Abstract
Polymorphisms in DNA repair genes may alter the repair mechanism which makes the person susceptible to DNA damage. Polymorphic variants in these DNA repair pathway genes such as Poly (ADP-ribose) polymerase- 1 (PARP1) have been associated with susceptibility of several types of cancer including thyroid. Many studies have been published on PARP1 gene polymorphisms and carcinogenesis with inconsistent results. The present study was designed to explore the link between the PARP1 polymorphisms and thyroid cancer risk. This case-control study was comprised of 456 thyroid cancer patients and 400 healthy controls. Three SNPs of PARP1 gene; rs1136410, rs1805414 and rs1805404 were analyzed using ARMS-PCR. The combined genotype and haplotype analysis were performed using haploview software 4.2. Major allele homozygote (CC) of rs1136410 and combined genotype (TT+TC) of rs180414 showed a significant association with thyroid cancer risk (OR = 1.30; 95% CI 0.99–1.77; P = 0.05) and (OR = 0.43; 95% CI = 0.27–0.67; P = 0.03). Histological subtype analysis showed the significant association of selected PARP1 SNPs with papillary, follicular and anaplastic subtypes in thyroid cancer patients. Haplotype analysis showed that TCT (p = 0.01), CTT (p = 0.02) and CTC (p = 0.03) were significantly higher in controls when compared to cases. However, TTC (p = 0.05) and TCC (p = 0.01) haplotype frequency was significantly higher in cases compared to controls. Global haplotype analysis showed that there was an overall significant difference between cases and controls (p = 0.001). Identification of these genetic risk markers may provide evidence for exploring insight into mechanisms of pathogenesis and subsequently aid in developing novel therapeutic strategies for thyroid cancer.
Introduction
Most frequent malignancy of thyroid gland is thyroid cancer which has most increasing trend in Pakistan and throughout the world [1]. The incidence of thyroid cancer in Pakistan is 1.2% of all malignancies consisting of papillary thyroid carcinoma 69–71%, follicular thyroid carcinoma 11–13%, medullary thyroid carcinoma 3–5% and anaplastic thyroid carcinoma 1–2% [2]. This trend is alarming in Asia and Europe [3], Canada [4], UK [5] and USA [6] despite good survival rate. Overall incidence of thyroid cancer is 1–2% globally [7].
Disruptions in DNA repair pathways predispose cells to DNA damage and accumulation of this damage can cause cancer and may promote the cancer growth [8]. Among the five different DNA repair pathways, base excision repair pathway (BER) is the main pathway involved in repairing of DNA damage [9, 10]. Variations in BER pathway and their mechanisms have been linked with increased risk of different cancers including thyroid cancer. BER pathway includes different molecules including Poly (ADP-ribose) polymerase- 1 (PARP1) also known as adenosine diphosphate ribosyl transferase, a significant component of BER system [11]. It is located on chromosome 1q41–q42, encoding a 113 KDa zinc finger DNA binding protein. Poly (ADP-ribosyl) transferase can modify various nuclear protein by poly (ADP-ribosylation [12]. A total of 1287 SNPs of PARP1 gene has been reported of which including 202 in coding known as coding region SNPs (cSNPs). In all cSNPs of PARP1 gene, Val762Ala polymorphism (rs1136410) is the most studied SNP but the cSNPs Ala284Ala (rs1805414) and Asp81Asp (rs1805404) are least investigated. PARP1 Val762Ale is based on T to C change at codon 762 at exon 17 in which valine is replaced with alanine in catalytic domain of PARP1 gene. PARP1 VAL762ALE has been reported to be associated with altered activity of PARP1 gene [11,13,14]. Change in amino acid results in reduced activity of PARP1 gene, which ultimately relates to increased risk of different cancers [12– 15]. Few studies have also shown positive association of PARP1 SNPs (rs180404 and rs180414 at position 81 and 284) with Alzheimer’s disease [16], glioblastoma [17] and protectively associated with colorectal cancer [18, 19]. Nevertheless, inconsistent results of all three SNPs have failed to clarify the complicated relationships between PARP1 and thyroid cancer risk. Additionally, the PARP1 Val762Ale has been showed combine effect with various SNPs in carcinogenesis of different region such as gastric region [20], colorectal region [21], breast region [22], brain region [23] and bladder region [24].
To best of our knowledge, limited number of studies have been reported on the association of PARP1 gene SNPs rs3611410 (Val762Ala), rs1805414 (Ala284Ala) and rs1805404(Asp81Asp) with thyroid cancer in Pakistani population. Therefore, to explore the reliable influence of these SNPs of PARP1 on thyroid cancer, mutational analysis in thyroid cancer patients and healthy controls has been planned to assess the active involvement of these polymorphisms in carcinogenesis.
Materials and methods
Study subjects and
Present study comprised of 456 confirmed patients of thyroid cancer and healthy control individuals of same age and gender (Table 1). Diagnosed thyroid cancer patients were confirmed through histopathological test at Nuclear medicine department of NORI (Nuclear Oncology Radiation Institute) Islamabad and PIMS (Pakistan institute of medicine Sciences). Normal individual who came for routine check-up were sampled as controls. Previously diagnosed patients of any cancer were excluded from this study. Written consent was taken from the individuals and questionnaire (S1 Annex) filled with data related to demographic factors, addictions, and eating habits was collected. Specimens of peripheral blood from all individuals were collected.
