polychlorinated biphenyls derived from the temporal evolution of cross-sectional biomonitoring data from the United Kingdom. Environ Health Perspect 2010;119:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolff MS, Zeleniuch-Jacquotte A, Dubin N, Toniolo P.. Risk of breast cancer and organochlorine exposure. Cancer Epidemiol Biomarkers Prev 2000;9:271–77. [PubMed] [Google Scholar]
- 35. Barber JL, Sweetman AJ, van Wijk D, Jones KC.. Hexachlorobenzene in the global environment: emissions, levels, distribution, trends and processes. Sci Total Environ 2005;349:1–44. [DOI] [PubMed] [Google Scholar]
- 36. Verner M-A, Sonneborn D, Lancz K. et al. Toxicokinetic modeling of persistent organic pollutant levels in blood from birth to 45 months of age in longitudinal birth cohort studies. Environ Health Perspect 2013;121:131–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Peñalver C, Rohde LA, Faraone SV.. Association between ADHD and obesity: a systematic review and meta-analysis. Am J Psychiatry 2016;173:34–43. [DOI] [PubMed] [Google Scholar]
- 38. Trnovec T, Dedík L, Jusko TA. et al. Assessment of exposure to PCB 153 from breast feeding and normal food intake in individual children using a system approach model. Chemosphere 2011;85:1687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iszatt N, Stigum H, Verner M-A. et al. Prenatal and postnatal exposure to persistent organic pollutants and infant growth: a pooled analysis of seven European birth cohorts. Environ Health Perspect 2015;123:730–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iszatt N, Stigum H, Govarts E. et al. Perinatal exposure to dioxins and dioxin-like compounds and infant growth and body mass index at seven years: a pooled analysis of three European birth cohorts. Environ Int 2016;94:399–407. [DOI] [PubMed] [Google Scholar]
- 41. Sagiv SK, Thurston SW, Bellinger DC, Altshul LM, Korrick SA.. Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environ Health Perspect 2012;120:904–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Korrick SA, Altshul LM, Tolbert PE, Burse VW, Needham LL, Monson RR.. Measurement of PCBs, DDE, and hexachlorobenzene in cord blood from infants born in towns adjacent to a PCB-contaminated waste site. J Expo Sci Environ Epidemiol 2000;10:743–54. [DOI] [PubMed] [Google Scholar]
- 43. Holene E, Nafstad I, Skaare JU, Sagvolden T.. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav Brain Res 1998;94:213–24. [DOI] [PubMed] [Google Scholar]
- 44. Sable HJK, Powers BE, Wang VC, Widholm JJ, Schantz SL.. Alterations in DRH and DRL performance in rats developmentally exposed to an environmental PCB mixture. Neurotoxicol Teratol 2006;28:548–56. [DOI] [PubMed] [Google Scholar]
- 45. Miller VM, Sanchez-Morrissey S, Brosch KO, Seegal RF.. Developmental coexposure to polychlorinated biphenyls and polybrominated diphenyl ethers has additive effects on circulating thyroxine levels in rats. Toxicol Sci 2012;127:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seegal RF, Brosch KO, Okoniewski RJ.. Coplanar PCB congeners increase uterine weight and frontal cortical dopamine in the developing rat: implications for developmental neurotoxicity. Toxicol Sci 2005;86:125–31. [DOI] [PubMed] [Google Scholar]
- 47. Rice DC. Parallels between attention deficit hyperactivity disorder and behavioral deficits produced by neurotoxic exposure in monkeys. Environ Health Perspect 2000;108:405–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johansson U, Fredriksson A, Eriksson P.. Low-dose effects of paraoxon in adult mice exposed neonatally to DDT: changes in behavioural and cholinergic receptor variables. Environ Toxicol Pharmacol 1996;2:307–14. [DOI] [PubMed] [Google Scholar]
- 49. Goldey ES, Taylor DH.. Developmental neurotoxicity following premating maternal exposure to hexachlorobenzene in rats. Neurotoxicol Teratol 1992;14:15–21. [DOI] [PubMed] [Google Scholar]
- 50. Johansen EB, Knoff M, Fonnum F. et al. Postnatal exposure to PCB 153 and PCB 180, but not to PCB 52, produces changes in activity level and stimulus control in outbred male Wistar Kyoto rats. Behav Brain Funct 2011;7:18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Johansen EB, Fonnum F, Lausund PL. et al. Behavioral changes following PCB 153 exposure in the spontaneously hypertensive rat—an animal model of attention-deficit/hyperactivity disorder. Behav Brain Funct 2014;10:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Banerjee TD, Middleton F, Faraone SV.. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr 2007;96:1269–74. [DOI] [PubMed] [Google Scholar]
- 53. Polańska K, Jurewicz J, Hanke W.. Review of current evidence on the impact of pesticides, polychlorinated biphenyls and selected metals on attention deficit/hyperactivity disorder in children. Int J Occup Med Environ Health 2013;26:16–38. [DOI] [PubMed] [Google Scholar]
- 54. Thapar A, Cooper M, Eyre O, Langley K.. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry 2013;54:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schuch V, Utsumi DA, Costa TVMM, Kulikowski LD, Muszkat M.. Attention deficit hyperactivity disorder in the light of the epigenetic paradigm. Front Psychiatry 2015;6:126.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.