Abstract
Introduction:
Cellular and humoral immune responses are both involved in protection against Plasmodium infections. The only malaria vaccine available, RTS,S, primarily induces short-lived antibodies and targets only a pre-erythrocytic stage antigen. Inclusion of erythrocytic stage targets and enhancing cellular immunogenicity are likely necessary for developing an effective second-generation malaria vaccine. Adenovirus vectors have been used to improve the immunogenicity of protein-based vaccines. However, the clinical assessment of adenoviral-vectored malaria vaccines candidates has shown the induction of robust Plasmodium-specific CD8+ but not CD4+ T cells. Signal peptides (SP) have been used to enhance the immunogenicity of DNA vaccines, but have not been tested in viral vector vaccine platforms.
Objectives:
The objective of this study was to determine if the addition of the SP derived from the murine IgGκ light chain within a recombinant adenovirus vector encoding a multistage P. vivax vaccine candidate could improve the CD4+ T cell response.
Methods:
In this proof-of-concept study, we immunized CB6F1/J mice with either the recombinant simian adenovirus 36 vector containing the SP (SP-SAd36) upstream from a transgene encoding a chimeric P. vivax multistage protein or the same SAd36 vector without the SP. Mice were subsequently boosted twice with the corresponding recombinant proteins emulsified in Montanide ISA 51 VG. Immunogenicity was assessed by measurement of antibody quantity and quality, and cytokine production by T cells after the final immunization.
Results:
The SP-SAd36 immunization regimen induced significantly higher antibody avidity against the chimeric P. vivax proteins tested and higher frequencies of IFN-γ and IL-2 CD4+ and CD8+ secreting T cells, when compared to the unmodified SAd36 vector.
Conclusions:
The addition of the murine IgGκ signal peptide significantly enhances the immunogenicity of a SAd36 vectored P. vivax multi-stage vaccine candidate in mice. The potential of this approach to improve upon existing viral vector vaccine platforms warrants further investigation.
Keywords: Malaria, Plasmodium vivax, Simian Adenovirus Vector, Adenoviral Vector, Signal Peptide, Signal Sequence, Heterologous Prime-Boost Immunization, Immunogenicity
1. Introduction
The life cycle of Plasmodium parasites is known for its complexity, and as a result, immunity to malaria infections in vertebrates relies on both humoral and cellular immune responses. Early passive transfer experiments demonstrated the protective role of IgG antibodies derived from malaria immune adults when used as a therapeutic intervention [1]. Clinical trials of sporozoite inoculation have revealed that IFN-γ producing T cells are associated with the protection from malaria [2]. Based on this evidence, a multistage vaccine capable of eliciting both cytophilic antibodies and antigen-specific T cells would likely enhance the protective efficacy of a comprehensive vaccination strategy.
The RTS,S/AS01 vaccine represents a significant breakthrough as the first P. falciparum malaria vaccine that has completed Phase 3 clinical trials [3]. However, RTS,S has reported low efficacy due in part to protection based primarily on antibodies against the circumsporozoite protein (CSP) central repeat region [4] present in pre-erythrocytic stage forms, which wane rapidly and require boosting immunizations to maintain efficacy [5]. The inclusion of erythrocytic stage targets to control parasites that evade liver clearance and enhancing cellular immunogenicity are likely necessary for developing an effective second generation of malaria vaccines.
Adenoviral vectored malaria vaccines have been able to improve the immunogenicity of protein-based vaccines [6–9] and induce protective Plasmodium-specific CD8+ T cells in pre-clinical and clinical studies [10–12], but low induction of CD4+ T cell suggests further improvements to adenoviral vectors should be investigated [11]. Recent studies examining the induction of CD4+ T cells following vaccination with an Ad5 vector have shown significantly lower frequencies of antigen-specific CD4+ T cells are induced when compared to acute infection, an effect that could be attributed to lower IL-2 signaling [13]. Increasing secretion or altering post-translational modifications of adenoviral transgene products might result in improved presentation of vaccine antigens to CD4+ T cells.
Signal peptides (SP), also referred to as signal sequences, are short peptides (~20–30 residues) that can influence the targeting pathway of the protein and promote protein secretion or specific post-translational modifications such as glycosylation [14]. As a result, SP from highly secreted proteins have been used to improve protein secretion levels of recombinant proteins in cell lines [15–17], as well as for ectopic expression of endogenous adenoviral genes [18]. Recently, the inclusion of an SP into a DNA vaccine targeting HPV oncogenes was found to induce potent cellular and humoral immune responses that protected against tumor challenge [19]. Of the signal peptides used to improve transgene expression, the sequence derived from the murine immunoglobulin kappa (IgGκ) light chain (METDTLLLWVLLLWVPGSTG), is one of the most well characterized [15–17].
We hypothesized that the addition of the signal peptide derived from murine IgGκ light chain upstream of a transgene delivered via a recombinant adenovirus vector would improve the CD4+ T cell response to the transgene product in comparison to vaccination with the same recombinant vector without the signal peptide [10]. Here we demonstrate that the addition of the murine IgGκ SP improves the immunogenicity of an adenoviral vectored P. vivax multistage vaccine [20, 21] in mice by significantly increasing IFN-γ and IL-2 secretion by CD4+ T cells, and improving antibody avidity. To our knowledge, this is the first report of the insertion of a signal peptide sequence as part of an adenoviral transgene with the goal of improving the immunogenicity of an adenoviral vectored vaccine candidate.
2. Material and Methods
2.1. Viral vectors.
The DNA sequence encoding the hybrid cPvCSP/cPvMSP1 protein containing the C-terminal six-His tag was codon-optimized for mammalian expression and incorporated into a pShuttle plasmid between the CMV promoter and BGH polyadenylation signal. The constructed plasmid was further modified to introduce the N-terminal SP into the hybrid cPvCSP/cPvMSP1 protein. The oligonucleotide duplex encoding IgGκ light chain SP was cloned into KpnI restriction site upstream of the cPvCSP/cPvMSP1 transgene resulting in additional three amino acids (Tyr-Pro-Thr) introduced between the signal peptidase consensus cleavage site and the first Met start codon of the cPvCSP/cPvMSP1 transgene. Both cPvCSP/cPvMSP1 or SP-cPvCSP/cPvMSP1 expression cassettes were excised with I-CeuI and PI-SceI restriction enzymes and ligated to plasmid carrying the SAd36 genome using unique I-CeuI and PI-SceI restriction sites introduced in place of E1 region, as previously described [10, 22].The ligated DNA was transformed into E. coli strain, XL10-Gold (Stratagene), to select the plasmids containing viral genomes carrying the CMV-driven cPvCSP/cPvMSP1 and SP-cPvCSP/cPvMSP1 expression cassettes. The constructed genomes were released from the plasmids by digestion with PacI restriction enzyme and were then transfected into HEK293 cells to rescue the replication incompetent SAd36 vector derivatives as described elsewhere [10]. Both vectors were upscaled in HEK293 cells and purified using double cesium chloride gradient centrifugation as previously described [23]. The purified vector preparations were dialyzed against PBS containing 10% glycerol, and viral particle (vp) titers were determined based on absorbance at 260 nm as described by Maizel et al. [24].
