Abstract
Background/objectives
To develop age- and sex-specific centile reference curves for fat-free mass (FFM) and fat mass (FM) adjusted for height in an adult Kenyan population and to investigate the association between FM, FFM and blood pressure (BP).
Subjects/methods
Measures of body composition from bioimpedance analyses and BP were collected in 1995 participants aged ≥50 years in Nakuru County, Kenya. Reference curves were produced using the LMS method. Multivariable linear regression models were used to test the cross-sectional association between body composition indexes and BP.
Results
The age- and sex-specific reference curves for body composition (FMI and FFMI) confirmed that FFMI is lower in both men and women with increasing age. FMI declines with age in women while among men the decline starts after 70 years. FFM was higher in men (47.4 ± 7.2 kg) than in women (38.8 ± 5.5 kg), while FM was lower in men (17.3 ± 8.1 kg) than in women (24.4 ± 10.2 kg). FMI, FFMI and BMI were all positively associated with systolic and diastolic BP, and after adjusting for body weight, FFMI remained positively associated with systolic BP and the FMI remained positively associated with diastolic BP. There was no evidence to suggest that FMI and FFMI were superior to measurement of BMI alone.
Conclusions
These body composition reference curves provide normative data on body composition for older adults in Kenya. Further research should consider the prospective associations with health, including frailty-related outcomes.
Subject terms: Risk factors, Epidemiology
Introduction
There is growing interest in the effect of body composition, beyond body weight, on health outcomes linked to aging [1, 2]. Traditionally, nutritional assessment has relied upon the body mass index (BMI) (weight/height2), which is easy, cheap and convenient to measure, but it is limited because the relative proportion of fat mass (FM) and fat free-mass (FFM) is unknown. Changes in body weight may be due to changes in FM, FFM or both. Although there is a positive correlation between FFM and body weight within populations, FFM cannot always be accurately predicted from weight or BMI at an individual-level [2], and changes in BMI may obscure underlying shifts in the proportion of FM and FFM.
Bioelectrical impedance analysis (BIA) is a simple, portable, inexpensive and non-invasive technique to assess FFM and FM [3]; however, the use of body composition measurements in routine health surveillance and in clinical practice is limited by the lack of reference data to allow the classification of individuals at risk across the age spectrum. Centile charts have been developed to describe changes in FM and FFM throughout childhood [4, 5] and for adults in high- and middle-income countries [6–9]. However, body composition varies by population [10, 11], and loss of FFM may be related to ethnicity, diet, physical inactivity or disease in older adults [12, 13]. Reference data are especially limited in African populations [12] and resource-poor settings [14]. Population-specific body composition reference data will help to elucidate age-related changes associated with ill-health, and will allow more accurate characterization of individuals at greatest risk of disability and morbidity so they can receive targeted support. This is especially important in low- and middle-income countries where the double burden of non-communicable diseases (NCDs) and infectious disease coupled with limited health systems means that healthcare resources need to be carefully prioritized [15–18].
There is growing evidence that FM and FFM are independent risk factors for NCDs. FM has been shown to be strongly associated with increased risk of chronic diseases such as hypertension, hyperlipidemia and insulin resistance [19]. Conversely, reductions in FFM are characteristic of aging and independently associated with worse health outcomes and disabilities [20, 21], leading to frailty, reduced life expectancy in healthy subjects and impaired quality of life associated with weakness, alongside increased personal and healthcare costs [22–24]. In addition, loss of FFM, or more specifically skeletal muscle mass (SMM) in combination with increased adiposity, known as sarcopenic obesity, is increasingly recognized as a major health concern in the aging population because of its association with a higher risk of cardiometabolic and functional abnormalities [25]. A previous study found an increased risk of hypertension among those with sarcopenic obesity [26].
This study aimed firstly to develop age- and sex-specific centile reference curves for FFM and FM derived from BIA in an adult Kenyan population. Secondly, we aimed to investigate the association between demographic characteristics and body composition measures; and to compare the predictive value of FFM and FM relative to BMI for the association with systolic and diastolic blood pressure (BP).
Methods
Study population
This study used data from a subsample of participants (n = 1995) from the longitudinal Nakuru Eye Disease Cohort Study [27]. The primary aim of the study was to establish a survey which formed the baseline to a 6-year prospective cohort study to estimate the incidence and progression of eye disease in this population and the data collection reflected these goals. The initial study population was determined following a baseline population-based survey conducted in 2007–2008 with the aim of recruiting a sample of ~5000 participants aged ≥50 years, based on an expected prevalence of a visual acuity of <6/12 in the better eye caused by posterior segment disease of 3.0%. A total of 100 clusters each of 50 participants were selected with a probability proportional to the size of the population across Nakuru district. Households were selected within clusters using a modified compact segment sampling method. In total, 4381 participants at baseline (87.4% response rate) underwent complete examination across 100 clusters. A follow-up examination was conducted 5.6 years later (January 2013 to March 2014), in which 2170 (50%) participants were followed up. Among these, 1995 participants (n = 950 men and n = 1045 women, 92% of the follow-up sample) had complete data on body composition and BP, which was used for the present analysis.
