Abstract
Many genes are expressed in embryonic brains, and some of them are continuously expressed in the brain after birth. For such persistently expressed genes, they may function to regulate the developmental process and/or physiological function in neonatal brains. To investigate neurobiological functions of specific genes in the brain, it is essential to inactivate genes in the brain. Here, we describe a simple stereotaxic method to inactivate gene expression in the striatum of transgenic mice at neonatal time windows. AAV-eGFP-Cre viruses were microinjected into the striatum of Ai14 reporter gene mice at postnatal day (P) 2 by stereotaxic brain surgery. The tdTomato reporter gene expression was detected in P14 striatum, suggesting a successful Cre-loxP mediated DNA recombination in AAV-transduced striatal cells. We further validated this technique by microinjecting AAV-eGFP-Cre viruses into P2Foxp2fl/fl mice. Double labeling of GFP and Foxp2 showed that GFP-positive cells lacked Foxp2 immunoreactivity in P9 striatum, suggesting the loss of Foxp2 protein in AAV-eGFP-Cre transduced striatal cells. Taken together, these results demonstrate an effective genetic deletion by stereotaxically microinjected AAV-eGFP-Cre viruses in specific neuronal populations in the neonatal brains of floxed transgenic mice. In conclusion, our stereotaxic technique provides an easy and simple platform for genetic manipulation in neonatal mouse brains. The technique can not only be used to delete genes in specific regions of neonatal brains, but it also can be used to inject pharmacological drugs, neuronal tracers, genetically modified optogenetics and chemogenetics proteins, neuronal activity indicators and other reagents into the striatum of neonatal mouse brains.
Keywords: Neuroscience, Issue 137, Stereotaxic surgery, striatum, neonatal mice, microinjection, AAV, Cre, loxP, gene manipulation
Introduction
Modern studies of the structure and function of the brain usually require genetic manipulation of specific genes in neuronal cells. To probe the functions of different genes, transgenic mice carrying mutant alleles, including knockout and knock-in alleles have been routinely generated. Stereotaxic brain surgery for adult rodents is a standard method to locally deliver drugs, viruses, tracers and other reagents to specific regions of rodent brains1,2. Applying the stereotaxic brain surgery to transgenic mice permits one to genetically manipulate the gene function and neuronal activity in specific neuronal populations of the mouse brain. The cell type-specific manipulation provides a powerful approach to decipher neuronal functions in complex neural circuits of the brain3,4,5.
Neural development of the nervous system begins at early embryonic stages, and the developmental processes continue after birth until the juvenile period. Postnatal maturation of the nervous system includes the precise synaptic wiring of neural circuits, which is essential for physiological and cognitive functions of the brain6. Therefore, studying developmental events that occur in neonatal time windows is important not only for understanding normal neural development, but it may also provide insights into the pathogenesis of neurodevelopmental and neuropsychiatric disorders7,8. Although the methods of stereotaxic brain surgery for adult rodents are readily available2,9, few protocols are available on the internet for stereotaxic brain surgery in neonatal mice10,11. In fact, stereotaxic microinjections of reagents into the brains of neonatal mouse pups are difficult, because the head of neonatal pup is too fragile to be fixed in the standard stereotaxic apparatus. Nonetheless, the application of stereotaxic brain surgery to transgenic mice is feasible for neonatal mice12. Here, we describe a simple method with a homemade setup to perform stereotaxic brain surgery in newborn mouse pups. We demonstrate that this technique allows one to conditionally delete floxed genes by microinjecting AAV-expressing Cre DNA recombinase into the striatum of reporter gene mice and conditionally floxed transgenic mice. This technique is also applicable to deliver reagents into the neonatal striatum of wild-type mice.
Protocol
The animal protocols described here have been approved by the Animal Care and Use Committees of National Yang-Ming University.
1. Preparation of The Holder for Neonatal Pups in The Stereotaxic Apparatus
Make the head tray: cut the bottom of a 1.5 mL centrifuge tube (15 mm long) into the shape that fits the head of neonatal pups by removing 1/5 of the wall of the tube.
Take a pipette tip box with the right size that fits with the pedestal of stereotaxic apparatus, and remove the top cover. Affix the head tray prepared in step 1.1 and a tissue embedding cassette onto the base of tip tray with hot-melting adhesive. The height of the tissue embedding cassette is 7 mm, which is used to support the pup's neck and body in the head-fixed position.
