Abstract
The goal of the present study was to examine differences in cortical thickness, cortical surface area, and subcortical volume between bilingual children who are highly proficient in two languages (i.e., English and Spanish) and bilingual children who are mainly proficient in one of the languages (i.e., Spanish). All children (N = 49) learned Spanish as a native language (L1) at home and English as a second language (L2) at school. Proficiency of both languages was assessed using the standardized Woodcock Language Proficiency Battery. Five-minute high-resolution anatomical scans were acquired with a 3-Tesla scanner. The degree of discrepancy between L1 and L2 proficiency was used to classify the children into two groups: children with balanced proficiency and children with unbalanced proficiency. The groups were comparable on language history, parental education, and other variables except English proficiency. Values of cortical thickness and surface area of the transverse STG, IFG-pars opercularis, and MFG, as well as subcortical volume of the caudate and putamen, were extracted from FreeSurfer. Results showed that children with balanced bilingualism had thinner cortices of the left STG, left IFG, left MFG and a larger bilateral putamen, whereas unbalanced bilinguals showed thicker cortices of the same regions and a smaller putamen. Additionally, unbalanced bilinguals with stronger foreign accents in the L2 showed reduced surface areas of the MFG and STS bilaterally. The results suggest that balanced/unbalanced bilingualism is reflected in different neuroanatomical characteristics that arise from biological and/or environmental factors.
1 | INTRODUCTION
Relative to adults, children usually attain better command of a second language (L2) and often reach native-like status (Patkowski, 1980). This initial observation has led some researchers to incorrectly conclude that aptitude does not play a significant role in the L2 proficiency of young learners (Harley & Hart, 1997). More recent studies have reported differences in L2 aptitude scores in children (Abrahamsson & Hyltenstam, 2008), in some cases explaining over 20% of the variance (Kiss & Nikolov, 2005). These findings thus show that language aptitude can significantly predict children’s L2 proficiency (Paradis, 2011). Because early bilingual children have about the same amount of experience with both languages, this creates a scenario where bilingual language experience is naturally held constant but aptitude continues to vary across children. In this experimentally ecological context, researchers can study discrepancies in the degree of bilingualism that may be attributed to aptitude and not experience. The study of aptitude in L2 learning has overlooked children and instead focused on adults with exceptional linguistic abilities who appear to have the capacity to overcome maturational effects (Bongaerts, Mennen, & van der Slik, 2000; Piller, 2002; White & Genesee, 1996). To bridge this gap of knowledge, the current investigation studied a sample of bilingual children and measured their language proficiency in the first and second language and classified them as having “balanced” or “unbalanced” bilingualism given the degree of discrepancy between language proficiencies, with the goal of examining the brain anatomy of these children in relation to their bilingual classification. To the extent that language proficiency in children depends on aptitude, the current study can provide indirect evidence regarding the role of aptitude in the neuroanatomy of bilingualism.
On the whole, language-learning aptitude is defined as an innate, relatively fixed talent for learning languages (Neufeld, 1979). However, this definition is largely outdated given the epigenetic and neuroscientific evidence gathered in the last few years. Numerous studies now suggest that biological factors initially deemed fixed, are actually dynamic and in continuous interaction with many levels of the environment (Beer, 1995; Chiel & Beer, 1997; Elman et al., 1996; Via & Lande, 1985). Thus, biological factors such as aptitude interrelate with experiential factors like second language (L2) age of acquisition (AoA), time spent using the L2, quality of input in the L2, and socioeconomic status. These constructs, among others, together contribute to the ultimate attainment of L2 proficiency (Dörnyei & Skehan, 2003; Novoa, Fein, & Obler, 1988; Ross, Yoshinaga, & Sasaki, 2002; Skehan, 1991). Given the potential confusability between aptitude and proficiency, it is worth clarifying that while these concepts are related, they are distinct. Aptitude refers to the ability to develop a skill, whereas proficiency refers to the degree of competence acquired after deliberate training of that skill. But again, as mentioned, the concepts are related; therefore, it would not be unusual to find individuals with high levels of proficiency in the L2 who also score high on language aptitude tests.
In a recent fMRI study conducted by Archila-Suerte, Munson, and Hernandez (2015), bilingual children were classified as balanced or unbalanced based on the degree of discrepancy between L1 and L2 proficiency. Although this study did not measure aptitude using traditional metacognitive tasks such as phonological working memory or analytical reasoning (Kiss & Nikolov, 2005; Paradis, 2011), the study did use standardized language assessments to measure receptive and expressive knowledge of the L1 and L2—which relate to the aforementioned metacognitive skills (Hummel, 2009; Kormos & Safar, 2008). An additional measure of foreign accent in the L2 enabled the researchers to strengthen the validity of children’s classification as balanced or unbalanced. Despite balanced and unbalanced bilingual children being comparable in age, AoA, years of education, parental education, L1 and L2 use, and L1 proficiency, they were significantly different in L2 proficiency. That is, while balanced bilinguals were highly proficient in both languages, unbalanced bilinguals were only proficient in the L1 but significantly less proficient in the L2. A classification of bilinguals as balanced or unbalanced according to the degree of discrepancy between L1 and L2 proficiencies demonstrates individual differences related to L2 learning in children. Thus, despite similarities across experiential variables, some children readily advance in the L2 and other children lag behind.
The results of the passive listening fMRI task employed by Archila-Suerte et al. (2015) revealed that unbalanced bilinguals have increased activity in the bilateral middle frontal gyrus relative to the balanced group, whereas balanced bilinguals have increased activity in the right middle temporal gyrus relative to the unbalanced group, when listening to L2 speech sounds. These brain regions are respectively associated with working memory or cognitive control (Derrfuss, Brass, & Yves von Cramon, 2004; Luk, Green, Abutalebi, & Grady, 2011; Roth, Serences, & Courtney, 2006) and sound processing (Vouloumanos, Kiehl, Werker, & Liddle, 2001). Therefore, it appears that balanced and unbalanced bilingual children rely on different neural mechanisms to process L2 speech sounds; unbalanced children, in particular, may recruit regions involved in executive function to facilitate the perception of L2 sounds. Although the findings from Archila-Suerte et al. (2015) prompted the analysis of brain anatomy in the bilingual children, the primary aim of the current study was to investigate neuroanatomical markers of differences in children’s degree of bilingualism (i.e., balanced or unbalanced), which we hypothesize derive from either structural changes due to learning or neurobiological predispositions. Here, we assessed two morphological indices of the cortex (i.e., thickness and surface area) and one index of the subcortex (i.e., volume) in regions known to be involved in language processing and cognitive control in bilinguals. Specifically, the transverse superior temporal gyrus (STG), inferior frontal gyrus—pars opercularis (IFG), middle frontal gyrus (MFG), and the dorsal striatum of the basal ganglia (i.e. the caudate and putamen)—were examined bilaterally.
Neuroanatomical changes related to bilingualism have been well documented, especially in bilingual adults (García-Pentón, Fernández García, Costello, Duñabeitia, & Carreiras, 2016; Li, Legault, & Litcofsky, 2014). For example, bilingual adults have more gray matter density in the left inferior parietal lobule (IPL) (Abutalebi, Canini, Della Rosa, Green, & Weekes, 2015; Mechelli et al., 2004), and in bilateral STG than monolingual adults (Abutalebi et al., 2014; Ressel et al., 2012). Bilinguals also exhibit greater thickness in the left IFG (Klein, Mok, Chen, & Watkins, 2014; Stein, Winkler, Kaiser, & Dierks, 2014), right anterior cingulate (Felton et al., 2017) and larger subcortical volume of the left putamen relative to monolinguals (Abutalebi et al., 2013). In addition, successful learners of L2 speech sounds have greater white matter density bilaterally (Golestani, Molko, Dehaene, LeBihan, & Pallier, 2007; Golestani, Paus, & Zatorre, 2002) and larger STGs in the left hemisphere (Wong et al., 2008). Poor perceivers of L2 speech contrasts have also been found to have more white matter density in the right IFG opercularis/insular region than good perceivers (Felton et al., 2017; Sebastián-Gallés et al., 2012). On the other hand, the anatomical characteristics of bilingual children’s brains are remarkably understudied. To our knowledge, only two studies have compared the anatomical microstructure of bilingual and monolingual children. Mohades (Mohades et al., 2012; Mohades et al., 2015) investigated 8–11-year-olds and found that the white matter tract connecting anterior regions of the frontal lobe with posterior regions of the temporo-occipital lobe in the left hemisphere (i.e., inferior occipitofrontal fasciculus, IFOF) had a higher fractional anisotropy (FA) value in simultaneous bilinguals relative to sequential bilinguals and monolinguals, suggesting that simultaneous bilingual children have more organized IFOF tracts that assist with faster processing of semantic information. Despite the dearth of studies investigating the neuroanatomy of bilingual children, findings from adult studies can suggest some initial predictions about younger populations. For example, the literature in adult second language learning would suggest that bilingual children should also have larger volume of the IPL when compared to monolingual children. However, it is also important to keep in mind that the effects of language experience may have different effects on the child and adult brains based on their developmental state.