Further details of all the procedures undertaken for all participants who attended an examination clinic at baseline and follow-up are available elsewhere [27]. Structured interviews were performed in the participant’s preferred language covering (i) demographic; (ii) past medical and ocular history including medication and relevant family history; (iii) known NCD risk factors; and (iv) socioeconomic status based on job, housing conditions, ownership of material goods and livestock which is translated in to a score based on previous work in the same population [28].
The study adhered to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of London School of Hygiene & Tropical Medicine (LSHTM Ref 6192) and by the African Medical and Research Foundation (AMREF) Ethics Committee (AMREF-ESRC P44/12).
Anthropometry and BIA
As per protocol, a nurse recorded measures for height (m) (Leicester Height Measure, Chasmors Ltd, London) and weight (kg) (Seca 761, Williams Medical Supplies, London). BMI was calculated as the weight (kg) divided by the squared height (m). BIA was performed in non-fasting and resting conditions, using a Tanita Segmental Body Composition Monitor (BC-601, Tanita Corp). This study used data on FM and FFM, calculated as the sum of the predicted total muscle mass (which includes skeletal and smooth muscle and the water contained in those) and bone mineral mass.
Consistent with previous studies [9, 29, 30] and in order to account for the fact that people with the same body weight and FM percentage who differ in height will have different body composition status, height-adjusted indexes for body composition were calculated: FMI (fat mass/height2) and FFMI (fat-free mass/height2).
Blood pressure
Three measurements of systolic and diastolic BP were taken using an Omron Digital Automatic Blood Pressure Monitor (HEM907, Omron) after the study participant had been seated for at least 10 min and each reading was taken 10 min apart. The mean of the three measurements was used.
Development of centile curves
Centile curves were constructed for men and women separately using the LMS method, which summarizes the data in terms of three smooth age-specific curves, namely L (lambda), M (mu) and S (sigma) [31]. The M and S curves correspond to the median and coefficient of variation of each body composition measure at each age, whereas the L curve allows for the age-dependent skewness in the distribution of each body composition measure. The LMS function from the R package GAMLSS was used to estimate age-specific centile curves [32, 33]. Following the same format of other growth reference curves [4, 5], centile curves for FFMI and FMI were calculated, from the 2nd to the 98th percentiles, spaced two-thirds of an SD score apart.
Statistical analyses
Stata version 14 was used for the statistical analyses. For descriptive analyses, means (SD) or samples (%) were used to describe demographic characteristics, anthropometric and health characteristics among men and women separately. Mean FMI and FFMI in each percentile were summarized for different age groups: 50–59, 60–69; 70–79; 80+. Four multivariable linear regression models were used to describe the population characteristics associated with each body composition index (i.e., FFMI and FMI) as well as for the BMI. Mean values were predicted for each demographic characteristic. The means presented are unconditional means calculated by averaging over the other variables in the model rather than estimating the means at fixed levels of the other covariates (reference levels). Four independent multivariable linear regression models were run to obtain beta coefficients (95% confidence interval (CI)) for the association between each measure (FFMI, FMI and BMI) and systolic and diastolic BP with adjustment for confounding variables such as age, gender, tribe, urban/rural, education, smoking and alcohol intake. In order to compare the predictive value between each body composition index (FFMI and FMI) and BMI, partial Pearson's correlation coefficients were obtained from the above multivariable models. Values of each body composition index and BMI were standardized into z-scores before running the models to allow comparison of their partial Pearson's correlation coefficients.
Results
Participant characteristics
From a total of 2170 Kenyan adults, a high proportion of participants had no education (17.1% men and 46.1% women) or only primary education (55.7% men and 31.8% women). The predominant tribal group was Kikuyu (Table 1).
Table 1.
