Abstract
Animals need to continuously adjust their water metabolism to the internal and external conditions. Homeostasis of body fluids thus requires tight regulation of water intake and excretion, and a balance between ingestion of water and solid food. Here, we investigated how these processes are coordinated in Drosophila melanogaster. We identified the first thirst-promoting and anti-diuretic hormone of Drosophila, encoded by the gene Ion transport peptide (ITP). This endocrine regulator belongs to the CHH (crustacean hyperglycemic hormone) family of peptide hormones. Using genetic gain- and loss-of-function experiments, we show that ITP signaling acts analogous to the human vasopressin and renin-angiotensin systems; expression of ITP is elevated by dehydration of the fly, and the peptide increases thirst while repressing excretion, promoting thus conservation of water resources. ITP responds to both osmotic and desiccation stress, and dysregulation of ITP signaling compromises the fly’s ability to cope with these stressors. In addition to the regulation of thirst and excretion, ITP also suppresses food intake. Altogether, our work identifies ITP as an important endocrine regulator of thirst and excretion, which integrates water homeostasis with feeding of Drosophila.
Author summary
Maintenance of energy and water balance is necessary for survival of all organisms. Even a mild dehydration triggers thirst, reduces appetite, and decreases diuresis (water excretion), thereby promoting conservation of water resources and survival under arid conditions. Homeostasis is regulated primarily by endocrine systems that utilize neuropeptides and peptide hormones. Whereas hormonal mechanisms that regulate the water balance in humans are relatively well understood, much less is known about these regulations in the fruit fly Drosophila melanogaster. Here, we describe the first thirst-promoting and anti-diuretic hormone of Drosophila, encoded by the gene Ion transport peptide (ITP). We show that ITP increases upon dehydration, and protects the animal from loss of body water by promoting thirst and repressing excretion. ITP also suppresses feeding, and can thus be considered as a master regulator integrating water and energy balance.
Introduction
Maintenance of homeostasis is based on ingestion and metabolism of water and nutrients in a manner that reflects the internal needs of the animal, but the precise regulatory mechanisms are incompletely understood [1]. Despite the strong evolutionary conservation of the main pathways underlying energy homeostasis [2–5], there is a considerable diversity in the strategies involved in the maintenance of water balance [6, 7]. In insects, this variability arises mainly from the diversity of their habitats and life history strategies. For example, some blood-sucking insects are able to ingest a blood meal that exceeds their body volume up to twelve-fold; their feeding is hence coupled to massive post-prandial diuresis of the excessive water and ions [8]. However, in most of the non-blood sucking terrestrial insects, water conservation is more important than water secretion [1, 9]. Studies on water balance in insects have historically focused mainly on the hormonal regulation of water excretion. These studies investigated the correlations between the hormone titers and diuresis, and analyzed the effects of injections or in vitro applications of the tested compounds (reviewed e.g. in [8–11]). These works contributed to a better understanding of water regulation at the level of fluid secretion by the Malpighian tubules and water reabsorption in the hindgut (reviewed e.g. in [8–11]). Later, development of genetic tools for Drosophila allowed analysis of diuretic hormones by direct genetic manipulations [12–14]. However, no anti-diuretic hormone has been identified in Drosophila until now.
Drosophila is under laboratory conditions raised on media that provide both nutrients and water, and flies therefore do not regulate food and water intake independently. Nevertheless, insects, including Drosophila, can sense water [15, 16] and exhibit hygrotactic behavior [17, 18]. If given the opportunity, flies differentiate between food and water sources, and are able to seek and drink free water [19, 20], or ingest media rich in water but devoid of nutrients [21]. Recently, a small group of neurons were identified in the Drosophila brain that antagonistically regulate thirst and hunger [22]. These neurons sense osmolarity cell-autonomously with the cation channel Nanchung, and internal nutrients indirectly via Adipokinetic hormone signaling [22]. Although several hormones have been shown to regulate feeding and satiety (reviewed in [23–27]), no endocrine regulator of thirst has been identified in Drosophila so far.
The mechanisms that orchestrate water sensing, water-seeking behavior and conservation of water remain unclear. We hypothesized that these processes are likely coordinated by endocrine signaling. Physiological roles of Drosophila hormones are mostly well characterized (reviewed e.g. in [23]); one of the few exceptions is Ion transport peptide (ITP), which belongs to the family of crustacean hyperglycemic hormones (CHH) [28, 29]. CHHs promote water reuptake and hence, act as an anti-diuretic hormones in crustaceans [30]. The locust homolog of ITP promotes water reabsorption by acting on chloride channels in the hindgut [31, 32]. Drosophila has a single ITP gene that gives rise to an amidated ITP hormone and to two longer forms called ITP-like peptides [28, 29]. The functions of Drosophila ITP have not been investigated so far, except for a study that has shown a role of ITP in modulation of evening activity by the circadian clock circuitry [33]. The findings from the crustacean [34] and locust [31, 32] members of the CHH family suggest that Drosophila ITP might be involved in the regulation of water balance as well. Here, we tested this hypothesis by investigating the effects of gain- and loss-of-function of ITP on key aspects of water homeostasis, such as body water content, desiccation and osmotic stress resistance, food and water intake, and excretion. Our work identified master regulatory roles of ITP in water homeostasis of Drosophila; ITP levels increase under desiccation stress and protect the fly from water loss by increasing thirst, reducing excretion rate, and promoting ingestion of water instead of food. Altogether, our work identifies the first anti-diuretic and drinking-promoting hormone in Drosophila, which also coordinates water balance with feeding behavior.
Results
ITP codes for an anti-diuretic peptide
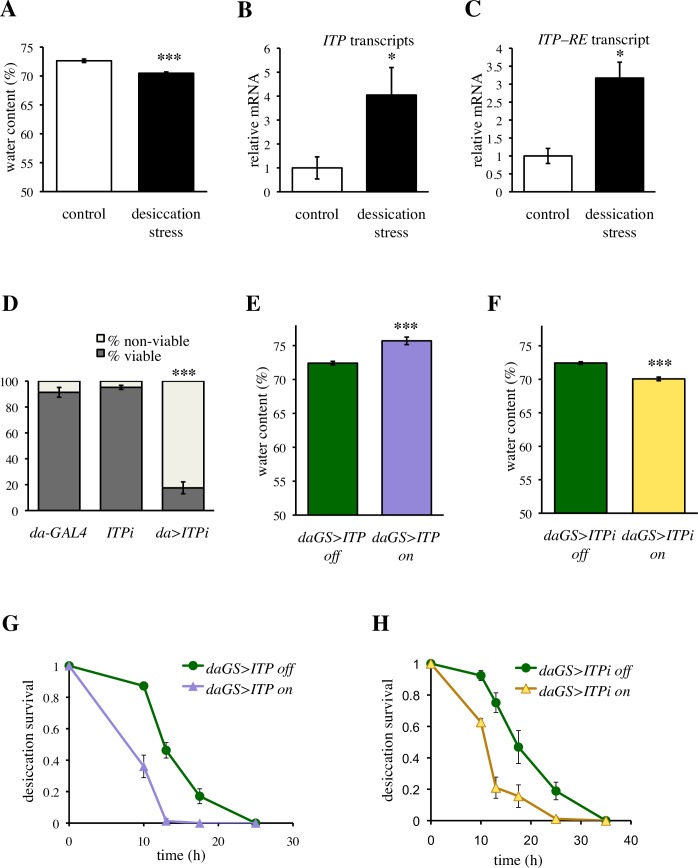
As the first step towards understanding the potential role of ITP in water homeostasis of Drosophila, we investigated whether expression of this gene reflects changes in the body water. We exposed standard (w1118) flies to a short-term (6 h) desiccation, which was sufficient to reduce body fluids (Fig 1A), and monitored expression of the ITP gene (CG13586) by quantitative PCR. Using a primer pair that covers all 5 known transcripts of the gene, we showed that desiccation stress increases expression of the ITP gene (Fig 1B), suggesting a role of ITP in water homeostasis. We confirmed that the transcriptional increase involves also the RE transcript (Fig 1C), the only transcript that gives rise to ITP (FlyBase FB2017_06), considered to be the only functional peptide produced by the ITP gene [28].
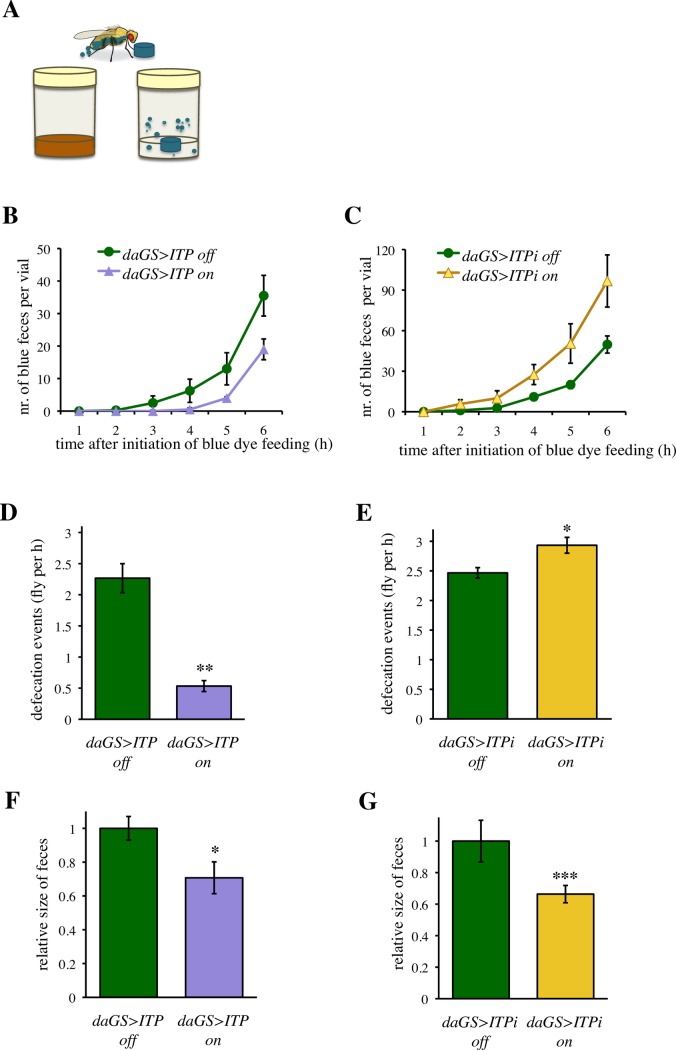
Fig 1. ITP regulates water homeostasis.