Changes in cortical thickness, specifically, have been found in relation to age and language proficiency. Age-related cortical thinning has been observed in healthy participants from 8 to 30 years (Tamnes et al., 2010) and 18 to 93 years (Salat et al., 2004). A study conducted by Fjell et al. (2009) examined multiple samples of participants to assess the consistency of age effects on cortical thickness. Their results showed that the STG, IFG, and MFG steadily diminish in thickness over time, while other regions like the inferior temporal lobe (ITL) and anterior cingulate cortices (ACC) appear to be less affected by age. In relation to language proficiency, Mårtensson et al. (Mårtensson et al., 2012) found that highly proficient bilinguals have thicker cortices in the left MFG, IFG, and STG relative to low proficient bilinguals. When examining the group of highly proficient individuals exclusively, the study found that the individuals experiencing more difficulty mastering the new language had greater gray matter density2 in the MFG. Other studies have found that L2 proficiency correlates with increased gray matter density in the IPL bilaterally (Abutalebi et al., 2014; Mechelli et al., 2004) Much less has been investigated in relation to cortical surface area and subcortical volume in bilinguals. A recent study found a negative correlation between surface area of the left precuneus and performance on a lexical decision task in bilingual adults (Burgaleta, Baus, Diaz, & Sebastian-Galles, 2014), and another study found that bilinguals have greater gray matter density of the left caudate nucleus relative to monolinguals (Zou, Ding, Abutalebi, Shu, & Peng, 2012). Given that cortical thickness and cortical surface area are genetically and phenotypically independent (Panizzon et al., 2009; Wierenga, Langen, Oranje, & Durston, 2014; Winkler et al., 2010), researchers have recommended considering each measure separately. Subcortical volume is also independent from any measure obtained in the cortex. Therefore, as cortical thickness, cortical surface, and subcortical volume are independent measures of brain morphology, the present study examined these anatomical characteristics in three separate MANCOVA models.
The cortical regions selected for analysis in the current study are two regions around the Sylvian fissure of the left hemisphere classically known to be involved in language processing: the STG and the IFG. The left STG is associated with early auditory processing of speech sounds (Hickok & Poeppel, 2007; Joanisse, Robertson, & Newman, 2007; Zevin & McCandliss, 2005), and the left IFG is associated with speech production/verbal fluency, and semantic processing (Friederici, Rueschemeyer, Hahne, & Fiebach, 2003; Poldrack et al., 1999). An additional area, the middle frontal gyrus (MFG), was included as a region of interest (ROI) due to its highlighted importance in studies of cognitive control in bilingualism research (Derrfuss et al., 2004; Guo, Liu, Misra, & Kroll, 2011; Luk et al., 2011) and the findings of Archila-Suerte et al. (2015). The subcortical regions, the bilateral caudate and putamen, were also selected based on findings that suggest the involvement of the basal ganglia in the cognitive control abilities of bilinguals (Abutalebi et al., 2013; Price, Green, & von Studnitz, 1999). In addition, it was necessary to investigate analogous regions in the right hemisphere to have a thorough understanding of the different structural profiles that characterize the brains of balanced and unbalanced bilingual children, since language experience has been shown to differentially affect thickness in each hemisphere (Felton et al., 2017).
The present study examined two different groups of bilingual children. One group comprised children who were highly proficient in both languages (i.e., balanced bilinguals), whereas the other group comprised children who were proficient in one of the languages but not the other (i.e., unbalanced bilinguals). For both groups of bilinguals, the L2 was learned in natural settings through immersion in the dominant language culture. Despite shared characteristics in age, years of education in the L2, parental education, and L1 proficiency across groups, group differences existed in L2 proficiency. Our main goal was to investigate differences between children with balanced vs. unbalanced bilingualism in various characteristics of brain anatomy (i.e., cortical thickness, cortical surface area, and subcortical volume). Based on the literature reviewed here, we hypothesized that successful/less successful learning of an L2 would be reflected in different brain morphology for these two bilingual groups. We expect that examining the relation between neuroanatomy and proficiency discrepancy across languages will help us identify the neural correlates of L2 learning success in emerging bilingual children.
2 | METHOD
2.1 | Participants
A total of 50 Spanish-English bilingual children between 6 and 13 years of age participated in this study (M = 9.26, SD = 1.74). All children began to learn Spanish from birth and English around 5 years of age. On average, children had 4 years of education in the L2 (M = 3.63, SD = 2.20) and reported speaking Spanish 50.32% and English 49.67% of the time at the time of testing. The large majority of children came from families whose parents had only completed an elementary education—on a scale of 1 to 6 (1 = some or less than elementary education, 6 = advanced degree)—M = 1.92, SD = 1.33. Sixty-seven percent of the children were born in the US and 29 percent arrived in the US before starting elementary school. Two families did not report the birthplace of their children. The children born outside the US came from a variety of Central and South American countries and had resided in the US an average of 6.7 years (SD = 2.44) at the time of testing.3 Children were not asked sociolinguistic or attitudinal questions about their experiences learning a second language. Children attended different types of schools with different approaches to second language learning. For the most part, however, children attended transitional bilingual programs where they are gradually shifted from Spanish to English instruction.
In the analyses, bilingual children were classified as having balanced or unbalanced bilingualism based on the degree of discrepancy between English and Spanish proficiency (described below). The mean discrepancy for balanced bilinguals was 5.14 (SD = 3.54) and the mean discrepancy for unbalanced bilinguals was 20.34 (SD = 7.13). Additional details regarding the classification of bilinguals are found in the results section. The sample of children used here include the same (38) children studied in Archila-Suerte et al. (2015), plus (12) additional participants with similar demographics, taken from the sample of Hernandez, Woods, and Bradley (2015). Two institutional review boards approved the present study. None of the parents reported cognitive impairments, language disabilities, or speech impediments for their children. (See Table 1 for participant characteristics.)
TABLE 1.
Demographic and behavioral data
| Overall Mean | Balanced | Unbalanced | F(1, 47) | Sig. | |
|---|---|---|---|---|---|
| N | 49 | 27 (19 born in US) | 22 (14 born in US) | N/A | N/A |
| Sex | 32 females | 15 females | 17 females | 2.55 | p = .117 |
| Age | 9.16 (1.75) | 9.33 (1.69) | 8.95 (1.84) | 0.56 | p = .456 |
| L2 AoA | 4.41 (2.03) | 4.44 (1.71) | 4.38 (2.41) | 0.01 | p = .920 |
| Parental education | 1.92 (1.34) | 1.74 (1.23) | 2.14 (1.46) | 1.06 | p = .307 |
| Total years of education | 5.09 (2.17) | 5.13 (2.50) | 5.05 (1.76) | 0.02 | p = .894 |
| Years of instruction in L2 | 3.63 (2.21) | 3.85 (2.28) | 3.36 (2.13) | 0.59 | p = .447 |
| English proficiency | 45.47 (13.30) | 52.31 (9.34) | 37.08 (12.75) | 23.23 | p < .0001* |
| Spanish proficiency | 53.62 (13.80) | 53.94 (12.45) | 53.22 (15.59) | 0.03 | p = .858 |
| Amount of L1 usea | 50.32 (21.63) | 48.80 (16.97) | 52.14 (26.48) | 0.26 | p = .607 |
| Amount of L2 usea | 49.67 (21.63) | 51.20 (16.97) | 47.85 (26.48) | 0.26 | p = .607 |
| Proficiency discrepancy | 11.97 (9.34) | 5.14 (3.54) | 20.34 (7.13) | 94.34 | p < .0001* |
| ICV | 1420768.82 (106876.02) | 1447317.61 (112688.70) | 1388186.23 (91476.11) | 3.94 | p = .053 |
Standard deviations are in parentheses.
Three participants did not report amount of L1 or L2 use.
2.2 | Standardized measures of language proficiency
2.2.1 | Woodcock-Johnson Language Proficiency Battery-Revised, English version (Woodcock, 1991)
The subtest of picture vocabulary assessed expressive word knowledge. This test required participants to overtly name pictures of objects or actions. The total number of items in the picture vocabulary subtest was 58. The subtest of listening comprehension assessed receptive knowledge of the language and it required orally completing incomplete sentences. The total number of items in the listening comprehension subtest was 38. The level of difficulty of each subtest gradually increased item by item.