Place the whole setup in a standard stereotaxic apparatus.
2. Preparation of 30G Injection Stainless Steel Needles
Pre-clean 30G syringe needles by soaking them in chloroform for 3 days.
Use forceps to carefully and slowly pull the needle out from its polypropylene hub in a chemical hood.
Wash the needles with absolute ethanol for 20 min followed by 70% ethanol rinses for 3 × 10 min on a shaker at 50 rpm. Let the needles air dry, and store the cleaned needles in a clean box at room temperature (RT) until use.
3. Prepare an Adapter for Microinjection Tubing
Connect the microliter syringe to a 30G injection needle with a PE10 polyethylene tube (PE10 tube). Prepare a PE20 polyethylene tubing (PE20 tube) adaptor for bridging the injection needle and the microliter syringe. The use of the adaptor is necessary, because the diameter of PE10 tube is much smaller than that of the needle of the 26G microliter syringe.
Prepare the tubing for the microliter syringe: connect the pre-cleaned 30G injection needle with 5 cm of PE20 tube, and seal the junction with instant glue. This tubing adaptor is reusable. NOTE: If the needle of microinjection syringe is 30G, the preparation (step 3.1) and usage (step 3.3) of this adaptor is not necessary.
Connect the tubing adaptor (prepared in step 3.1) with a microliter syringe (10 µL).
Connect one end of PE10 tube (no longer than 60 cm) onto the 30G needle of the PE20 tubing adaptor. Mount a new 30G injection needle onto the other end of the PE10 tube.
4. Load the Microinjection Tube with Autoclaved Distilled Water, Dye and Viruses
Remove the plunger of the microliter syringe. Use a 25G syringe to load the microliter syringe and its connected PE tube with autoclaved distilled water to remove air from the tubing.
Place the plunger back to the microliter syringe, and push the plunger until 2 µL of distilled water remains in the barrel. Do not let the volume of distilled water become lower than 2 µL.
Mount carefully the microliter syringe onto the micro flow rate syringe pump.
Pipette small amounts of 0.1% fast green dye (prepared in 0.9% saline and filtered with 0.22 µm filter) and the virus liquids to a piece of parafilm.
Withdraw a small amount of air to make an air bubble visible at the junction between the 30G injection needle and PE10 tube followed by loading 0.7 µL of filtered fast green into the tube to test the flow of fluids in the microinjection tubing.
Withdraw another small amount of air to make a second air bubble followed by loading the virus liquids into the microinjection tubing.
Attach and secure the 30G microinjection needle to the arm of stereotaxic apparatus.
5. Anesthesia of Neonatal Mice by Hypothermia
Place the pup in a latex glove sleeve and immerse it in crushed ice up to the neck for 5 min.
Pinch the pup's feet with forceps to make sure no withdrawal response of its feet.
Place the pup with latex glove sleeve in the head tray and put some crushed ice around the latex sleeve to keep it cold for hypothermia anesthesia.
Apply vet ointment on pup's eyes to prevent dryness while under anesthesia if possible.
6. Microinjection
Prepare sterile surgery by wiping the stereotaxic instrument thoroughly with 70% ethanol. Sterilize the surgical instrument by immersing them in 70% ethanol.
Scrub the pup's head with 70% ethanol. Locate the landmark lambda on the skull and mark the lambda with a marker pen. Aim the needle tip to the lambda, and set the anterior-posterior (AP) and medial-lateral (ML) coordinates as zero.
Move the injection arm to the target site according to the X and Y coordinates of the target site. For the striatum of postnatal day (P) 2 pups, the coordinates are: AP, +2.4 mm anterior to the lambda; ML, ±1.0 mm lateral from the midline; dorsal-ventral (DV), −1.7 mm from the skull. Mark the position of fast green dye in PE10 tube with a pen.
Bring down the 30G injection needle slowly to penetrate through the skin and skull, and then bring up the needle tip until it stops at the surface of the skull. Set the DV coordinate as zero.
Bring down the 30G injection needle slowly until it reaches the DV coordinate of the target site. Wait for 1 min to allow the parenchyma resuming its normal shape. Run the microinjection program (100 nL/min).