Table 1. Demographic characteristics for controls and cases.
| Variables | Case (n = 456) | Controls (n = 400) | P*-value |
|---|---|---|---|
| Age (Y) | |||
| <42, n(%) | 208 (45.6) | 179 (44.75) | 0.87 |
| >42, n(%) | 248 (54.38) | 221 (55.25) | 0.89 |
| Sex | |||
| Male, n(%) | 107 (23.4) | 70 (17.5) | 0.08 |
| Female, n(%) | 349 (76.53) | 330 (82.2) | 0.46 |
| Grade of cancer | |||
| Grade I | 211 (46%) | N/A | 0.08 |
| Grade II | 162 (36%) | N/A | |
| Grade III | 72 (15%) | N/A | |
| Grade IV | 11 (3%) | N/A | |
| Family History | |||
| Yes, n(%) | 32 (7.2) | 6 (1.5) | 0.0004 |
| No, n(%) | 427 (93.7) | 394 (98.5) | 0.0004 |
n = total number; P* = χ2-test.
Ethical approval
The study was conducted with a prior approval from the institutional ethical review board of COMSTAS Institute of Information Technology (CIIT) Islamabad. Members of this committee include Dean ORIC (Office of Research Innovation and Commercialization) Prof. Dr. Raheel Qamar (convener), Prof. Dr. Mahmood A Kayani (Chairman Deptt of Biosciences), Dr. Faheem Tahir (Deputy Director, NIH) and Dr. Tayyaba Yasmin (Associate Head of department). All the samples were collected after a signed, informed consent from all participants of the study. The ethical review board approved the consent procedure &execution of project on thyroid cancer.
DNA extraction
About 3-4ml blood from all individuals were taken. Extraction of DNA from whole blood was performed according to Phenol chloroform method with minor modifications [25]. Quantification of extracted DNA was done through 2% ethidium bromide gels and spectrophotometrically using Nano Drop (Thermoscientifiv, USA). DNA samples were stored at -20°C.
SNPs selection
Three functional polymorphisms of PARP1 gene were selected using a set of web-based SNP selection tools (http://snpinfo.niehs.nih.gov/snpinfo/snpfunc.htm). Following criteria was followed for selection of functional SNPs: (1) Minor allele frequency of validated SNPs > 5% in Asian population; (2) validated SNPs in important functional domain of PARP1 gene such as Val762Ala (rs3611410, catalytic domain), Ala284Ala (rs1805414, PARD1 domain) and Asp81Asp (rs1805404, Zinc figure domain).
Genotyping
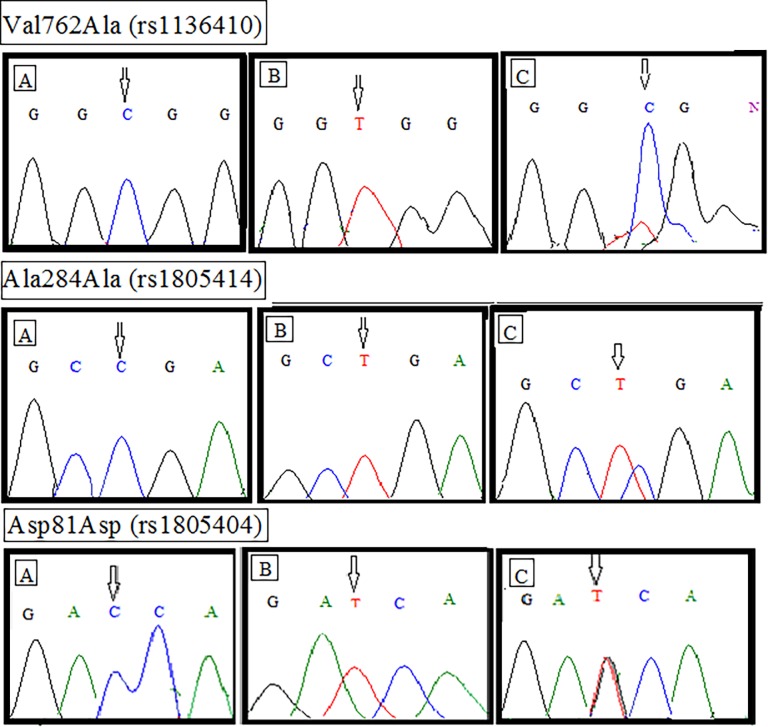
Allele- specific polymerase chain reaction (ARMS-PCR) was performed for analyzing the changes in genetic make-up. Primers specific for the amplification of selected gene SNPs were designed using WASP (web-based allele specific primer designing tool) (Liu et al., 2012). Designed primers for each specific polymorphism are enlisted in S1 Table. Constituents of the PCR reaction were 50-100ng genomic DNA, 100μM of each primer and Solis BioDyne master mix whereas the total volume of the reaction was 10μl. Conditions for the thermal cycler reaction were: denaturation at 94°C for 30 secs, optimized annealing temperature for 45 secs, extension at 72°C for 1 min and final extension for 7 mins. Visualization of the PCR products was carried out through 2% agarose gel electrophoresis (100V, 300A for 45 mins). Results were anticipated based on the appearance of required bands for wild and mutant primers by UV trans illuminator (Gel Doc BioRad, USA). β-Actin was used as a positive control in each PCR reaction. Furthermore, the saples were sequenced to confirm the anticipated genotypes of each PCR product (wild, mutant and heterozygous) as shown in Fig 1.
Fig 1. Electropherograms of selected polymorphism of PARP1 gene in thyroid cancer patients and controls.
Genotype pattern of first selected SNP, Val762Ala (rs3611410), (A) homozygous wild (B) homozygous mutant (C) heterozygous mutant. Genotype pattern of second selected SNP, Ala284Ala (rs1805414), (A) homozygous wild (B) homozygous mutant (C) heterozygous mutant. Genotype pattern of third selected SNP, Asp81Asp (rs1805404), (A) homozygous wild (B) homozygous mutant (C) heterozygous mutant.