(A) Short term (6 h) desiccation stress efficiently reduces body fluids. Two-tailed Student’s t–test: P < 0.001. (B) Desiccation stress increases expression of the ITP gene. A primer pair that detects all 5 isoforms (transcripts RC, RD, RE, RF and RG, FlyBase FB2017_06) was used. Two-tailed Student’s t–test: P < 0.05. (C) Desiccation stress increases the abundance of the RE transcript. Primer pair that detects solely this isoforms was used. Two-tailed Student’s t–test: P < 0.05. (D) Daughterless-GAL4 driven ITP RNAi (da>ITPi) results in developmental lethality. Fischer’s exact test: P < 0.001 for comparison with each control. Animals were analyzed in three replicates, Fischer’s exact test was done on pooled data. Sample size: da-GAL4 n = 595; ITPi n = 439; da>ITPi n = 566. (E) Daughterless-GeneSwitch-driven over-expression of ITP (daGS>ITP) increases the proportion of body fluids. Two-tailed Student’s t–test: P < 0.001. (F) daGS driven ITPi (daGS>ITPi) decreases the proportion of body fluid. Two-tailed Student’s t–test: P < 0.001. (G) Over-expression of ITP decreases desiccation resistance. Log-rank test: P < 0.001. Sample size: daGS>ITP off n = 83; daGS>ITP on = 70. (H) ITP RNAi decreases desiccation resistance. Log-rank test: P < 0.001. Sample size: daGS>ITPi off n = 88; daGS>ITPi on n = 89.
It has been shown that an ITP mutation is embryonically lethal [35], and RNAi driven by the ubiquitous daughterless-GAL4 (da-GAL4) also resulted in considerable developmental lethality (Fig 1D). Therefore, to investigate the role of ITP in water balance, we used the GeneSwitch system [36, 37], which allowed circumventing the developmental lethality of ITP and studying the gain- and loss-of function of ITP specifically during the adult stage. In addition, this system enabled investigation of genetically identical animals, thereby avoiding any confounding effects of genetic backgrounds. The system is switched on by feeding flies the drug RU-486, which in itself does not affect water balance (S1 Fig). The expression pattern of ITP is complex and involves several distinct neuron types in the central nervous system and periphery, but the hormone is supposed to be released into the hemolymph [28, 29]. Therefore, we used the ubiquitous daughterless-GeneSwitch (daGS) [38] driver for both RNAi (ITPi) and over-expression of ITP. The daGS-driven over-expression of ITP resulted in increased (Fig 1E), and RNAi in decreased water content (Fig 1F), demonstrating that ITP has anti-diuretic function. We reproduced this effect also using an independent RNAi line targeting an alternative part of the ITP transcript (S2 Fig), and confirmed that ITP has anti-diuretic activity in female flies as well (S3 Fig). However, despite their higher initial water content, animals with increased ITP levels were more sensitive to desiccation (Figs 1G and S4), with their survival reduced by over 30%. Interestingly, animals with reduced ITP levels had moderately increased sensitivity to desiccation as well (Figs 1H and S4), suggesting that survival under arid conditions depends on a tightly regulated expression of ITP.
Taken together, these experiments revealed that ITP codes for a hormone that is regulated by internal water content and has an anti-diuretic function.
ITP is required to cope with desiccation stress
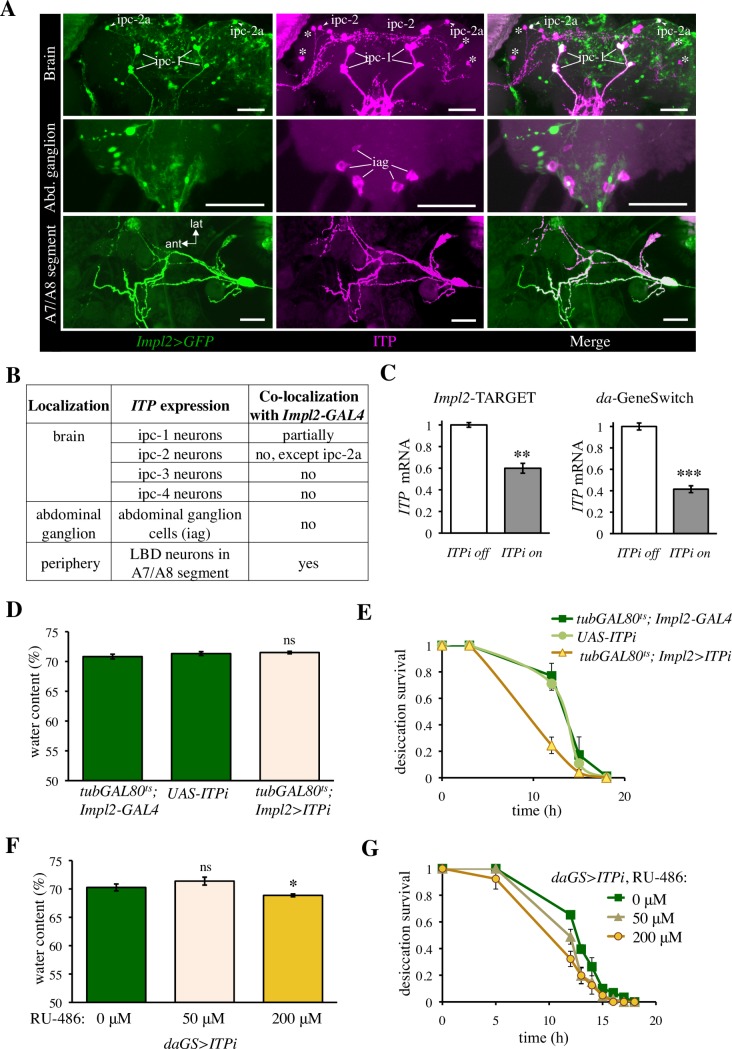
Next, we asked if ITP regulates the response to desiccation, or whether it determines desiccation resistance only by influencing the initial water content prior to the desiccation. The daGS>ITPi manipulations from the experiments described above could not answer these questions, because they resulted in reduced body water already before the onset of desiccation. Thus, we looked for a weaker genetic manipulation of ITP, which would allow testing the desiccation resistance without affecting the initial water content. Using an ITP-specific antibody, we confirmed previous results [28, 29] showing that the gene is expressed in the neurosecretory cells of the brain termed ipc-1 and ipc-2, in the interneurons termed ipc-3 and ipc-4, in the abdominal ganglion cells (iag cells), and in the lateral bipolar dendrite neurons (LBD neurons) of abdominal segments A7/A8 (Figs 2A, 2B and S5). To achieve a weaker genetic manipulation of ITP, we used the Impl2-GAL4 driver, which targets only a subpopulation of the ITP-producing neurons: the neurosecretory neurons in the brain (ipc-1 cells and the ipc-2a cells) and the LBD neurons in the periphery (Figs 2A, 2B and S5). To avoid potential developmental effects, we took advantage of the TARGET switch (temporal and regional gene expression targeting, [39]), by which the temperature sensitive tubGAL80ts allows switching on the RNAi specifically in the adult flies. Although we did not test the RNAi efficiency in a cell-autonomous manner, the Impl2-based TARGET effectively decreased the global ITP mRNA (Fig 2C). Consistently, with targeting only a limited number of ITP-expressing neurons, the effect on the global ITP mRNA was approximately 20% weaker than the effect of the ubiquitous daGS-driven ITPi (Fig 2C). Importantly, the ITPi driven by Impl2-based TARGET was not sufficient to impair body fluids (Figs 2D and S6). Thus, this driver allowed us to disentangle the effect of ITP on water storage before the onset of desiccation from its role during the desiccation exposure. ITPi driven by the Impl2-based TARGET resulted in a reduced survival under desiccation (Figs 2E and S6), suggesting that ITP is required to cope with the desiccation stress via an additional mechanism, not only by regulating water storage prior to desiccation. An effect on desiccation survival, similar to Impl2-driven ITPi, was obtained also by daGS-driven ITPi, when the system was switched by a low RU-486 dose. This low dose (50 μM) was not sufficient to affect the body water content (Fig 2F), but was sufficient to reduce survival upon desiccation stress, although to a lower extent than the standard dose of 200 μM RU-486 (Fig 2G) used in the rest of the GeneSwitch-based experiments.
Fig 2. ITP regulates desiccation survival independently of its action on the water storage under ad libitum feeding.
(A) Impl2-GAL4 is a suitable driver to target several of the ITP-expressing neurons. Impl2-GAL4-driven expression of GFP partially overlaps with the ITP immunoreactivity (ITPir); scale bars 50 μm. Upper panel: brain neurons. Note the ipc-1 and ipc-2a neurosecretory cells are covered by the Impl2 expression pattern, whereas the rest of the ipc-2 (marked in middle figure) and ipc-3 interneurons (asterisks) are not. Median panel: neurons in the terminal abdominal ganglia that express ITP do not express of Impl2>GFP. iag = ITPir abdominal ganglia neurons. Lower panel: peripheral lateral bipolar dendrite (LBD) neurons in the seventh and eighth abdominal segment express both Impl2>GFP and ITP (ant anterior, lat lateral). (B) Summary table listing partially overlapping expression pattern of Impl2-GAL4 and ITP. (C) ITPi driven by both Impl2-based TARGET and da-GeneSwitch effectively decreases ITP mRNA. Two-tailed Student’s t–test: P < 0.01 for the TARGET manipulations and P < 0.001 for the GeneSwitch manipulation. F1 generation of the cross between the ITPi line and w1118 was used as a control (off conditions) for the TARGET-based experiment. (D) ITPi driven by the Impl2- based TARGET does not affect the proportion of body fluids. Two-tailed Student’s t–test: P > 0.05 for both comparisons with controls. (E) ITPi driven by the Impl2-based TARGET reduces survival under desiccation. Log-rank test: P < 0.001 for comparisons with both controls. Sample size: tub-GAL80ts; Impl2-GAL4 n = 87; ITPi n = 83; tub-GAL80ts; Impl2>ITPi n = 49. (F) Mild ITPi driven by the daGS induced by 50 μM RU-486 does not affect body fluids. Two-tailed Student’s t–test: P > 0.05 for comparisons with the non-induced conditions. Strong ITPi driven by the daGS induced by 200 μM RU-486 reduces body water. Two-tailed Student’s t–test: P < 0.05. (G) Mild ITPi driven by the daGS induced by 50 μM RU-486 reduces survival under desiccation. Log-rank test: P < 0.05. The detrimental effect of ITPi on desiccation resistance is dose-dependent; induction by 50 μM RU-486 has a milder effect than induction by the 200 μM RU-486. Log-rank test: P < 0.01. Sample size: 0 μM RU-486 n = 61; 50 μM RU-486 n = 45; 200 μM RU-486 n = 40.
