Abstract
Objective:
Anorexia nervosa (AN), a psychiatric disorder characterized by restriction of food intake despite severe weight loss, is associated with increased comorbid anxiety and depression. Secretion of oxytocin, an appetite-regulating neurohormone with anxiolytic and antidepressant properties, is abnormal in AN. The link between oxytocin levels and psychopathology in AN has not been well explored.
Methods:
We performed a cross-sectional study of 79 women aged 18–45 years (19 AN, 26 AN in partial recovery [ANPR], and 34 healthy controls [HC]) investigating the relationship between basal oxytocin levels and disordered eating psychopathology, anxiety, and depressive symptoms. AN diagnoses were based on DSM-5 criteria. Data acquisition took place between December 2008 and March 2014. Fasting serum oxytocin levels were obtained, and the following self-report measures were used to assess psychopathology: Eating Disorder Examination Questionnaire, State-Trait Anxiety Inventory, and Beck Depression Inventory-II.
Results:
Fasting oxytocin levels were low in ANPR compared to HC (p=0.0004). In ANPR but not AN, oxytocin was negatively associated with disordered eating psychopathology (r=−0.39, p=0.0496) and anxiety symptoms (state anxiety: r=−0.53, p=0.006; trait anxiety: r=−0.49, p=0.01). Furthermore, ANPR with significant disordered eating psychopathology, anxiety symptoms, or depressive symptoms had lower oxytocin levels compared to those with minimal or no symptoms (p=0.04, 0.02, and 0.007, respectively).
Conclusion:
We speculate that a dysregulation of oxytocin pathways may contribute to persistent psychopathology after partial weight recovery from anorexia nervosa.
Introduction
Anorexia nervosa (AN) is a psychiatric disorder affecting mostly young females that is characterized by distorted body image, intense fear of gaining weight, and food restriction despite a low body mass index (BMI). The estimated incidence of AN is 8 per 100,000 persons per year1. Comorbid psychiatric disorders are common in AN, with an estimated lifetime prevalence of up to 65% for anxiety disorders and 80% for affective disorders2, with these comorbidities predicting a more severe variant of AN, especially with respect to psychological symptoms and suicide risk3.
Recent evidence suggests that dysregulation of the anorexigenic neurohormone oxytocin may be one of many factors underlying the psychopathology of AN. Administration of oxytocin reduces food intake in rodents, nonhuman primates, and humans4–6. In addition to promoting satiety, oxytocin has been linked to disordered eating thoughts and behaviors7 and has been shown to have anxiolytic8–12 and antidepressant13, 14 properties. Administration of oxytocin reduces anxiety and depression behaviors in rodents13, 15–18. Pilot studies in humans have demonstrated that intranasal oxytocin reduces amygdala activity and amygdala-midbrain connectivity and may reduce anxiety in response to psychosocial stress19–21. A genetic polymorphism of the oxytocin receptor was shown to moderate the association of maternal depression in early childhood and depressive symptoms in adolescence22. Furthermore, peripheral oxytocin levels are low in depressed females23. In 14 patients with major depression who did not respond to treatment with 40 mg of escitalopram for at least 8 weeks, the addition of 8 IU of intranasal oxytocin twice per day over 4 weeks significantly reduced depressive symptoms24.
Oxytocin secretion is dysregulated in AN, and the pattern of dysregulation and its possible connection to comorbid psychopathology is not fully understood. Mean overnight oxytocin levels are low in women ill with AN compared to healthy controls (HC)25, likely as an adaptive response to nutritional deprivation. Limited data exist on oxytocin levels in subjects recovered from AN. The most recent study, investigating a homogeneous sample of 9 individuals meeting the DSM-5 criteria for full recovery from AN, including full (100.500B11.5% ideal body weight [IBW]) and stable (37.2±6.4 months) weight recovery for at least 12 months, showed persistence of low oxytocin levels in the fully recovered AN subjects compared to healthy controls7. The significance of low basal oxytocin levels after weight recovery from AN is not well understood, but suggests an underlying dysfunction of oxytocin pathways.
The only study to date examining the relationship between oxytocin and comorbid psychopathology in AN focused on postprandial oxytocin levels7. Postprandial oxytocin levels were high in low-weight AN subjects and low in fully recovered AN subjects compared to HC. Furthermore, oxytocin levels were positively associated with the severity of disordered eating psychopathology across low-weight and fully recovered AN subjects and with functional magnetic resonance imaging (fMRI) hypoactivation of food motivation neurocircuitry (hypothalamus, amygdala, hippocampus, orbitofrontal cortex, and insula in low-weight AN subjects and insula in fully recovered AN subjects compared to HC)7. Higher postprandial oxytocin levels were associated with increased anxiety and depressive symptoms across low-weight and fully recovered AN subjects26. However, in the postprandial state, these changes in oxytocin levels may be directly explained by food intake or psychopathology (stress, anxiety) related to food consumption. Whether abnormal basal fasting levels of oxytocin are related to psychopathology in AN and AN in recovery has not been explored. Furthermore, the association between oxytocin levels and psychopathology has not been examined separately in AN, in which low weight may underlie changes in oxytocin secretion, versus AN in full or partial recovery, in which low weight is no longer a factor. We hypothesized that lower levels of fasting oxytocin would be associated with increased psychopathology in women with AN regardless of weight status, suggesting that low oxytocin levels may contribute to disordered eating psychopathology, anxiety, and depression.