Statistical analyses
Chi square (χ2) test was used for comparing the demographical factors between cancer patients and controls for each SNP. Odd ratio (ORs) and 95% confidence intervals (CIs) were calculated according to the gender, age, smoking status and family history of cancer. Statistically significant P-value was <0.05. Multiplicative interaction effects of polymorphisms were estimated using adjusted logistic regression model for SNP-SNP interactions. Results were analyzed statistically by means of GraphPad prism software v 6.0.
From the genotypic data, haplotypes were generated. Examination of linkage disequilibrium (LD) and haplotype was performed using Haploview 4.2, which examines the data through expectation maximization (EM) algorithm. It gives the results of both haplotype and global data by estimating the haplotype frequency separately both in samples and controls. Odds ratios (ORs) and 95% CIs were assessed using unconditional logistic regression for each haplotype. Haplotype analysis was performed without accounting for the variables of the study because the haplotype analysis uses more degrees of freedom than single locus analysis. Moreover, statistical power decreases by adjusting the additional covariates. In haplotype analysis, each haplotype was evaluated separately versus all other haplotypes. In present study, genetic haplotype approach was used to analyze the influence of common cis ordered SNPs at the PARP1 locus to thyroid cancer risk among Pakistani population. The order of variants in the inferred haplotypes was Val762Ala (rs3611410), Ala284Ala (rs1805414) and Asp81Asp (rs1805404), corresponding to the physical location of these variants in the PARP1 gene. For examination, threshold frequency of haplotypes for inclusion was set at 1%. Power calculation was performed using Power Calculator for case-control Genetic Association Analysis (PGA), to assess the power value of the study. Input variables e.g. genetic mode of inheritance (co-dominant), disease allele frequency, marker allele frequency, control to case ratio and relative risk (RR) were used for power calculation at α = 0.05.
Results
Association of PARP1 SNPs and risk of thyroid cancer
Genotype and allele frequencies of all the three selected SNPs of PARP1 gene are listed in Table 2 and were calculated using Hardy-Weinberg equilibrium. For SNP rs3611410, frequency of minor allele homozygote (CC) was observed significantly higher in cancer patients (OR = 1.30; 95%CI 0.99–1.71; p = 0.05) compared to controls. For second SNP rs1805414, combined genotype (TC+CC) was observed significantly lower in thyroid cancer patients (OR = 0.43, 95% CI = 0.27–0.67; p = 0.003) compared to controls. Frequency of minor allele heterozygote (TC) was also observed significantly higher in healthy controls (OR = 0.80, 95% CI = 0.65–1.00; p = 0.05) compared to thyroid cancer patients. For SNP rs1805404, minor allele heterozygote (CT) was observed significantly higher in controls (OR = 0.63, 95% CI = 0.40–1.00; p = 0.05) compared to cancer patients as shown in Table 2.
Table 2. Allele and genotype frequencies of selected SNPs of PARP1 gene in cases and controls.
| Polymorphisms | Case, n(%) | Control, n(%) | OR (95% CI) | pa | Powerb | |
|---|---|---|---|---|---|---|
| PARP1 | ||||||
| rs3611410 | TT | 82 (17.98) | 93 (23.25) | 1.0 (Reference) |
0.83 |
|
| TC | 97 (21.27) | 91 (22.75) | 1.17 (0.85–1.62) | 0.31 | ||
| CC | 276 (60.52) | 216 (54.0) | 1.30 (0.99–1.71) | 0.05 | ||
| CC+TC | 373 (81.7) | 307 (76.75) | 1.36 (0.97–1.89) | 0.06 | ||
| rs1805414 | TT | 70 (15.3) | 29 (7.25) | 1.0 (Reference) |
0.39 |
|
| TC | 268 (58.49) | 291 (72.0) | 0.80 (0.65–1.00) | 0.05 | ||
| CC | 118 (25.8) | 80 (20.0) | 1.29 (0.94–1.77) | 0.10 | ||
| CC+TC | 386 (84.6) | 371 (92.7) | 0.43 (0.27–0.67) | 0.003 | ||
| rs1805404 | CC | 316 (69.2) | 254 (63.5) | 1.0 (Reference) |
0.05 |
|
| CT | 105 (23.2) | 98 (24.5) | 0.93 (0.69–1.27) | 0.69 | ||
| TT | 35 (7.6) | 48 (12.0) | 0.63 (0.40–1.00) | 0.05 | ||
| TT+CT | 140 (30.7) | 146 (36.5) | 0.77 (0.57–1.02) | 0.07 | ||
OR, odds ratio; CI, confidence interval; OR, CI and pa-value calculated by regression analysis.
Powerb Statistical power analysis using PGA1
Histological subtype analysis showed that minor allele homozygote CC (p = 0.02) of polymorphism rs1136410 was found significantly higher in papillary thyroid carcinoma compared to other subtypes of thyroid cancer. In case of polymorphism rs1805414, frequency of minor allele homozygote CC (p = 0.05) and minor allele heterozygote TC (p = 0.0001) was noted significantly higher in papillary thyroid carcinoma compared to follicular subtype, medullary and anaplastic subtype. In case of third polymorphism rs1805404, frequency of minor allele homozygote (TT) was observed significantly higher in papillary thyroid carcinoma (p = 0.008) and anaplastic carcinoma (p = 0.003) compared to other subtypes of thyroid carcinoma as shown in Table 3.