Demographic characteristics of the study population
| Men (n=950) | Women (n=1045) | |||
|---|---|---|---|---|
| Demographic characteristics | Mean/n | SD/% | Mean/n | SD/% |
| Age (years) | 67.8 | 8.7 | 66.6 | 8.9 |
| Maximum education level | ||||
| None | 162 | 17.1 | 482 | 46.1 |
| Primary | 525 | 55.3 | 409 | 39.1 |
| Secondary/college/university | 252 | 26.5 | 130 | 12.4 |
| Missing | 11 | 1.2 | 24 | 2.3 |
| Socioeconomic status | ||||
| 1 quartile (poorest) | 244 | 25.7 | 220 | 21.1 |
| 2 quartile | 194 | 20.4 | 228 | 21.8 |
| 3 quartile | 186 | 19.6 | 241 | 23.1 |
| 4 quartile (richest) | 187 | 19.7 | 183 | 17.5 |
| Missing | 139 | 14.6 | 173 | 16.6 |
| Tribe | ||||
| Mixed | 132 | 13.9 | 115 | 11.0 |
| Kikuyu | 559 | 58.8 | 702 | 67.2 |
| Kalenjin | 259 | 27.3 | 228 | 21.8 |
Mean BMI was within the healthy range for men: 23.1 ± 4.4 and in the overweight range for women: 26.1 ± 5.6 g/m2 (Table 2). The prevalence of obesity was lower in men (7.2%) compared to women (25.5%). FFM was higher in men (47.4 ± 7.2 kg) than in women (38.8 ± 5.5 kg), while FM was lower in men (17.3 ± 8.1 kg) than in women (24.4 ± 10.2 kg). Mean systolic BP for men was 134.0 ± 23.1 mm Hg and 130.8 ± 24.2 mm Hg for women. Mean diastolic BP was 82.7 ± 13.1 mm Hg for men and 82.6 ± 12.9 mm Hg for women. The majority of men and women never smoked (73.7% and 98.4%, respectively). A higher proportion of men consumed alcohol in the past or currently (55.7% and 25.3%, respectively) compared to a higher proportion of women who never consumed alcohol (60.1%).
Table 2.
Health-related participant characteristics
| Men (n=950) | Women (n=1045) | |||
|---|---|---|---|---|
| Mean/n | SD/% | Mean/n | SD/% | |
| Weight (kg) | 64.7 | 13.5 | 63.2 | 14.8 |
| Height (cm) | 167.2 | 7.0 | 155.4 | 6.6 |
| BMI (kg/m2) | 23.1 | 4.4 | 26.1 | 5.6 |
| BMI category | ||||
| Underweight (<20 kg/m2) | 126 | 13.3 | 76 | 7.3 |
| Normal weight (20–25 kg/m2) | 530 | 55.8 | 400 | 38.3 |
| Overweight (25–30 kg/m2) | 226 | 23.8 | 303 | 29.0 |
| Obese (>30 kg/m2) | 68 | 7.2 | 266 | 25.5 |
| FFM (kg) | 47.4 | 7.2 | 38.8 | 5.5 |
| FFMI (kg/m2) | 16.9 | 2.1 | 16.1 | 1.9 |
| FM (%) | 25.6 | 7.7 | 37.1 | 8.2 |
| FM (kg) | 17.3 | 8.1 | 24.4 | 10.2 |
| FMI (kg/m2) | 6.2 | 2.9 | 10.1 | 4.1 |
| FFM to FM index | 3.4 | 1.8 | 1.9 | 1.0 |
| Blood pressure | ||||
| Systolic (mm Hg) | 134.0 | 23.1 | 130.8 | 24.2 |
| Diastolic (mm Hg) | 82.7 | 13.1 | 82.6 | 12.9 |
| Smoking status | ||||
| Never | 700 | 73.7 | 1,028 | 98.4 |
| Former | 125 | 13.2 | 3 | 0.3 |
| Current | 97 | 10.2 | 5 | 0.5 |
| Alcohol consumption | ||||
| Never | 167 | 17.6 | 628 | 60.1 |
| Former | 529 | 55.7 | 332 | 31.8 |
| Current | 240 | 25.3 | 65 | 6.2 |
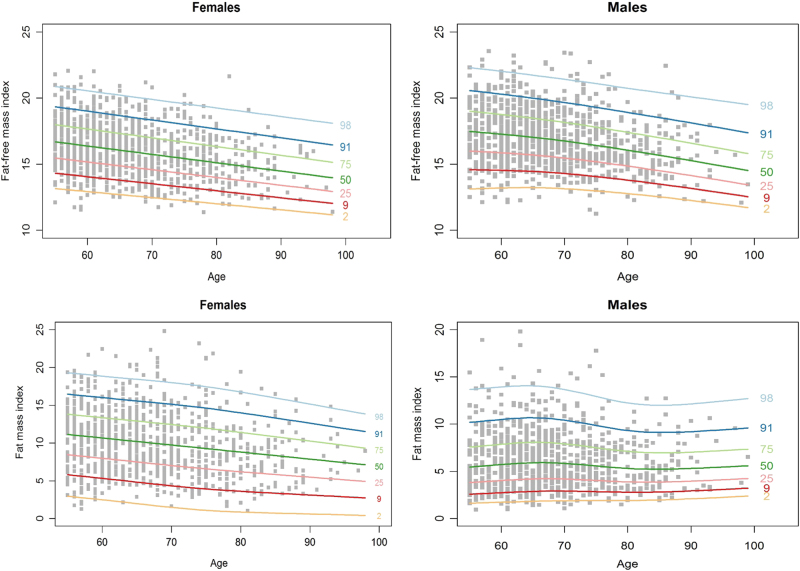
Body composition reference values
Figure 1a–d shows age- and gender-specific centiles curves for FFMI and FMI. FFMI demonstrated a relatively consistent and significant rate of decline with age for both men and women (Fig. 1a, b). At the median, FFMI for men is 17.3 kg/m2 at 60 years and declines to 15.3 kg/m2 at 90 years (Supplemental Table 1). The younger women began with a lower FFMI than for men but followed a similar pattern with a median FFMI at 60 years of 16.4 kg/m2 declining to 14.5 kg/m2 at 90 years.