2.2. In vitro viral vector culture and western blot analysis.
To assess the expression levels of cPvCSP/cPvMSP1 and SP-cPvCSP/cPvMSP1 transgenes, monolayers of A549 cells grown in 6-well plates were incubated for 1 hour with either vector at the multiplicity of infection (MOI) of 2,500 vp/cell. Infection medium (DMEM/F-12, 1:1) containing 2% FBS was replaced with fresh culture medium containing 5% FBS and cells were incubated at 37°C and 5% CO2 for at least 48 hours to allow transgene expression. The samples of cell lysates and culture medium supernatants were collected and analyzed by Western blot using anti-six-His tag mAb Penta-His (QIAGEN) and polyclonal IgG purified from sera of rabbits immunized with the cPvCSP or the cPvMSP1 chimeric proteins (Convance Inc.).
2.3. Chimeric protein vaccine design and peptide pools.
We have previously described the synthetic genes encoding the chimeric P. vivax CSP (cPvCSP) [20] and the chimeric merozoite surface protein 1(cPvMSP1) [21]. These chimeric proteins include several promiscuous T cell epitopes (PTE) capable of binding to multiple human HLA alleles and at least one B cell epitope, with each region separated by GPGPG spacers to enhance stability. cPvCSP contains 1) two PTE from the C-terminal region of P. vivax CSP; 2) the conserved region I of P. vivax CSP; 3) VK210 type 1 repeat sequence variants, and 4) three copies of the 9-mer peptide representing the VK247 type 2 repeat sequence variant [20]. cPvMSP1 includes 1) five PTE from PvMSP1; 2) an extended PvMSP119 fragment that includes two T helper epitopes derived from PvMSP133; and 3) six copies of the CSP repeat region NANP derived from P. falciparum included as a purification tag [21]. Production of the transgenes and proteins have been described previously [20, 21].
Peptide libraries containing 15-mer synthetic peptides, overlapping by 11 residues each and spanning the complete sequence of both cPvCSP and cPvMSP1 were commercially synthesized (Sigma-Aldrich), and used to characterize T cell reactivity to specific protein regions as described [20, 21]. The cPvCSP peptide library was separated into 4 pools, with pool A representing the first PTE in cPvCSP; pool B representing the second PTE and the region I; pool C representing the VK210 repeat sequences; and pool D representing the VK247 repeat sequences [20]. The two cPvMSP1 peptide pools represented the cPvMSP1 PTE (pool 1) and the PvMSP-133 and the PvMSP-119 protein fragments (pool 2) [21].
2.4. Mouse immunizations.
Female CB6F1/J (H-2d/b) mice, aged 6–8 weeks were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed in micro-isolation cages. We have previously assessed the immune response of six inbred mouse strains with different H-2 alleles to the cPvCSP protein and found that the chimeric protein was able to induce robust antibody responses in all of the strains tested [20]. Similar immunogenicity was also observed in both BALB/c and C57BL/6 mice in response to the cPvMSP1 peptide in previous assessments by our group [21]. The CB6F1/J mice were selected as a TH1/TH2 neutral strain to characterize the induction of CD4 response by vaccination, as the parent strains C57BL6 and BALB/c have been reported to be skewed to TH1 or TH2 responses, respectively [25]. All animal experiments and procedures were performed in accordance with guidelines and approved by the Emory University Institutional Animal Care and Use Committee. Two groups of 10 mice were primed intramuscularly with 108 viral particles (v.p.) of either the SAd36-cPvCSP/cPvMSP1 or the SP-SAd36-cPvCSP/cPvMSP1 vector diluted in PBS (Table 1). Mice were boosted on days 20, and 40 post-priming with 10 μg of each protein emulsified in Montanide 51 ISA VG (Seppic) adjuvant subcutaneously. A group of 10 naïve mice was used as a control, as we have not found significant differences between naïve and adjuvant immunized mice. Mice were bled 24 hours before each immunization and 20 days after the final immunization (day 60) for determination of antibody responses. Five days after the final immunization, 5 mice per group were euthanized for flow cytometric analysis of T cell responses.
Table 1. Immunization Regimens.
|
Regimen |
Prime Day 0 |
Boost Day 20 |
Boost Day 40 |
|||
|---|---|---|---|---|---|---|
| Ad-transgene | Dose | Protein | Dose | Protein | Dose | |
|
Unmodified
SAd36 |
SAd36- cPvCSP/cPvMSP1 |
108
v.p. |
cPvCSP+cPvMSP1 | 10 μg/each |
cPvCSP+cPvMSP1 | 10 μg/each |
|
Signal Peptide
SAd36 |
SP-SAd36- cPvCSP/cPvMSP1 |
108
v.p. |
cPvCSP+cPvMSP1 | 10 μg/each |
cPvCSP+cPvMSP1 | 10 μg/each |
| Naïve Control | No immunization | No Immunization | No Immunization | |||
Mice received an intramuscular prime at day 0 with the adenovirus in PBS at a dose of 108 v.p. Mice were boosted subcutaneously with a mixture of 10 μg of the individual cPvCSP and cPvMSP1 proteins emulsified in Montanide ISA 51 VG in a 1:1 volume ratio at days 20 and 40. Blood was drawn in the 24 hours preceding each immunization and 20 days after the final immunization for the analysis of antibody responses by ELISA and immunofluorescence assays (n=5). Splenocytes were obtained 5 days after the final immunization for analysis of T cell functionality in response to stimulation with cPvCSP or cPvMSP1 peptide pools (n=5).
2.5. Serological Assays.
Total IgG antibody titers and IgG1 and IgG2a subclass titers against cPvCSP and cPvMSP1 were determined via ELISA, as described previously [21]. Antibody avidity indices were determined using ammonium thiocyanate elution ELISA for each group using pooled sera, with each sample run in quadruplicate, as described previously [21]. The avidity index was calculated as the ratio between the antilog of the absorbance curves obtained with (x1) and without (x2) NH4SCN, following procedures described previously [21, 26]. Sera obtained at day 60 were pooled by group to evaluate antibody reactivity against native PvCSP and PvMSP1 via indirect immunofluorescence assay as described previously using pooled sera at a 1:500 dilution in PBS with 1% BSA [21].