Methods
Subjects
We studied 79 women, age 18–45 years: 19 AN subjects, 26 ANPR subjects, and 34 HC, recruited from the community. Women with AN met DSM-5 criteria as determined by the Structured Clinical Interview for DSM Disorders (SCID)27, with IBW less than 85% by 1983 Metropolitan Life tables. Women with AN in recovery met DSM-5 criteria for AN in the past, with current weight between 90% and 120% of IBW. The definition of this group follows the DSM-5 criteria for partial recovery. Accordingly, we will refer to this group as AN in partial recovery (ANPR). Exclusion criteria for AN, ANPR, and HC subjects were current hormone use, 3,4-methylenedioxymethamphetamine (MDMA) use, pregnancy or breastfeeding, and diabetes mellitus.
HC were 90%−120% of IBW with regular menstrual cycles and no pubertal delay. These subjects had no eating disorder history as assessed by the SCID and no excessive exercise within 3 months (having run more than 25 miles or exercised more than 10 hours in any 1 week).
Procedures
This study was approved by the Partners Human Research Committee. Written informed consent was obtained prior to procedures. A medical history, physical examination, and urine pregnancy test were performed. Height, metabolic weight, and elbow breadth were measured by research dieticians, and BMI (weight/height2, kg/m2) and percentage of IBW were calculated. Frame size was determined by comparing elbow breadth to race-specific norms derived from the US Health and Nutritional Examination Survey-I28. We used the Eating Disorder Examination Questionnaire (EDEQ)29, State-Trait Anxiety Inventory (STAI)30, and Beck Depression Inventory-II (BDI-II)31 to assess psychopathology. Fasting serum was drawn for oxytocin at approximately 8 AM. Data acquisition took place between December 2008 and March 2014.
Psychopathology Measures
The EDEQ is a well-validated self-report measure composed of 28 items based on the Eating Disorder Examination Interview29. It is used to assess attitudes and behaviors related to eating patterns and body image over the past 28 days. Four scales are derived: dietary restraint (EDEQ-DR), eating concern (EDEQ-EC), shape concern (EDEQ-SC), and weight concern (EDEQ-WC). A global score (EDEQ-GS) can be calculated to render a dimensional assessment of eating disorder psychopathology. We considered EDEQ global scores above 2.5 (approximately +1 SD above the healthy population mean32–36) to be significant for active disordered eating psychopathology. The STAI is a well-validated, reliable instrument for assessing anxiety symptoms. The State scale assesses how subjects feel “right now, at this moment,” and the Trait scale assesses how they feel “generally”30. Scores of > 46 on the State and > 45 on the Trait scales (1 SD > mean) were considered significant for active anxiety in our analysis37. The BDI-II is a validated, reliable questionnaire for assessing depressive symptoms. It assesses the severity of depression over the prior 2 weeks, using DSM-IV criteria31. The following cutoffs were used: 0–13 minimal depressive symptoms, 14–19 mild depression, 20–28 moderate depression, and 29–63 severe depression38.
Biochemical Analysis
Serum was stored in a −80°C freezer until analysis. Oxytocin was measured in unextracted serum by enzyme-linked immunosorbent assay (ELISA; Assay Designs, Inc., Ann Arbor, Michigan). We have previously reported a robust correlation between extracted and unextracted serum oxytocin levels39. The limit of detection was 12.5 pg/mL. In-house quality control samples had a mean of 152 and 338 pg/mL, respectively, and a between-assay coefficient of variation of 15% and 18%, respectively.
Data Analysis
JMP Pro statistical discovery software (version 11; SAS Institute) was used for statistical analyses. Clinical characteristics, oxytocin levels, and psychopathology were compared by Fisher least significant difference test: first, variables were compared using overall analysis of variance (ANOVA); pairwise comparisons were then made only for variables that were significantly different (i.e., pairwise comparisons were only made when the overall ANOVA rejected the null hypothesis). Effect sizes for pairwise comparisons were determined using Hedges g. Additional correction for multiple comparisons is not indicated when this method is used for 3-group comparisons40. Given evidence that oxytocin levels may be different between the binge/purge and restricting subtypes of AN41, a secondary post hoc analysis of oxytocin levels excluded patients with binge/purge subtype. We also controlled for potential confounders (i.e., BMI and amenorrhea) using multivariate least-square analyses. Pearson correlations were used to investigate associations between oxytocin levels and psychopathology. Statistical significance was defined as a 2-tailed p-value < 0.05. Data are reported as mean±SEM.
ANPR subjects were divided into subgroups with and without significant psychopathology based on predetermined EDEQ, STAI, and BDI-II cutoffs, and mean fasting oxytocin levels were compared between these subgroups using Student t test.
Results
Subject Characteristics
Table 1 presents subject characteristics. Mean age was 23.9±0.6 years and did not differ across groups. Per study design, percentage of IBW was low in AN compared to ANPR and HC, but did not significantly differ between ANPR and HC. Mean number of months since self-reported “recovery” in ANPR was 37.2±6.4, with values ranging from 5 to 94 months. The following psychiatric comorbidities were self-reported by the participants: depression in 11 AN (57.9%) and 15 ANPR (57.7%), anxiety in 7 AN (36.8%) and 13 ANPR (50.0%), obsessive-compulsive disorder in 5 ANPR (19.2%), attention-deficit/hyperactivity disorder in 3 AN (15.8%) and 5 ANPR (19.2%), and borderline personality disorder in one AN (5.3%). Four of 19 AN (21.1%) and 5 of 26 (19.2%) ANPR subjects reported significant current bingeing/purging behavior as defined by at least 1 bingeing/purging episode per week42. One additional AN (5.3%) and two additional ANPR subjects (7.7%) reported a history of regular binging/purging without current occurrence. Fourteen of 19 (73.7%) AN reported amenorrhea, compared to 3 of 26 (11.5%) ANPR and no HC.