Make sure that the mark of fast green dye is moving in the PE tube to ensure the virus liquid is injected into the brain.
Wait for 1 min after the termination of the microinjection, and then slowly and progressively bring up the needle to 1/2 height of DV depth within 30 s. After 30 s, slowly withdraw the needle from the pup's head.
Repeat step 6.2 to 6.7 until the microinjections of all targeted sites are completed.
7. Post-surgical Recovery of The Pup
Warm up the pup for 20 min in a 33 °C incubator. Check the recovery of the pup from hypothermia anesthesia every 5 min until the pup has regained sufficient consciousness to maintain sternal recumbency.
Return the pup back to the dam after the pup is fully recovered.
Representative Results
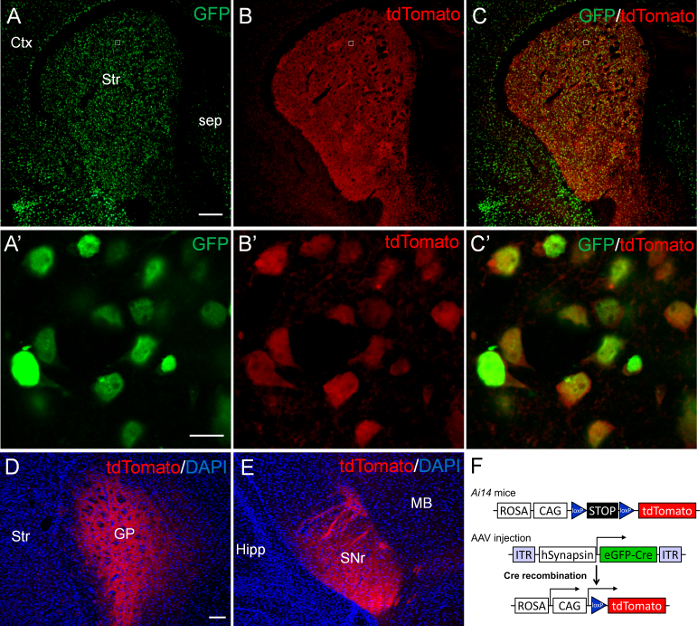
For the first set of experiment, we microinjected 200 nL of AAV9.hSynapsin.HI.eGFP-Cre.WPRE.SV40 viruses (AAV-eGFP-Cre, 1/10 dilution in Dulbecco's phosphate buffered saline) that express the Cre DNA recombinase fused with GFP into P2 striatum of Ai14 mice. The Ai14 mice express tdTomato reporter gene upon Cre-mediated deletion of loxP-flanked (floxed) STOP cassette (Figure 2F). The brains were harvested at P14 for immunostaining of GFP and tdTomato. Many AAV transduced GFP-positive cells were present throughout the striatum (Figure 2A, C), indicating an extensive infection of striatal cells by AAV-eGFP-Cre viruses. Similar extensive expression of tdTomato was also found in the striatum (Figure 2B, C). Upon microscopic examination at high magnification, we found that nearly all GFP-positive striatal cells co-expressed the tdTomato reporter gene (Figure 2A'-C'). Moreover, tdTomato signals were detected in presumably axon terminals in the globus pallidus (Figure 2D) and the substantia nigra pars reticulata (Figure 2E), the target regions of striatonigral and striatopallidal projection neurons13. These results suggest a successful Cre-loxP mediated DNA recombination induced by AAV-mediated expression of Cre activity in neonatal striatal neurons.