2.6. Ex vivo stimulation with peptide pools and analysis of cytokine production by flow cytometry.
CD4+ and CD8+ T cells functionality was defined as their ability to produce IFN-γ, IL-2, and TNF-α. Flow cytometry analysis of ex vivo stimulated T cells derived from splenocytes obtained five days after the final immunization was performed as described previously [27]. Briefly, splenocytes were stimulated for 6 hours with 1 μg/ml of a single peptide pool, with GolgiPlug (BD Biosciences). Cells were Fc blocked (BD Biosciences) and stained with Live/Dead Fixable Yellow dye (Life Technologies) and anti-CD3, CD4, CD8, IFN-γ, IL-2, and TNF-α antibodies (BioLegend) according to the manufacturers’ protocol. Flow cytometry was performed using an LSRII cytometer (BD Biosciences) and analyzed with FlowJo V10.1 software. Cytokine positive cells were identified based on gating of unstimulated cells, with the threshold set above background. Cytokine production values for all cells that did not meet the threshold were set to zero. The sample gating strategy is provided in Supplementary Figure 1. Analyses of multifunctional T cell responses were performed using a Boolean analysis in FlowJo and data analysis in SPICE software [57].
2.7. Germinal Center B cell responses in draining lymph nodes after priming.
Mice (n=6/group) were immunized intramuscularly bilaterally into the quadriceps femoris muscles with the unmodified SAd36-cPvCSP/cPvMSP1 or Signal Peptide- SAd36-cPvCSP/cPvMSP1 vectors at 108 v.p. for the assessment of germinal center B cell responses in draining lymph nodes 9 days after priming. Lymph nodes were removed [28], and one lymph node per mouse was processed for assessment via flow cytometry as described previously [29]. Lymphocytes were stained for viability, Fc blocked, and surface stained with anti-CD95 BV605 (clone SA367H8), anti-GL7 PE (clone GL7), and anti-B220 Alexa Fluor 647 (clone RA3–6B2) antibodies (Biolegend) according to manufacturer’s instructions. Flow cytometry was performed using an LSRII cytometer (BD Biosciences) and analyzed with FlowJo V10.1 software. The germinal center B cell populations were identified as described previously [30], with FMO samples used to set gates for positive populations for CD95, B220, and GL7. A sample gating strategy is shown in Supplementary Figure 6.
Germinal centers were also visualized via immunofluorescent assay in tissue sections prepared from inguinal lymph nodes obtained from the same cohort of mice. Briefly, lymph nodes were frozen in optimal cutting temperature compound (VWR International) as described previously [31]. Frozen tissues were cut into four sections of 10 μ m. Sections were stained with unconjugated GL7 (clone GL7, Biolegend) and B220 (clone RA3–6B2, Biolegend) antibodies for chromogenic staining prior to staining of replicate sections with anti-B220 and GL7 fluorochrome-conjugated antibodies listed previously. Slides were visualized as described previously using an Olympus FV1000 confocal microscope and Olympus Fluoview V4.2 software for image capture [21, 30].
2.8. Statistics.
GraphPad Prism Software V5.0 was used to perform statistical analysis and generate graphs. Mann-Whitney test was used for analysis of differences in antibody titers between the SAd36 and SP-SAd36 regimens. Unpaired t-tests were used for analysis of differences in antibody avidity. Kruskal-Wallis test with Dunn’s post-test was used to determine the differences in cytokine production between the two immunization groups and the naïve mice following stimulation with the peptide pools. Analysis of the differences in triple, double, and single cytokine producing CD4 and CD8 T cells between the SAd36 and SP-SAd36 regimens was assessed using student’s t-test. Statistical analysis for germinal center B cell assessment was conducted using Kruskal-Wallis with Dunn’s post-test to determine differences between the immunization regimens.
3. Results
3.1. The impact of the murine IgGκ signal peptide on protein secretion in vitro.
The simian adenovirus 36 (SAd36) vector was selected for assessment of the effect of the insertion of the murine IgGκ light chain derived signal peptide on the adenoviral vector immunogenicity, as we have previously found SAd36 to exhibit higher immunogenicity than the standard Ad5 vector [10]. The SAd36 vector is replication deficient due to deletion of the E1 gene. A hybrid transgene encoding a chimeric P. vivax CSP (cPvCSP) linked to a chimeric P. vivax MSP1 (cPvMSP1) protein (cPvCSP/cPvMSP1), expressed under the control of the CMV promoter, was inserted in place of the deleted E1 gene. The signal peptide derived from the murine IgGκ light chain was inserted after the CMV promoter and upstream of the cPvCSP/cPvMSP1 transgene (Figure 1A).
Figure 1.
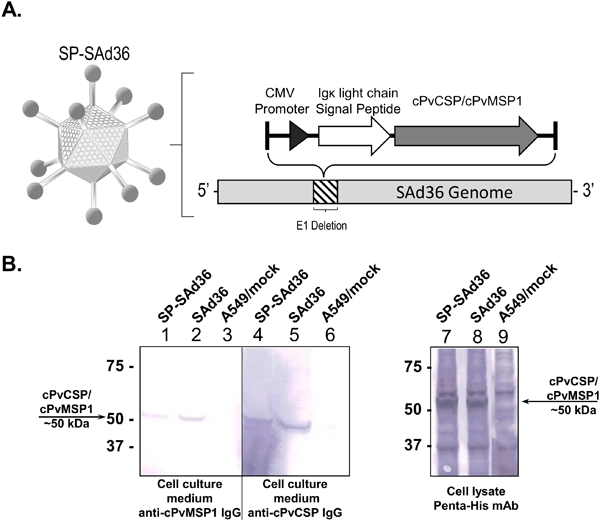
Signal Peptide Simian Adenovirus 36 schematic and protein expression. A) Schematic of the signal peptide simian adenovirus 36 vector (SP-SAd36-cPvCSP/cPvMSP1). The SP-SAd36 vector is replication deficient due to deletion of the E1 gene. Inserted in the place of the deleted E1 gene is the immunoglobulin kappa light chain signal peptide and the cPvCSP/cPvMSP1 transgene, which is under control of the cytomegalovirus (CMV) promoter. B) Analysis of cPvCSP/cPvMSP1 expression following Ad vector infection. Western blot analysis of A549 cells 2 days post infection with the indicated Ad vectors at the MOI of 2,500 vp/cell. The major protein bands close to 51 kDa correspond to the expected mass for cPvCSP/cPvMSP1. The positions of molecular weight markers (in thousands of daltons) are indicated to the left of each gel. Left: The secretory proteins were detected in culture medium collected from cells infected with SP-SAd36-cPvCSP/cPvMSP1 (lane 1) and SAd36-cPvCSP/cPvMSP1 (lane 2) using antibodies raised against the cPvMSP1 protein in rabbits. Antibodies raised against the cPvCSP protein in rabbits were used to detect secretory proteins in tissue culture medium collected from cells infected with SP-SAd36-cPvCSP/cPvMSP1 (lane 4) and SAd36-cPvCSP/cPvMSP1 (lane 5). Mock-infected A549 cells (lane 3 and 6) are also shown as controls. Right: The hybrid cPvCSP/cPvMSP1 protein containing a 6x-His Tag, was detected in samples of cell lysates obtained from cells infected with SP-SAd36-cPvCSP/cPvMSP1 (lane 7) and SAd36-cPvCSP/cPvMSP1 (lanes 8) using a Penta-His mAb. The negative control sample of mock-infected A549 cells (lane 9) is also shown.