Table 1. Participant characteristics.
| Characteristic | AN | ANPR | HC | AN vs. ANPR | AN vs. HC | ANPR vs. HC | Overall ANOVA |
|---|---|---|---|---|---|---|---|
| Mean±SEM | P Value, Hedges g | ||||||
| Age (years) | 23.8±1.3 | 23.8±1.0 | 23.9±0.8 | 0.99 | |||
| Weight (kg) | 46.7±0.9 | 61.0±1.4 | 61.5±0.9 | <0.0001,−2.38 | <0.0001,−2.99 | 0.7,−0.08 | <0.0001 |
| Body mass index (kg/m2) | 17.6±0.2 | 22.5±0.4 | 22.6±0.3 | <0.0001,−3.20 | <0.0001,−3.49 | 0.8,−0.06 | <0.0001 |
| % Ideal body weight | 80.2±0.9 | 100.5±1.5 | 101.2±1.1 | <0.0001,−3.20 | <0.0001,−3.60 | 0.7,−0.10 | <0.0001 |
| Oxytocin (pg/mL) | 1244±85 | 974±92 | 1501±114 | 0.1,0.62 | 0.1,−0.44 | 0.0004,−0.88 | 0.002 |
| EDEQ-DR | 0.8,0.07 | <0.0001,1.91 | <0.0001,1.40 | <0.0001 | |||
| EDEQ-EC | 2.4±0.3 | 2.3±0.4 | 0.5±0.1 | 0.04,0.46 | <0.0001,3.02 | <0.0001,1.68 | <0.0001 |
| EDEQ-SC | 2.6±0.3 | 1.9±0.3 | 0.1±0.03 | 0.9,0.05 | <0.0001,1.96 | <0.0001,1.75 | <0.0001 |
| EDEQ-WC | 3.6±0.3 | 3.5±0.3 | 0.9±0.2 | 0.5,−0.18 | <0.0001,1.58 | <0.0001,1.61 | <0.0001 |
| EDEQ-GS | 2.6±0.4 | 3.0±0.4 | 0.6±0.2 | 0.7,0.09 | <0.0001,2.34 | <0.0001,1.78 | <0.0001 |
| STAI State | 2.8±0.3 | 2.7±0.3 | 0.5±0.1 | 0.7,0.11 | <0.0001,1.69 | <0.0001,1.15 | <0.0001 |
| STAI Trait | 43.7±2.5 | 42.2±3.2 | 28.0±1.4 | 0.1,0.42 | <0.0001,1.94 | <0.0001,1.27 | <0.0001 |
| BDI-II Total Score | 53.3±2.9 | 47.3±2.9 | 31.4±1.7 | 0.3,0.23 | <0.0001,1.74 | <0.0001,1.32 | <0.0001 |
| 18.2±2.8 | 15.3±2.4 | 3.1±1.0 | |||||
Boldface indicates p < 0.05. AN, active anorexia nervosa; ANOVA, analysis of variance; ANPR, anorexia nervosa in partial recovery; BDI-II, Beck Depression Inventory-II; DR, dietary restraint; EC, eating concern; EDEQ, Eating Disorder Examination Questionnaire; GS, global score; HC, healthy control; SC, shape concern; SEM, standard error of the mean; STAI, State-Trait Anxiety Inventory; WC, weight concern.
Psychopathology
Table 1 presents EDEQ, STAI, and BDI-II results. EDEQ global and all subscale scores were higher in AN and ANPR than HC (p<0.0001). While EDEQ-EC scores were higher in AN than ANPR (p=0.04), there were no significant differences in other subscale or global scores between AN and ANPR. Symptoms of anxiety and depression were higher in AN and ANPR than HC (p<0.0001), and did not significantly differ between AN and ANPR. Of note, the mean differences in EDEQ-GS, STAI State, STAI Trait, and BDI-II scores between ANPR versus HC and AN versus HC were all of large magnitude (Hedges g=1.15–2.34), whereas the mean differences between the AN and ANPR groups were of low magnitude (Hedges g=0.09–0.42).
Oxytocin
Oxytocin levels were low in ANPR compared to HC (p=0.0004), with the difference displaying a large effect size (Hedges g=−0.88; Table 1). This difference remained significant after controlling for BMI and estrogen status, as assessed by presence or absence of amenorrhea (p=0.002). Levels in AN were intermediate, but differences between AN and HC (p=0.1) or AN and ANPR (p=0.1) did not achieve statistical significance, even though the mean differences in oxytocin levels were of medium effect size (Hedges g of 0.62 and −0.44 for AN vs. ANPR and AN vs. HC, respectively). When the AN and ANPR subjects who reported current bingeing and/or purging were excluded from the analyses, oxytocin levels still differed between groups (p=0.01) with lower oxytocin in ANPR compared to HC (p=0.004) and no significant differences between AN and ANPR (p=0.29) or AN and HC (p=0.14).