2.2.2 | Woodcock-Muñoz Language Proficiency Battery-Revised, Spanish version (Woodcock & Muñoz-Sandoval, 1995)
The subtests of Vocabulario Sobre Dibujos and Comprensión de Lectura were analogous to the English subtests described above, thus aligning with the English version to assess expressive and receptive knowledge, respectively. The total number of items in the picture vocabulary subtest was 58 and the total number of items in the listening comprehension subtest was 35. None of the items in the Spanish version were direct translations or cognates of the English version. All items were unique and gradually increased in difficulty.
2.3 | Procedure
Participants completed two sessions. The first session took place in a private room in the laboratory. After a verbal description of the goals and risks of the study, parents and children signed the consent forms in the language of their choice. Parents proceeded to complete a questionnaire describing their child’s demographic information including birthplace, ethnicity, and health, as well as rating their own parental education. Parents then completed a language history questionnaire reporting their child’s L2 AoA, total number of years of education, years of instruction in L2, and amount of daily language use. Finally, a trained research assistant assessed children’s language proficiency in Spanish and English using the standardized measures of the Woodcock battery in counterbalanced order.
A subset of (37) children read 144 English words that contained various English vowels (e.g., a, o, u). Their productions were recorded using an external tabletop microphone (Omnidirectional Condenser, MX391/0). After the recordings, four English monolingual judges rated the degree of foreign accent in English using a 9-point scale (1 = native-like accent, 9 = strong non-native accent). Recordings were presented in random order to prevent bias.
The second session took place at the Human Neuroimaging Laboratory of Baylor College of Medicine in the Texas Medical Center.4 Once children were found to be clear of metal in their bodies, they were escorted to the scanner and instructed to remain as still as possible.
2.4 | Whole-brain MRI acquisition
High spatial resolution 3D T1-weighted images were acquired with a 3-Tesla magnetom TIM Trio scanner (Siemens AG, Germany) and a 12-channel head coil. A Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence was implemented (TR = 1.2s, TE = 2.66 ms, 256 × 224 matrix, 1 mm3 isotropic voxel size). To prevent motion, children were provided with extra padding to hold their heads in place. Anatomical scans lasted approximately 5 minutes.
2.5 | Cortical parcellation and subcortical segmentation
FreeSurfer 5.3.0 software (http://surfer.nmr.mgh.harvard.edu/, Center for Biomedical Imaging, Charlestown, MA) was used to measure cortical thickness, cortical surface area, and subcortical volume. FreeSurfer automated processing stream corrects for motion and strips the skull of each T1-weighted image using a hybrid watershed/surface deformation procedure (Ségonne et al., 2004), transforms images into Talairach space, and segments cortical and subcortical tissue into cerebrospinal fluid (CSF), gray matter/subcortical nuclei, and white matter based on intensity gradients. During subcortical processing and segmentation, FreeSurfer yields an automatic labeling of subcortical structures. The cortex is displayed as a surface model with a mesh of triangles (i.e., vertices). After reconstruction, deformable procedures such as surface inflation are smoothed with a full-width-half-maximum Gaussian kernel of 30 mm and averaged across participants using a non-rigid high-dimensional spherical averaging method to align cortical folding patterns (Fischl & Dale, 2000; Fischl, Sereno, Tootell, & Dale, 1999). This is followed by the parcellation of the cerebral cortex into respective gyral and sulcal structure (Desikan et al., 2006; Fischl et al., 2004), along with the generation of curvature and sulcal maps. Intensity and continuity information is used from the entire 3D MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white matter boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl & Dale, 2000). The default FreeSurfer template was used for the processing of our data because participants were above 5 years of age. Several child studies with similar participant age groups have also opted for using the standard FreeSurfer template and obtained valid results. See Almeida et al. (2010), Kirk et al. (2009) and Wolosin, Richardson, Hennessey, Denckla, and Mostofsky (2009) as example child studies with a similar data processing approach.
After automatic reconstruction of MR images, participants’ brain images were visually checked in 2D using Freeview 1.0. Each of the volume’s slices was scrolled through on the coronal, sagittal, and horizontal planes to ensure correct surface extraction and labeling of the white matter, pial surface, and subcortical regions. In case of defective labels, images were manually corrected and examined after a second reconstruction. Nine participants were dropped from data analysis due to excessive banding in the images produced by head motion (original N was 59 children).
2.6 | Statistical analyses
Cortical thickness, cortical surface area, and subcortical volume values obtained from the Destrieux and Aseg atlases in FreeSurfer were imported into SPSS v.22. All cortical regions (i.e., STG, IFG, and MFG) and subcortical nuclei (caudate and putamen) were selected a priori based on published literature suggesting the involvement of these areas in language processing and cognitive control. The specific parcellations examined from each of the cortical regions were the transverse STG, IFG pars opercularis, and MFG (not further sub-divided). See Destrieux, Fischl, Dale, and Halgren (2010) for details regarding parcellation and anatomical nomenclature. Two multivariate analyses of covariance (MANCOVA), one per hemisphere, were conducted to test differences in cortical thickness between children with balanced or unbalanced bilingualism. Similarly, two MANCOVAs were conducted to test differences in cortical surface area. And finally, two separate MANCOVAs were conducted to test differences in subcortical volume between the bilingual groups. Age and Intracranial Volume (ICV) were included as covariates of interest to remove any lingering effect that could explain the differences in thickness, surface area, or subcortical volume between balanced and unbalanced bilinguals. Left vs. right and cortical thickness vs. cortical surface vs. subcortical volume analyses were conducted separately because the dependent variables must be correlated in order to meet one of the MANCOVA’s assumptions. As stated in the literature review, these morphological measures have been demonstrated to be independent (Wierenga et al., 2014; Winkler et al., 2010). The statistical assumptions of normality, independence of observations, homogeneity of variances, univariate and multivariate outliers, multicollinearity, equality of covariance between the groups, and the relationship between the independent variable and covariates needed for proper examination of the data using MANCOVA were checked. Additional correlational analyses between brain morphology measures (thickness, surface area, subcortical volume) and foreign accent rating were conducted on the subset of bilingual children that had speech recordings available.
3 | RESULTS
3.1 | Language proficiency
Raw scores from picture vocabulary and listening comprehension subtests in English and Spanish were converted to percent correct scores prior to conducting all analyses. To calculate proficiency, we first examined the correlation between picture vocabulary and listening comprehension within each language. The assessments of picture vocabulary and listening comprehension were significantly correlated within the English language (r = .83, p < .001) and within the Spanish language (r = .87, p < .001). Hence, the percent correct scores from each subtest were averaged within each language to obtain a global measure of proficiency for each language. To calculate the discrepancy between English and Spanish proficiency and thus determine which children were more balanced or unbalanced across languages, we subtracted the global score of English proficiency from the global score of Spanish proficiency and used the absolute value. Based on the mean discrepancy between languages (M = 11.97, SD = 9.34), children with larger discrepancy scores between English and Spanish proficiency (i.e., above the mean) were classified as unbalanced (n = 22) and children with smaller discrepancy scores (i.e., below the mean) were classified as balanced (n = 27). Note that the current study does not strictly examine language dominance. That is, participant classification was not based on proficiency of one language versus the other. Children were classified based on their overall degree of bilingualism. Generally, children in the balanced group were considered to be more bilingual than children in the unbalanced group. However, being classified as a balanced bilingual did not inevitably indicate high proficiency in both languages. Some balanced bilinguals had low proficiency in both languages; therefore, while the discrepancy between Spanish and English scores was minimal, overall language proficiency was still below average. To be exact, our sample contained 17 balanced bilinguals who were highly proficient in both languages and eight balanced bilinguals who were low proficient in both languages (n = 8).