Fig. 1.
Age- and sex-specific centile reference curves for fat-free mass (FFM) and fat mass (FM) adjusted for height
FMI for men peaked between 60 and 70 years, followed by a gentle decline before stabilizing between 80 and 90 years (Fig. 1c). In women, FM shows an on-going decline as age increases (Fig. 1d). The decline in men began at 60 years with a lower FMI of 5.7 kg/m2, decreasing to 5.3 kg/m2 at 90 years. Women however started at 60 years with a median FMI of 10.7 kg/m2 and reduced to 7.9 kgm2 at 90 years.
Sociodemographic correlates of body composition
Multivariable linear regression models were used to investigate associations between each body composition measure and demographic and lifestyle characteristics (Table 3). Consistent with the results shown in the centile curves, mean FFMI was significantly lower in older age groups in both men and women, while FMI was significantly lower in older ages only among women. BMI was significantly lower in older age groups among men and women.
Table 3.
Estimated marginal mean (SE)a from descriptive models of participant characteristics in association with body composition status
| FFMI | FMI | FFM to FM | BMI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |||||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Age group | ||||||||||||||||
| 60 (n = 1314 from 55–69 years) | 17.3 | 0.1 | 16.3 | 0.1 | 6.3 | 0.1 | 10.3 | 0.2 | 3.4 | 0.1 | 1.9 | 0.0 | 23.5 | 0.2 | 26.7 | 0.2 |
| 70 (n = 466 from 70–79 years) | 16.8 | 0.1* | 15.9 | 0.1* | 6.2 | 0.1 | 9.9 | 0.1* | 3.4 | 0.1 | 1.9 | 0.0 | 23.0 | 0.1* | 25.9 | 0.2* |
| 80 (n = 188 from 70–79 years) | 16.4 | 0.1* | 15.5 | 0.1* | 6.1 | 0.2 | 9.5 | 0.2* | 3.3 | 0.1 | 2.0 | 0.1 | 22.5 | 0.3* | 25.1 | 0.3* |
| 90 (n = 27 from 70–79 years) | 16.0 | 0.2* | 15.1 | 0.2* | 6.0 | 0.3 | 9.1 | 0.4* | 3.2 | 0.2 | 2.0 | 0.1 | 22.0 | 0.4* | 24.3 | 0.5* |
| Tribe | ||||||||||||||||
| Mixed (n = 247) | 17.1 | 0.2 | 16.0 | 0.2 | 5.8 | 0.3 | 9.6 | 0.4 | 3.7 | 0.2 | 2.0 | 0.1 | 22.9 | 0.4 | 25.6 | 0.6 |
| Kikuyu (n = 1261) | 17.1 | 0.1 | 16.1 | 0.1 | 6.4 | 0.1* | 10.2 | 0.1 | 3.3 | 0.1* | 1.8 | 0.0 | 23.5 | 0.2 | 26.3 | 0.2 |
| Kalenjin (n = 487) | 16.6 | 0.1* | 16.0 | 0.1 | 5.8 | 0.2 | 9.9 | 0.3 | 3.5 | 0.1 | 2.0 | 0.1 | 22.4 | 0.3 | 25.9 | 0.4 |
| Residence | ||||||||||||||||
| Rural (n = 1506) | 16.7 | 0.1 | 15.9 | 0.1 | 5.8 | 0.1 | 9.7 | 0.1 | 3.5 | 0.1 | 1.9 | 0.0 | 22.5 | 0.2 | 25.6 | 0.2 |
| Urban (n = 489) | 17.5 | 0.1* | 16.5 | 0.1* | 7.3 | 0.2* | 11.3 | 0.3* | 3.0 | 0.1* | 1.9 | 0.1 | 24.8 | 0.3* | 27.9 | 0.4* |
| Education | ||||||||||||||||
| None (n = 644) | 16.5 | 0.2 | 15.8 | 0.1 | 6.1 | 0.2 | 9.3 | 0.2 | 3.4 | 0.2 | 2.0 | 0.0 | 22.6 | 0.4 | 25.1 | 0.3 |
| Primary (n = 934) | 16.9 | 0.1* | 16.3 | 0.1* | 6.0 | 0.1 | 10.5 | 0.2* | 3.5 | 0.1 | 1.8 | 0.1* | 22.9 | 0.2 | 26.8 | 0.3* |
| Secondary/college/university (n = 382) | 17.4 | 0.1* | 16.5 | 0.2* | 6.6 | 0.2 | 11.5 | 0.4* | 3.2 | 0.1 | 1.6 | 0.1* | 24.0 | 0.3* | 28.1 | 0.5* |
| Smoking | ||||||||||||||||
| Never (n = 1728) | 17.1 | 0.1 | 16.1 | 0.1 | 6.4 | 0.1 | 10.1 | 0.1 | 3.3 | 0.1 | 1.9 | 0.0 | 23.5 | 0.2 | 26.2 | 0.2 |
| Former (n = 128) | 16.8 | 0.2 | 15.3 | 1.0 | 6.3 | 0.3 | 10.1 | 2.2 | 3.5 | 0.2 | 1.7 | 0.6 | 23.1 | 0.4 | 25.4 | 3.0 |
| Current (n = 102) | 15.6 | 0.2* | 15.2 | 0.8 | 4.5 | 0.3* | 8.6 | 1.7 | 4.1 | 0.2* | 2.8 | 0.4* | 20.2 | 0.4* | 23.8 | 2.3 |