Oxytocin and Disordered Eating Psychopathology
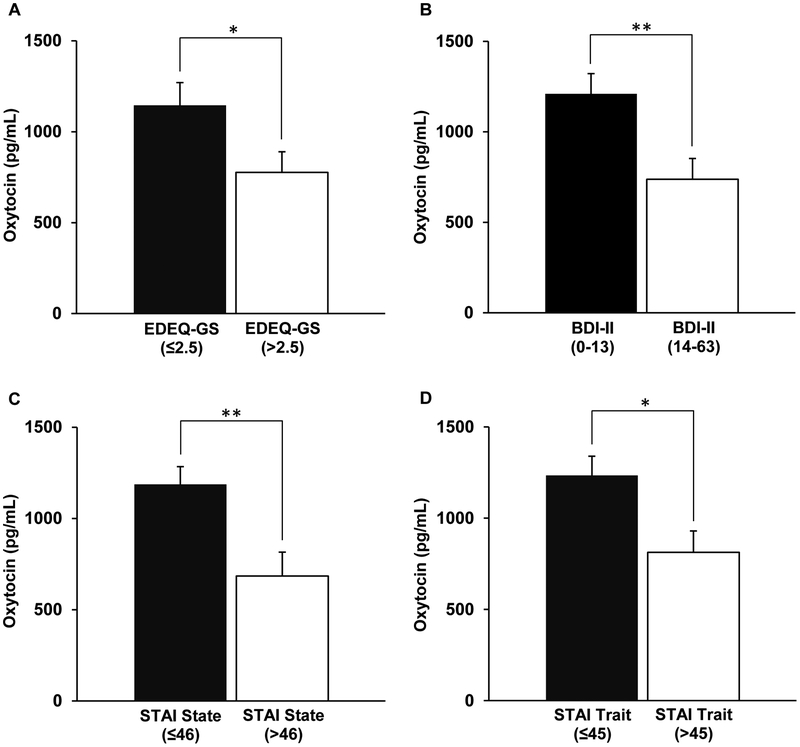
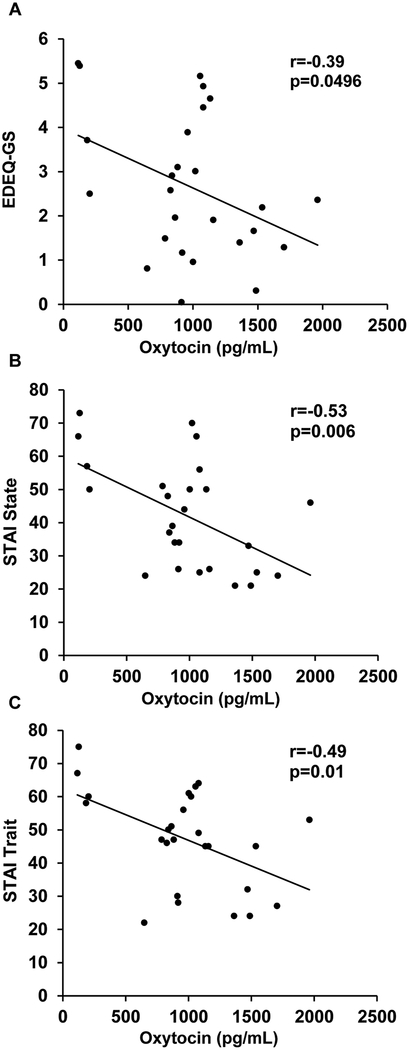
The relationship between fasting serum oxytocin levels and the severity of disordered eating psychopathology in AN and ANPR is shown in Table 2. In ANPR, fasting oxytocin levels were negatively associated with EDEQ-WC (p=0.04) and global scores (p=0.0496; Figure 11). The correlations between oxytocin and EDEQ-DR (p=0.06), EDEQ-EC (p=0.09), and EDEQ-SC (p=0.12) subscales were also negative in ANPR, but did not achieve statistical significance. Even though some correlations only reached only trend levels, in ANPR all correlations between serum oxytocin levels and EDEQ scores had a moderate effect size (0.31≤|r|≤0.40). There were no associations between oxytocin levels and EDEQ scores in AN (all p values ≥ 0.21). Mean fasting oxytocin levels were lower in ANPR subjects with significant disordered eating psychopathology (EDEQ-GS>2.5, n=12) versus those without (EDEQ-GS≤2.5, n=14) (776±114 vs. 1144±126 pg/mL, p=0.04; Figure 2).
Table 2. Relationship between fasting serum oxytocin levels and psychopathology in AN and ANPR.
| Fasting Oxytocin (pg/mL) | ||||
|---|---|---|---|---|
| AN | ANPR | |||
| Correlation Coefficient | P Value | Correlation Coefficient | P Value | |
| EDEQ-DR | 0.14 | 0.57 | −0.38 | 0.06 |
| EDEQ-EC | −0.01 | 0.98 | −0.34 | 0.09 |
| EDEQ-SC | 0.30 | 0.21 | −0.31 | 0.12 |
| EDEQ-WC | 0.30 | 0.21 | −0.40 | 0.04 |
| EDEQ-GS | 0.22 | 0.36 | −0.39 | 0.0496 |
| STAI State | 0.15 | 0.54 | −0.53 | 0.006 |
| STAI Trait | 0.11 | 0.66 | −0.49 | 0.01 |
| BDI-II Total Score | −0.05 | 0.85 | −0.34 | 0.09 |
Boldface indicates p value < 0.05. AN, active anorexia nervosa; ANPR, anorexia nervosa in partial recovery; BDI-II, Beck Depression Inventory-II; DR, dietary restraint; EC, eating concern; EDEQ, Eating Disorder Examination Questionnaire; GS, global score; SC, shape concern; STAI, State-Trait Anxiety Inventory; WC, weight concern.