Before determining the immunogenicity of the SAd36 vector containing the signal peptide (SP-SAd36), the expression of the cPvCSP/cPvMSP1 transgene was analyzed in the cell lysates and tissue culture medium of A549 cells collected 2 days post-infection with 2,500 v.p. of the recombinant SAd36 and SP-SAd36 via western blot. The hybrid cPvCSP/cPvMSP1 protein is expressed as a single protein with a mass of 51 kDa (Figure 1B).
When the secretion of the cPvCSP/cPvMSP1 protein was assessed via western blot, we observed a slight reduction in the amount of soluble cPvCSP/cPvMSP1 protein released into the tissue culture medium from A549 cells infected with the SP-SAd36 vector as compared to SAd36 control, appearing as a medium intensity band at 51kDa using anti-cPvMSP1 or anti-cPvCSP antibodies (Figure 1B, Left). Therefore, the use of murine IgGκ light chain leader sequence was not able to increase either expression or secretion efficiency of our multistage P. vivax vaccine candidate following infection of A549 cells in vitro. However, this does not exclude possible alterations in post-translational processing of the peptides due to the SP. Assessment of protein levels within the infected A549 cell lysates revealed bands of similar intensity at ~50 kDa for cells infected with either SP-SAd36 or SAd36 vectors expressing the chimeric P. vivax transgene when an anti-Penta-His monoclonal antibody was used for detection (Figure 1B, Right panel), suggesting that similar levels of the protein are retained inside the cell independent of the SP.
3.2. SP-SAd36 induces high avidity antibodies.
We assessed the humoral immunogenicity through the analysis of antibody kinetics induced by priming immunization with either the recombinant SP-SAd36 or unmodified SAd36 vector followed by two protein boost immunizations (Table 1). The dose of 108 v.p. of SAd36 was selected based on our previous studies with the recombinant SAd36-cPyCSP/cPyMSP1 vector expressing orthologous P. yoelii sequences [10]. When antibody titers elicited by the immunization regimens was assessed, no significant differences in antibody titers against the cPvCSP protein were observed between the two regimens at any time point (Figure 2A). Assessment of the anti-cPvMSP1 antibody responses revealed similar kinetics and final antibody titers between regimens. However, mice immunized with SP-SAd36 displayed significantly higher anti-PvMSP1 titers post-priming compared to unmodified SAd36 primed mice (Figure 2D).
Figure 2.

Antibody response to cPvCSP and cPvMSP1 proteins following priming immunization at day 0 with either the unmodified SAd36-cPvCSP/cPvMSP1 recombinant vector or the recombinant vector including the signal peptide sequence (SP-SAd36-cPvCSP/cPvMSP1) and two subsequent recombinant proteins boosts at days 20 and 40. A) Kinetics of antibody titers to cPvCSP. B) IgG1 and IgG2a antibody titers elicited against cPvCSP, 20 days after the final immunization. C) Antibody avidity against cPvCSP, determined by ammonium thiocyanate ELISAs 20 days after the final immunization. D) Kinetics of antibody titers to cPvMSP1. E) IgG1 and IgG2a antibody titers elicited against cPvMSP1, 20 days after the final immunization. F) Antibody avidity against cPvMSP1, determined by ammonium thiocyanate ELISAs 20 days after the final immunization. Statistical analysis was conducted using Mann Whitney test for antibody titers, and unpaired t-test for avidity to determine differences between the immunization regimens. Statistically significant differences are denoted by *(p < 0.05), **(p < 0.01), and *** (p<0.001).
The quality of the antibodies induced by immunization was assessed through the analysis of IgG subclasses since anti-CSP [32] and MSP1 [33] cytophilic antibodies, which correspond with IgG2a in mice, as they have been found to be associated with protection. Analysis of the IgG1 and IgG2a titers 20 days after the final immunization (day 60), revealed no significant differences in the subclasses elicited by either vector against the two P. vivax chimeric proteins (Figure 2B, E). Antibody avidity 20 days after the final immunization was assessed as another measure of antibody quality. We observed that mice immunized with the SP-SAd36 regimen produced anti-cPvCSP and anti-cPvMSP1 antibodies of significantly higher avidity than those induced by the unmodified SAd36 regimen (Figure 2C, F).
3.3. Antibodies induced by vaccination recognize Plasmodium native proteins.
The ability of the anti-cPvCSP and anti-cPvMSP1 antibodies to recognize the native structure of CSP and MSP1 was assessed by IFA. P. berghei sporozoites transgenic for the P. vivax CSP VK210 repeat region [34] were used for the assessment of the anti-cPvCSP antibodies, as the VK210 repeat region is present within the cPvCSP protein. We observed that antibodies elicited by either immunization regimen recognized the P. vivax CSP VK210 transgenic sporozoites (Figure 3A). Slides prepared with blood obtained from a P. vivax infected Saimiri boliviensis monkey were used for the assessment of anti-cPvMSP1 antibodies. We observed that sera obtained from mice immunized with either regimen bound to P. vivax schizonts (Figure 3B). Combined, these data indicate that antibodies elicited against the cPvCSP protein and cPvMSP1 protein recognize the native structure of the P. vivax CSP and MSP1, respectively.
Figure 3.

Immunofluorescence assays of P. vivax sporozoites and blood stage schizonts. Reactivity of immunized mouse sera to sporozoites (A) and blood stage schizonts (B). Top panels show Alexa 488 labeled anti-mouse IgG secondary antibodies binding to sera obtained from mice immunized with either the regimen that included priming with the unmodified SAd36-cPvCSP/cPvMSP1 or SP-SAd36-cPvCSP/cPvMSP1 recombinant vectors 20 days after the final immunization. Middle panels show the DAPI stained nuclei of sporozoites and blood stage schizonts. The bottom panels show the merged images.