| Alcohol intake | ||||||||||||||||
| Never (n = 795) | 17.1 | 0.2 | 16.2 | 0.1 | 6.3 | 0.2 | 10.2 | 0.2 | 3.5 | 0.1 | 1.9 | 0.0 | 23.3 | 0.3 | 26.4 | 0.2 |
| Former (n = 861) | 17.0 | 0.1 | 16.0 | 0.1 | 6.2 | 0.1 | 10.1 | 0.2 | 3.3 | 0.1 | 1.9 | 0.1 | 23.2 | 0.2 | 26.0 | 0.3 |
| Current (n = 305) | 16.8 | 0.1 | 15.4 | 0.2* | 6.0 | 0.2 | 8.6 | 0.5* | 3.5 | 0.1 | 2.3 | 0.1* | 22.7 | 0.3 | 24.0 | 0.7* |
*P < 0.05
a Results are estimated marginal means (SE) from multivariable linear regression models that included each body composition measure or BMI as the outcome and the demographic characteristics as predictors
Men and women from urban areas had a significantly higher FFMI, FMI and BMI than those from rural areas. Among participants with secondary education and above compared to those with no education, men had significantly higher FFMI and BMI, whereas women had significantly higher FFMI, FMI and BMI. Compared to never smokers, men who currently smoked had significantly lower FFMI, FMI and BMI. Among women, those currently consuming alcohol had a significantly lower FFMI, FMI and BMI compared to those who never consumed alcohol.
Associations of body composition measures and BMI with BP
Table 4 shows standardized beta estimates and partial correlation coefficients for the association between each body composition measure and BP after adjustment for age, gender, tribe, urban/rural, education, smoking and alcohol intake. BMI, FFMI and FMI are all positively associated with both systolic and diastolic BP with correlation coefficients ranging from 0.13 to 0.22. After adjusting for body weight the associations were weakened. However, FFMI remained significantly positively associated with higher systolic BP and FMI with higher diastolic BP.
Table 4.
Standardized beta coefficients and partial correlationsa relating each body mass measure with BP
| Systolic BP | Diastolic BP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | Beta | 95% CI | P | r | Beta | 95% CI | P | |||
| Model 1b | ||||||||||
| BMI | 0.15 | 3.98 | 2.81 | 5.16 | 0.000 | 0.22 | 3.23 | 2.59 | 3.87 | 0.000 |
| FFMI | 0.15 | 3.64 | 2.55 | 4.74 | 0.000 | 0.18 | 2.39 | 1.79 | 2.99 | 0.000 |
| FMI | 0.13 | 3.22 | 2.13 | 4.31 | 0.000 | 0.21 | 2.86 | 2.26 | 3.45 | 0.000 |
| Model 2c | ||||||||||
| FFMI | 0.07 | 2.61 | 1.03 | 4.18 | 0.001 | 0.04 | 0.68 | −0.18 | 1.54 | 0.119 |
| FMI | 0.03 | 1.51 | −0.61 | 3.63 | 0.162 | 0.06 | 1.60 | 0.45 | 2.75 | 0.006 |
a Results are r (partial Pearson's correlation coefficient) and standardized beta coefficients (95% CI)
b Model 1 adjust for age, gender, tribe, urban/rural, education, smoking and alcohol intake
c Model 2 includes body weight (kg) plus all the other covariates included in model 1
Discussion
This analysis presents novel age- and sex-specific reference curves for body composition (FMI and FFMI) in an older Kenyan population. This study confirms that FFM is lower in both men and women at older ages. FM also declines with age in women while among men the decline starts after 70 years. FMI, FFMI and BMI were all positively associated with systolic and diastolic BP, and after adjusting for body weight, the FFMI remained positively associated with systolic BP whereas the FMI remained positively associated with diastolic BP.