Figure 2. Fasting oxytocin and psychopathology in anorexia in partial recovery (ANPR).
A. ANPR with EDEQ-GS>2.5 (significant disordered eating psychopathology) had lower oxytocin levels compared to ANPR with EDEQ-GS≤2.5 (no or minimal disordered eating psychopathology). B. ANPR with BDI-II 14–63 (clinical depressive symptoms) had significantly lower oxytocin levels compared to ANPR with BDI-II 0–13 (minimal or no depressive symptoms). C. ANPR with STAI State>46 (significant anxiety symptoms) had lower oxytocin levels compared to ANPR with STAI State≤46. D. ANPR with STAI Trait>45 (significant anxiety symptoms) had lower oxytocin levels compared to ANPR with STAI Trait≤45. BDI-II Beck Depression Inventory-II; EDEQ, Eating Disorder Examination Questionnaire; GS, global score; STAI, State-Trait Anxiety Inventory. **, p<0.01; *, p<0.05.
Oxytocin and Anxiety Symptoms
In ANPR, fasting oxytocin levels were negatively associated with STAI State (r=−0.53, p=0.006) and Trait (r=−0.49, p=0.01) with large effect sizes (Figure 1; Table 2). There were no associations between fasting oxytocin levels and anxiety symptoms in AN (STAI State: r=0.15, p=0.54; STAI Trait: r=0.11, p = 0.66).
Figure 1. Correlations between fasting oxytocin levels and psychopathology in anorexia in partial recovery (ANPR).
A. Fasting oxytocin levels and EDEQ-GS. B. Fasting oxytocin levels and STAI State score. C. Fasting oxytocin levels and STAI Trait score. EDEQ, Eating Disorder Examination Questionnaire; GS, global score; STAI, State-Trait Anxiety Inventory.
Figure 1 shows mean oxytocin levels in ANPR with versus without significant anxiety symptoms. Mean fasting oxytocin levels were low in ANPR with current anxiety symptoms (STAI State>46, n=11) compared to those without current anxiety symptoms (STAI State≤46, n=15) (685±130 vs. 1186±98 pg/mL, p=0.004). Mean fasting oxytocin levels were also low in ANPR with significant trait anxiety (STAI Trait>45, n=16) compared to those without (STAI Trait≤45, n=10) (812±118 vs. 1233±106 pg/mL, p=0.02).
Oxytocin and Depressive Symptoms
Fasting oxytocin levels in ANPR were negatively associated with BDI-II scores at the trend level (r=−0.34, p=0.09; Table 2). Again, even though short of significance, the effect size was moderate. There were no associations between fasting oxytocin levels and depressive symptoms in AN (r=−0.05, p=0.85). Mean fasting oxytocin levels were low in those ANPR subjects with clinical depressive symptoms (BDI-II 14–63, n=13) compared to those with minimal or no symptoms (BDI-II 0–13, n=13; 738±115 vs. 1210±112 pg/mL, p=0.007; Figure 2).
Discussion
To our knowledge, this is the first investigation linking peripheral oxytocin levels to increased disordered eating psychopathology and symptoms of anxiety and depression in women who are partially recovered from AN. Importantly, these data suggest that dysregulation of oxytocin pathways in women following partial recovery from AN may relate to persistent psychopathology.
Prior studies demonstrated oxytocin dysregulation in women with active AN. Low nocturnal serum and fasting cerebrospinal fluid (CSF) levels of oxytocin, as well as failure of oxytocin levels to appropriately increase in response to stimulation in AN indicate a possible oxytocin deficiency in the setting of chronic starvation25, 41, 43. Differences in methylation status of the oxytocin receptor between AN and HC have been reported, although it remains unclear whether these epigenetic differences confer risk or are secondary to the illness44. There are limited data on oxytocin regulation following partial recovery in AN, with studies to date showing inconclusive results. In a study of 7 women with restricting AN (with 3 subjects followed longitudinally through weight recovery), CSF oxytocin levels normalized following 3 weeks of weight restoration to 84.7±2.0% of average body weight41. Chiodera et al. similarly found that oxytocin levels in response to stimulation with insulin or oral ethinylestradiol normalized in 7 low-weight AN subjects who achieved a mean weight of 95.3±1.4% of average body weight over a period of 16–17 weeks43. Neither study assessed the relationship between oxytocin and psychophathology. These data suggest that oxytocin secretion may normalize acutely following short-term partial weight restoration in AN. In contrast, in a study of 13 AN subjects, 9 fully recovered AN subjects (98.3±3.8% IBW, weight-restored for 44.4±12.0 months) and 13 HC, we previously found that fasting and postprandial serum oxytocin levels were low in fully recovered AN compared to HC7. Our data in a larger and more heterogeneous group of women meeting DSM-5 criteria for ANPR now confirm low fasting serum oxytocin levels in ANPR, independent of BMI or presence/absence of amenorrhea. Whether low oxytocin levels in ANPR represent a trait of AN or a scar from chronic starvation is unknown.