Thus, ITP regulates desiccation survival not only by accumulating proper levels of body water prior to the desiccation challenge, but it is also required to cope with the arid conditions.
ITP signaling regulates response to osmotic stress
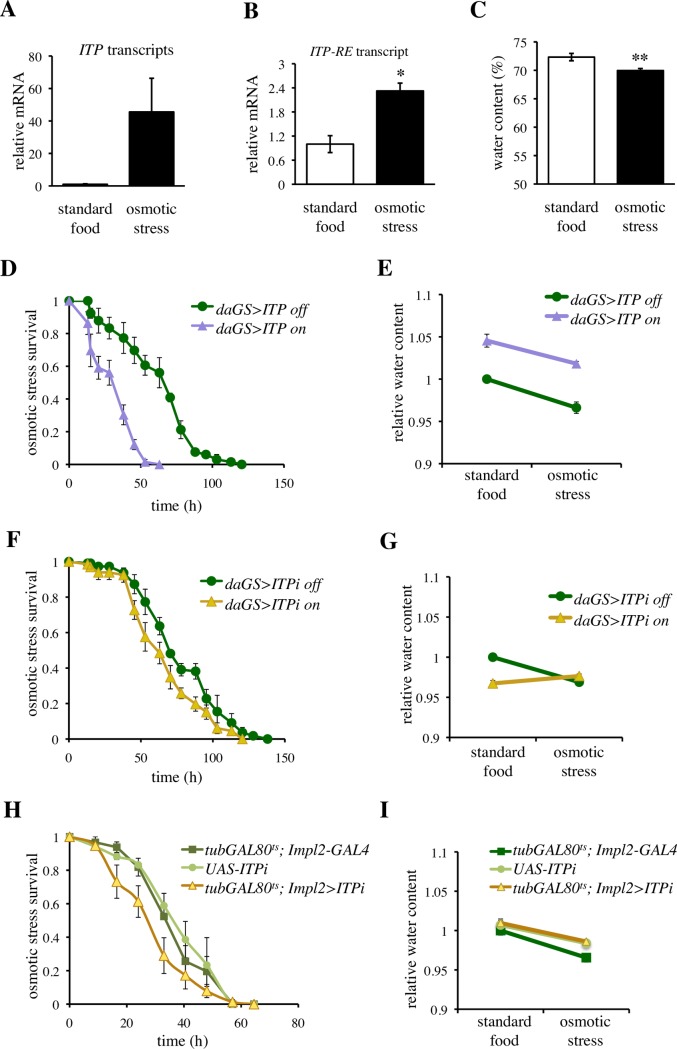
Regulation of water balance is important especially under ionic stress. Therefore, we monitored ITP expression after feeding on a medium containing 4% NaCl, using a primer pair that covers expression of all 5 known transcripts of the gene (Fig 3A), and a primer pair specific for the ITP-RE transcript, the only transcript that gives rise to ITP [28]. Osmotic stress indeed increased expression of ITP-RE (Fig 3B). However, this treatment also reduced the amount of body water (Fig 3C) and hence we cannot differentiate whether the increase in ITP expression was driven by the changes in the osmolarity or the volume of body fluids. Genetic over-expression of ITP decreased survival during osmotic stress (Fig 3D), without affecting the osmolarity-induced changes in the body water (Fig 3E and S1 Table).
Fig 3. ITP regulates survival under osmotic stress.
(A) Osmotic stress appears to increase expression of the ITP gene. However, when a primer pair that detects all 5 isoforms (transcripts RC, RD, RE, RF and RG, FlyBase FB2017_06) is used, a clear tendency is observed but the P value does not reach statistical significance. Two-tailed Student’s t–test: P > 0.05. (B) Osmotic stress increases abundance of the ITP RE transcript. A primer pair that detects solely this isoform was used. Two-tailed Student’s t–test: P < 0.05. (C) Osmotic stress decreases body fluid. Two-tailed Student’s t–test: P < 0.01. (D) Over-expression of ITP decreases survival during osmotic stress. Log-rank test: P < 0.001. Sample size: daGS>ITP off n = 66; daGS>ITP on = 66. (E) Over-expression of ITP increases body water, but does not affect the osmotic stress-induced loss of body water. Two-way ANOVA, ITP over-expression and osmotic treatment as fixed factors. Effect of ITP: P < 0.001, effect of osmotic stress: P < 0.001, effect of ITP × osmotic stress interaction: P > 0.05. See S1 Table for further details. (F) daGS-driven ITP RNAi (ITPi) decreases resistance to osmotic stress. Log-rank test: P < 0.05. Sample size: daGS>ITPi off n = 66; daGS>ITPi on n = 66. (G) daGS-driven ITPi decreases water content under standard conditions (two-tailed Student’s t–test: P < 0.001), and interacts with the effect of osmotic stress. Two-way ANOVA, ITPi and osmotic treatment as fixed factors; effect of ITPi: P < 0.01, effect of osmotic stress: P < 0.05, effect of the interaction: P < 0.001. See S2 Table for further details. (H) ITPi driven by the Impl2-based TARGET decreases osmotic stress resistance. Log-rank test: P < 0.01 for each comparison with controls. Sample size: tub-GAL80ts; Impl2-GAL4 n = 69; ITPi n = 66; tub-GAL80ts; Impl2>ITPi n = 75. (I) ITPi driven by the Impl2-based TARGET does not affect water content under standard conditions (two-tailed Student’s t–test: P > 0.05 for each comparison with controls), nor interacts with the effect of osmotic stress (two-way ANOVA, ITPi and osmotic treatment as fixed factors; effect of the interaction: P > 0.05. See S3 Table for further details. Thus, the Impl2-based TARGET enables disentangling the requirement for ITP under osmotic stress from its requirement for water preservation.
Similar to ITP over-expression, ITPi driven by the daGS and the Impl2-GAL4 lines resulted in a weak, but statistically significant reduction of osmotic resistance (Fig 3F and 3H), suggesting that both up-and down-regulations of ITP impair osmotic tolerance. The daGS-driven ITPi reduced water levels to an extent comparable to that seen under osmotic stress (Fig 3G). Subsequent exposure to osmotic stress did not decrease the body water of the daGS>ITPi flies any further (Fig 3G and S2 Table). The weaker Impl2-driven ITPi neither affected water content nor its reduction by osmotic stress (Fig 3I and S3 Table), suggesting that ITP is required to cope with osmotic stress independently of the regulations of water content.
Taken together, we show that survival under osmotic challenge requires tight regulation of ITP expression, as both up- and down-regulation of this gene resulted in a reduced survival on a food medium with a high salt content.
ITP regulates food intake
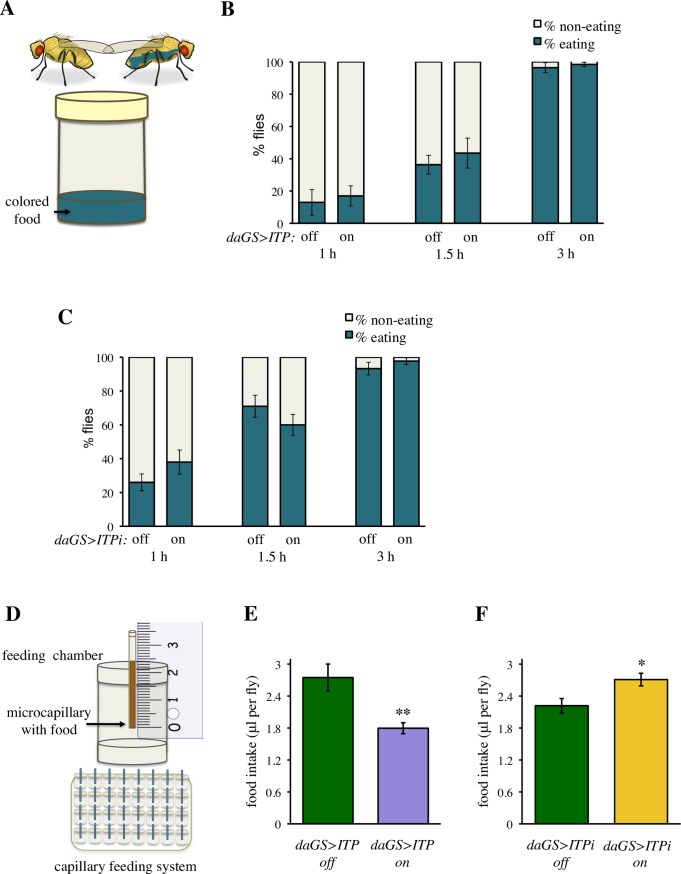
Next, we investigated the functional mechanism by which ITP regulates water balance. Under standard laboratory conditions, Drosophila obtains water from the food. Thus, we first asked whether ITP regulates food consumption. We tested whether ITP manipulations affect frequency of eating, measured as propensity to start spontaneous feeding. We transferred fed flies to fresh food supplemented with blue dye, which allows monitoring the time when animals initiate feeding (Fig 4A). Neither ITP over-expression nor ITP RNAi affected the propensity of flies to start spontaneous feeding (Fig 4B and 4C). Subsequently, we measured the total volume of food consumed (Fig 4D), using a modification of the capillary feeding (CAFE) assay [40, 41]. This assay revealed that ITP is an anorexigenic factor; an increase in ITP reduced the volume of consumed food (Fig 4E), whereas ITP RNAi increased the total food intake (Figs 4F and S7).