Table 3. Distribution of genotypes and odds ratios (OR) for different histological subtypes of thyroid carcinoma and controls.
| Genotypes PARP1 |
Controls (n = 400) | Papillary carcinoma | *P- value | Follicular | *P- value | Medullary | *P- value | Anaplastic | *P- value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (n = 351) | n | carcinoma | n | carcinoma | n | (n = 7) | ||||||
| OR (95% CI) | (n = 82) | (n = 16) | OR (95% CI) | ||||||||||
| OR (95% CI) | OR (95% CI) | ||||||||||||
| rs1136410 | |||||||||||||
| TT | 93 | 64 | 1.00(Ref) | 15 | 1.00(Ref) | 3 | 1.00(Ref) | 4 | 1.00(Ref) | ||||
| TC | 91 | 76 | 0.93(0.66–1.32) | 0.71 | 17 | 0.88(0.49–1.59) | 0.68 | 4 | 1.13(0.35–3.59) | 0.83 | 1 | 0.56(0.06–4.76) | 0.60 |
| CC | 216 | 217 | 1.37(1.03- 1.84) | 0.02 | 48 | 1.20(0.74–1.94) | 0.45 | 9 | 1.09(0.40–2.99) | 0.85 | 2 | 0.340.06–1.77) | 0.207 |
| rs1805414 | |||||||||||||
| TT | 29 | 57 | 1.00(Ref) | 13 | 1.00(Ref) | 3 | 1.00(Ref) | 1 | 1.00(Ref) | ||||
| TC | 291 | 209 | 0.55(0.40–0.74) | 0.0001 | 47 | 0.50(0.30–0.82) | 0.006 | 9 | 0.48(0.17–1.32) | 0.15 | 4 | 0.49 (0.11–2.26) | 0.36 |
| CC | 80 | 91 | 1.40(0.99–1.97) | 0.05 | 20 | 1.29(0.73–2.25) | 0.37 | 4 | 1.33(0.41–4.24) | 0.62 | 2 | 1.60(0.30–8.39) | 0.57 |
| rs1805404 | |||||||||||||
| CC | 254 | 249 | 1.00(Ref) | 55 | 1.00(Ref) | 11 | 1.00(Ref) | 2 | 1.00(Ref) | ||||
| CT | 98 | 86 | 1.00(0.71–1.39) | 0.99 | 18 | 0.86(0.48–1.53) | 0.62 | 4 | 1.02(0.32–3.25) | 0.96 | 1 | 0.51(0.06–4.31) | 0.53 |
| TT | 48 | 22 | 0.49(0.28–0.83) | 0.008 | 7 | 0.68(0.29–1.57) | 0.37 | 01 | 0.48(0.06–3.78) | 0.49 | 4 | 9.77(2.12–45.0) | 0.003 |
OR, odds ratio; CI, confidence interval; OR, CI and *p-value calculated by regression analysis.
Haplotype analysis results
Eight haplotypes were generated for three selected SNPs (rs1136410, rs1805414 and rs1805404) of PARP1 gene among thyroid cases and controls. Haplotypes CTC has 25%, CTT has 46% and TCT has 28% reducing effect in thyroid cancer patients. TCC (OR = 1.33; 95%CI = 1.07–1.66; p = 0.01) and TTC (OR = 1.23; 95%CI = 0.99–1.52; p = 0.05) haplotypes were associated with increased risk of thyroid cancer as shown in Table 4. Global haplotype analysis showed that there was an overall significant difference in cases and controls (p = 0.001).
Table 4. Distribution of haplotype analysis in study cohort.
| Haplotype | Cases | Controls | Chi2 | Fisher’s P | Pearson’s P | OR (95% CI) |
|---|---|---|---|---|---|---|
| TTT | 82.87 (0.091) | 69.08 (0.086) | 0.107 | 0.743 | 0.743 | 1.05 (0.75–1.47) |
| TTC | 268.1 (0.294) | 201.9 (0.252) | 3.702 | 0.054 | 0.054 | 1.23 (0.99–1.52) |
| TCT | 48.93 (0.054) | 67.02 (0.084) | 6.123 | 0.013 | 0.013 | 0.62 (0.42–0.90) |
| TCC | 252.0 (0.276) | 177.9(0.222) | 6.588 | 0.010 | 0.010 | 1.33 (1.07–1.66) |
| CTT | 23.88 (0.026) | 37.71 (0.047) | 5.399 | 0.020 | 0.020 | 0.54 (0.32–0.91) |
| CTC | 134.1(0.147) | 148.2 (0.185) | 4.535 | 0.033 | 0.033 | 0.75 (0.58–0.97) |
| CCT | 22.32 (0.024) | 18.18 (0.023) | 0.056 | 0.812 | 0.812 | 1.07 (0.57–2.01) |
| CCC | 79.68 (0.087) | 79.82 (0.100) | 0.777 | 0.3780 | 0.378 | 0.86 (0.62–1.19) |
| Global | 23.175 | 0.0016 | 0.0015 |
Abbreviations: SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval; OR, CI and p-value calculated by regression analysis.
Genotype-genotype interaction
After haplotype analysis, interaction between the selected SNP of PARP1 gene was calculated by logistic regression model shown in Table 5. The analysis revealed that SNP 1 vs SNP 2 (Val762Ala vs Ala284Ala) and SNP2 vs SNP3 (Ala284Ala vs Asp81Asp) had a positive correlation with increased risk of thyroid cancer (OR = 1.099; 95% CI = 0.068–17.846; OR = 2.33; 95% CI = 0.142–37.366). However, SNP1 vs SNP3 (Val762Ala vs Asp81Asp) had a negative correlation with thyroid cancer risk (OR = 0.88; 95% CI = 0.532–1.486). All correlations were found statistically non-significant. These results were further confirmed by -2 log likelihood ratios of reduced model and no signification association between SNPs was observed (Table 6).