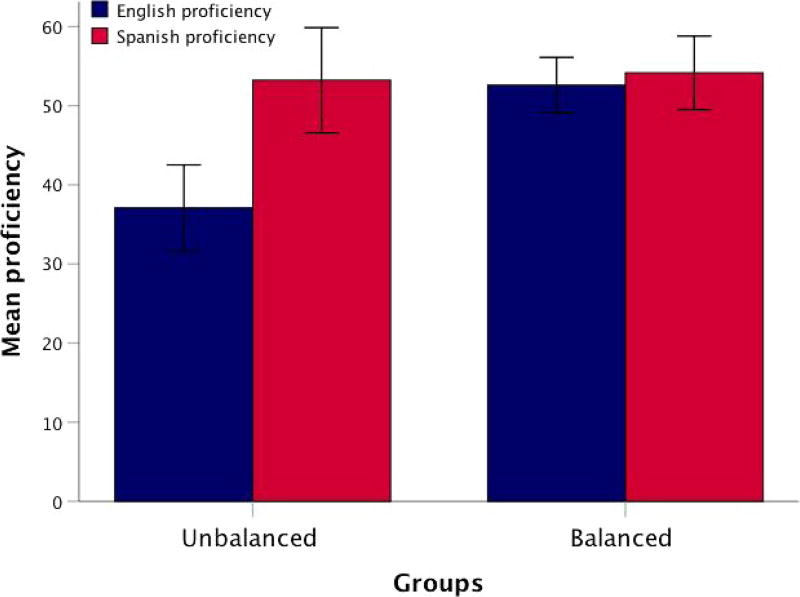
Significance tests showed that, on average, balanced bilingual children were highly proficient in both English and Spanish (t(26) = −1.39, p = .17), whereas unbalanced children were more proficient in Spanish than in English (t(21) = −5.20, p < .001). (See Figure 1.) A one-way ANOVA revealed that balanced and unbalanced bilinguals were not significantly different in sex, age, AoA, parent-rated amount of language use, years of education in the L2, Spanish proficiency, parental education, or ICV. The groups only significantly differed in English proficiency (F(1, 47) = 23.23, p < .0001). (See Table 1.) A frequency table reporting the number of children in each age group and bilingualism group has also been provided. (See Table 2.)
FIGURE 1.
Mean language proficiency in English and Spanish in groups of balanced and unbalanced bilingual children. Error bars represent standard error
TABLE 2.
Age group frequency
| Overall frequency |
Balanced | Unbalanced | |
|---|---|---|---|
| Total N | 49 | 27 | 22 |
| 6-year-olds | 5 | 1 | 4 |
| 7-year-olds | 4 | 3 | 1 |
| 8-year-olds | 6 | 4 | 2 |
| 9-year-olds | 11 | 6 | 5 |
| 10-year-olds | 16 | 9 | 7 |
| 11-year-olds | 1 | 0 | 1 |
| 12-year-olds | 5 | 3 | 2 |
| 13-year-olds | 1 | 1 | 0 |
Bilingual children born outside the US had higher proficiency in Spanish than children born in the US (F(1, 45) = 8.82, p < .005). English proficiency did not differ between children born inside or outside the country (F(1, 45) = 1.51, p = .22). Frequency analyses revealed approximately equal distributions for children born outside the US [balanced (n = 6) and unbalanced (n = 8)] and children born in the US [balanced (n = 19) and unbalanced (n = 14)].5 Thus, birthplace did not appear to bias classification, as children born inside or outside the US were evenly distributed between groups of balanced and unbalanced bilinguals. (See Table 1.) Plus, a 2 × 2 ANOVA examining birthplace × classification for English and Spanish proficiency revealed no interactions between birthplace and classification (English F(1, 43) = .04, p = .84 and Spanish F(1, 43) = .165, p = .68).
Finally, even though children’s proficiency in English and Spanish increased with age (English r = .59, p < .001; Spanish r = .71, p < .001), age did not positively correlate with the proficiency classification of balanced/unbalanced bilinguals (r = .109, p = .45). Moreover, a 2 × 2 ANOVA examining birthplace × age for English and Spanish proficiency revealed no interactions between the independent variables (English F(5, 35) = 1.96, p = .10 and Spanish F(5, 35) = 1.22, p = .31). Therefore, akin to birthplace, age did not impact classification of children as balanced or unbalanced bilinguals.
For the subset of 37 bilingual children6 with speech recordings, there was a significant negative correlation between foreign accent and L2 proficiency (r = −.62 p < .001); thus, children with higher proficiency in English had diminished foreign accents in the language.
3.2 | Multivariate analysis of covariance (MANCOVA)
To ensure a homogeneous dataset of comparably sized brains, one child with an ICV of 2 standard deviations above the mean was excluded from analyses (new N = 49, ICV M = 1420768.82).
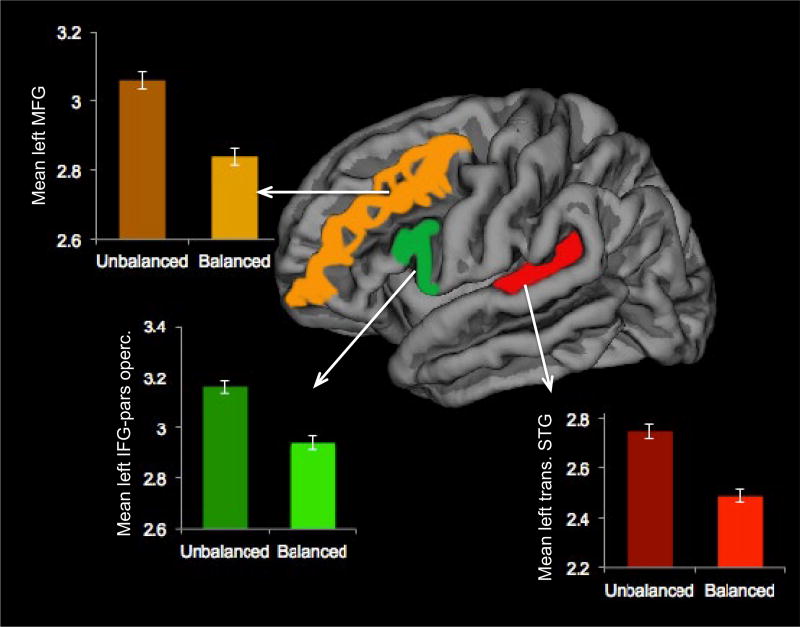
In the first pair of MANCOVA models, cortical thickness of the transverse STG, IFG-pars opercularis and MFG were included as dependent variables, proficiency classification (balanced/unbalanced) as the independent variable, and age and ICV as covariates.7 There was an interaction between the independent variable (i.e., balanced/unbalanced proficiency) and the covariate age in the right hemisphere (F(6, 78) = 2.61, p = .02). This interaction violated one of MANCOVA’s assumptions; therefore, no multivariate or between-subject effects of cortical thickness were scrutinized in the right hemisphere. On the other hand, multivariate tests showed a significant effect of proficiency classification in the left hemisphere (F(3, 42) = 3.37, p = .02, Wilk’s Lambda (Λ) = .80, partial eta square (η2) = .19, observed power = .72). Approximately 19% of the multivariate variance in cortical thickness in the left hemisphere ROIs was associated with proficiency classification. Between-subject effects showed that the groups significantly differed in thickness of transverse STG (F(1, 44) = 5.58, p = .02), IFG-pars opercularis (F(1, 44) = 9.55, p = .003), and MFG (F(1, 44) = 6.51, p = .01), with balanced bilinguals having thinner cortices than unbalanced bilinguals in all of these regions. (See Figure 2.)
FIGURE 2.
Differences in cortical thickness in the left transverse STG, IFG-pars opercularis, and MFG between groups of balanced and unbalanced bilingual children. Balanced bilinguals show reduced thickness in these regions relative to unbalanced bilinguals
In the second pair of MANCOVA models, cortical surface area of the transverse STG, IFG-pars opercularis and MFG were included as dependent variables, proficiency classification (balanced/unbalanced) as the independent variable, and age and ICV as covariates. Overall multivariate tests were not significant in the right (F(3, 43) = .86, p = .46, Wilk’s Lambda (Λ) = .94) or left hemispheres (F(3, 43) = .71, p = .54, Wilk’s Lambda (Λ) = .95). Therefore, between-subject effects were not scrutinized further.