Fig 4. ITP regulates feeding.
(A) Schematic drawing of the method to measure hunger as propensity to initiate feeding. (B) Over-expression of ITP does not affect the propensity to start feeding. Fischer’s exact test: P > 0.05 at all tested time points. Animals were analyzed in four replicates, Fischer’s exact test was conducted using pooled data. Sample size: daGS>ITP off: n = 100 (1 h), n = 96 (1.5 h), n = 78 (3 h); daGS>ITP on: n = 100 (1 h), n = 86 (1.5 h), n = 76 (3 h). (C) ITPi does not affect the propensity to start feeding. Fischer’s exact test: P > 0.05 at all tested time points. Animals were analyzed in four replicates, Fischer’s exact test was done using pooled data. Sample size: daGS>ITPi off: n = 100 (1 h), n = 100 (1.5 h), n = 87 (3 h); daGS>ITPi on: n = 100 (1 h), n = 100 (1.5 h), n = 85 (3 h). (D) Schematic drawing of the capillary feeding system, which measures the total volume of food eaten during a given period of time. (E) Over-expression of ITP decreases food intake. Two-tailed Student’s t–test: P < 0.01. (F) ITPi increases food intake. Two-tailed Student’s t–test: P < 0.05.
These experiments indicate that ITP is a negative regulator of food intake. Thus, increased water levels in the daGS>ITP and reduced levels in the daGS>ITPi animals suggest that ITP acts downstream of feeding to conserve body water.
ITP regulates excretion
The ureter of Drosophila feeds into the hindgut, and water that is not re-absorbed by the hindgut epithelium is excreted by the same route as the feces [9]. Thus, we investigated whether the ITP manipulations affect excretion. Since our previous experiments (Fig 4B and 4C) had shown that genetic manipulations of ITP do not affect the propensity to initiate feeding, we monitored the speed of food transit throughout the digestive tract as the time from initiation of feeding until excretion of the blue dye in the feces (Fig 5A). We transferred flies on the food with blue dye, and measured the time-dependent increase in the blue-dyed feces. The ITP gain-of-function reduced the speed of the food transition throughout the digestive tract (Fig 5B and S4 Table), whereas ITP RNAi increased it (Figs 5C and S8 and S5 Table).
Fig 5. ITP regulates the pace of transit through the digestive tract and the number of defecation events.
(A) Schematic drawing of the assay to measure defecation rate. All flies started to feed on the blue-dyed food at the same time, and the numbers of colored feces were counted every hour after the transfer on the blue dye food. (B) Over-expression of ITP decreases defecation rate. Two-way ANOVA, ITP and time as fixed factors; effect of ITP P < 0.01, effect of time: P < 0.001, effect of the interaction: P > 0.05. See S4 Table for further details. (C) ITPi increases defecation rate. Two-way ANOVA, ITPi and time as fixed factors; effect of ITPi P < 0.001, effect of time: P < 0.001, effect of the interaction: P > 0.05. See S5 Table for further details. (D) Over-expression of ITP decreases defecation events per fly. Two-tailed Student’s t–test: P < 0.01. (E) ITPi increases defecation events per fly. Two-tailed Student’s t–test: P < 0.05. (F) Over-expression of ITP decreases the size of feces. Two-tailed Student’s t–test: P < 0.05. (G) ITPi decreases the size of feces. Two-tailed Student’s t–test: P < 0.001.
Subsequently, we tested whether ITP regulates also the frequency of the defecation events. Thus, we continuously fed flies with the blue-dyed food for two days and observed defecation events under conditions when intake and excretion of the dye were at equilibrium. The frequency of defecation events was decreased by ITP over-expression (Fig 5D), and increased by ITPi (Fig 5E). Hence, deficiency for ITP leads to a phenotype reminiscent of human diarrhea. The size of individual feces was reduced by both manipulations of ITP (Fig 5F and 5G). Nevertheless, we were not able to detect significant differences in the color intensity of feces that might be indicative of differences in the water content (S9 Fig).
Altogether, the above experiments indicate that ITP regulates the rate of excretion. Deficiency in ITP results in a faster transit through the digestive tract and an increased number of defecation events, reminiscent of diarrhea, a common cause of dehydration in humans.
ITP regulates thirst
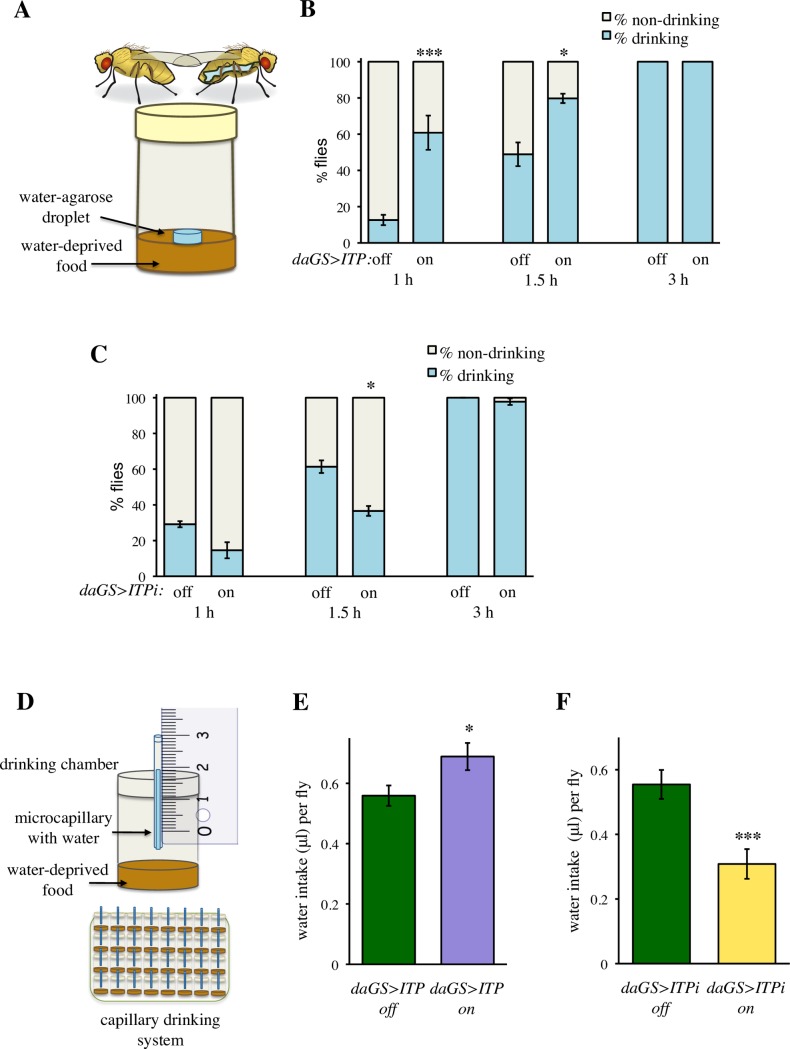
Under standard experimental conditions, flies obtain water from their food, and the classical food intake assays do not distinguish between thirst and hunger. To differentiate the role of ITP in water versus food intake, we modified a recent method by Lau et al. [19]. We reared flies on a medium poor in water (‘dry food’), and provided access to a separate, blue-dyed source of water (Fig 6A). Flies with increased ITP levels started to drink faster than controls (Fig 6B), and vice versa, ITPi resulted in a delayed time to the onset of water intake (Fig 6C). In order to test whether ITP also regulates the total volume of ingested water, we modified the CAFE assay monitors from the food intake experiment (Fig 4D); water was provided in microcapillaries in the presence of food poor in water (Fig 6D). Consistent with their increased propensity to start drinking, ITP over-expressing flies also drank more (Fig 6E), whereas ITPi flies drank less water than controls (Figs 6F and S10).
Fig 6. ITP regulates thirst and water intake.
(A) Schematic drawing of a system to measure thirst as propensity to initiate water intake. (B) Over-expression of ITP increases thirst, i.e. reduces the time to start spontaneous drinking. Significant differences are indicated by asterisk symbols. Fischer’s exact test: * P < 0.05; *** P < 0.001. Animals were analyzed in three replicates. Fischer’s exact test was done on pooled data. Sample size: daGS>ITP off: n = 47 (1 h), n = 45 (1.5 h), n = 45 (3 h); daGS>ITP on: n = 46 (1 h), n = 44 (1.5 h), n = 43 (3 h). (C) ITPi reduces thirst, i.e. extends the time until flies initiate drinking. Fischer’s exact test: * P < 0.05. Animals were analyzed in three replicates. Fischer’s exact test was done using pooled data. Sample size: daGS>ITPi off: n = 48 (1 h), n = 42 (1.5 h), n = 43 (3 h); daGS>ITPi on: n = 48 (1 h), n = 44 (1.5 h), n = 43 (3 h). (D) Schematic drawing of the capillary drinking system, which measures the total volume of water ingested during a given period of time. Panels (E) and (F) show the mean water intake per day of feeding on a water-deprived food. (E) Over-expression of ITP increases the amount of ingested water. Two-tailed Student’s t–test: P < 0.05. (F) ITPi decreases the amount of ingested water. Two-tailed Student’s t–test: P < 0.001.
These experiments revealed that ITP is the first known hormonal regulator of thirst in Drosophila.
In summary, in this study we identified ITP as a neuroendocrine factor central to regulation of water homeostasis. ITP increases in response to hypovolemia, and triggers drinking, while repressing feeding and water excretion, promoting thus conservation of water resources and protection from dehydration (Fig 7).
Fig 7. Scheme summarizing the roles of ITP in Drosophila, as revealed by this study.