3.4. Immunization with the SP-SAd36 regimen induces higher IFN-γ and IL-2 production by CD4+ and CD8+ T cells.
The cellular response against cPvCSP and cPvMSP1 was assessed by determining the frequency of IFN-γ, IL-2 and TNF-α secreting T cells following ex vivo stimulation with peptides pools representing the complete amino acid sequence of the chimeric proteins [20, 21]. When the frequencies of cytokine-secreting T cells obtained from mice immunized with the SP-SAd36 regimen were compared to those of naïve mice, we observed significantly higher frequencies of cytokine-secreting CD4+ and CD8+ T cells obtained from the SPSAd36 regimen in response to all cPvCSP and cPvMSP1 peptide pools (not shown). Significantly higher frequencies of cytokine-secreting T cells in mice immunized with the SAd36 regimen in comparison to naïve mice were only observed for IFN-γ – secreting CD8+ T cells in response to cPvCSP pool B and cPvMSP1 pool 2; for IL-2-secreting CD8+ T cells in response to cPvCSP pool A; for TNF-α secreting CD4+ T cells in response to cPvMSP1 pool 1; and for TNF-α secreting CD8+ T cells in response to both cPvMSP1 pools (Supplementary Figures 2 and 3).
Analyses between the SP-SAd36 and SAd36 immunization groups revealed significantly higher frequencies of IFN-γ secreting CD4+ T cells from mice immunized with the SP-SAd36 regimen following stimulation with cPvCSP pools A and B (Figure 4A). Significantly higher frequencies of IL-2-secreting CD4+ T cells were observed in mice immunized with the SP-SAd36 regimen in response to all four cPvCSP pools (Figure 4B). Analysis of CD8+ T cells revealed significantly higher frequencies of IFN-γ secreting cells in response to pool D in mice immunized with the SP-SAd36 regimen (Figure 4D). We observed no differences in the frequency of TNF-α secreting T cells between the immunization regimens (Figure 4C and F). When T cell multifunctionality was assessed, we observed that mice immunized with SP-SAd36 had significantly higher frequencies of IL-2 and TNF-α co-expressing CD4+ T cells when stimulated with cPvCSP Pool B (Supplementary Figure 4).
Figure 4.

Cytokine-secreting T cells after ex vivo stimulation with cPvCSP peptide pools 5 days after the final immunization and assessed by flow cytometry. A-C) Frequency of cytokine-secreting CD4+ T cells following stimulation with cPvCSP peptide pools A, B, C, or D. D-F) Frequency of cytokine-secreting CD8+ T cells following stimulation with cPvCSP peptide pools A, B, C, or D. Interferon-γ-secreting T cells are shown in Figures A and D. Interleukin-2-secreting T cells are shown in B and E. Tumor necrosis factor-α-secreting T cells are shown in C and F. Values represent the percentage of either CD4+ or CD8+T cells positive for the individual cytokine. Kruskal-Wallis test with Dunn’s post-test was used to determine differences in the production of cytokines in response to a stimulus with an individual peptide pool between mice immunized with a regimen that included a priming with SAd36-cPvCSP/cPvMSP1 or priming with SP-SAd36-cPvCSP/cPvMSP1. Statistically significant differences between the SAd36 and SP-SAd36 regimens in response to individual pools are denoted by *(p < 0.05) and **(p < 0.01) within the SP-SAd36 bar.
When the frequencies of IFN-γ, IL-2, and TNF-α secreting CD4+ and CD8+ T cells in response to the cPvMSP1 peptide pools were assessed, we observed a significantly higher frequency of IL-2 –secreting CD4+ T cells obtained from the SP-SAd36 regimen mice when compared to the unmodified SAd36 regimen (Figure 5B). No other significant differences were observed in the production of cytokines by CD4+ or CD8+ T cells in response to the cPvMSP1 peptide pools (Figure 5). When T cell multifunctionality was assessed, we observed that mice immunized with SP-SAd36 had significantly higher frequencies of IFN-γ and IL-2 co-expressing cells CD8+ T cells when stimulated with cPvMSP1 Pool 2 (Supplementary Figure 5).
Figure 5.
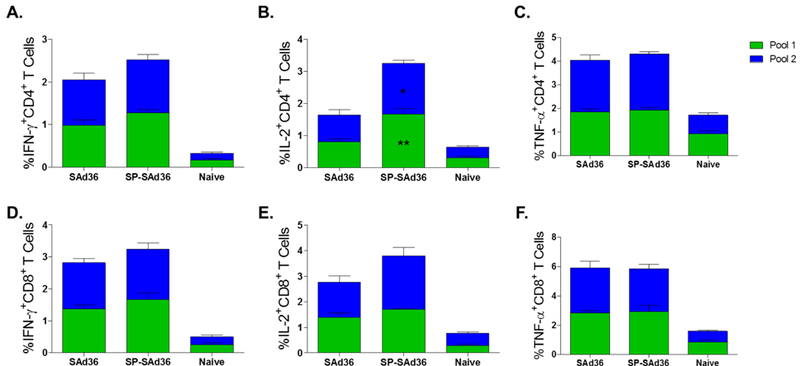
Cytokine-secreting T cells after ex vivo stimulation with cPvMSP1 peptide pools 5 days after the final immunization and assessed by flow cytometry. A-C) Frequency of cytokine-secreting CD4+ T cells following stimulation with cPvMSP1 peptide pools 1 or 2. D-F) Frequency of cytokine-secreting CD8+ T cells following stimulation with cPvMSP1 peptide pools 1 or 2. Interferon-γ-secreting T cells are shown in Figures A and D. Interleukin-2-secreting T cells are shown in B and E. Tumor necrosis factor-α-secreting T cells are shown in C, and F. Values presented represent the percentage of either CD4+ or CD8+ T cells positive for the individual cytokine. Statistical analysis was conducted using Kruskal Wallis with Dunn’s post-test was used to determine differences in the production of cytokines in response to a stimulus with an individual peptide pool between mice immunized with a regimen that included priming with SAd36-cPvCSP/cPvMSP1 or priming with SP-SAd36-cPvCSP/cPvMSP1. Statistically significant differences between the SAd36 and SP-SAd36 regimens in response to individual pools are denoted by *(p < 0.05) and **(p < 0.01) within the SP-SAd36 bar.
3.5. Higher frequencies of germinal center B cells are observed in draining lymph nodes following immunization with SP-SAd36.
To further understand the mechanisms involved in the increased antibody avidity and IL-2 production by CD4+ T cells observed following immunization with the SP-SAd36 immunization regimen, we assessed the frequency of germinal center B cells (B220+CD95+GL7+) in draining lymph nodes via flow cytometry analysis. The experiments were conducted nine days after priming with 108 v.p. of either the SAd36-cPvCSP/cPvMSP1 or SP-SAd36-cPvCSP/cPvMSP1 vector intramuscularly (Figure 6). We observed significantly higher frequencies of B220+CD95+GL7+ germinal center B cells in mice immunized with the SP-SAd36 vector when compared to naïve mice (Figure 6A). However, no significant differences were observed between naïve mice and SAd36 immunized mice at this timepoint. Similarly, when we compared the total number of the B220+CD95+GL7+ triple positive population, we observed similar differences between the groups, with significant differences between the SP-SAd36 and naïve groups (Figure 6B). To confirm these differences in the GC B cell response, we conducted immunostaining using fluorescent microscopy of LNs obtained from the same animals at day 9 post-priming with either the SAd36 or SP-SAd36. The draining inguinal LN sections were stained with anti-GL7 and anti-B220 antibodies, to identify GCs and B cell follicles respectively. Germinal centers were clearly stained in the dLN sections obtained from both SAd36 and SP-SAd36 when visualized by fluorescence microscopy with a distinctly higher number of GL7+ cells in lymph nodes from mice immunized with SP-SAd36 and very low number of positive cells observed in lymph nodes from unvaccinated mice (Figure 6C).