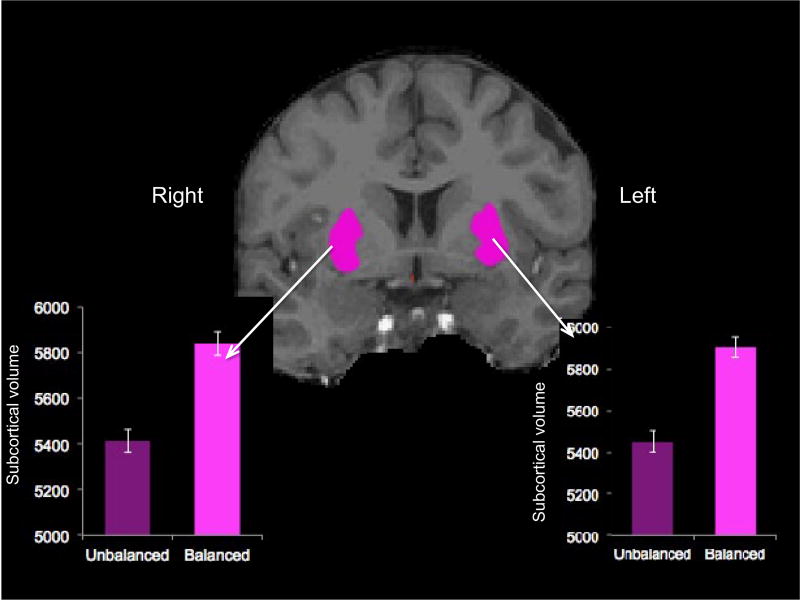
In the third pair of MANCOVAs, subcortical volumes of the caudate and putamen were included as dependent variables, proficiency classification (balanced/unbalanced) as the independent variable, and age and ICV as covariates. Multivariate tests showed a significant effect of proficiency classification in the right (F(2, 44) = 3.34, p = .04, Wilk’s Lambda (Λ) = .86, partial eta square (η2) = .13, observed power = .60) and left hemispheres (F(2, 44) = 3.48, p = .03, Wilk’s Lambda (Λ) = .86, partial eta square (η2) = .13, observed power = .62). Therefore, approximately 13% of the multivariate variance in volume of subcortical regions in each hemisphere is associated with proficiency classification. Between-subject effects specifically showed that the groups only significantly differed in the volume of the putamen (right: F(1, 45) = 6.79, p = .01 and left: F(1, 45) = 7.03, p = .01), with balanced bilinguals having larger volumes than unbalanced bilinguals in this region. (See Figure 3.) There were no significant differences in the caudate.
FIGURE 3.
Differences in subcortical volume in the bilateral putamen between groups of balanced and unbalanced bilingual children. Balanced bilinguals show a significantly larger volume of the putamen compared to unbalanced bilinguals
3.3 | Bivariate correlations
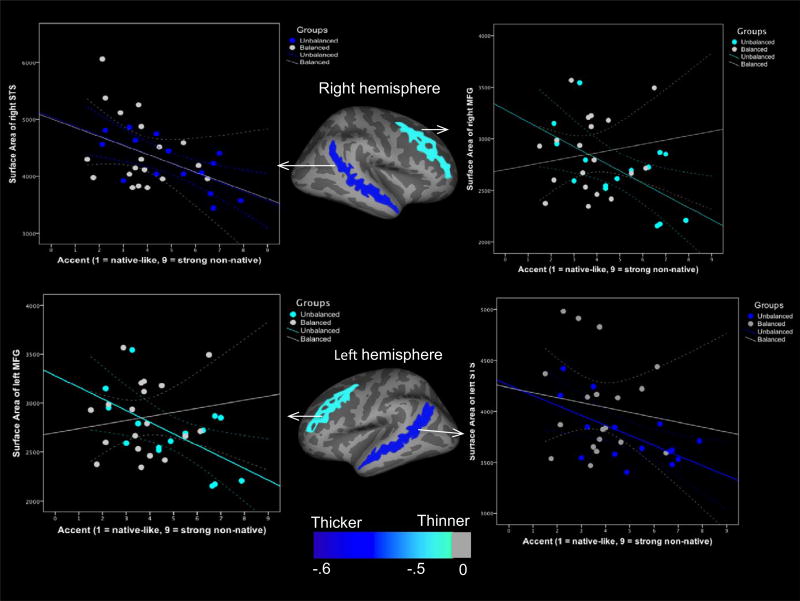
We were additionally interested in the relationship between several morphological measures (thickness, surface area, and subcortical volume) and accent in the L2 for the subgroup of 37 bilingual children whose accent in the L2 was rated. Here we found that unbalanced bilinguals showed significant negative correlations between cortical surface area of the bilateral MFG and foreign accent (right: r = −.59, p = .01 and left: r = −.59, p = .01) and between cortical surface area of bilateral superior temporal sulcus (STS) and foreign accent (right: r = −.67, p = .006 and left: r = −.61, p = .01). (See Figure 4.) Those with greater foreign accent had reduced surface areas in these regions. There were no significant correlations between thickness or subcortical volume and foreign accent in unbalanced bilinguals. Furthermore, no significant correlations were found between thickness, surface area, or subcortical volume of any of the brain regions of interest (transverse STG, IFG-pars opercularis, MFG, caudate, or putamen) and foreign accent in balanced bilinguals. The fact that the correlations differed across groups suggests that these are more tenuous than other effects reported. These are intriguing, yet secondary, results that need to be investigated in depth in the future.
FIGURE 4.
Correlations between foreign accent and cortical surface area of the bilateral MFG and STS in unbalanced bilingual children. Unbalanced bilinguals with stronger foreign accents have smaller cortical surfaces of the MFG and STS bilaterally. There are no significant correlations between cortical surface area and accent in balanced bilingual children
4 | DISCUSSION
The present study identified neuroanatomical differences between children with balanced vs. unbalanced bilingualism. Specifically, balanced bilinguals had thinner cortices of the left transverse STG, IFG-pars opercularis, and MFG compared to unbalanced bilinguals. Balanced bilinguals also showed a larger bilateral putamen than unbalanced bilinguals. There were no significant differences in cortical surface area between the two groups. However, for children with unbalanced bilingualism, a thicker foreign accent in the L2 negatively correlated with cortical surface area of the MFG and STS. That is, those with stronger accents had smaller surface areas of the MFG and STS. In summary, children with balanced bilingualism showed thinner cortices of the left STG, left IFG, left MFG, and a larger bilateral putamen; unbalanced bilinguals showed thicker cortices of the same regions and a smaller putamen. Furthermore, unbalanced bilinguals with heavier accents in the L2 showed reduced surface areas of the MFG and STS bilaterally.
As demonstrated by our results, being born inside or outside the US was unrelated to classification of children as balanced or unbalanced bilinguals. More importantly, cortical thickness was significantly different between the groups after controlling for age. Therefore, cortical thinning of the transverse STG, IFG-pars opercularis, and MFG in balanced bilinguals does not appear to be related to the maturational processes of aging but rather is related to increased proficiency in both languages. This could suggest that bilingualism is influencing brain development. Contrary to previous studies that show thickening of the STG, IFG, and MFG with higher L2 proficiency (Mårtensson et al., 2012), our results show the opposite relationship in these same regions. Two variables that may help explain such different results are that Mårtensson et al. (2012) collected a small sample of adult bilinguals highly trained as interpreters and examined L2 proficiency alone, whereas our sample included young children with less developed language skills and examined overall proficiency within each language. However, Mårtensson et al. (2012) did find that individuals who had more difficulty mastering the L2 had thicker cortices in these regions, which matches our findings of children with unbalanced bilingualism having thicker transverse STG, IFG-pars opercularis, and MFG. More importantly, the brain morphology results presented here align with the fMRI results presented in Archila-Suerte et al. (2015), as children with unbalanced bilingualism demonstrated thicker MFG and also increased neural activity in this region in response to listening to L2 speech sounds.
Our results did not find differences in cortical surface area between our two groups of bilingual children. We did find, however, a negative relationship between cortical surface area and foreign accent in unbalanced bilingual children. Unbalanced bilinguals with stronger accents in English showed smaller surface areas of the MFG and STS bilaterally. These results show that only one aspect of proficiency (i.e., degree of accent) is associated with surface area. This is intriguing to us because while accent may not be completely independent of proficiency, accent appears to play a unique role in some aspects of brain anatomy. In light of previous findings that have demonstrated that variations in cortical thickness and surface area of the STG are linked to sound perception ability in both speech (Wong et al., 2008) and music domains (Wengenroth et al., 2014), and that such morphological variability is partially heritable (Cai et al., 2014; Thompson et al., 2001), these results could suggest that a biological predisposition may be influencing the degree of success with which children acquire the phonology of the L2. The results related to surface area and foreign accent must be taken with caution, however, due to the much smaller sample analyzed. These are secondary results that need to be investigated further in future studies.
The results of subcortical volume, showing that children with unbalanced bilingualism have smaller bilateral putamen, parallel functional imaging findings from various language tasks. For example, increased activity in the left putamen has been reported in multilinguals when reading or producing words in the non-proficient language (Abutalebi et al., 2013; Meschyan & Hernandez, 2006). Neural activity has also been reported in the left putamen in response to degraded speech (Meyer, Steinhauer, Alter, Friederici, & von Cramon, 2004) and in late talkers (Preston et al., 2010). Moreover, left putaminal damage has been associated with foreign accent syndrome (Berthier et al., 2015). And finally, a recent study found that children with poor phonological skills in the L1 and L2 have less gray matter density in the putamen, bilaterally (Cherodah, Rao, Midha, & Sumathi, 2016). All this evidence points to the putamen as an area necessary for optimal language processing. A small bilateral putamen in unbalanced bilinguals may partly explain why these children have difficulties learning the L2, although deficient language skills may also result in lesser development of the putamen. Overall, the morphological differences observed in the neuroanatomical profiles of children with balanced vs. unbalanced bilingualism are sensible given the involvement of these structures (STG, IFG, MFG, putamen) in cognitive control and language processing.