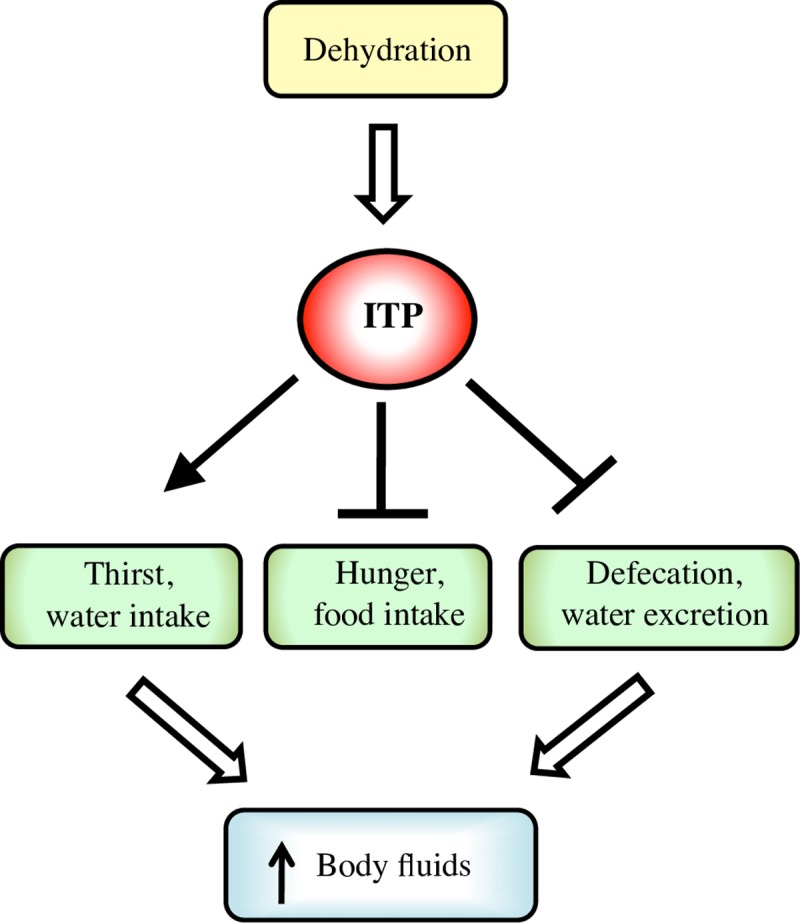
ITP is a central regulator of water balance. Conditions leading to reduced volume of body fluids result in increased expression of ITP gene. ITP subsequently promotes water intake, while inhibiting feeding and water loss by excretion, promoting thus the increase in body fluids and restoration of water balance.
Discussion
ITP is a functional analog of the vasopressin and renin-angiotensin systems
With the colonization of dry land and evolution of terrestrial life, conservation, rather than elimination of water became the main challenge for the maintenance of water homeostasis [42]. Despite the differences in the organization of the endocrine systems, the main principles of fluid homeostasis are the same in vertebrates and invertebrates; these include thirst, compensation for the feeding-induced increase in osmolarity by water intake, and water re-absorption by the excretory systems [1, 9, 10, 42]. In humans, water homeostasis is regulated primarily by an osmostat located in the hypothalamus [43]. This osmostat increases water levels by triggering thirst, and reduces the water loss by inducing release of the anti-diuretic hormone vasopressin [43]. In addition to the regulation by osmolarity, thirst is also induced by the changes in the blood volume both via vasopressin [44, 45] and the renin-angiotensin system [42, 46]. Even though thirst and water retention are physiologically coupled, their regulation occurs independently [43, 47]. We show here that these regulations are simplified in Drosophila, where the same hormone promotes thirst, reduces appetite, and increases water storage. Thus, ITP acts as a functional analog of both vasopressin and renin-angiotensin. Interestingly, like the vasopressin [44, 45] and renin-angiotensin system [42, 46], also ITP is regulated by body water content.
Over-expression of ITP increases water content by 4.5%, whereas RNAi dehydrates the fly by 3.3%. The physiological consequences of such mild changes of water levels are not known in Drosophila, but for comparison, in human patients, loss of as little as 2% water significantly impairs cognitive abilities [48], and liquid overload and hypervolemia represent harmful conditions as well [49].
Our findings show that knockdown of ITP leads to increased water excretion similar to human disorders caused by defective water re-absorbance in kidney, such as diabetes insipidus [43, 50]. Conversely, ITP over-expression results in increased water retention reminiscent of the human syndrome of inappropriate anti-diuretic hormone secretion (SIADH) [43]. ITP manipulations may thus become useful tools to induce and study pathologies associated with these human disorders in Drosophila.
Role of ITP in drinking
ITP is the first identified hormone that regulates drinking in Drosophila. Thus, it acts as a functional analog of the renin-angiotensin system of mammals. Similar to the renin-angiotensin system, ITP is most likely activated by hypovolemia. The neural circuits that control drinking and are regulated by ITP, however, remain to be investigated. Neurons that repress drinking in Drosophila have already been identified in the suboesophageal zone [22]. These neurons are regulated cell autonomously by an ion channel that senses osmolarity [22]. ITP-knockdown flies do not have the drive to drink despite their state of dehydration, whereas ITP over-expressing flies drink despite their excessive water content. Thus, unlike the Nanchung-expressing repressors of drinking [22], the ITP-regulated neurons are not regulated by the volume of body water, but rather by ITP itself.
Role of ITP in excretion
In insects, primary urine is produced by the Malpighian tubules that are functional analogs of mammalian kidneys [9]. Water enters the lumen of these tubules by passive diffusion along the ionic gradient maintained by the vacuolar V-H+-ATPase [9]. The function of the Malpighian tubules is hormonally regulated by diuretic hormones [9], which in Drosophila include products of the genes capa [13, 51], DH31 [52], DH44 and leucokinin [14]. Urine then enters the hindgut, where it mixes with the gut contents. Importantly, considerable parts of the water and ions are subsequently re-absorbed in the ileum and rectum [9, 32, 53]. Here, we show that ITP reduces excretion of water by reducing the defection rate. Thus, it is likely that Drosophila ITP promotes water reabsorption in the hindgut similar to its homologs in the desert locust Schistocerca gregaria [31, 32] or in the European green crab Carcinus maenas [34]. It is noteworthy that ITP-expressing neurons in the abdominal ganglia innervate Drosophila hindgut [29], suggesting that in addition to the hormonal regulation [29], the hindgut may also be regulated by ITP in a paracrine fashion. In crabs and in the red flour beetle Tribolium castaneum¸ CHH- or ITP-producing endocrine cells, respectively, have even been detected in gut epithelia [34, 54]. Thus, whether produced in the neurosecretory cells or in the endocrine cells of the gut, the actions of CHHs and ITPs on the hindgut appear to be evolutionarily conserved.
Role of ITP in feeding
In mammals, an increase in osmolarity due to food intake results in postprandial thirst, and conversely, dehydration inhibits feeding when water is not available [55] and this is likely also the case in Drosophila. Our findings of the ITP-driven positive regulation of water intake, concomitant with a negative regulation of feeding likely represents another level of regulation of thirst and hunger, acting in parallel to that of the four drink-repressing neurons in the suboesophageal zone [22].
Roles of ITP under desiccation and osmotic stress
Whereas many terrestrial arthropods frequently experience arid conditions, salt stress is not very common in non-blood feeding terrestrial insects. Nevertheless, desiccation and salt stress resistance have been traditional tests in the studies of Drosophila diuretic hormones. RNAi against diuretic hormones increases desiccation resistance, as shown for capa [13], DH44 [14] and leucokinin [12] genes. However, it remains unclear whether these hormones contribute to the natural response to the desiccation and osmotic stress. For example, desiccation does not change expression of diuretic hormones DH44 and leucokinin [14]. In contrast, ITP seems to be a natural component of the desiccation and osmotic stress responses, since both stressors trigger an increase in ITP expression. The role of ITP in thirst, hunger and excretion suggest that the ITP-regulated changes in behavior and physiology represent natural responses to cope with the reduction of body water. Consistently, knockdown of ITP reduces survival under desiccation and osmotic stress. However, it is unclear why over-expression of ITP reduces resistance to desiccation and osmotic stress. The UAS-GAL4 based manipulations may increase ITP levels far beyond the physiological range, which—although not lethal under standard feeding—might reduce survival under stressful conditions. Given the role of ITP in the ion transport across the hindgut epithelia of locusts [31, 32], it is tempting to speculate that a similar mechanism exists in Drosophila. In such a scenario, the non-physiological doses of ITP might considerably increase osmolarity of hemolymph. This would be toxic when feeding on a food medium with a high salt content, as well as under desiccation conditions (which further increase osmolarity).
Possible pleiotropic actions of ITP, further unresolved questions and future directions
Although ITP has been known for a long time [56], its function has remained enigmatic in Drosophila. Our pioneering work on its roles in Drosophila physiology suggests that ITP codes for a master regulator of water balance, which also integrates the water homeostasis with energy metabolism. Thus, our study not only shows that this member of the CHH family has an evolutionarily conserved anti-diuretic role in Drosophila as it has in other arthropods [34], but also reveals novel functions of this peptide family in food and water intake. It remains to be investigated to what extent these roles are conserved in other insect species or even in crustaceans, but the strong evolutionary conservation of the gene structure [30] suggests that this might be the case. It is possible that the fly ITP regulates, in addition to its here-described role in water balance, other processes that are known to be CHH-regulated in crustaceans [34]. For example, the high developmental lethality of ITP RNAi, together with the previously described lethality of ITP mutants [35] imply that Drosophila ITP plays a critical role during development, perhaps analogous to the role of CHHs in crustacean molting [34].