Table 5. Logistic regression model of SNP-SNP interactions and thyroid cancer risk.
| Polymorphisms | B | S. E | Wald | Sig | OR | 95% CI |
|---|---|---|---|---|---|---|
| rs3611410 vs rs1850414 | 0.095 | 1.42 | 0.004 | 0.947 | 1.099 | 0.068–17.846 |
| rs3611410 vs rs1805404 | -0.117 | 0.262 | 0.200 | 0.655 | 0.88 | 0.532–1.486 |
| rs1850414 vs rs1805404 | 0.833 | 1.422 | 0.343 | 0.558 | 2.33 | 0.142–37.366 |
Abbreviations: SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval; OR, CI and p-value calculated by logistic regression analysis.
Table 6. Likelihood ratio analysis of SNP-SNP interaction in thyroid cancer patients.
| Polymorphisms | -2 log likelihoods of reduced model |
Chi-square | Sig |
|---|---|---|---|
| rs3611410 vs rs1850414 | 20.629 | 0.129 | 0.938 |
| rs3611410 vs rs1805404 | 20.701 | 0.201 | 0.654 |
| rs1850414 vs rs1805404 | 25.316 | 1.743 | 0.418 |
Abbreviations: SNP, single nucleotide polymorphism; significance level calculated by Likelihood ratio
Analysis
Discussion
PARP1 is an important DNA repair gene that binds with DNA and promote Poly (ADP-ribosylation) of many proteins. It plays a significant role in detecting the DNA damage and repair [26]. In this study, we investigated the association of three PARP1 gene polymorphisms i-e Val762Ala (rs1136410), Ala284Ala (rs1805414) and Asp81Asp (rs1805404) with thyroid cancer susceptibility. We also explored whether the three polymorphisms were in linkage disequilibrium, and if any common haplotypes of these SNPs are found associated with thyroid carcinogenesis. At the end, we examined the combined effect of all three selected SNPs on thyroid cancer risk. Among these selected SNPs Val762Ala has been extensively studied. Substitution of an amino acid within carboxyl terminal of catalytic domain of the enzyme is responsible for PARP1 Val762Ala variation. This variant can reduce the enzymatic activity by producing a steric modification in catalytic domain [27] and interaction with XRCC1 is also limited [28]. These alterations can decrease the BER pathway capacity and hence increase the cancer susceptibility in PARP1 Ala762 carriers. Therefore, genetic variants of PARP1 that contribute to PARP1 activity may be a risk factor for cancer development and progression. In thyroid cancer patients, significant higher minor homozygote (CC) frequency of Val762Ala was observed as compared to controls in present study. These results are consistent with previous studies that showed the rs1136410 as a risk factor for carcinomas of the brain, head and neck in Chinese population, esophagus, lung, breast, stomach, bladder, colorectal in European population, prostate and skin in USA [29–34]. However, contradictory results have also been obtained in glioma where rs1136410 plays a protective role [35]. In Caucasian populations, the PARP1 Val762Ala gene variant minimizes the risk of development of some cancers [36–40], while in Chinese populations PARP1 762Ala gene variant increases the risk of cancer in numerous studies [41–43].
For second selected SNP of PARP1 gene Ala284Ala (rs1805414), in cancer patients there was higher minor allele frequency than in controls and has significant effect on increasing the risk of thyroid cancer in current study. Previous studies have found a positive association between the Ala284Ala SNP and increased risk of breast cancer in Swedish population [44], Alzheimer’s disease in USA [16], Glioblastoma in German population [17], inversely associated with colorectal cancer in Chines population [18, 19]. A base substitution (T-allele) at Ala282Ala is not responsible for disrupting the protein function itself because, it does not alter the sequence of amino acid of that protein. So, the hypothesis of association of this variant with colorectal adenoma is because of the linkage disequilibrium with another variant in PARP1 promoter is supported by our findings in current study [18]. Since regulation of PARP1 gene is controlled by its promoter therefore, variation in this region at transcription binding sites may affect its expression [18]. Milani et al., (2007) [45] has also found that PARP1 promoter region has functional regulatory polymorphisms and in cancerous cells, allelic imbalance influences the expression level of SNP rs1805414. In case of third polymorphism of PARP1 gene Asp81Asp (rs1805404), frequency of minor allele homozygote was observed significantly higher in controls compared to thyroid cancer patients and showed inverse association with the said disease. Similar trend has earlier been reported by Jin et al. (2010) [46] where mutant allele of Asp81Asp has showed inverse association non-hedgehog lymphoma in males.
In second part of the study, the genotype frequency of three selected SNPs was compared with different histological subtypes of thyroid cancer and frequency of minor allele homozygote/heterozygote of Val762Ala (rs1136410), Ala284Ala (rs1805414) and Asp81Asp (rs1805404) was observed significantly higher in papillary thyroid carcinoma compared to other histological subtype of said disease. Similar trend for minor allele homozygote/heterozygote of DNA repair genes polymorphisms and increased incidence of papillary thyroid carcinoma compared to other histological subtype of thyroid cancer has been reported in different studies [47–50]. However, till now only one study has been reported with respect to PARP1 gene polymorphism and thyroid cancer subtype and no significant change in crude/adjusted OR of Val762Ala polymorphism of PARP1 gene, has been revealed in either papillary or follicular thyroid cancer subgroups [50].