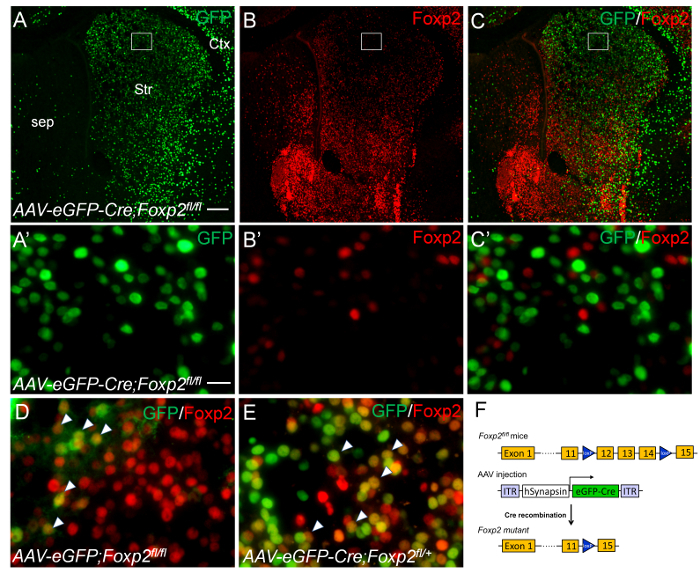
For the second set of experiment, we microinjected 50 nL of AAV-eGFP-Cre viruses into the striatum of Foxp2fl/fl mice carrying loxP-flanked Foxp2 alleles at P2 (Figure 3A-C, F), and harvested the brain at P9. No Foxp2+;GFP+ double-labeled cells were found in the striatum, i.e., Foxp2 protein was absent in GFP-positive cells (Figure 3A'-C'). In control experiments, Foxp2+;GFP+ double-labeled cells were present in the striatum of Foxp2fl/fl mice with AAV-eGFP control viruses (Figure 3D) and in the striatum of heterozygous Foxp2fl/+ mice with AAV-eGFP-Cre viruses (Figure 3E). These results indicate that Foxp2 gene is specifically deleted in AAV-eGFP-Cre transduced cells of neonatal striatum.
Taken together the results of AAV-eGFP-Cre;Ai14 and AAV-eGFP-Cre;Foxp2fl/fl mice, the technique of stereotaxic neonatal brain surgery that we developed is amenable for conditionally delete genes in specific regions of neonatal mouse brains.
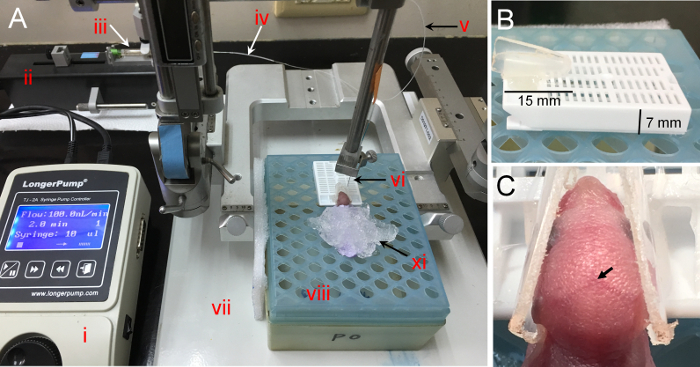
Figure 1. Stereotaxic apparatus and homemade head-fixed holder for neonatal mice. (A) The stereotaxic injection system consists of the following devices: i. syringe pump (controller); ii. syringe pump (drive unit); iii. microliter syringe (10 µL); iv. PE20 tubing adaptor; v. PE10 tube; vi. 30G injection needle; vii. stereotaxic apparatus; viii. a pipette tips box converted platform for neonatal pups; xi: crushed ice. (B) The height of the tissue embedding cassette is about 7 mm. The button of 1.5 mL centrifuge tube is about 15 mm long. (C) The mouse head is secured in the homemade head-fixed holder. The arrow indicates the landmark lambda in the head of neonatal mice. Please click here to view a larger version of this figure.
Figure 2. Expression of tdTomato in the striatum of P14 Ai14 mice after AAV-eGFP-Cre mediated Cre-loxP DNA recombination. AAV-eGFP-Cre viruses were sterotaxically injected into P2 striatum of Ai14 mice. The brain was analyzed at P14. (A) Many GFP-positive cells are present throughout the striatum (Str). (B) Many tdTomato-positive cells are also present in the striatum. (C) The merged images show an extensive co-localization of GFP and tdTomato in the striatum. Confocal images of the boxed regions in A-C are shown at high magnification in A'-C', respectively. All GFP-positive cells (green) co-express tdTomato (red) in the striatum. (D, E) The tdTomato signals are detected in the target regions of striatal projection neurons, including the globus pallidus (GP; D) and the substantia nigra pars reticulata (SNr; E). (F) Schematic drawings of AAV-eGFP-Cre and Ai14 transgenic constructs. Ctx: cortex; Sep: septum; Hipp: Hippocampus; MB: midbrain. Scale bar in A (for A-C), 200 µm; A' (for A'-C'), 10 µm; D (for D and E), 100 µm. Please click here to view a larger version of this figure.