Although identification of the cellular sources of ITP that are responsible for the here-described functions of this hormone was beyond the scope of this manuscript, the expression pattern of the gene already provides some tempting hints. Previous in situ-hybridizations and immunohistochemistry experiments based on a locust anti-ITP antibody showed that Drosophila ITP is expressed in several neuronal types [28, 29]. Here, using an antibody specific to Drosophila ITP, we confirmed that these cells include ipc-1 and ipc-2a neurosecretory neurons in the brain, ipc-3 and ipc-4 interneurons, three pairs of iag cells in the abdominal ganglia, and the LBD neurons in abdominal segments A7 and A8. As described previously [28, 29], although ITP is expressed in several interneurons, the most prominent cells of the brain that express ITP are the neurosecretory protocerebral ipc-1 and the ipc-2a neurons, which send axons towards neurohemal release sites in the corpora cardiaca, corpora allata, and aorta. Our experiments based on the Impl2 driver showed that a proper response to desiccation and osmotic stress requires production of ITP in the ipc-1 neurons, ipc-2a neurons, or LBD neurons, or in their combination. The ITP production in these cells becomes nevertheless critical only under desiccation and osmotic stress. In contrast to the global manipulations, ITPi targeted to these neurons is not sufficient to impair water balance under standard conditions. Thus, water content is regulated either via ITP produced by cells outside of the Impl2 expression pattern, or the ITP-producing neurons are redundant in their ability to produce sufficient ITP to maintain water homeostasis under standard conditions. Altogether, additional cell type-specific manipulations are required to differentiate whether thirst, excretion and food intake are regulated by specific neurons, or whether different ITP-producing neurosecretory cells act redundantly to produce sufficient amount of the hormone to regulate physiology of the fly.
Another key step towards understanding the ITP actions is the identification of the hitherto unknown Drosophila ITP receptor. This will facilitate cell- and tissue-specific manipulations to unravel the neural circuit(s) responsible for the roles of ITP in the control of thirst and hunger, and allow more detailed studies of the peripheral roles of ITP in defecation and water excretion.
Materials & methods
Fly lines and husbandry
Flies were reared under a 12 h light–12 h dark cycle on a standard Drosophila medium consisting of 6 g agar, 50 g yeast, 100 g sugar, 5.43 mL propionic acid, and 1.3 g methyl 4-hydroxybenzoate per 1 L of medium. Adult flies were collected within 24 h after eclosion, flipped on fresh media, and housed in groups of around 50 females + 50 males per vial. Flies for the TARGET experiments developed at 18°C and on the third day after eclosion were transferred to 29°C for the RNAi induction. Flies for the GeneSwitch experiments developed at 25°C on standard medium, and were kept from the third day after adult eclosion on a standard medium supplemented with RU-486 and reared further at 25°C. All GeneSwitch experiments were conducted with 0 and 200 μM RU-486, and experiments described in Fig 2F and 2G were performed also with 50 μM RU-486. After the switch induction, both TARGET and GeneSwitch flies were flipped every second day onto fresh media. If not stated otherwise, male flies were used for experiments 6–7 days after the induction of the transgene expression. Controls for the non-GeneSwitch experiments were generated by crossing the UAS and GAL4 lines to the w1118 strain. Experiments on the desiccation and osmotic stress–induced changes in the ITP expression were performed on the w1118 strain. The list of used fly stocks is available in the S1 File.
Viability determination
Viability was expressed as egg-to-adult survival, i.e. as the percentage of eggs that gave rise to adult flies. Three independent egg collections (each at least 120 eggs) were tested for each genotype. Eggs were counted, allowed to develop at 25°C at 12 h light/12 h dark cycle on standard medium, and eclosed flies were collected and counted.
Water content measurements
Water content was expressed as percentage of fresh body weight. Flies were weighed using a Mettler MT5 analytical microbalance (Mettler Toledo). Fresh weight was determined, then flies were desiccated for 2 days at 65°C and weighed again. The amount of water was calculated as the difference between the fresh and the dry weight, and expressed as % of the fresh body weight. At least 5 replicates (each consisting of 5 flies) were tested per treatment / genotype.
Desiccation resistance assay
Desiccation resistance was estimated as survival of flies in empty vials without any water source. Experiments were done in 3–4 replicates. TARGET-based experiments took place at 29°C, GeneSwitch-based experiments took place at 25°C.
Osmotic stress assay
Osmotic stress resistance was determined as survival of flies on food medium containing 4% NaCl. Experiments were done in triplicates. TARGET-based experiments took place at 29°C, GeneSwitch-based experiments took place at 25°C. The food contained the same concentration of RU-486 (200 μM) or ethanol vehicle control as during the pre-feeding period.
Food intake measurement by capillary feeding assay (CAFE)
The volume of ingested food was measured by a modification of the CAFE assay [40] in a feeder device constructed out of 24-well-plates, similar to the one described before [41]. Capillaries with food (Hirschmann minicaps, 5μ) were exchanged daily. Food intake of at least 15 animals per treatment was measured during 3 days, and corrected for the evaporation rate. The liquid food contained the same concentration of RU-486 (200 μM) or ethanol vehicle control as during the pre-feeding period.
Measurement of hunger as the propensity to start feeding
Flies were transferred into a vial with a drop (approximately 0.2 mL) of food medium containing 0.5% Brilliant Blue (Sigma), and the proportion of flies that started feeding (blue dye was observable in their body after inspection under a stereomicroscope) was counted 1 h, 1.5 h and 3 h after the transfer. Flies were separated into the tested groups 1 day before the experiment to avoid potential interference of CO2 anesthesia with the food intake. Each time point was tested in 4 replicates, each consisting of at least 16 flies.
Measurement of the speed of the food transit throughout the digestive tract
Flies were transferred into vials with a drop (approximately 0.2 mL) of food medium containing 0.5% Brilliant Blue (Sigma) and allowed to feed continuously. The cumulative numbers of feces that contained the blue dye were counted in the vial every hour, until 6 h after the switch to the blue-dyed medium. Feces were counted in three replicates, each vial containing 20 flies. For testing the statistical significance by two-way ANOVA, the number of new feces that were deposited within the given period was used.
Measurement of the excretion rate at equilibrium
Excretion rate was measured as the number of defecation events (number of feces) per fly per hour. Flies were fed for 48 h on standard food (with or without RU-486) with 0.5% Brilliant Blue (Sigma). Flies were subsequently transferred into a new vial with a small drop of colored food, and the number of feces produced per fly per vial was counted. Experiments were performed in three replicates, each consisting of 20 flies.
Analysis of the size and color intensity of the feces
Flies were fed for 48 h on standard food (with or without RU-486) with 0.5% Brilliant Blue (Sigma). Subsequently, a new transparent plastic lid was put on top of the vials, and feces collected on this lid within 2.5 h and were photographed using Leica WILD M32 stereomicroscope with Leica DFC290 camera. The area and lightness were measured using the T.U.R.D. software [57].
Measurement of thirst / propensity to start drinking
Flies were separated into tested groups 1 day before the experiment to avoid potential effect of CO2 exposure on the water intake. Flies were transferred into vials containing approximately 2 mL of the water-deprived food, and after 30 min into new vials with 2 mL of the water-deprived food and a 0.2 mL of a water-rich agar droplet (0.6% agarose, 0.5% Brilliant Blue) and allowed to eat and drink. Flies that started to drink were identified based on the blue color in their abdomina after inspection under a stereomicroscope. The proportion of flies that started to drink was checked 1 h, 1.5 h and 3 h after transferring flies to the water source. Experiments were done in triplicates, and at each time point, at least 42 flies were tested. Water-deprived food medium contained 75% less water and agar than the standard medium, consisting of: 0.6 g agar, 20 g yeast, 40 g sugar, 0.54 mL propionic acid, 0.13 g methyl 4-hydroxybenzoate and 1 mL of 20 mM RU-486 or ethanol per 100 mL of medium.
Measurement of water intake by capillary drinking assay
The capillary drinking assay was performed in a device similar to the CAFE assay feeder, with the following modifications: the bottom of each chamber contained approximately 0.8 mL of water-deprived food with 200 μM RU-486 or ethanol as a vehicle control. Water-deprived food medium contained 75% less water and agar than the standard medium, as described above. Flies were allowed to drink water from the capillaries. To make the measurements of the ingested water easier, water was colored with 0.05% Brilliant Blue (Sigma). The volume of ingested water was measured over one day, and corrected for the evaporation rate. At least 18 flies were tested for each genetic manipulation.
Immunohistochemistry
Adult flies were dissected in ice-cold Drosophila Ca2+ free saline. After removing wings and legs, brain-thoracic/abdominal ganglia complexes were quickly excised from head and thorax. All preparations were fixed overnight in Zamboni's fixative overnight at room temperature, washed and treated as described in detail earlier [29]. The only modifications concerned the use of two different primary and secondary antibodies always at the same time of incubations. Primary antibodies were a polyclonal rabbit anti-DrmITP diluted 1:10,000 [33] and a monoclonal mouse anti-GFP (against Jelly fish GFP; Invitrogen) diluted 1:1,000. Secondary antibodies were goat anti-rabbit Alexa 546 and goat anti-mouse Alexa 488, respectively (Invitrogen), both diluted 1:1,000. Preparations were imaged with a Zeiss LSM 780 confocal microscope by use of 10× or 20× objectives. Confocal images were processed with Zeiss ZEN software, version 8.1 2012, for maximum intensity projections of z-stacks. Brightness and contrast was adjusted using Corel Photopaint X7 during plate-mounting using Corel Draw X7.
Quantitative PCR (qPCR)
RNA was extracted using the Zymo Research QuickRNA MicroPrep kit according to the manufacturer’s instructions. cDNA was synthesized by the QuantiTect Reverse Transcription Kit (Qiagen) using 1 μg of the total RNA. Quantitative real-time PCR was performed using SensiFAST SYBR Hi-ROX Kit (Bioline) and StepOne Real-Time PCR System (Applied Biosystems). Expression levels were normalized to Actin 5C (Act5C). Information on the primers is available in the S1 File.
Statistical analyses
Measurement variables were analyzed by two-tailed Student’s t-test, one-way or two-way ANOVA. Nominal variables were analyzed by two-tailed Fischer’s exact test. Survival data were analyzed by log-rank test. P values are indicated by asterisk symbols (* P < 0.05, ** P < 0.01, *** P < 0.001). Error bars represent SEM. Data on the measurement variables were analyzed using Excel or PAST [58]: http://palaeo-electronica.org/2001_1/past/issue1_01.htm. Survival data were analyzed using PAST. Data on nominal variables were analyzed by Graphpad QuickCalcs (https://www.graphpad.com/quickcalcs/).
Supporting information
Water content of the w1118 strain reared for one week on food enriched with 200 μM RU-486 does not differ from the controls reared with the carrier (ethanol). Two-tailed Student’s t test: P > 0.05.