Most reference values for body composition are focused on predominately white populations from Sweden, Switzerland, the United Kingdom and the United States, with only a limited number of other non-white populations, including Japanese, African Americans and Mexican Americans [9, 11, 12, 14, 34, 35]. To our knowledge, there are no reference values for body composition specifically derived for Black African populations. The Japanese women have the lowest overall FFMI followed by similar FFMI for each age category from the Kenyan and Swedish populations; both the White and African American populations have the overall highest FFMI across the age categories. For men, the Kenyan population has lowest mean FFMI for each age category followed closely by the Japanese population. The male Swedish and both American populations have significantly higher FFMI for each age category. All ethnicities show a trend towards gradual decreasing FFMI for men, whereas those of United States and Sweden show a more static trend for women with a gradual decline in the Kenyan and Japanese populations. The overall lower FFMI and more rapid decline with age observed in the Kenyan cohort for both men and women compared to other studies may be due in part to poorer nutrition, particularly protein intake; between 2005 and 2007, dietary protein consumption for Kenya was 58 g/person/day and for United Kingdom, Sweden, United States and Japan was 104, 107, 114 and 92 g/person/day respectively [36]. Other potential explanations may include higher incidence of disease both infectious and non-communicable, and lower physical activity levels which may be related to comorbidity restriction. However, the differences in the trends observed highlights the need for ethnic-specific reference data on body composition.
The Swedish data show the population FMI to increase between 50 and 61 years and over 75 years [14]. In a slightly older Japanese population of women there was little change in FMI from 65 years to 85+ years, and this is in contrast to our Kenyan women aged over 55 years in whom FMI progressively declines. Among Swedish men, the FMI increases by 1 kg/m2 between the two age groups, whereas the Kenyan men exhibited a marginal decline in FMI with age and are more similar to the Japanese older male population who demonstrated a marginal decrease by 85+ years following a small increase at 75 years [11]. However, the sample size is small, limiting a more detailed interpretation.
Our second objective was to explore the relationship between FFMI and FMI with BP. Our models suggest both FFMI and FMI are positively associated with diastolic and systolic BP. However, there was no evidence to suggest that FMI and FFMI were superior to measurement of BMI alone. These findings are consistent with others studies that show that simple anthropometric measures such as BMI or waist circumference are strongly associated with obesity-related health risk factors such as high BP [37, 38]. BMI remains an inexpensive, easy to implement measure that is strongly associated with increased risk of components of the metabolic syndrome [39, 40] and thus an important screening tool.
The main strength of this study is the focus on older people in Kenya, while previous studies including African ethnicities have all included African Americans. We are unable to account for any bias that may have been imposed in using a follow-up subsample of the cohort, and generally those not included were those whom had died, moved away, were not located or were located but did not come to the examination. This may have affected the representativeness of the initial population sample. BIA was used to measure body composition because it is cheaper and easier to use in the field than reference techniques. BIA has been compared against reference methods such as whole-body dual energy X-ray absorptiometry showing high correlations [41–43], but it is less accurate at an individual level, especially at very high BMIs [43]. Moreover, the bioimpedance measures of body composition rely on predictive algorithms developed using data from diverse populations, mostly Caucasians. However, uncertainty remains over whether these estimates are less accurate for this Kenyan population [44]. Also, the reference values presented in this study are specific to the particular scale used.
An additional limitation is that we used measures of FFM rather than SMM. FFM includes SMM but also organ weight and other non-fat components such as bone mass, while SMM specifically is a more important tissue for the maintenance of glucose homeostasis and potentially a stronger biomarker for metabolic health [45]. While FFM and SMM are strongly correlated, we can only infer that the individuals in the lower centiles may have sarcopenia, a known risk factor for disability, independent of morbidity [1]. Nonetheless, this type of population-specific reference data for body composition in the elderly could potentially be used in health checks to identify individuals who may be at risk of sarcopenia, or to monitor changes in body composition over time to identify people with disproportionate losses of FFM, which may lead to frailty. Further research is needed into the relationship between FMI or FFMI and clinical outcomes in prospective cohorts, especially among people of non-Caucasian ethnicity given the known discrepancies between BMI and body composition across different ethnic groups [46] in order to identify clinical thresholds for intervention.
Conclusion
This study presents novel reference data for FM and FFM in an older Kenyan population and confirmed that FFM is lower in both men and women with increasing age. It also shows a significant decline in FM in women with a smaller decline in men. FMI, FFMI and BMI were all positively associated with systolic and diastolic BP. Both FFMI and FMI show similar associations to those observed with BMI. The trends observed in the Kenyan population may be related to ethnic differences in body composition and highlights the need for ethnic-specific reference data on body composition. Further research should also consider the prospective associations with health, including frailty-related outcomes.
Electronic supplementary material
Acknowledgments
Funding
This study was supported by the Medical Research Council, Fight for Sight, British Council for the Prevention of Blindness and International Glaucoma Association. MJB is supported by the Wellcome Trust (Grant Number 098481/Z/12/Z). SAJ and CP are funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care Oxford at Oxford Health NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (10.1038/s41430-018-0177-z) contains supplementary material, which is available to authorized users.