In third part of the study, we successfully established haplotypes for the PARP1 gene from the different combinations of the three SNPs. In global haplotype analysis, generally the significant difference was shown among patients and control individuals. This difference was because of the TTC and TCC haplotypes, which was more frequent in cases compared to controls, conferring increased effect against the development of thyroid cancer. Additionally, as far as we know, neither any study was carried out to analyze the combined effect in the form of haplotype analysis of Val762Ala (rs1136410), Ala284Ala (rs1805414) and Asp81Asp (rs1805404) SNPs of PARP1 gene in thyroid cancer previously. When combining putative risk alleles in the form of haplotype, increase in risk of developing thyroid cancer was observed. Overall, this study findings propose a synergistic interaction among the susceptibility genotypes. It is suggested that increased effect observed, due to these susceptibility SNPs, is in agreement with poly allelic model, according to this model susceptibility is influenced by the combination of several alleles in a population, where each allele effect is responsible for a slight genotypic risk. Cancer growth and development is frequently affected by the SNPs with low penetrance. However, the combined effect of these variants is more effective than a single variant effect. [51]. The combined effect of PARP1 polymorphism Val762Ala with other polymorphism have already be reported in different cancer such as lung cancer [43], noncardia gastric cancer [52], esophageal squamous cell carcinoma [36], skin cancer [53], breast cancer [32], colorectal cancer [39] and cancer [54].
To conclude, our findings indicate that selected SNPs of PARP1 gene especially Val762Ala and some haplotypes of the said gene may play a role in increasing the thyroid carcinogenesis risk and its genetic susceptibility. However, the major coding SNPs, i.e., rs1805414 and rs1805404 polymorphism, of the PARP1 showed inverse association with thyroid pathogenesis among Pakistani population. For confirming the contributions of single variation, gene-gene interactions and gene-environment interactions with thyroid cancer comprehensive studies should carried out in distinct ethnic groups because genetic polymorphisms differ between distinct groups.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors are thankful to the Nuclear Medicine Oncology and Radiotherapy Institute (NORI) for the blood samples collections.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Bukhari U, Sadiq S, Memon J, Baig F: Thyroid carcinoma in Pakistan: a retrospective review of 998 cases from an academic referral center. Hematol Oncol Stem Cell Ther 2009; 2:345–348. [DOI] [PubMed] [Google Scholar]
- 2.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R: Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013; 2013:965212 10.1155/2013/965212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukhari MH, Niazi S, Hanif G, Qureshi SS, Munir M, Hasan M, Naeem S: An updated audit of fine needle aspiration cytology procedure of solitary thyroid nodule. Diagn Cytopathol 2008; 36:104–112. 10.1002/dc.20731 [DOI] [PubMed] [Google Scholar]
- 4.Smailyte G, Miseikyte-Kaubriene E, Kurtinaitis J, Sakoda L, Horn-Ross P, Feldt-Rasmussen U: Increasing thyroid cancer incidence in Lithuania in 1978–2003. BMC Cancer 2006; 6:284 10.1186/1471-2407-6-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N: International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 2009; 20:525–531. 10.1007/s10552-008-9260-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw A, Semenciw R, Mery L: Cancer in Canada Fact Sheet Series# 1: Thyroid cancer in Canada. Chronic Dis Inj Can 2014; 34:64–68. [PubMed] [Google Scholar]
- 7.Brown P, Jiang H, Ezzat S, Sawka AM, Guay B, Johnson-Obaseki S: A detailed spatial analysis on contrasting cancer incidence patterns in thyroid and lung cancer in Toronto women. BMC Public Health 2016; 16:950 10.1186/s12889-016-3634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001; 411:366–374. 10.1038/35077232 [DOI] [PubMed] [Google Scholar]
- 9.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004; 7:1111–30. [DOI] [PubMed] [Google Scholar]
- 10.Lockett KL, Hall MC, Xu J, Zheng SL, Berwick M. et al. The ADPRT V762A genetic variant contributes to prostate cancer susceptibility and deficient enzyme function. Cancer research. 2004; 64(17):6344–8. 10.1158/0008-5472.CAN-04-0338 [DOI] [PubMed] [Google Scholar]
- 11.Wood RD, Mitchell M, Sgouros J and Lindahl T. Human DNA repair genes. Science. 2001; 5507:1284–9. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhang X, Park TS, Gidday JM. Cerebral endothelial cell apoptosis after ischemia–reperfusion: role of PARP activation and AIF translocation. Journal of Cerebral Blood Flow & Metabolism. 2005;25(7):868–77. [DOI] [PubMed] [Google Scholar]
- 13.Wang XG, Wang ZQ, Tong WM, Shen Y. PARP1 Val762Ala polymorphism reduces enzymatic activity. Biochemical and biophysical research communications. 2007;354(1): 122–6. 10.1016/j.bbrc.2006.12.162 [DOI] [PubMed] [Google Scholar]