Figure 3. Conditional deletion of Foxp2 gene in the neonatal striatum by intrastriatal injecions of AAV-eGFP-Cre viruses. Intrastriatal injections of AAV-eGFP-Cre viruses were performed in P2 Foxp2fl/fl (A-C') and Foxp2fl/+(E) mice. The brains were analyzed at P9. (A-C) GFP-positive cells (A, C, green) lack Foxp2 immunoreactivity (B, C, red) in the striatum of Foxp2fl/fl mice with AAV-eGFP-Cre viruses. The boxed regions in A-C are shown at high magnification in A'-C', respectively. (D) Striatal cells co-express GFP and Foxp2 are present in the striatum that was injected with AAV-eGFP control viruses. (E) GFP-positive cells co-express Foxp2 (arrowheads) are present in the striatum of Foxp2fl/+ mice with AAV-eGFP-Cre viruses. (F) Schematic drawings of AAV-eGFP-Cre virus and Foxp2fl/fl transgenic mice. Scale bar in A (for A-C), 200 µm; A' (for A'-C', D and E), 20 µm. Please click here to view a larger version of this figure.
Discussion
In the present study, we demonstrate a simple and reliable stereotaxic method for injecting AAV viruses into the striatum of neonatal mouse brains. We microinjected AAV-eGFP-Cre viruses into the striatum of Ai14 reporter mice at P2 and then analyzed the reporter gene expression at P14. We found AAV transduced GFP-positive cells throughout the striatum at rostrocaudal levels. Moreover, nearly all GFP-positive cells co-expressed the tdTomato reporter gene in striatal cells, suggesting a successful Cre-loxP DNA recombination induced by AAV-mediated expression of Cre activity. We further confirmed the efficacy of this approach by microinjections of AAV-eGFP-Cre into the striatum Foxp2fl/fl mice at P2. Double labeling showed that many AAVs-transduced GFP-positive cells lacked Foxp2 immunoreactivity in P9 striatum, suggesting a successfully conditional deletion of Foxp2 gene in neonatal striatum. The volumes of microinjected AAVs are correlated with the size of the infected brain regions. With 200 nL of the injected volume, GFP-positive cells were found extensively throughout the neonatal striatum, and some scattered GFP-positive cells were also present in the cortex adjacent to the striatum. With 50 nL of the injected volume, GFP-positive cells were locally restricted to the neonatal striatum. Therefore, titrating the volumes of injected AAVs is important for optimal transduction of neuronal populations in specific brain regions. Moreover, the transduction rate is also determined by the titer of viral preparation. It is conceivable that precise targeting specific cell types can be achieved by using transgenic mice with different promoters.
There are several surgical tips for successful injecting AAVs to the interested brain regions. First, because of the small size of mouse pups and the difficulty in securing the position of the mouse head with hands during surgery, it is difficult to perform stereotaxic brain surgery in neonatal mice10. Although a previous study has demonstrated neonatal stereotaxic surgery, but it requires two people to perform the microinjection during the surgery11. We have overcome these problems by developing a custom-made head-fixed device that can firmly secure the pup head during brain surgery. The whole procedures can be performed by a single person. The brain sizes of neonatal mice are varied due to different genetic backgrounds, maternal care and the ages of pups. An advantage of our homemade device is that it allows a gentle but secured fixation of neonatal mouse head, which is essential for performing high precision stereotaxic brain surgery. Second, owing to the elasticity of the soft skin and the thin wall of skull, skin incision followed by skull drilling in the head is difficult to perform in neonatal pups. To overcome this problem, it is necessary to make a puncture of the skin and the skull with a needle tip prior to lowering the injection needle to the targeted brain region. Third, it is important to check the flow of visible dye in the PE tube to make sure the microinjection is in progression during injection. If the dye does not move in the PE tube, efforts should be taken to trouble shoot the problems, including a possible leakage of fluid in the tubing and clogged needles. Note that this method applies to the neonatal pups whose lambda are visible through the skin before P5. For pups older than P5, it may need an incision in the skin to expose the lambda. Note that light illumination onto P4 and P5 mouse heads may help locate the landmark lambda. Although we have not performed this technique of stereotaxic surgery in neonatal rats, we assume that the technique is applicable to rat pups with some modifications.