While the correspondence between brain structure and function may not be exact, researchers generally agree that there is a strong coupling between structural and functional networks (Das et al., 2014; Mišić et al., 2016; Wang, Dai, Gong, Zhou, & He, 2015; Zhou, Zemanová, Zamora-Lopez, Hilgetag, & Kurths, 2007). Accordingly, the anatomical results presented here may help us to broadly hypothesize about the brain function of balanced and unbalanced bilingual children. Thicker cortex of the STG, IFG, MFG, a smaller bilateral putamen, and reduced cortical surface area of the MFG and STS related to a strong foreign accent in unbalanced bilinguals might suggest that their cognitive system is dealing with a laborious task that requires increased mental effort to improve performance. Learning an L2 may be more taxing and demanding for children who do not have the skills or experience to process new linguistic information. In line with our interpretation, thickening of the cortex has been reported in bilinguals who have more difficulty in the L2 (Mårtensson et al., 2012); reduced cortical surface area has been reported in children with dyslexia, ADHD, and autism (Frye et al., 2010; Raznahan et al., 2010; Shaw et al. 2012; Wolosin et al., 2009), and subcortical volume reductions have been noted in children with language disorders (Badcock, Bishop, Hardiman, Barry, & Watkins, 2012; Mayes, Reilly, & Morgan, 2015). A thicker cortex in children with unbalanced bilingualism might also relate to difficulties in language switching, especially as the brain regions examined in this study (IFG, MFG, STG) have been found in studies of bilinguals switching between their respective languages (Hernandez, 2009; Kovelman, Shalinsky, Berens, & Petitto, 2008; Kovelman et al., 2009). Future studies should investigate how degree of bilingualism relates to language switching abilities.
Functions of the basal ganglia have been associated with habit formation and implicit learning (Packard & Knowlton, 2002; Seger, 2006). Based on the literature mentioned above related to putaminal function, it is possible that an atypical development of subcortical structures in children with unbalanced bilingualism may result in more use of an alternative route for L2 learning—one that requires cortical areas to be involved. If subcortical regions are not efficiently managing information from the L2 in unbalanced bilinguals, then another mechanism involving explicit sub-articulation and cognitive control may be necessary to assist in L2 learning. While unbalanced bilinguals may still learn various aspects of the L2 implicitly, the function of subcortical regions could be potentially constrained by the suboptimal anatomical characteristics described in children with unbalanced bilingualism (i.e., small putamen). Several studies have proposed two pathways in L2 learning, one in which individuals use an implicit system and another one in which individuals use an explicit system to learn the L2 (Chandrasekaran, Yi, & Maddox, 2014; DeKeyser, 2008; Ellis, 1994; Hernandez & Li, 2007; Ullman, 2001); thus, allowing the possibility that more or less successful acquisition of the L2 is associated with the extent to which individuals engage in implicit or explicit learning. In the present study, our results seem to suggest that unbalanced bilinguals who may have reduced subcortical/ implicit systems readily available for the acquisition of an L2 draw on cortical/explicit systems as an alternative way to learn an L2.
The results of this study support our hypothesis that bilingual language outcomes (balanced vs. unbalanced) are differentially reflected in brain morphology in bilingual children. An enduring debate in psychology and many other sciences is whether developmental changes are due to nature vs. nurture. While the results of this study cannot ascertain whether anatomical changes were caused by linguistic experiences or whether anatomical differences were originally present in these children at birth, they do take us a step closer to unraveling the complexities of L2 learning. It is important to remember that children are continuing to develop their linguistic proficiency in both languages, and other sociolinguistic and pedagogical variables not examined here may also play a role in children’s neuroanatomy. In all, we expect that understanding the morphology and function of the brain and its impact on language learning can help parents and educators make informed decisions regarding bilingualism.
4.1 | Limitations and future directions
Due to the lack of a Spanish monolingual group, we can only conjecture that our participants were highly proficient in L1. A group of English monolingual children (not included here) had significantly higher English proficiency than the unbalanced bilingual group, but not the balanced bilingual group. However, we cannot extrapolate that a similar pattern of proficiency would be found if bilingual children were compared to a Spanish monolingual group because the groups of bilinguals assessed in this study do not live in a Spanish-speaking country. More likely, the bilingual children studied here have lower Spanish proficiency than Spanish monolingual children. An additional related limitation is that the language assessments employed here are somewhat dated and normed on monolingual populations. Future studies should assess language proficiency in bilinguals considering monolingual norms in each language and bilingual norms.
It is important to note that the MANCOVAs controlled for age only. Sex differences have also been found to relate to cortical thickness (Sowell et al., 2007). It is thus possible that taking the additional variable of sex into account could either increase or decrease the statistical significance of the results. Future studies ought to examine sex differences in groups of balanced and unbalanced bilinguals in depth. Finally, we acknowledge that while the two bilingual groups were comparable in age, years of education in the L2, and SES, they may not have been comparable in other aspects. For example, language input quality, personality, and genetic makeup were not assessed so it is possible that some of the differences in L2 learning between groups may be explained by these variables.
It is also important to highlight that the data gathered for this study only addressed information pertinent to the child at the time of testing. Children were not assessed longitudinally and there were not enough participants in each age group for a cross-sectional approach. However, the demonstration of neurostructural differences related to childhood bilingual language experience provides a foundational step towards future explorations within a broader developmental context. Studying L2 proficiency in children is particularly difficult as they are in the midst of an ongoing trajectory of cognitive and linguistic development. Given the tight coupling between aptitude and experience that becomes ever more entrenched throughout human development, it would be ideal to conduct a prospective longitudinal study of brain morphology and L2 learning whereby monolingual children of varying aptitude levels are tracked as they become bilingual to attempt to disentangle aptitude from experience.
5 | CONCLUSIONS
Different neuroanatomical profiles characterize children with balanced vs. unbalanced bilingualism. Balanced bilinguals had thinner cortices of the STG, IFG, MFG, and a larger bilateral putamen, whereas unbalanced bilinguals had thicker cortices and a smaller putamen. In addition, unbalanced bilinguals with stronger foreign accents in English had less cortical surface area in the MFG and STS. These results suggest that brain anatomy, which may have been shaped by experience, is related to language function and may play a role in how well children learn an L2. It is possible that children with unbalanced bilingualism are using alternative mechanisms to manage input in the L2. The findings presented here contribute, not only to the field of bilingualism, but also to the wider literature on experience-based plasticity.
RESEARCH HIGHLIGHTS.
This study examined the brain anatomy of bilingual children.
Spanish-English bilingual children of comparable age, L2 AoA, parental education, years of education, and L1 proficiency were classified as balanced or unbalanced bilinguals. Children only differed in L2 proficiency.
Three measures of brain morphology were studied: cortical thickness, cortical surface area, and subcortical volume.
Results showed significantly different neuroanatomical profiles for children with balanced and unbalanced bilingualism.
Acknowledgments
We want to thank the research assistants who helped with pre-viewing and editing of brain images: Matthew Spruiell, Matthew Rodriguez, and Shelby Ivy. This work was supported by the Institute for Biomedical Imaging Science (IBIS) for Plasticity in Speech Perception in Early Bilingual Children and grant number R21HD059103-01 for the Neural Correlates of Lexical Processing in Child L2 Learners from the National Institutes of Health (NIH).
Footnotes
All authors collaborated in the development of this project. Specifically, Dr Archila-Suerte was in charge of data collection, training of research assistants, data analyses, interpretation of results, creation of figures, manuscript write up, and revision; Dr Woods assisted with the creation of Table 1 and original manuscript editing; Dr Chiarello provided comments and insight on neuroanatomical analyses, and Dr Hernandez assisted with the interpretation of results as well as providing comments and funding for data collection.
Cortical thickness and gray matter density are different morphological measures but are related.
Length of US residency analysis was only conducted with participants from the Archila-Suerte et al. (2015) study (N = 9), as such data were not collected for additional participants from Hernandez et al. (2015).
The Human Neuroimaging Laboratory is now known as the Core for Advanced Magnetic Resonance Imaging (CAMRI).
Parental report regarding birthplace was missing for two participants.
The subsample of 37 children was selected from the overall pool of participants. No additional details are provided about this group because their characteristics are virtually identical to that of the entire sample.