References
- 1.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 2.Kyle UG, Schutz Y, Dupertuis YM, Pichard C. Body composition interpretation. Contributions of the fat-free mass index and the body fat mass index. Nutrition. 2003;19:597–604. doi: 10.1016/S0899-9007(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 3.Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GI. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. 1985;41:810–7. doi: 10.1093/ajcn/41.4.810. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes (Lond) 2006;30:598–602. doi: 10.1038/sj.ijo.0803232. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy HD, Samani-Radia D, Jebb SA, Prentice AM. Skeletal muscle mass reference curves for children and adolescents. Pediatr Obes. 2014;9:249–59. doi: 10.1111/j.2047-6310.2013.00168.x. [DOI] [PubMed] [Google Scholar]
- 6.Dey DK, Bosaeus I, Lissner L, Steen B. Body composition estimated by bioelectrical impedance in the Swedish elderly. Development of population-based prediction equation and reference values of fat-free mass and body fat for 70- and 75-y olds. Eur J Clin Nutr. 2003;57:909–16. doi: 10.1038/sj.ejcn.1601625. [DOI] [PubMed] [Google Scholar]
- 7.Saragat B, Buffa R, Mereu E, De Rui M, Coin A, Sergi G, et al. Specific bioelectrical impedance vector reference values for assessing body composition in the Italian elderly. Exp Gerontol. 2014;50:52–56. doi: 10.1016/j.exger.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Zeng P, Wu S, Han Y, Liu J, Zhang Y, Zhang E, et al. Differences in body composition and physical functions associated with sarcopenia in Chinese elderly: reference values and prevalence. Arch Gerontol Geriatr. 2015;60:118–23. doi: 10.1016/j.archger.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Franssen FM, Rutten EP, Groenen MT, Vanfleteren LE, Wouters EF, Spruit MA. New reference values for body composition by bioelectrical impedance analysis in the general population: results from the UK Biobank. J Am Med Dir Assoc. 2014;15:448.e441–448.e446. doi: 10.1016/j.jamda.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6. doi: 10.1046/j.1467-789X.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 11.Seino S, Shinkai S, Iijima K, Obuchi S, Fujiwara Y, Yoshida H, et al. Reference values and age differences in body composition of community-dwelling older Japanese Men and women: a pooled analysis of four cohort studies. PLoS ONE. 2015;10:e0131975. doi: 10.1371/journal.pone.0131975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obisesan TO, Aliyu MH, Bond V, Adams RG, Akomolafe A, Rotimi CN. Ethnic and age-related fat free mass loss in older Americans: the Third National Health and Nutrition Examination Survey (NHANES III) BMC Public Health. 2005;5:41. doi: 10.1186/1471-2458-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hull HR, Thornton J, Wang J, Pierson RN, Jr, Kaleem Z, Pi-Sunyer X, et al. Fat-free mass index: changes and race/ethnic differences in adulthood. Int J Obes (Lond) 2011;35:121–7. doi: 10.1038/ijo.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson I, Lissner L, Samuelson G, Fors H, Lantz H, Naslund I, et al. Body composition through adult life: Swedish reference data on body composition. Eur J Clin Nutr. 2015;69:837–42. doi: 10.1038/ejcn.2014.268. [DOI] [PubMed] [Google Scholar]
- 15.Walley J, Lawn JE, Tinker A, de Francisco A, Chopra M, Rudan I, et al. Primary health care: making Alma-Ata a reality. Lancet. 2008;372:1001–7. doi: 10.1016/S0140-6736(08)61409-9. [DOI] [PubMed] [Google Scholar]
- 16.Nyirenda MJ. Non-communicable diseases in sub-Saharan Africa: understanding the drivers of the epidemic to inform intervention strategies. Int Health. 2016;8:157–8. doi: 10.1093/inthealth/ihw021. [DOI] [PubMed] [Google Scholar]
- 17.Thoma ME, Hediger ML, Sundaram R, Stanford JB, Peterson CM, Croughan MS, et al. Comparing apples and pears: women’s perceptions of their body size and shape. J Womens Health. 2012;21:1074–81. doi: 10.1089/jwh.2012.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amuna P, Zotor FB. Epidemiological and nutrition transition in developing countries: impact on human health and development. Proc Nutr Soc. 2008;67:82–90. doi: 10.1017/S0029665108006058. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3:73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 20.Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S40–47. doi: 10.1038/sj.ejcn.1601024. [DOI] [PubMed] [Google Scholar]
- 21.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 22.Rizzoli R, Reginster JY, Arnal JF, Bautmans I, Beaudart C, Bischoff-Ferrari H, et al. Quality of life in sarcopenia and frailty. Calcif Tissue Int. 2013;93:101–20. doi: 10.1007/s00223-013-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42:203–9. doi: 10.1093/ageing/afs194. [DOI] [PubMed] [Google Scholar]