(TIF)
ITPi driven by an alternative RNAi strain (VDRC #43848) with a differential target region recapitulates the decrease in the proportion of body water observed with the ITPi line VDRC#330029 (see Fig 1F). Two-tailed Student’s t–test: P < 0.05.
(TIF)
(A) Over-expression of ITP increases proportion of body water. Two-tailed Student’s t–test: P < 0.001. (B) ITPi decreases the proportion of body water. Two-tailed Student’s t–test: P < 0.01.
(TIF)
(A) Over-expression of ITP decreases desiccation resistance. Log-rank test: P < 0.001. Sample size: daGS>ITP off n = 60; daGS>ITP on n = 60. (B) ITP RNAi decreases survival during desiccation. Log-rank test: P < 0.05. Sample size: daGS>ITPi off n = 60; daGS>ITPi on n = 45.
(TIF)
(A) Schematic drawing of the expression pattern of the Impl2 driver (green), which covers the ipc-1 and ipc-2a cells, insulin-producing neurosecretory cells (IPCs), hugin cells (hc), cells of corpora cardiaca, some neurons in the abdominal ganglia, and the LBD neurons at the border of the dorsal abdominal segments A7/A8 next to the heart. Note that some of the Impl2>GFP cells in the brain and the abdominal ganglia are shown in partially reduced number for clarity and not to scale, including IPCs (around 14 cells), hugin cells (22), and adipokinetic hormone producing cells in corpora cardiaca (>8). (B) Schematic drawing of the ITP producing cells. ITP-expressing neurons that are covered by the Impl2 driver are depicted in yellow (ipc-1 cells, ipc-2a cells and LBD neurons). Cells that express ITP but not Impl2 are depicted in magenta (ipc-2, ipc-3 and ipc-4 brain neurons and the iag-cells in the abdominal ganglia).
(TIF)
(A) ITPi driven by the Impl2-based TARGET does not affect the proportion of body water. Two-tailed Student’s t–test: P > 0.05 for both comparisons with controls. (B) ITPi driven by the Impl2-based TARGET reduces survival under desiccation. Log-rank test: P < 0.001 for both comparisons with controls. Sample size: tub-GAL80ts; Impl2-GAL4 n = 67; ITPi n = 66; tub-GAL80ts; Impl2-GAL4>ITPi n = 63.
(TIF)
ITPi driven by an alternative RNAi strain (VDRC #43848) with a differential target region recapitulates the increase in food intake observed with the ITPi line VDRC#330029 (see Fig 4F). Two-tailed Student’s t–test: P < 0.05.
(TIF)
ITPi driven by an alternative RNAi strain (VDRC #43848) with a differential target region recapitulates the increase in the defecation rate observed with the ITPi line VDRC#330029 (see Fig 5C). Two-way ANOVA, ITP and time as fixed factors; effect of ITPi P < 0.01, effect of time: P < 0.001.
(TIF)
(A) Over-expression of ITP does not affect the lightness of feces. Two-tailed Student’s t–test: P > 0.05. (B) ITPi does not affect the lightness of feces. Two-tailed Student’s t–test: P > 0.05. In both (A) and (B), the measured lightness was normalized to the lightness of the controls.
(TIF)
ITPi driven by an alternative RNAi strain (VDRC #43848) with a differential target region recapitulates the decrease in the water intake observed with the ITPi line VDRC#330029 (see Fig 4F). Two-tailed Student’s t–test: P < 0.05.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
(XLSX)
Acknowledgments
We are very grateful to Dr. Peter Klepsatel for helpful comments and help with the data analyses. We thank to Véronique Monnier, Hervé Tricoire, Charlotte Helfrich-Förster, Hugo Stocker, Martin Schmid, Dan Liu, the Vienna Drosophila Resource Center and the Bloomington Drosophila Stock Center (NIH P40OD018537) for fly stocks. The title of this paper is inspired by Vincent Dethier’s “The Hungry Fly”, a great book on the feeding behavior of the blowfly [59].
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from the Swedish Research Council (Vetenskapsrådet; 2015-04626; https://www.vr.se/english.html) and Carl Trygger’s Foundation (http://www.carltryggersstiftelse.se), both to DRN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jourjine N. Hunger and thirst interact to regulate ingestive behavior in flies and mammals. Bioessays. 2017;39(5). 10.1002/bies.201600261 [DOI] [PubMed] [Google Scholar]
- 2.Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6(4):257–66. 10.1016/j.cmet.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das R, Dobens LL. Conservation of gene and tissue networks regulating insulin signalling in flies and vertebrates. Biochem Soc Trans. 2015;43(5):1057–62. 10.1042/BST20150078 [DOI] [PubMed] [Google Scholar]
- 4.Owusu-Ansah E, Perrimon N. Modeling metabolic homeostasis and nutrient sensing in Drosophila: implications for aging and metabolic diseases. Dis Model Mech. 2014;7(3):343–50. 10.1242/dmm.012989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padmanabha D, Baker KD. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrin Met. 2014;25(10):518–27. 10.1016/j.tem.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 6.Beyenbach KW. The plasticity of extracellular fluid homeostasis in insects. J Exp Biol. 2016;219(Pt 17):2596–607. 10.1242/jeb.129650 [DOI] [PubMed] [Google Scholar]
- 7.Gullan PSC P. J. The Insects: An Outline of Entomology, 5th edition. John Wiley & Sons, 2014. 2014. [Google Scholar]
- 8.Coast GM. Neuroendocrine control of ionic homeostasis in blood-sucking insects. J Exp Biol. 2009;212(Pt 3):378–86. 10.1242/jeb.024109 [DOI] [PubMed] [Google Scholar]
- 9.Gäde G. Regulation of intermediary metabolism and water balance of insects by neuropeptides. Annu Rev Entomol. 2004;49:93–113. 10.1146/annurev.ento.49.061802.123354 [DOI] [PubMed] [Google Scholar]
- 10.Coast G. The endocrine control of salt balance in insects. Gen Comp Endocrinol. 2007;152(2–3):332–8. 10.1016/j.ygcen.2007.02.018 [DOI] [PubMed] [Google Scholar]
- 11.Coast GM, Garside CS. Neuropeptide control of fluid balance in insects. Ann N Y Acad Sci. 2005;1040:1–8. 10.1196/annals.1327.001 [DOI] [PubMed] [Google Scholar]
- 12.Zandawala M, Marley R, Davies SA, Nässel DR. Characterization of a set of abdominal neuroendocrine cells that regulate stress physiology using colocalized diuretic peptides in Drosophila. Cell Mol Life Sci. 2018;75(6):1099–115. 10.1007/s00018-017-2682-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terhzaz S, Teets NM, Cabrero P, Henderson L, Ritchie MG, Nachman RJ, et al. Insect capa neuropeptides impact desiccation and cold tolerance. Proc Natl Acad Sci USA. 2015;112(9):2882–7. 10.1073/pnas.1501518112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannell E, Dornan AJ, Halberg KA, Terhzaz S, Dow JAT, Davies SA. The corticotropin-releasing factor-like diuretic hormone 44 (DH44) and kinin neuropeptides modulate desiccation and starvation tolerance in Drosophila melanogaster. Peptides. 2016;80:96–107. 10.1016/j.peptides.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465(7294):91–5. 10.1038/nature09011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen ZJ, Wang QX, Wang ZR. The Amiloride-Sensitive Epithelial Na+ Channel PPK28 Is Essential for Drosophila Gustatory Water Reception. J Neurosci. 2010;30(18):6247–52. 10.1523/JNEUROSCI.0627-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji F, Zhu Y. A novel assay reveals hygrotactic behavior in Drosophila. PLoS One. 2015;10(3):e0119162 10.1371/journal.pone.0119162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enjin A, Zaharieva EE, Frank DD, Mansourian S, Suh GS, Gallio M, et al. Humidity Sensing in Drosophila. Curr Biol. 2016;26(10):1352–8. 10.1016/j.cub.2016.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau MT, Lin YQ, Kisling S, Cotterell J, Wilson YA, Wang QP, et al. A simple high throughput assay to evaluate water consumption in the fruit fly. Sci Rep. 2017;7(1):16786 10.1038/s41598-017-16849-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanson BG, Yap S, Taylor PW. Geometry of compensatory feeding and water consumption in Drosophila melanogaster. J Exp Biol. 2012;215(Pt 5):766–73. 10.1242/jeb.066860 [DOI] [PubMed] [Google Scholar]
- 21.Ja WW, Carvalho GB, Zid BM, Mak EM, Brummel T, Benzer S. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc Natl Acad Sci USA. 2009;106(44):18633–7. 10.1073/pnas.0908016106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jourjine N, Mullaney BC, Mann K, Scott K. Coupled Sensing of Hunger and Thirst Signals Balances Sugar and Water Consumption. Cell. 2016;166(4):855–66. 10.1016/j.cell.2016.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nässel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92(1):42–104. 10.1016/j.pneurobio.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 24.Schoofs L, De Loof A, Van Hiel MB. Neuropeptides as Regulators of Behavior in Insects. Ann Rev Entomol, Vol 62. 2017;62:35–52. 10.1146/annurev-ento-031616-035500 [DOI] [PubMed] [Google Scholar]
- 25.Itskov PM, Ribeiro C. The dilemmas of the gourmet fly: the molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front Neurosci-Switz. 2013;7 10.3389/fnins.2013.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melcher C, Bader R, Pankratz MJ. Amino acids, taste circuits, and feeding behavior in Drosophila: towards understanding the psychology of feeding in flies and man. J Endocrinol. 2007;192(3):467–72. 10.1677/JOE-06-0066 [DOI] [PubMed] [Google Scholar]
- 27.Pool AH, Scott K. Feeding regulation in Drosophila. Curr Opin Neurobiol. 2014;29:57–63. 10.1016/j.conb.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dircksen H. Insect ion transport peptides are derived from alternatively spliced genes and differentially expressed in the central and peripheral nervous system. J Exp Biol. 2009;212(Pt 3):401–12. 10.1242/jeb.026112 [DOI] [PubMed] [Google Scholar]
- 29.Dircksen H, Tesfai LK, Albus C, Nässel DR. Ion transport peptide splice forms in central and peripheral neurons throughout postembryogenesis of Drosophila melanogaster. J Comp Neurol. 2008;509(1):23–41. Epub 2008/04/18. 10.1002/cne.21715 [DOI] [PubMed] [Google Scholar]
- 30.Webster SG, Keller R, Dircksen H. The CHH-superfamily of multifunctional peptide hormones controlling crustacean metabolism, osmoregulation, moulting, and reproduction. Gen Comp Endocrinol. 2012;175(2):217–33. 10.1016/j.ygcen.2011.11.035 [DOI] [PubMed] [Google Scholar]
- 31.Audsley N, Mcintosh C, Phillips JE. Actions of Ion-transport peptide from locust corpus cardiacum on several hindgut transport processes. J Exp Biol. 1992;173:275–88 [DOI] [PubMed] [Google Scholar]
- 32.Phillips JE, Wiens C, Audsley N, Jeffs L, Bilgen T, Meredith J. Nature and control of chloride transport in insect absorptive epithelia. J Exp Zool. 1996;275(4):292–9. [DOI] [PubMed] [Google Scholar]
- 33.Hermann-Luibl C, Yoshii T, Senthilan PR, Dircksen H, Helfrich-Förster C. The ion transport peptide is a new functional clock neuropeptide in the fruit fly Drosophila melanogaster. J Neurosci. 2014;34(29):9522–36. 10.1523/JNEUROSCI.0111-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung JS, Dircksen H, Webster SG. A remarkable, precisely timed release of hyperglycemic hormone from endocrine cells in the gut is associated with ecdysis in the crab Carcinus maenas. Proc Natl Acad Sci USA. 1999;96(23):13103–7. 10.1073/pnas.96.23.13103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park Y, Kim, H., Li, D., Adams, M. A Novel function of ion transport peptide in Drosophila ecdysis. Program and Abstracts 45th Annual Drosophila Research Conference, Washington, DC 2004;Abstract 774C (Flybase ID: FBrf0174159).