- 14.Ye F, Cheng Q, Hu Y, Zhang J, Chen H. PARP-1 Val762Ala polymorphism is associated with risk of cervical carcinoma. PLoS One. 2012; 18:3746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu H, Ma H, Yin M, Wei Q. Association between PARP‐1 V762A polymorphism and cancer susceptibility: a meta‐analysis. Genetic epidemiology. 2012;36(1):56–65. 10.1002/gepi.20663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu HP, Lin WY, Wu BT, Liu SH, Wang WF, Tsai CH, Lee CC, Tsai FJ. Evaluation of the poly (ADP‐ribose) polymerase‐1 gene variants in Alzheimer's disease. Journal of clinical laboratory analysis. 2010;24(3):182–186. 10.1002/jcla.20379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller A, Harz C, Matzas M, Meder B, Katus HA, Ludwig N, Fischer U, Meese E. Identification of novel SNPs in glioblastoma using targeted resequencing. PloS one. 2011;6(6):18158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berndt SI, Platz EA, Fallin MD, Thuita LW, Hoffman SC, Helzlsouer KJ. Mismatch repair polymorphisms and the risk of colorectal cancer. International journal of cancer. 2007;120(7):1548–54. 10.1002/ijc.22510 [DOI] [PubMed] [Google Scholar]
- 19.Ogino S and Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. Journal of the National Cancer Institute. 2010; 2: s172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen YY, Pan XF, Loh M, Tian Z, Yang SJ, Lv SH, Huang WZ, Huang H, Xie Y, Soong R, Yang CX. ADPRT Val762Ala and XRCC1 Arg194Trp polymorphisms and risk of gastric cancer in Sichuan of China. Asian Pacific Journal of Cancer Prevention. 2012;13(5):2139–44. [DOI] [PubMed] [Google Scholar]
- 21.Alhadheq AM, Purusottapatnam Shaik J, Alamri A, Aljebreen AM, Alharbi O, Almadi MA, Alhadeq F, Azzam NA, Semlali A, Alanazi M, Bazzi MD. The Effect of Poly (ADP-ribose) Polymerase-1 Gene 3′ Untranslated Region Polymorphism in Colorectal Cancer Risk among Saudi Cohort. Disease markers. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao WH, Wang X, Frappart L, Rigal D, Wang ZQ, Shen Y, Tong WM (2007) Analysis of genetic variants of the poly(ADP-ribose) polymerase-1 gene in breast cancer in French patients. Mutat Res 632:20–28. 10.1016/j.mrgentox.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 23.Rajaraman P, Hutchinson A, Wichner S, Black PM, Fine HA, Loeffler JS, Selker RG, Shapiro WR, Rothman N, Linet MS, Inskip PD (2010) DNA repair gene polymorphisms and risk of adult meningioma, glioma, and acoustic neuroma. Neuro Oncol 12:37–48. 10.1093/neuonc/nop012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueroa JD, Malats N, Real FX, Silverman D, Kogevinas M, Chanock S, Welch R, Dosemeci M, Tardon A, Serra C, Carrato A, Garcia-Closas R, Castano-Vinyals G, Rothman N, GarciaClosas M (2007) Genetic variation in the base excision repair pathway and bladder cancer risk. Hum Genet 121:233–242. 10.1007/s00439-006-0294-y [DOI] [PubMed] [Google Scholar]
- 25.Sarwar R, Bashir K, Saeed S, Mahjabeen I, Kayani MA. Association of Promoter Polymorphisms in Xrcc2 Gene Involved in DNA Double Strand Break Repair and Increased Susceptibility to Thyroid Cancer Risk in Pakistani Population. J Carcinog Mutagen. 2016; 7: 26–40. [Google Scholar]
- 26.Jiang JC, Zhang X, Yang H and Wang W. Polymorphisms in DNA repair genes: ADPRT, XRCC1 and XPD and cancer risk in genetic epidemiology. Methods Mol Biol. 2009; 471:305–333. 10.1007/978-1-59745-416-2_16 [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 32:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 29.Lockett K.L. Hall M.C. Xu J. Zheng S.L. Berwick M. et al. (2004) The ADPRT V762A genetic variant contributes to prostate cancer susceptibility and deficient enzyme function. Cancer research. 64(17), 6344–8. 10.1158/0008-5472.CAN-04-0338 [DOI] [PubMed] [Google Scholar]
- 30.Huang D. W., Sherman B. T., & Lempicki R. A. (2008). Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research, 37(1), 1–13. 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C., Hu Z., Lu J., Liu Z., Wang L. E., El‐Naggar A. K., & & Wei Q. (2007). Genetic polymorphisms in DNA base‐excision repair genes ADPRT, XRCC1, and APE1 and the risk of squamous cell carcinoma of the head and neck. Cancer, 110(4), 867–875. 10.1002/cncr.22861 [DOI] [PubMed] [Google Scholar]
- 32.Smith T. R., Levine E. A., Freimanis R. I., Akman S. A., Allen G. O., Hoang K. N., & & Hu J. J. (2008). Polygenic model of DNA repair genetic polymorphisms in human breast cancer risk. Carcinogenesis, 29(11), 2132–2138. 10.1093/carcin/bgn193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H.P. Lin W.Y. Wu B.T. Liu S.H. Wang W.F. Tsai C.H. Lee C.C. Tsai F.J. (2010) Evaluation of the poly (ADP‐ribose) polymerase‐1 gene variants in Alzheimer's disease. Journal of clinical laboratory analysis. 24(3), 182–186. 10.1002/jcla.20379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajaram N., Reichenberg J. S., Migden M. R., Nguyen T. H., & Tunnell J. W. (2010). Pilot clinical study for quantitative spectral diagnosis of non‐melanoma skin cancer. Lasers in surgery and medicine, 42(10), 876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen A. Y., Jemal A., & Ward E. M. (2009). Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer, 115(16), 3801–3807. 10.1002/cncr.24416 [DOI] [PubMed] [Google Scholar]