The onset and maximal expression of viruses carrying gene expression take several days or weeks after injections into the brains14,15. In the present study, we found that AAV-eGFP-Cre viruses-mediated Cre-loxP DNA recombination in neonatal striatal neurons occurred as early as 8 days after viral transduction. The previous study has reported that AAV-mediated Cre-dependent reporter activity can be detected in the olfactory bulb of newborn mouse pups as early as 3 days after the bulk injection of AAVs15. It is conceivable that the early onset of AAV-mediated genetic activity is an advantage for studying developmental events that continuously occur in the postnatal brains.
In summary, the neonatal stereotaxic technique that we developed is not only for conditionally delete genes in specific brain regions, but it is also amenable to inject pharmacological reagents and neuronal tracers to specific brain regions in neonatal mice. Although we only made microinjections into the neonatal striatum in the present study, in principle, our stereotaxic neonatal brain surgery can be applied to target specific brain regions if the coordinates of specific regions in neonatal brains are available. Given the variability in the size of neonatal mouse brains with different genetic backgrounds at different stages, it is essential to empirically determine the precise coordinates of the targeted sites by the investigators. Finally, by stereotaxic microinjections of AAVs expressing genetically modified optogenetic3 and chemogenetic proteins4 and neuronal activity indicators5 into specific brain regions, one may explore the role of neuronal activity on the postnatal development of the brain at the resolution of cell type- and circuit-specific levels.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the Ministry of Science and Technology grants MOST104-2311-B-010-010-MY3, MOST106-2321-B-010-012, the National Health Research Institutes grant NHRI-EX106-10429NI, and the featured Areas Research Center Program grant from the Ministry of Education through Brain Research Center, National Yang-Ming University in Taiwan, and Postdoctoral Fellowship grants MOST106-2811-B-010-031 (S.-Y.C.), MOST105-2811-B-010-036 and MOST106-2811-B-010-030 (H.-Y.K.).
References
- Athos J, Storm DR. High precision stereotaxic surgery in mice. Current Protocols in Neuroscience. 2001. pp. A.4A.1–A.4A.9. Appendix 4, Wiley Online Library. [DOI] [PubMed]
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 2006;1(6):3166–3173. doi: 10.1038/nprot.2006.450. [DOI] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci. 2012;13(4):251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. DREADDs for neuroscientists. Neuron. 2016;89(4):683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopfel T. Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci. 2012;13(10):687–700. doi: 10.1038/nrn3293. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacol. 2010;35(1):147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ. The genetics of neurodevelopmental disease. Curr. Opin. Neurobiol. 2011;21(1):197–203. doi: 10.1016/j.conb.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Sahin M, Sur M. Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science. 2015;350(6263) doi: 10.1126/science.aab3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierberl KC, Rajadhyaksha AM. Stereotaxic microinjection of viral vectors expressing Cre recombinase to study the role of target genes in cocaine conditioned place preference. J. Vis. Exp. 2013. p. e50600. [DOI] [PMC free article] [PubMed]
- Mathon B, et al. Increasing the effectiveness of intracerebral injections in adult and neonatal mice: a neurosurgical point of view. Neurosci. Bull. 2015;31(6):685–696. doi: 10.1007/s12264-015-1558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Truong H, Nakagawa Y, Giesler GJ., Jr A microinjection technique for targeting regions of embryonic and neonatal mouse brain in vivo. Brain Res. 2010;1307:43–52. doi: 10.1016/j.brainres.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, et al. Foxp2 controls synaptic wiring of corticostriatal circuits and vocal communication by opposing Mef2c. Nat. Neurosci. 2016;19(11):1513–1522. doi: 10.1038/nn.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu. Rev. Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Karra D, Dahm R. Transfection techniques for neuronal cells. J. Neurosci. 2010;30(18):6171–6177. doi: 10.1523/JNEUROSCI.0183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham CE, Grier BD, Belluscio L. Bulk regional viral injection in neonatal mice enables structural and functional interrogation of defined neuronal populations throughout targeted brain areas. Front. Neural Circuit. 2015;9:72. doi: 10.3389/fncir.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]