Additional analysis included sex as a covariate. This did not add or change the core of our results.
References
- Abrahamsson N, Hyltenstam K. The robustness of aptitude effects in near-native second language acquisition. Studies in Second Language Acquisition. 2008;30:481–509. [Google Scholar]
- Abutalebi J, Canini M, Della Rosa PA, Green DW, Weekes BS. The neuroprotective effects of bilingualism upon the inferior parietal lobule: A structural neuroimaging study in aging Chinese bilinguals. Journal of Neurolinguistics. 2015;33:3–13. [Google Scholar]
- Abutalebi J, Canini M, Della Rosa PA, Sheung LP, Green DW, Weekes BS. Bilingualism protects anterior temporal lobe integrity in aging. Neurobiology of Aging. 2014;35:2126–2133. doi: 10.1016/j.neurobiolaging.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Della Rosa PA, Gonzaga AK, Keim R, Costa A, Perani D. The role of the left putamen in multilingual language production. Brain and Language. 2013;125:307–315. doi: 10.1016/j.bandl.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Almeida LG, Ricardo-Garcell J, Prado H, Barajas L, Fernández-Bouzas A, Ávila D, Martínez RB. Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: A cross-sectional study. Journal of Psychiatric Research. 2010;44:1214–1223. doi: 10.1016/j.jpsychires.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Archila-Suerte P, Munson B, Hernandez A. Cognitive control and consequences of multilingualism (Bilingual processing and acquisition 2) Amsterdam: John Benjamins; 2015. The role of executive function in the perception of L2 speech sounds in young balanced and unbalanced dual language learners; pp. 71–96. [Google Scholar]
- Badcock NA, Bishop DVM, Hardiman MJ, Barry JG, Watkins KE. Co-localisation of abnormal brain structure and function in specific language impairment. Brain and Language. 2012;120:310–320. doi: 10.1016/j.bandl.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer RD. A dynamical systems perspective on agent–environment interaction. Artificial Intelligence. 1995;72:173–215. [Google Scholar]
- Berthier ML, Dávila G, Moreno-Torres I, Beltrán-Corbellini Á, Santana-Moreno D, Roé-Vellvé N, Ruiz-Cruces R. Loss of regional accent after damage to the speech production network. Frontiers in Human Neuroscience. 2015;9:610. doi: 10.3389/fnhum.2015.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongaerts T, Mennen S, van der Slik F. Authenticity of pronunciation in naturalistic second language acquisition: The case of very advanced late learners of Dutch as a second language. Studia Linguistica. 2000;54:298–308. [Google Scholar]
- Burgaleta M, Baus C, Diaz B, Sebastian-Galles N. Brain structure is related to speech perception abilities in bilinguals. Brain Structure and Function. 2014;219:1405–1416. doi: 10.1007/s00429-013-0576-9. [DOI] [PubMed] [Google Scholar]
- Cai DC, Fonteijn H, Guadalupe T, Zwiers M, Wittfeld K, Teumer A, Buitelaar J. A genome-wide search for quantitative trait loci affecting the cortical surface area and thickness of Heschl’s gyrus. Genes, Brain and Behavior. 2014;13:675–685. doi: 10.1111/gbb.12157. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B, Yi H-G, Maddox WT. Dual-learning systems during speech category learning. Psychonomic Bulletin and Review. 2014;21:488–495. doi: 10.3758/s13423-013-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherodah S, Rao C, Midha R, Sumathi TA. A role for putamen in phonological processing in children. Bilingualism: Language and Cognition. 2016;20:318–326. [Google Scholar]
- Chiel HJ, Beer RD. The brain has a body: Adaptive behavior emerges from interactions of nervous system, body and environment. Trends in Neurosciences. 1997;20:553–557. doi: 10.1016/s0166-2236(97)01149-1. [DOI] [PubMed] [Google Scholar]
- Das T, Abeyasinghe P, Crone J, Sosnowski A, Laureys S, Owen A, Soddu A. Highlighting the structure–function relationship of the brain with the ising model and graph theory. BioMed Research International. 2014;2014:237898. doi: 10.1155/2014/237898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKeyser R. Implicit and explicit learning. In: Doughty CJ, Long MH, editors. The handbook of second language acquisition. Oxford: Blackwell; 2008. pp. 313–348. [Google Scholar]
- Derrfuss J, Brass M, Yves von Cramon D. Cognitive control in the posterior frontolateral cortex: Evidence from common activations in task coordination, interference control, and working memory. NeuroImage. 2004;23:604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Hyman BT. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörnyei Z, Skehan P. Individual differences in second language learning. In: Doughty CJ, Long MH, editors. The handbook of second language acquisition. Oxford: Blackwell; 2003. pp. 589–630. [Google Scholar]
- Ellis NC, editor. Implicit and explicit learning of languages. New York: Academic Press; 1994. [Google Scholar]
- Elman JL, Bates EA, Johnson MH, Karmiloff-Smith A, Parisi D, Plunkett K. Rethinking innateness. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- Felton A, Vazquez D, Ramos-Nunez AI, Greene MR, Macbeth A, Hernandez AE, Chiarello C. Bilingualism influences structural indices of interhemispheric organization. Journal of Neurolinguistics. 2017;42:1–11. doi: 10.1016/j.jneuroling.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Kennedy D. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Fischl B. High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Rueschemeyer S-A, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cerebral Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Frye RE, Liederman J, Malmberg B, McLean J, Strickland D, Beauchamp MS. Surface area accounts for the relation of gray matter volume to reading-related skills and history of dyslexia. Cerebral Cortex. 2010;20:2625–2635. doi: 10.1093/cercor/bhq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pentón L, Fernández García Y, Costello B, Duñabeitia JA, Carreiras M. The neuroanatomy of bilingualism: How to turn a hazy view into the full picture. Language, Cognition and Neuroscience. 2016;31:301–327. [Google Scholar]
- Golestani N, Molko N, Dehaene S, LeBihan D, Pallier C. Brain structure predicts the learning of foreign speech sounds. Cerebral Cortex. 2007;17:575–582. doi: 10.1093/cercor/bhk001. [DOI] [PubMed] [Google Scholar]
- Golestani N, Paus T, Zatorre RJ. Anatomical correlates of learning novel speech sounds. Neuron. 2002;35:997–1010. doi: 10.1016/s0896-6273(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Guo T, Liu H, Misra M, Kroll JF. Local and global inhibition in bilingual word production: fMRI evidence from Chinese-English bilinguals. NeuroImage. 2011;56:2300–2309. doi: 10.1016/j.neuroimage.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley B, Hart D. Language aptitude and second language proficiency in classroom learners of different starting ages. Studies in Second Language Acquisition. 1997;19:379–400. [Google Scholar]
- Hernandez AE. Language switching in the bilingual brain: What’s next? Brain and Language. 2009;109:133–140. doi: 10.1016/j.bandl.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Li P. Age of acquisition: Its neural and computational mechanisms. Psychological Bulletin. 2007;133:638–650. doi: 10.1037/0033-2909.133.4.638. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Woods EA, Bradley KAL. Neural correlates of single word reading in bilingual children and adults. Brain and Language. 2015;143:11–19. doi: 10.1016/j.bandl.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hummel KM. Aptitude, phonological memory, and second language proficiency in nonnovice adult learners. Applied Psycholinguistics. 2009;30:225–249. [Google Scholar]
- Joanisse MF, Robertson EK, Newman RL. Mismatch negativity reflects sensory and phonetic speech processing. NeuroReport. 2007;18:901–905. doi: 10.1097/WNR.0b013e3281053c4e. [DOI] [PubMed] [Google Scholar]
- Kirk GR, Haynes MR, Palasis S, Brown C, Burns TG, McCormick M, Jones RA. Regionally specific cortical thinning in children with sickle cell disease. Cerebral Cortex. 2009;19:1549–1556. doi: 10.1093/cercor/bhn193. [DOI] [PubMed] [Google Scholar]
- Kiss C, Nikolov M. Developing, piloting, and validating an instrument to measure young learners’ aptitude. Language Learning. 2005;55:99–150. [Google Scholar]
- Klein D, Mok K, Chen J-K, Watkins KE. Age of language learning shapes brain structure: A cortical thickness study of bilingual and monolingual individuals. Brain and Language. 2014;131:20–24. doi: 10.1016/j.bandl.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Kormos J, Safar A. Phonological short-term memory, working memory and foreign language performance in intensive language learning. Bilingualism: Language and Cognition. 2008;11:261–271. [Google Scholar]