- 36.Hao B, Wang H, Zhou K, Li Y, Chen X. et al. Identification of genetic variants in base excision repair pathway and their associations with risk of esophageal squamous cell carcinoma. Cancer Res. 2004; 64:4378–4384. 10.1158/0008-5472.CAN-04-0372 [DOI] [PubMed] [Google Scholar]
- 37.Kang SLL, Min Y, He, Ting W, Tao L, Xun L. et al. Association between parp-1 polymorphisms and susceptibility to gastric cancer. World J Gastroenterology. 2010;18: 1434–1441. [Google Scholar]
- 38.Miao X, Zhang X, Zhang L, Guo Y, Hao B. et al. Adenosine diphosphate ribosyl transferase and x-ray repair cross-complementing 1 polymorphisms in gastric cardia cancer. Gastroenterology. 2006; 131:420–427. 10.1053/j.gastro.2006.05.050 [DOI] [PubMed] [Google Scholar]
- 39.Stern MC, Conti DV, Siegmund KD, Corral R, Yuan JM, et al. DNA repair single-nucleotide polymorphisms in colorectal cancer and their role as modifiers of the effect of cigarette smoking and alcohol in the Singapore Chinese health study. Cancer Epidemiol Biomarkers Prev. 2007; 16:2363–2372. 10.1158/1055-9965.EPI-07-0268 [DOI] [PubMed] [Google Scholar]
- 40.Wang M, Qin C, Zhu J, Yuan L, Fu G, Zhang Z, Yin C. Genetic variants of xrcc1, ape1, and adprt genes and risk of bladder cancer. DNA Cell Biol. 2010; 29(6):303–311. 10.1089/dna.2009.0969 [DOI] [PubMed] [Google Scholar]
- 41.Zhai X, Liu J, Hu Z, Wang S, Qing J, et al. Polymorphisms of ADPRT Val762Ala and XRCC1 Arg399Glu and risk of breast cancer in Chinese women: a case control analysis. Oncol Rep. 2006; 15:247–252. [PubMed] [Google Scholar]
- 42.Zhang Q, Li Y, Li X, Zhou W, Shi B, Chen H, Yuan W. Parp-1 val762ala polymorphism, Pylori infection and risk for gastric cancer in Han Chinese population. Mol Biol. 2009; 36(6):1461–1467. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Miao X, Liang G, Hao B, Wang Y, Tan W, Li Y, Guo Y, He F, Wei Q, Lin D. Polymorphisms in DNA base excision repair genes ADPRT and XRCC1 and risk of lung cancer. Cancer Res. 2005; 65:722–726 [PubMed] [Google Scholar]
- 44.Alanazi MS, Parine NR, Shaik JP, Alabdulkarim HA, Ajaj SA, Khan Z. Association of single nucleotide polymorphisms in Wnt signaling pathway genes with breast cancer in Saudi patients. PloS one. 2013; 8(3):e59555 10.1371/journal.pone.0059555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milani L, Gupta M, Andersen M, Dhar S, Fryknäs M, Isaksson A, Larsson R, Syvänen AC. Allelic imbalance in gene expression as a guide to cis-acting regulatory single nucleotide polymorphisms in cancer cells. Nucleic acids research. 2007; 35(5):e34 10.1093/nar/gkl1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin XM, Kim HN, Lee IK, Park KS, Kim HJ, Choi JS, Juhng SW, Choi C. PARP-1 Val762Ala polymorphism is associated with reduced risk of non-Hodgkin lymphoma in Korean males. BMC medical genetics. 2010; 11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bastos H, Antao M, Silva S, Azevedo A, Manita I, Pina JE, Ferreira T, Linbert E, Rueff J, Jorge G. Association of polymorphisms in genes of the homologous recombination DNA repair pathway and thyroid cancer risk. Thyroid. 2009; 19: 1067–1075. 10.1089/thy.2009.0099 [DOI] [PubMed] [Google Scholar]
- 48.Yan L, Li Q, Li X, Ji H, Zhang L. Association studies between XRCC1, XRCC2, XRCC3 polymorphisms and differentiated thyroid carcinoma. Cellular Physiology and Biochemistry. 2016;38(3):1075–84. 10.1159/000443058 [DOI] [PubMed] [Google Scholar]
- 49.Sarwar R, Mahjabeen I, Bashir K, Saeed S, Kayani MA. Haplotype based analysis of XRCC3 gene polymorphisms in thyroid cancer. Cellular Physiology and Biochemistry. 2017;42(1):22–33. 10.1159/000477109 [DOI] [PubMed] [Google Scholar]
- 50.Santos LS, Branco SC, Silva SN, Azevedo AP, Gil OM, Manita I, Ferreira TC, Limbert E, Rueff J, Gaspar JF. Polymorphisms in base excision repair genes and thyroid cancer risk. Oncology reports. 2012. November 1;28(5):1859–68. 10.3892/or.2012.1975 [DOI] [PubMed] [Google Scholar]
- 51.Chiang FY, Wu CW, Hsiao PJ, Kuo WR, Lee KW. et al. Association between polymorphisms in DNA base excision repair genes XRCC1, APE1, and ADPRT and differentiated thyroid carcinoma. Clinical Cancer Research. 2008; 14(18):5919–5924. 10.1158/1078-0432.CCR-08-0906 [DOI] [PubMed] [Google Scholar]
- 52.Pan XF, Xie Y, Loh M, Yang SJ, Wen YY, Tian Z, Huang H, Lan H, Chen F, Soong R, Yang CX. Polymorphisms of XRCC1 and ADPRT genes and risk of noncardia gastric cancer in a Chinese population: a case-control study. Asian Pacific Journal of Cancer Prevention. 2012;13(11):5637–42. [DOI] [PubMed] [Google Scholar]
- 53.Li C, Liu Z, Wang LE, Strom SS, Lee JE, Gershenwald JE, Ross MI, Mansfield PF, Cormier JN, Prieto VG, Duvic M, Grimm EA, Wei Q (2006) Genetic variants of the ADPRT, XRCC1 and APE1 genes and risk of cutaneous melanoma. Carcinogenesis 27:1894–1901. 10.1093/carcin/bgl042 [DOI] [PubMed] [Google Scholar]
- 54.Qin Q, Lu J, Zhu H, Xu L, Cheng H, Zhan L, Yang X, Zhang C, Sun X. PARP-1 Val762Ala polymorphism and risk of cancer: a meta-analysis based on 39 case-control studies. PloS one. 2014. May 22;9(5):e98022 10.1371/journal.pone.0098022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.