Figure 6.
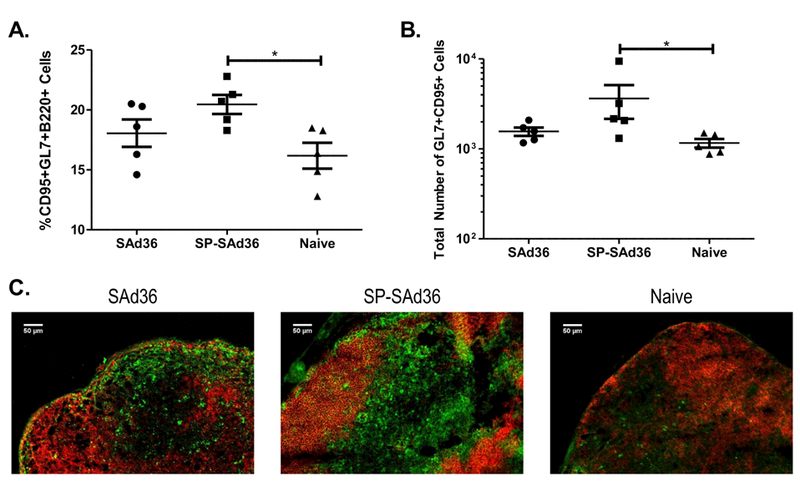
Frequency and total number of germinal center B cells in the draining lymph nodes nine days post priming. Frequency (A) and total number (B) of germinal center B cells, identified by positive staining with anti-B220, CD95, and GL7 antibodies via flow cytometry for the SP-SAd36-cPvCSP/cPvMSP1 and SAd36-cPvCSP/cPvMSP1 immunizations, and naïve mice. Statistical analysis was conducted using Kruskal-Wallis with Dunn’s post-test to determine differences between the immunization regimens. Statistically significant differences are denoted by *(p < 0.05). C) Draining inguinal lymph node from the same mice were obtained 9 days after priming with either the SP-SAd36-cPvCSP/cPvMSP1 or SAd36-cPvCSP/cPvMSP1 vectors, and sectioned and stained with the same fluorochrome-conjugated anti-B220 and GL7 antibodies used for flow cytometry assessment. A representative section from each group is shown at 20x magnification.
4. Discussion
The development of an effective malaria vaccine able to induce strong and balanced CD4+ and CD8+ T cell responses, as well as cytophilic antibodies, remains elusive. At present, multiple vaccine platforms and delivery systems are being investigated to determine the optimal vaccination regimen to induce broad and long-lasting immunity to malaria. Clinical studies of the protein-based vaccine, RTS,S, have shown protective efficacy mainly mediated through the induction of antibodies [35]. However, suboptimal CD8+ T cell induction has been a concern for RTS,S [4]. Experimental and clinical evidence has demonstrated that adenoviral vectors induce strong CD8+ T cell responses to Plasmodium antigens while maintaining a good safety profile [36–40]. However, poor induction of CD4+ T cell responses by an adenoviral-vectored malaria vaccine in clinical trials indicates that improvements in CD4+ T cell induction should be investigated [11].
Here we describe a strategy to further optimize the delivery of adenoviral transgenic products through the insertion of a murine IgGκ derived signal peptide. Although we observed no differences in the secretion of the cPvCSP/cPvMSP1 transgene product into the A549 tissue culture medium between the SP-SAd36 and unmodified SAd36 vectors, signal peptides have a variety of other effects on protein synthesis which may influence the selection of protein targeting pathways within the SP-SAd36 infected cells. Interactions between the signal peptide and the translocon at the endoplasmic reticulum (ER), as well as downstream events within the ER, including the potential cleavage of the IgGκ signal peptide, may also affect antigen presentation (reviewed in [14]). Our observations of increased antibody responses are consistent with those observed in recent studies on the effect of signal peptides on the immunogenicity of the HIV glycoprotein gp120, which found that the addition of a signal peptide impacted the glycan profile of gp120 increasing the antigenicity of the mature protein [41]. Additionally, the use of a signal peptide in an HPV DNA vaccine based on the protein E7 was found to significantly increase antibody titers to E7, a tumor-associated antigen which typically exhibits low immunogenicity, when compared to mice immunized with the same antigen without the signal peptide. The authors concluded that this was due to the conserved function of the signal peptide and its ability to alter the default protein trafficking pathway; this modulation in the sorting of the heterologous protein in mammalian cells resulted in the increased humoral immune response to the DNA vaccine [42]. However, the changes in immunogenicity may also vary depending on the adenoviral vector used, as comparative assessments of Ad5 and Ad26 vectored gp120 vaccinations have found that the viral vector used can skew the Fc-effector profiles of vaccine-induced antibodies and glycosylation profiles [43–46]. The contribution of the murine IgGκ signal peptide on these processes as part of the SP-SAd36 vector transgene, therefore, requires further investigation.
Comparison of the antibody response induced by the SP-SAd36 and unmodified SAd36 regimens revealed that the SP-SAd36 vector induced significantly higher antibody avidity in comparison to the unmodified vector. These findings are significant as increased avidity may translate to enhanced efficacy based on observations from preclinical studies of P. falciparum CSP vaccines which reported that avidity indices above 0.80 provided sterilizing protection against P. falciparum CSP transgenic P. berghei challenge [47]. P. falciparum vaccine clinical trials have also demonstrated antibody avidity to be associated with improved ability to inhibit parasite growth [48].
Assessment of differences in cellular immunogenicity revealed significantly higher frequencies of IFN-γ-secreting CD4+ T cells in response to the peptide pools representing the promiscuous T cell epitopes within cPvCSP protein chimera. This improvement is encouraging based on reports that high frequencies of IFN-γ-secreting CD4+ T cells provide protection from malaria [49, 50]. Moreover, IFN-γ producing CD4+ cells play a crucial role in promoting B cell class switching activity [51] and have also been associated with protection from P. falciparum in clinical trials [2].