- 24.Boyd CM, Xue QL, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–31. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 25.Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J Cell Biochem. 2015;116:1171–8. doi: 10.1002/jcb.25077. [DOI] [PubMed] [Google Scholar]
- 26.Park SH, Park JH, Song PS, Kim DK, Kim KH, Seol SH, et al. Sarcopenic obesity as an independent risk factor of hypertension. J Am Soc Hypertens. 2013;7:420–5. doi: 10.1016/j.jash.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Bastawrous A, Mathenge W, Peto T, Weiss HA, Rono H, Foster A, et al. The Nakuru eye disease cohort study: methodology & rationale. BMC Ophthalmol. 2014;14:60. doi: 10.1186/1471-2415-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuper H, Polack S, Eusebio C, Mathenge W, Wadud Z, Foster A. A case-control study to assess the relationship between poverty and visual impairment from cataract in Kenya, the Philippines, and Bangladesh. PLoS Med. 2008;5:e244. doi: 10.1371/journal.pmed.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–9. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 30.Bosy-Westphal A, Muller MJ. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease--there is need for a unified definition. Int J Obes (Lond) 2015;39:379–86. doi: 10.1038/ijo.2014.161. [DOI] [PubMed] [Google Scholar]
- 31.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–19. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 32.Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale and shape. J R Stat Soc Ser C (Appl Stat) 2005;54:507–54. doi: 10.1111/j.1467-9876.2005.00510.x. [DOI] [Google Scholar]
- 33.Rigby RA, Stasinopoulos DM. Automatic smoothing parameter selection in GAMLSS with an application to centile estimation. Stat Methods Med Res. 2014;23:318–32. doi: 10.1177/0962280212473302. [DOI] [PubMed] [Google Scholar]
- 34.Schutz Y, Kyle U, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18-98 y. Int J Obes. 2002;26:953–60. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 35.Tengvall M, Ellegard L, Malmros V, Bosaeus N, Lissner L, Bosaeus I. Body composition in the elderly: reference values and bioelectrical impedance spectroscopy to predict total body skeletal muscle mass. Clin Nutr. 2009;28:52–58. doi: 10.1016/j.clnu.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Chartsbin Collector Team. Daily protein intake per capita. Chartsbin.com, 2011. Accessed 01 May 2017.
- 37.Mooney SJ, Baecker A, Rundle AG. Comparison of anthropometric and body composition measures as predictors of components of the metabolic syndrome in a clinical setting. Obes Res Clin Pract. 2013;7:e55–66. doi: 10.1016/j.orcp.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Spiegelman D, Israel RG, Bouchard C, Willett WC. Absolute fat mass, percent body fat, and body-fat distribution: which is the real determinant of blood pressure and serum glucose? Am J Clin Nutr. 1992;55:1033–44. doi: 10.1093/ajcn/55.6.1033. [DOI] [PubMed] [Google Scholar]
- 39.Bray M, Pomeroy J, Knowler WC, Bersamin A, Hopkins S, Brage S, et al. Simple anthropometrics are more correlated with health variables than are estimates of body composition in Yup’ik people. Obesity (Silver Spring) 2013;21:E435–438. doi: 10.1002/oby.20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Z, Zheng L, Detrano R, Zhang X, Xu C, Li J, et al. Incidence and predictors of hypertension among rural Chinese adults: results from Liaoning Province. Ann Fam Med. 2010;8:19–24. doi: 10.1370/afm.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C. Single prediction equation for bioelectrical impedance analysis in adults aged 20–94 years. Nutrition. 2001;17:248–53. doi: 10.1016/S0899-9007(00)00553-0. [DOI] [PubMed] [Google Scholar]
- 42.Smith-Ryan A, Fukuda D, Moon J, Stout J, Cramer J, Kendall K, et al. Tracking fat-free mass changes in elderly men and women using single-frequency bioimpedance and dual-energy X-ray absorptiometry: a four-compartment model comparison. Eur J Clin Nutr. 2013;67(S1):S40. doi: 10.1038/ejcn.2012.163. [DOI] [PubMed] [Google Scholar]
- 43.Roubenoff R, Baumgartner RN, Harris TB, Dallal GE, Hannan MT, Economos CD, et al. Application of bioelectrical impedance analysis to elderly populations. J Gerontol A Biol Sci Med Sci. 1997;52:M129–136. doi: 10.1093/gerona/52A.3.M129. [DOI] [PubMed] [Google Scholar]
- 44.Ward LC, Heitmann BL, Craig P, Stroud D, Azinge EC, Jebb S, et al. Association between ethnicity, body mass index, and bioelectrical impedance. Implications for the population specificity of prediction equations. Ann N Y Acad Sci. 2000;904:199–202. doi: 10.1111/j.1749-6632.2000.tb06449.x. [DOI] [PubMed] [Google Scholar]
- 45.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12:427–32. doi: 10.1007/BF02982703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord. 2000;24:1011–7. doi: 10.1038/sj.ijo.0801353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.