Abnormal oxytocin secretion has previously been linked to disordered eating psychopathology in AN. In a prior study, we found a strong association between postprandial oxytocin levels and EDEQ scores in a group of low-weight and fully recovered AN. Furthermore, in a visual food-related fMRI paradigm, oxytocin levels accounted for fMRI hypoactivation of brain regions involved in food motivation in low-weight AN compared to HC, as well as in fully recovered AN compared to HC7. Kim et al. recently demonstrated that a single dose of intranasal oxytocin in women with AN reduced selective attention to images of food and fat body parts, indicating that oxytocin administration may improve eating disorder psychopathology45. We now demonstrate that lower fasting serum oxytocin levels in ANPR are associated with greater severity of disordered eating psychopathology, including higher weight concern and global EDEQ scores. In addition, those ANPR with significant disordered eating psychopathology as assessed by the EDEQ have lower oxytocin levels compared to those without psychopathology. Although we cannot infer causality, these results suggest that oxytocin dysregulation may reflect or contribute to disordered eating psychopathology in ANPR.
Similarly, abnormal oxytocin secretion may in part explain anxiety symptoms in AN. In studies of female oxytocin knockout mice tested in an exposed to psychogenic stressors task, the animals spent less time in the open arms of the maze than controls, indicative of greater anxiety-like behavior10, as did wild-type female mice administered an oxytocin receptor antagonist into the lateral ventricles9. Furthermore, administration of oxytocin into the lateral ventricles of oxytocin deficient mice reduced anxiety-like behaviors10. Treatment of wild type rats with oxytocin, administered peripherally or infused into the central nucleus of the amygdala, also resulted in anxiolytic effects8, 15. The mechanisms underlying the anxiolytic effects of oxytocin are not yet fully understood, but may include inhibition of the hypothalamic-pituitary-adrenal (HPA) axis21, 46, 47 and modulation of serotonergic neurons in the amygdala and hippocampal complex, dorsal raphe nucleus, insula, and orbitofrontal cortex48. In humans, lower plasma and CSF oxytocin levels are associated with greater symptoms of anxiety in children49, and in healthy men, 24 IU intranasal oxytocin reduced anticipatory anxiety in a public speaking task19. When 16 socially anxious males and 26 healthy controls were administered 24 IU of intranasal oxytocin or placebo, oxytocin administration reduced the difference between socially anxious versus non-socially anxious subjects in attentional bias for emotional faces as measured by the dot-probe task50. We previously reported a relationship between postprandial levels of oxytocin and anxiety symptoms in a combined group of low-weight and fully recovered AN26. We now show that low fasting peripheral oxytocin levels, which reflect basal unstimulated oxytocin, are associated with more severe anxiety symptoms in ANPR. Furthermore, those ANPR with significant anxiety symptoms as assessed by STAI State and Trait have lower oxytocin levels compared to those without such symptoms, supporting the concept that oxytocin dysregulation following partial recovery in AN may contribute to persistent symptoms of anxiety.
Oxytocin dysregulation may also contribute to depressive symptoms in AN. Depressive disorders are frequently precipitated by stressful social experiences, which activate the HPA axis, and the antidepressant effects of oxytocin may in part stem from its ability to inhibit the HPA axis46. In wild type mice, mating induces oxytocin release and reduces depression-like behaviors measured by duration of immobility in the forced swim test; this antidepressant effect of mating is absent in oxytocin receptor-deficient mice14. Intraperitoneal injection of oxytocin in mice has been shown to reduce the duration of immobility, with antidepressant effects comparable to those of imipramine13. In a study of 6 women and 8 men who experienced treatment failure with 2 different antidepressant classes and did not respond to escitalopram, 16 IU of intranasal oxytocin daily in conjunction with escitalopram resulted in significant improvement in depressive symptoms as early as day 8 of treatment24. We previously showed a relationship between postprandial oxytocin levels and depressive symptoms in a group of women with low-weight and fully recovered AN26. We now demonstrate that ANPR with significant depressive symptoms have lower fasting peripheral oxytocin levels than those with no or minimal depressive symptoms. Whether oxytocin dysregulation leads to depressive symptoms is unknown and warrants investigation.
It is interesting that although we detected correlations between oxytocin and disordered eating psychopathology and anxiety symptoms in ANPR, these associations were not observed in AN. One possibility is that the effects of nutritional depletion and/or other endocrine abnormalities on psychopathology in the setting of starvation outweigh the effects of oxytocin deficiency. For example, chronic starvation leads to low levels of leptin, a hormone with anxiolytic and antidepressant properties and high levels of the stress hormone cortisol, both of which have been linked to psychopathology in AN.51–53 These signals to increase psychopathology may attenuate the redundant effects of oxytocin deficiency to do the same. Future studies defining the role of oxytocin in disordered eating psychopathology, anxiety, and depression in AN during starvation will be important.
Further studies are also required to address the role of menstrual cycle functioning in the reported link between oxytocin and disordered eating pathology, anxiety, and depression. In the current sample, menstrual status, while recorded, reported, and controlled for in the analyses, and menstrual phase at time of data acquisition were not standardized. Given that estrogen is a positive regulator of oxytocin secretion, the higher proportion of subjects with regular menstrual cycle function in the ANPR compared to the AN group is likely to make us underestimate the actual oxytocin difference that would occur when keeping menstrual cycle functioning constant. Our prior study demonstrated low oxytocin in eumenorrheic AN in full recovery versus HC, supporting the concept that the low oxytocin levels are independent of menstrual status7, 26. As an additional potential limitation, this study measures peripheral oxytocin levels, which may not represent oxytocin concentrations in relevant brain regions. However, the robust correlations between peripheral oxytocin and psychopathology suggests a link between oxytocin measured in the blood and central oxytocin activity.