Immunization with the SP-SAd36 regimen also resulted in increased frequencies of IL-2 secreting CD4+ T cells observed in response to all peptide pools of cPvCSP and cPvMSP1. This feature may represent a significant improvement for adenoviral vectors based on a recent study by Lee et al. [13] which found that vaccination with an Ad5 recombinant vector induced significantly lower frequencies of antigen-specific CD4+ TH1 cells compared to acute infection, an effect attributed low IL-2 signaling [13]. The increased IL-2 production we observed also has the potential to improve the overall T cell immunogenicity, as IL-2 promotes proliferation and differentiation of naïve CD8+ T cells and the survival of antigen-experienced T cells [52]. These improvements in the cellular immune responses are consistent with reports demonstrating enhanced immunogenicity of a DNA vaccine due to the inclusion of the IgGκ signal peptide when compared to an unmodified vaccine [19].
When we assessed germinal center B cell responses, we observed the highest frequency of germinal center B cells in mice immunized with the SP-SAd36 vector, suggesting that the improvements in antibody avidity observed for the SP-SAd36 regimen may be due to the induction of better germinal center responses. Previous studies of germinal center B cells and T follicular helper (TFH) responses in mouse models of Plasmodium infection have found that increased frequencies of these cell types are associated with increased titers of anti-parasite antibodies and improvements in parasite control and clearance [53, 54]. Moreover, in the murine P. chabaudi model the germinal centers are essential for the resolution of chronic infection [55, 56].
Overall, we observed that the inclusion of an IgGκ signal in the sequence of an adenoviral vector insert, improved the functionality of CD4+ cells, the antibody avidity and the frequency of germinal center B cells. Our results highlight the potential of this method to improve existing viral vector vaccine platforms and warrants further investigation.
Supplementary Material
Acknowledgments.
The authors would like to thank Dr. James Wilson for providing us with the SAd36 vector; Dr. Fidel Zavala for providing our group with P. vivax CSP VK210 repeat region transgenic P. berghei sporozoites for IFA slides; Dr. Mary Galinski for providing our group with P. vivax infected Saimiri boliviensis blood for IFA slides; Evan Dessasau III from Yerkes Histology Core for sectioning of murine lymph nodes; Dr. Deepa Kodandera from the Yerkes Molecular Pathology Core for sectioning, staining, and technical assistance with the lymph node IFA slides; Dr. April Reedy from the Emory Integrated Cellular Imaging core for assistance in fluorescence microscopy and image capture; and Monica Cabrera-Mora for technical support.
Funding. This research was supported by the National Institutes of Health, NIAID grants R56-AI103382–01A1 and R21-AI117459–01A1, and the Yerkes National Primate Research Center Base Grant No RR00165 awarded by the National Center for Research Resources of the National Institutes of Health. This project was supported in part by ORIP/OD P51OD011132.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest. None.
References
- [1].Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature 1961;192:733–7. [DOI] [PubMed] [Google Scholar]
- [2].Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 2009;361:468–77. [DOI] [PubMed] [Google Scholar]
- [3].Gosling R, von Seidlein L. The Future of the RTS,S/AS01 Malaria Vaccine: An Alternative Development Plan. PLoS Med 2016;13:e1001994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malaria journal 2009;8:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Greenwood B, Dicko A, Sagara I, Zongo I, Tinto H, Cairns M, et al. Seasonal vaccination against malaria: a potential use for an imperfect malaria vaccine. Malaria journal 2017;16:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Coughlan L, Mullarkey C, Gilbert S. Adenoviral vectors as novel vaccines for influenza. J Pharm Pharmacol 2015;67:382–99. [DOI] [PubMed] [Google Scholar]
- [7].Appaiahgari MB, Vrati S. Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther 2015;15:337–51. [DOI] [PubMed] [Google Scholar]
- [8].Majhen D, Calderon H, Chandra N, Fajardo CA, Rajan A, Alemany R, et al. Adenovirus-based vaccines for fighting infectious diseases and cancer: progress in the field. Hum Gene Ther 2014;25:301–17. [DOI] [PubMed] [Google Scholar]
- [9].Capone S, D’Alise AM, Ammendola V, Colloca S, Cortese R, Nicosia A, et al. Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Expert Rev Vaccines 2013;12:379–93. [DOI] [PubMed] [Google Scholar]
- [10].Fonseca JA, McCaffery JN, Kashentseva E, Singh B, Dmitriev IP, Curiel DT, et al. A prime-boost immunization regimen based on a simian adenovirus 36 vectored multi-stage malaria vaccine induces protective immunity in mice. Vaccine 2017;35:3239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ewer KJ, O’Hara GA, Duncan CJ, Collins KA, Sheehy SH, Reyes-Sandoval A, et al. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nature communications 2013;4:2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, et al. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infection and immunity 2010;78:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee J, Hashimoto M, Im SJ, Araki K, Jin HT, Davis CW, et al. Adenovirus Serotype 5 Vaccination Results in Suboptimal CD4 T Helper 1 Responses in Mice. J Virol 2017;91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hegde RS, Bernstein HD. The surprising complexity of signal sequences. Trends Biochem Sci 2006;31:563–71. [DOI] [PubMed] [Google Scholar]
- [15].Guler-Gane G, Kidd S, Sridharan S, Vaughan TJ, Wilkinson TC, Tigue NJ. Overcoming the Refractory Expression of Secreted Recombinant Proteins in Mammalian Cells through Modification of the Signal Peptide and Adjacent Amino Acids. PloS one 2016;11:e0155340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu H, Zou X, Li T, Wang X, Yuan W, Chen Y, et al. Enhanced production of secretory glycoprotein VSTM1-v2 with mouse IgGkappa signal peptide in optimized HEK293F transient transfection. J Biosci Bioeng 2016;121:133–9. [DOI] [PubMed] [Google Scholar]
- [17].Wang X, Liu H, Yuan W, Cheng Y, Han W. Efficient production of CYTL1 protein using mouse IgGkappa signal peptide in the CHO cell expression system. Acta Biochim Biophys Sin (Shanghai) 2016;48:391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bair CR, Kotha Lakshmi Narayan P, Kajon AE. The tripartite leader sequence is required for ectopic expression of HAdV-B and HAdV-E E3 CR1 genes. Virology 2017;505:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chandra J, Dutton JL, Li B, Woo WP, Xu Y, Tolley LK, et al. DNA Vaccine Encoding HPV16 Oncogenes E6 and E7 Induces Potent Cell-mediated and Humoral Immunity Which Protects in Tumor Challenge and Drives E7-expressing Skin Graft Rejection. J Immunother 2017;40:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cabrera-Mora M, Fonseca JA, Singh B, Oliveira-Ferreira J, Lima-Junior Jda C, Calvo-Calle JM, et al. Induction of Multifunctional Broadly Reactive T Cell Responses by a Plasmodium vivax Circumsporozoite Protein Recombinant Chimera. Infect Immun 2015;83:3749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fonseca JA, Cabrera-Mora M, Singh B, Oliveira-Ferreira J, da Costa Lima-Junior J, Calvo-Calle JM, et al. A chimeric protein-based malaria vaccine candidate induces robust T cell responses against Plasmodium vivax MSP119. Sci Rep 2016;6:34527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Roy S, Medina-Jaszek A, Wilson MJ, Sandhu A, Calcedo R, Lin J, et al. Creation of a panel of vectors based on ape adenovirus isolates. J Gene Med 2011;13:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kashentseva EA, Douglas JT, Zinn KR, Curiel DT, Dmitriev IP. Targeting of adenovirus serotype 5 pseudotyped with short fiber from serotype 41 to c-erbB2-positive cells using bispecific single-chain diabody. J Mol Biol 2009;388:443–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maizel JV Jr., White DO, Scharff MD. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 1968;36:115–25. [DOI] [PubMed] [Google Scholar]