- 36.Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci USA. 2001;98(22):12596–601. 10.1073/pnas.221303298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98(22):12602–7. 10.1073/pnas.221303998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tricoire H, Battisti V, Trannoy S, Lasbleiz C, Pret AM, Monnier V. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech Ageing Dev. 2009;130(8):547–52. 10.1016/j.mad.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 39.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302(5651):1765–8. 10.1126/science.1089035 [DOI] [PubMed] [Google Scholar]
- 40.Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007;104(20):8253–6. 10.1073/pnas.0702726104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gáliková M, Klepsatel P, Xu YJ, Kühnlein RP. The obesity-related adipokinetic hormone controls feeding and expression of neuropeptide regulators of Drosophila metabolism. Eur J Lipid Sci Tech. 2017;119(3). 10.1002/ejlt.201600138 [DOI] [Google Scholar]
- 42.Danziger J, Zeidel ML. Osmotic homeostasis. Clin J Am Soc Nephrol. 2015;10(5):852–62. 10.2215/CJN.10741013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verbalis JG. Disorders of water metabolism: diabetes insipidus and the syndrome of inappropriate antidiuretic hormone secretion. Handb Clin Neurol. 2014;124:37–52. 10.1016/B978-0-444-59602-4.00003-4 [DOI] [PubMed] [Google Scholar]
- 44.Arima H, Kondo K, Kakiya S, Nagasaki H, Yokoi H, Yambe Y, et al. Rapid and sensitive vasopressin heteronuclear RNA responses to changes in plasma osmolality. J Neuroendocrinol. 1999;11(5):337–41. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi M, Arima H, Goto M, Banno R, Watanabe M, Sato I, et al. Vasopressin gene transcription increases in response to decreases in plasma volume, but not to increases in plasma osmolality, in chronically dehydrated rats. Am J Physiol Endocrinol Metab. 2006;290(2):E213–7. 10.1152/ajpendo.00158.2005 [DOI] [PubMed] [Google Scholar]
- 46.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78(3):583–686. 10.1152/physrev.1998.78.3.583 [DOI] [PubMed] [Google Scholar]
- 47.Baylis PH, Thompson CJ. Osmoregulation of vasopressin secretion and thirst in health and disease. Clin Endocrinol. 1988;29(5):549–76. 10.1111/j.1365-2265.1988.tb03704.x PubMed PMID: WOS:A1988Q717500010. [DOI] [PubMed] [Google Scholar]
- 48.Grandjean AC, Grandjean NR. Dehydration and cognitive performance. J Am Coll Nutr. 2007;26(5 Suppl):549S–54S [DOI] [PubMed] [Google Scholar]
- 49.McGuire MD, Heung M. Fluid as a Drug: Balancing Resuscitation and Fluid Overload in the Intensive Care Setting. Adv Chronic Kidney D. 2016;23(3):152–9. 10.1053/j.ackd.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 50.Lu HA. Diabetes Insipidus. Adv Exp Med Biol. 2017;969:213–25. 10.1007/978-94-024-1057-0_14 [DOI] [PubMed] [Google Scholar]
- 51.Davies SA, Cabrero P, Povsic M, Johnston NR, Terhzaz S, Dow JA. Signaling by Drosophila capa neuropeptides. Gen Comp Endocrinol. 2013;188:60–6. 10.1016/j.ygcen.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 52.Coast GM, Webster SG, Schegg KM, Tobe SS, Schooley DA. The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J Exp Biol. 2001;204(10):1795–804 [DOI] [PubMed] [Google Scholar]
- 53.Phillips JE. Excretion in insects: function of gut and rectum in concentrating and diluting the urine. Fed Proc. 1977;36(11):2480–6 [PubMed] [Google Scholar]
- 54.Begum K, Li B, Beeman RW, Park Y. Functions of ion transport peptide and ion transport peptide-like in the red flour beetle Tribolium castaneum. Insect Biochem Mol Biol. 2009;39(10):717–25. 10.1016/j.ibmb.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 55.Zimmerman CA, Leib DE, Knight ZA. Neural circuits underlying thirst and fluid homeostasis. Nat Rev Neurosci. 2017;18(8):459–69. 10.1038/nrn.2017.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hewes RS, Taghert PH. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome research. 2001;11(6):1126–42 10.1101/gr.169901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wayland MT, Defaye A, Rocha J, Jayaram SA, Royet J, Miguel-Aliaga I, et al. Spotting the differences: probing host/microbiota interactions with a dedicated software tool for the analysis of faecal outputs in Drosophila. J Insect Physiol. 2014;69:126–35. 10.1016/j.jinsphys.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica. 2001;4(1). http://palaeo-electronica.org/2001_1/past/issue1_01.htm [Google Scholar]
- 59.Dethier VG. The Hungry Fly A Physiological Study of the Behavior Associated with Feeding. Harvard University Press, 1974 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Water content of the w1118 strain reared for one week on food enriched with 200 μM RU-486 does not differ from the controls reared with the carrier (ethanol). Two-tailed Student’s t test: P > 0.05.
(TIF)
ITPi driven by an alternative RNAi strain (VDRC #43848) with a differential target region recapitulates the decrease in the proportion of body water observed with the ITPi line VDRC#330029 (see Fig 1F). Two-tailed Student’s t–test: P < 0.05.
(TIF)
(A) Over-expression of ITP increases proportion of body water. Two-tailed Student’s t–test: P < 0.001. (B) ITPi decreases the proportion of body water. Two-tailed Student’s t–test: P < 0.01.
(TIF)
(A) Over-expression of ITP decreases desiccation resistance. Log-rank test: P < 0.001. Sample size: daGS>ITP off n = 60; daGS>ITP on n = 60. (B) ITP RNAi decreases survival during desiccation. Log-rank test: P < 0.05. Sample size: daGS>ITPi off n = 60; daGS>ITPi on n = 45.
(TIF)
(A) Schematic drawing of the expression pattern of the Impl2 driver (green), which covers the ipc-1 and ipc-2a cells, insulin-producing neurosecretory cells (IPCs), hugin cells (hc), cells of corpora cardiaca, some neurons in the abdominal ganglia, and the LBD neurons at the border of the dorsal abdominal segments A7/A8 next to the heart. Note that some of the Impl2>GFP cells in the brain and the abdominal ganglia are shown in partially reduced number for clarity and not to scale, including IPCs (around 14 cells), hugin cells (22), and adipokinetic hormone producing cells in corpora cardiaca (>8). (B) Schematic drawing of the ITP producing cells. ITP-expressing neurons that are covered by the Impl2 driver are depicted in yellow (ipc-1 cells, ipc-2a cells and LBD neurons). Cells that express ITP but not Impl2 are depicted in magenta (ipc-2, ipc-3 and ipc-4 brain neurons and the iag-cells in the abdominal ganglia).
(TIF)
(A) ITPi driven by the Impl2-based TARGET does not affect the proportion of body water. Two-tailed Student’s t–test: P > 0.05 for both comparisons with controls. (B) ITPi driven by the Impl2-based TARGET reduces survival under desiccation. Log-rank test: P < 0.001 for both comparisons with controls. Sample size: tub-GAL80ts; Impl2-GAL4 n = 67; ITPi n = 66; tub-GAL80ts; Impl2-GAL4>ITPi n = 63.
(TIF)
ITPi driven by an alternative RNAi strain (VDRC #43848) with a differential target region recapitulates the increase in food intake observed with the ITPi line VDRC#330029 (see Fig 4F). Two-tailed Student’s t–test: P < 0.05.
(TIF)
ITPi driven by an alternative RNAi strain (VDRC #43848) with a differential target region recapitulates the increase in the defecation rate observed with the ITPi line VDRC#330029 (see Fig 5C). Two-way ANOVA, ITP and time as fixed factors; effect of ITPi P < 0.01, effect of time: P < 0.001.
(TIF)
(A) Over-expression of ITP does not affect the lightness of feces. Two-tailed Student’s t–test: P > 0.05. (B) ITPi does not affect the lightness of feces. Two-tailed Student’s t–test: P > 0.05. In both (A) and (B), the measured lightness was normalized to the lightness of the controls.
(TIF)
ITPi driven by an alternative RNAi strain (VDRC #43848) with a differential target region recapitulates the decrease in the water intake observed with the ITPi line VDRC#330029 (see Fig 4F). Two-tailed Student’s t–test: P < 0.05.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.