In summary, low fasting oxytocin levels in women who have partially recovered from AN are associated with increased severity of disordered eating psychopathology and anxiety symptoms. Furthermore, oxytocin levels are low in those with significant disordered eating psychopathology, anxiety symptoms, or depressive symptoms compared to those without such symptoms. Future studies will be important to investigate whether low oxytocin levels following partial recovery in AN contribute to psychopathology and impact prognosis. Oxytocin pathways may include novel treatment targets in eating disorder patients.
Clinical Points.
-
-
Serum oxytocin levels are low in women with anorexia nervosa in partial weight recovery (ANPR), and low oxytocin levels are associated with more severe psychopathology.
-
-
Oxytocin dysregulation may contribute to persistent psychopathology in ANPR. Oxytocin pathways may include novel treatment targets in eating disorder patients.
Acknowledgments:
We gratefully acknowledge Anne Klibanski, MD1 for her constructive review of the manuscript. We also thank the MGH Clinical Research Center staff and study participants.
Funding Source:
The study was supported by NIH Grants P30 DK40561, UL1 RR025758, and K23 MH092560. The funding agencies had no direct role in the performance of the reported study and write-up of this manuscript.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
References
- 1.Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Current opinion in psychiatry 2006. Jul;19(4):389–394. [DOI] [PubMed] [Google Scholar]
- 2.Herzog DE KT. Psychiatric comorbidity in eating disorders Annual Review of Eating Disorders. Oxford, United Kingdom: Radcliffe Publishing, Ltd; 2007: p. 35–50. [Google Scholar]
- 3.Brand-Gothelf A, Leor S, Apter A, Fennig S. The impact of comorbid depressive and anxiety disorders on severity of anorexia nervosa in adolescent girls. The Journal of nervous and mental disease 2014. October;202(10):759–762. [DOI] [PubMed] [Google Scholar]
- 4.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides 1989. Jan-Feb;10(1):89–93. [DOI] [PubMed] [Google Scholar]
- 5.Blevins JE, Graham JL, Morton GJ, et al. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. American journal of physiology Regulatory, integrative and comparative physiology 2015. March 1;308(5):R431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ. Oxytocin reduces caloric intake in men. Obesity 2015. May;23(5):950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson EA, Holsen LM, Santin M, et al. Oxytocin secretion is associated with severity of disordered eating psychopathology and insular cortex hypoactivation in anorexia nervosa. The Journal of clinical endocrinology and metabolism 2012. October;97(10):E1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 2001. April 1;21(7):2546–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology 2003. June;144(6):2291–2296. [DOI] [PubMed] [Google Scholar]
- 10.Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. Journal of neuroendocrinology 2004. April;16(4):319–324. [DOI] [PubMed] [Google Scholar]
- 11.Blume A, Bosch OJ, Miklos S, et al. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. The European journal of neuroscience 2008. April;27(8):1947–1956. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida M, Takayanagi Y, Inoue K, et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009. February 18;29(7):2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arletti R, Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life sciences 1987. October 5;41(14):1725–1730. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita H, Tomizawa K, Okimoto N, Nishiki T, Ohmori I, Matsui H. Oxytocin mediates the antidepressant effects of mating behavior in male mice. Neuroscience research 2010. October;68(2):151–153. [DOI] [PubMed] [Google Scholar]
- 15.Uvnas-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacology, biochemistry, and behavior 1994. September;49(1):101–106. [DOI] [PubMed] [Google Scholar]
- 16.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 1997. July;138(7):2829–2834. [DOI] [PubMed] [Google Scholar]
- 17.Ring RH, Malberg JE, Potestio L, et al. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology 2006. April;185(2):218–225. [DOI] [PubMed] [Google Scholar]
- 18.Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 2014. April;42:225–236. [DOI] [PubMed] [Google Scholar]
- 19.de Oliveira DC, Zuardi AW, Graeff FG, Queiroz RH, Crippa JA. Anxiolytic-like effect of oxytocin in the simulated public speaking test. Journal of psychopharmacology (Oxford, England) 2012. April;26(4):497–504. [DOI] [PubMed] [Google Scholar]
- 20.Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience 2005. December 7;25(49):11489–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological psychiatry 2003. December 15;54(12):1389–1398. [DOI] [PubMed] [Google Scholar]
- 22.Thompson SM, Hammen C, Starr LR, Najman JM. Oxytocin receptor gene polymorphism (rs53576) moderates the intergenerational transmission of depression. Psychoneuroendocrinology 2014. May;43:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuen KW, Garner JP, Carson DS, et al. Plasma oxytocin concentrations are lower in depressed vs. healthy control women and are independent of cortisol. Journal of psychiatric research 2014. April;51:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scantamburlo G, Hansenne M, Geenen V, Legros JJ, Ansseau M. Additional intranasal oxytocin to escitalopram improves depressive symptoms in resistant depression: An open trial. European psychiatry : the journal of the Association of European Psychiatrists 2014. October 1. [DOI] [PubMed] [Google Scholar]