- [25].Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000;164:6166–73. [DOI] [PubMed] [Google Scholar]
- [26].Ferreira MU, Katzin AM. The assessment of antibody affinity distribution by thiocyanate elution: a simple dose-response approach. Journal of immunological methods 1995;187:297–305. [DOI] [PubMed] [Google Scholar]
- [27].Fonseca JA, Cabrera-Mora M, Kashentseva EA, Villegas JP, Fernandez A, Van Pelt A, et al. A Plasmodium Promiscuous T Cell Epitope Delivered within the Ad5 Hexon Protein Enhances the Protective Efficacy of a Protein Based Malaria Vaccine. PLoS One 2016;11:e0154819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Matheu MP, Parker I, Cahalan MD. Dissection and 2-photon imaging of peripheral lymph nodes in mice. J Vis Exp 2007:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mac-Daniel L, Buckwalter MR, Gueirard P, Menard R. Myeloid Cell Isolation from Mouse Skin and Draining Lymph Node Following Intradermal Immunization with Live Attenuated Plasmodium Sporozoites. J Vis Exp 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li Y, Leneghan DB, Miura K, Nikolaeva D, Brian IJ, Dicks MD, et al. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci Rep 2016;6:18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McGowan JW, Bidwell GL 3rd.The Use of Ex Vivo Whole-organ Imaging and Quantitative Tissue Histology to Determine the Bio-distribution of Fluorescently Labeled Molecules. J Vis Exp 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schwenk R, DeBot M, Porter M, Nikki J, Rein L, Spaccapelo R, et al. IgG2 antibodies against a clinical grade Plasmodium falciparum CSP vaccine antigen associate with protection against transgenic sporozoite challenge in mice. PloS one 2014;9:e111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infection and immunity 2009;77:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Espinosa DA, Yadava A, Angov E, Maurizio PL, Ockenhouse CF, Zavala F. Development of a chimeric Plasmodium berghei strain expressing the repeat region of the P. vivax circumsporozoite protein for in vivo evaluation of vaccine efficacy. Infection and immunity 2013;81:2882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kazmin D, Nakaya HI, Lee EK, Johnson MJ, van der Most R, van den Berg RA, et al. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A 2017;114:2425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Antrobus RD, Coughlan L, Berthoud TK, Dicks MD, Hill AV, Lambe T, et al. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Molecular therapy : the journal of the American Society of Gene Therapy 2014;22:668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rodrigues EG, Zavala F, Nussenzweig RS, Wilson JM, Tsuji M. Efficient induction of protective anti-malaria immunity by recombinant adenovirus. Vaccine 1998;16:1812–7. [DOI] [PubMed] [Google Scholar]
- [38].Stewart VA, McGrath SM, Dubois PM, Pau MG, Mettens P, Shott J, et al. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infection and immunity 2007;75:2283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rodriguez A, Mintardjo R, Tax D, Gillissen G, Custers J, Pau MG, et al. Evaluation of a prime-boost vaccine schedule with distinct adenovirus vectors against malaria in rhesus monkeys. Vaccine 2009;27:6226–33. [DOI] [PubMed] [Google Scholar]
- [40].O’Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. The Journal of infectious diseases 2012;205:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yolitz J, Schwing C, Chang J, Van Ryk D, Nawaz F, Wei D, et al. Signal peptide of HIV envelope protein impacts glycosylation and antigenicity of gp120. Proc Natl Acad Sci U S A 2018;115:2443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Massa S, Paolini F, Curzio G, Cordeiro MN, Illiano E, Demurtas OC, et al. A plant protein signal sequence improved humoral immune response to HPV prophylactic and therapeutic DNA vaccines. Hum Vaccin Immunother 2017;13:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gunn BM, Alter G. Modulating Antibody Functionality in Infectious Disease and Vaccination. Trends Mol Med 2016;22:969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, et al. Dissecting Polyclonal Vaccine-Induced Humoral Immunity against HIV Using Systems Serology. Cell 2015;163:988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mahan AE, Jennewein MF, Suscovich T, Dionne K, Tedesco J, Chung AW, et al. Antigen-Specific Antibody Glycosylation Is Regulated via Vaccination. PLoS Pathog 2016;12:e1005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Davies J, Jiang L, Pan LZ, LaBarre MJ, Anderson D, Reff M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol Bioeng 2001;74:288–94. [PubMed] [Google Scholar]
- [47].Porter MD, Nicki J, Pool CD, DeBot M, Illam RM, Brando C, et al. Transgenic parasites stably expressing full-length Plasmodium falciparum circumsporozoite protein as a model for vaccine down-selection in mice using sterile protection as an endpoint. Clin Vaccine Immunol 2013;20:803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Druilhe P, Spertini F, Soesoe D, Corradin G, Mejia P, Singh S, et al. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLoS Med 2005;2:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, et al. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS One 2013;8:e61395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].King T, Lamb T. Interferon-gamma: The Jekyll and Hyde of Malaria. PLoS pathogens 2015;11:e1005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].McCall MB, Sauerwein RW. Interferon-gamma--central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukoc Biol 2010;88:1131–43. [DOI] [PubMed] [Google Scholar]
- [52].Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013;38:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 2011;13:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Figueiredo MM, Costa PAC, Diniz SQ, Henriques PM, Kano FS, Tada MS, et al. T follicular helper cells regulate the activation of B lymphocytes and antibody production during Plasmodium vivax infection. PLoS Pathog 2017;13:e1006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Perez-Mazliah D, Nguyen MP, Hosking C, McLaughlin S, Lewis MD, Tumwine I, et al. Follicular Helper T Cells are Essential for the Elimination of Plasmodium Infection. EBioMedicine 2017;24:216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Perez-Mazliah D, Ng DH, Freitas do Rosario AP, McLaughlin S, Mastelic-Gavillet B, Sodenkamp J, et al. Disruption of IL-21 signaling affects T cell-B cell interactions and abrogates protective humoral immunity to malaria. PLoS Pathog 2015;11:e1004715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011;79:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