- Kovelman I, Shalinsky MH, Berens MS, Petitto L-A. Shining new light on the brain’s “bilingual signature”: A functional near infrared spectroscopy investigation of semantic processing. NeuroImage. 2008;39:1457–1471. doi: 10.1016/j.neuroimage.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman I, Shalinsky MH, White KS, Schmitt SN, Berens MS, Paymer N, Petitto L-A. Dual language use in sign-speech bimodal bilinguals: fNIRS brain-imaging evidence. Brain and Language. 2009;109:112–123. doi: 10.1016/j.bandl.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Legault J, Litcofsky KA. Neuroplasticity as a function of second language learning: Anatomical changes in the human brain. Cortex. 2014;58:301–324. doi: 10.1016/j.cortex.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Luk G, Green DW, Abutalebi J, Grady C. Cognitive control for language switching in bilinguals: A quantitative meta-analysis of functional neuroimaging studies. Language and Cognitive Processes. 2011;27:1479–1488. doi: 10.1080/01690965.2011.613209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J, Eriksson J, Bodammer NC, Lindgren M, Johansson M, Nyberg L, Lövdén M. Growth of language-related brain areas after foreign language learning. NeuroImage. 2012;63:240–244. doi: 10.1016/j.neuroimage.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Mayes AK, Reilly S, Morgan AT. Neural correlates of childhood language disorder: A systematic review. Developmental Medicine and Child Neurology. 2015;57:706–717. doi: 10.1111/dmcn.12714. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, Price CJ. Neurolinguistics: Structural plasticity in the bilingual brain. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Meschyan G, Hernandez AE. Impact of language proficiency and orthographic transparency on bilingual word reading: An fMRI investigation. NeuroImage. 2006;29:1135–1140. doi: 10.1016/j.neuroimage.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Meyer M, Steinhauer K, Alter K, Friederici AD, von Cramon DY. Brain activity varies with modulation of dynamic pitch variance in sentence melody. Brain and Language. 2004;89:277–289. doi: 10.1016/S0093-934X(03)00350-X. [DOI] [PubMed] [Google Scholar]
- Mišić B, Betzel RF, de Reus MA, van den Heuvel MP, Berman MG, McIntosh AR, Sporns O. Network-level structure-function relationships in human neocortex. Cerebral Cortex. 2016;26:3285–3296. doi: 10.1093/cercor/bhw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohades SG, Struys E, Van Schuerbeek P, Mondt K, Van De Craen P, Luypaert R. DTI reveals structural differences in white matter tracts between bilingual and monolingual children. Brain Research. 2012;1435:72–80. doi: 10.1016/j.brainres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Mohades SG, Van Schuerbeek P, Rosseel Y, Van De Craen P, Luypaert R, Baeken C. White-matter development is different in bilingual and monolingual children: A longitudinal DTI study. PLoS ONE. 2015;10:e0117968. doi: 10.1371/journal.pone.0117968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld GG. Towards a theory of language learning ability. Language Learning. 1979;29:227–241. [Google Scholar]
- Novoa L, Fein D, Obler LK. Talent in foreign languages: A case study. In: Obler LK, Fein D, editors. The exceptional brain: Neuropsychology of talent and special abilities. New York: Guilford Press; 1988. pp. 294–302. [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annual Review of Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Franz CE. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis J. Individual differences in child English second language acquisition: Comparing child-internal and child-external factors. Linguistic Approaches to Bilingualism. 2011;1:213–237. [Google Scholar]
- Patkowski MS. The sensitive period for the acquisition of syntax in a second language 1. Language Learning. 1980;30:449–468. [Google Scholar]
- Piller I. Passing for a native speaker: Identity and success in second language learning. Journal of Sociolinguistics. 2002;6:179–208. [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Preston JL, Frost SJ, Mencl WE, Fulbright RK, Landi N, Grigorenko E, Pugh KR. Early and late talkers: School-age language, literacy and neurolinguistic differences. Brain. 2010;133:2185–2195. doi: 10.1093/brain/awq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Green DW, von Studnitz R. A functional imaging study of translation and language switching. Brain. 1999;122:2221–2235. doi: 10.1093/brain/122.12.2221. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, Murphy DG. Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cerebral Cortex. 2010;20:1332–1340. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- Ressel V, Pallier C, Ventura-Campos N, Diaz B, Roessler A, Avila C, Sebastian-Galles N. An effect of bilingualism on the auditory cortex. Journal of Neuroscience. 2012;32:16597–16601. doi: 10.1523/JNEUROSCI.1996-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Yoshinaga N, Sasaki M. Aptitude-exposure interaction effects on Wh-movement violation detection by pre-and-post-critical period Japanese bilinguals. In: Robinson P, editor. Individual differences and instructed language learning (Language learning and language teaching 2) Amsterdam: John Benjamins; 2002. pp. 267–299. [Google Scholar]
- Roth JK, Serences JT, Courtney SM. Neural system for controlling the contents of object working memory in humans. Cerebral Cortex. 2006;16:1595–1603. doi: 10.1093/cercor/bhj096. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sebastián-Gallés N, Soriano-Mas C, Baus C, Díaz B, Ressel V, Pallier C, Pujol J. Neuroanatomical markers of individual differences in native and non-native vowel perception. Journal of Neurolinguistics. 2012;25:150–162. [Google Scholar]
- Seger CA. The basal ganglia in human learning. Neuroscientist. 2006;12:285–290. doi: 10.1177/1073858405285632. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Dale A, Busa E, Glessner M, Salat D, Hahn H, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2012;72:191–197. doi: 10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehan P. Individual differences in second language learning. Studies in Second Language Acquisition. 1991;13:275–298. [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Winkler C, Kaiser A, Dierks T. Structural brain changes related to bilingualism: Does immersion make a difference? Frontiers in Psychology. 2014;5:1116. doi: 10.3389/fpsyg.2014.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, Van Erp T, Poutanen V-P, Huttunen M, Khaledy M. Genetic influences on brain structure. Nature Neuroscience. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: The declarative/ procedural model. Nature Reviews Neuroscience. 2001;2:717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Via S, Lande R. Genotype–environment interaction and the evolution of phenotypic plasticity. Evolution. 1985;39:505–522. doi: 10.1111/j.1558-5646.1985.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Kiehl KA, Werker JF, Liddle PF. Detection of sounds in the auditory stream: Event-related fMRI evidence for differential activation to speech and nonspeech. Journal of Cognitive Neuroscience. 2001;13:994–1005. doi: 10.1162/089892901753165890. [DOI] [PubMed] [Google Scholar]
- Wang Z, Dai Z, Gong G, Zhou C, He Y. Understanding structural-functional relationships in the human brain: A large-scale network perspective. Neuroscientist. 2015;21:290–305. doi: 10.1177/1073858414537560. [DOI] [PubMed] [Google Scholar]
- Wengenroth M, Blatow M, Heinecke A, Reinhardt J, Stippich C, Hofmann E, Schneider P. Increased volume and function of right auditory cortex as a marker for absolute pitch. Cerebral Cortex. 2014;24:1127–1137. doi: 10.1093/cercor/bhs391. [DOI] [PubMed] [Google Scholar]
- White L, Genesee F. How native is near-native? The issue of ultimate attainment in adult second language acquisition. Second Language Research. 1996;12:233–265. [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH. Abnormal cerebral cortex structure in children with ADHD. Human Brain Mapping. 2009;30:175–184. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Warrier CM, Penhune VB, Roy AK, Sadehh A, Parrish TB, Zatorre RJ. Volume of left Heschl’s gyrus and linguistic pitch learning. Cerebral Cortex. 2008;18:828–836. doi: 10.1093/cercor/bhm115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW. Woodcock Language Proficiency Battery-Revised (WLPB-R) Itasca, IL: Riverside Publishing; 1991. [Google Scholar]
- Woodcock RW, Muñoz-Sandoval A. Woodcock-Johnson Language Proficiency Battery-Revised (Spanish) Itasca, IL: Riverside Publishing; 1995. [Google Scholar]
- Zevin JD, McCandliss BD. Dishabituation of the BOLD response to speech sounds. Behavioral and Brain Functions. 2005;1:4. doi: 10.1186/1744-9081-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Zemanová L, Zamora-Lopez G, Hilgetag CC, Kurths J. Structure–function relationship in complex brain networks expressed by hierarchical synchronization. New Journal of Physics. 2007;9:178. [Google Scholar]
- Zou L, Ding G, Abutalebi J, Shu H, Peng D. Structural plasticity of the left caudate in bimodal bilinguals. Cortex. 2012;48:1197–1206. doi: 10.1016/j.cortex.2011.05.022. [DOI] [PubMed] [Google Scholar]