- 25.Lawson EA, Donoho DA, Blum JI, et al. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. The Journal of clinical psychiatry 2011. November;72(11):1546–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawson EA, Holsen LM, Santin M, et al. Postprandial oxytocin secretion is associated with severity of anxiety and depressive symptoms in anorexia nervosa. The Journal of clinical psychiatry 2013. May;74(5):e451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL,Gibbon M, &William JBW Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 28.Frisancho AR, Flegel PN. Elbow breadth as a measure of frame size for US males and females. Am J Clin Nutr 1983. February;37(2):311–314. [DOI] [PubMed] [Google Scholar]
- 29.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? The International journal of eating disorders 1994. December;16(4):363–370. [PubMed] [Google Scholar]
- 30.Spielberger CE C; Mantoun J; Lushene R; NFER-Nelson. The State-Trait Inventory Windsor; 1987. [Google Scholar]
- 31.Beck AT, Steer RA, & Brown GK Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996a. [Google Scholar]
- 32.Aardoom JJ, Dingemans AE, Slof Op’t Landt MC, Van Furth EF. Norms and discriminative validity of the Eating Disorder Examination Questionnaire (EDE-Q). Eating behaviors 2012. December;13(4):305–309. [DOI] [PubMed] [Google Scholar]
- 33.Kelly NR, Cotter EW, Mazzeo SE. Eating Disorder Examination Questionnaire (EDE-Q): norms for Black women. Eating behaviors 2012. December;13(4):429–432. [DOI] [PubMed] [Google Scholar]
- 34.Luce KH, Crowther JH, Pole M. Eating Disorder Examination Questionnaire (EDE-Q): norms for undergraduate women. The International journal of eating disorders 2008. April;41(3):273–276. [DOI] [PubMed] [Google Scholar]
- 35.Mond JM, Chen A, Kumar R. Eating-disordered behavior in Australian and Singaporean women: a comparative study. The International journal of eating disorders 2010. December;43(8):717–723. [DOI] [PubMed] [Google Scholar]
- 36.Nakai Y, Nin K, Fukushima M, et al. Eating disorder examination questionnaire (EDE-Q): norms for undergraduate Japanese women. European eating disorders review : the journal of the Eating Disorders Association 2014. November;22(6):439–442. [DOI] [PubMed] [Google Scholar]
- 37.Vautier S A longitudinal SEM approach to STAI data:two comprehensive multitrait-multistate models. Journal of personality assessment 2004. October;83(2):167–179. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of personality assessment 1996b. December;67(3):588–597. [DOI] [PubMed] [Google Scholar]
- 39.Lawson EA, Ackerman KE, Estella NM, et al. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. European journal of endocrinology / European Federation of Endocrine Societies 2013. March;168(3):457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meier U A note on the power of Fisher’s least significant difference procedure. Pharmaceutical statistics 2006. Oct-Dec;5(4):253–263. [DOI] [PubMed] [Google Scholar]
- 41.Demitrack MA, Lesem MD, Listwak SJ, Brandt HA, Jimerson DC, Gold PW. CSF oxytocin in anorexia nervosa and bulimia nervosa: clinical and pathophysiologic considerations. The American journal of psychiatry 1990. July;147(7):882–886. [DOI] [PubMed] [Google Scholar]
- 42.Association AP. Diagnostic and statistical manual of mental disorders. 5th ed Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 43.Chiodera P, Volpi R, Capretti L, et al. Effect of estrogen or insulin-induced hypoglycemia on plasma oxytocin levels in bulimia and anorexia nervosa. Metabolism: clinical and experimental 1991. November;40(11):1226–1230. [DOI] [PubMed] [Google Scholar]
- 44.Kim YR, Kim JH, Kim MJ, Treasure J. Differential methylation of the oxytocin receptor gene in patients with anorexia nervosa: a pilot study. PloS one 2014;9(2):e88673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YR, Kim CH, Cardi V, Eom JS, Seong Y, Treasure J. Intranasal oxytocin attenuates attentional bias for eating and fat shape stimuli in patients with anorexia nervosa. Psychoneuroendocrinology 2014. June;44:133–142. [DOI] [PubMed] [Google Scholar]
- 46.Neumann ID, Kromer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regulatory peptides 2000. December 22;96(1–2):31–38. [DOI] [PubMed] [Google Scholar]
- 47.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. The Journal of neuroscience : the official journal of the Society for Neuroscience 2004. March 24;24(12):2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mottolese R, Redoute J, Costes N, Le Bars D, Sirigu A. Switching brain serotonin with oxytocin. Proceedings of the National Academy of Sciences of the United States of America 2014. June 10;111(23):8637–8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carson DS, Berquist SW, Trujillo TH, et al. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Molecular psychiatry 2014. November 4. [DOI] [PubMed] [Google Scholar]
- 50.Clark-Elford R, Nathan PJ, Auyeung B, et al. Effects of oxytocin on attention to emotional faces in healthy volunteers and highly socially anxious males. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 2014;18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawson EA, Donoho D, Miller KK, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. The Journal of clinical endocrinology and metabolism 2009. December;94(12):4710–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawson EA, Eddy KT, Donoho D, et al. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. European journal of endocrinology / European Federation of Endocrine Societies 2011. February;164(2):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawson EA, Miller KK, Blum JI, et al. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (Oxf) 2012. April;76(4):520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]