Abstract
Predicting ecological responses to climate change requires an understanding of the mechanisms that influence species’ tolerances to temperature. Based on the idea that air and water breathing animals are differentially suited to life in either medium due to differences in their respiratory morphology, we examined the possibility that the thermal tolerances of co-existing intertidal pulmonate and patellogastropod limpets may differ in different breathing media. We tested this by determining each species’ median lethal temperature (LT50) and cardiac Arrhenius breakpoint temperature (ABT) as measures of upper thermal tolerance limits, in air and water. Although all these species can survive in air and water, we hypothesised that the pulmonate limpets, Siphonaria capensis and S. serrata, would have higher thermal limits than the patellogastropod limpets, Cellana capensis and Scutellastra granularis, in air and vice versa in water. The results did not support our hypotheses, since C. capensis had similar thermal tolerance limits to the pulmonate limpets in air and the pulmonate limpets had thermal tolerance limits similar to or higher than S. granularis in water. Thus, considering pulmonate and patellid limpets as groups, we found no differences in their collective upper thermal tolerance limits in either medium. We conclude that differences between these two limpet groups in their respiratory morphology do not influence thermal tolerance, but that tolerances are species-specific.
Introduction
Climate change is a statistically significant change in the long-term state of the global climate, caused by a combination of natural and external anthropogenic activity [1]. One of the most important consequences of climate change is the perceived change in environmental temperatures, which are likely to have numerous consequences for ecosystem level processes [2–6]. It has, therefore, become important to improve our understanding of the impact that climate change may have on individual organisms and thus overall ecosystems [7]. The rocky shore and its inhabitants have frequently been used to examine the ecological effects that global changes in temperature may have [8–11] because of the extreme thermal conditions normally experienced across the shore [12–16].
Intertidal limpets are ecologically important due to their activity as grazers and their interactions with other intertidal organisms [17], while their ability to tolerate the many environmental challenges of the intertidal zone has made them important physiological study subjects [18–21]. Like other intertidal animals, limpets contend with continuously alternating breathing media as the tide ebbs and floods. Linked to this is the evolution of different respiratory structures, for example, the pallial gills of patellogastropod limpets and, the mantle cavity lungs and secondary gills of pulmonate limpets [17, 22, 23]. Structural differences between the respiratory anatomies of these two groups’ can influence their ecology [24–26]. While the pallial gills can be used during aerial respiration, they are primarily suited to aquatic respiration and the pulmonate limpets’ secondary gills are not as adept as pallial gills at aiding aquatic respiration [19, 25, 27]. Therefore, while most patellogastropod limpets are suited to an aquatic lifestyle the pulmonate lung is an advantage to life in air [19, 25, 28, 29]. In fact, Marshall and McQuaid [26] found that the pulmonate system may promote a higher thermal tolerance than that of patellogastropods in air. On this basis, we hypothesized that respiratory morphology influences the metabolism and therefore, thermal tolerance of organisms.
Several measures of thermal tolerance have previously been used to help gain an understanding of how organisms respond to thermal variation in their immediate environment. These include whole organism lethal limit measures, which involve determining an organisms’ critical temperatures and LT50 values [30–33] and sub-lethal limit measures such as the detection of heat shock proteins and the measurement of cardiac ABTs [34–37]. In this paper, the response to temperature variation of the patellogastropods, Scutellastra granularis and Cellana capensis, and the pulmonates, Siphonaria capensis and S. serrata, was compared. The upper thermal limits were estimated by measuring LT50 values as a direct measure of mortality rates and the ABT for heart rate under increasing temperature [17, 18]. These species were chosen as model organisms because of their overlapping geographical (S1 Fig) and vertical distributions (S2 Fig) across the shore [38–42]. While both pulmonate species are more common in intertidal zones above the low mid-shore, the patellids are ubiquitous from the subtidal fringe to the high mid-shore [38, 39].
Given the differences in their respiratory anatomy, we postulated that these two groups of limpets would exhibit different thermal tolerances in air and water. Recognising that neither group is exclusively air or water breathing, we hypothesised that: 1. the pulmonate limpets would have higher thermal limits than the patellogastropod limpets in air, and vice versa in water; 2. The thermal limits of pulmonate limpets would be greater in air than water, while patellogastropod limpets would show the reverse, with higher thermal limits in water than air.
Materials and methods
Ethics statement
Only invertebrate marine molluscs (limpets) were used. All work was conducted under the research permits (RES2014/12 and RES2015/04) for collection and practical experiments issued by the Department of Agriculture, forestry and fishery of the Republic of South Africa.
Sample sites and collection
A total of 400 individuals of each species were collected from high mid-shore rocks on the south-east coast of South Africa at Kenton-on-Sea (33.68° S, 26.67° E), Port Alfred (33.59° S, 26.89° E) and Cintsa West (32.82° S, 28.12° E), during low tide in austral winter (June–July) in 2014 and 2015. Similar sized individuals (20–30 mm) of each species were collected during low tide by quickly sliding a coarse scalpel between the muscular foot and the substratum. Any limpets not detached at the first attempt were left, to avoid using animals that may have been injured. Specimens were transported to the laboratory within 3 hours in small containers, moistened with sea water and kept inside an insulated box. In the laboratory, the specimens were housed in a 20L glass tank filled with 5L of aerated seawater at 22°C for a minimum of 24h and a maximum of 48h before use. Before experimentation, limpets were submerged in 500mL containers filled with constantly aerated seawater for 1 hour to ensure they were fully hydrated.
Determining the median lethal temperature (LT50)
Thermal limits were determined first by finding the LT50 values, using a protocol based on Clarke et al. [43]. LT50 measurements were carried out on 150 individuals per species in each medium, over three trials (50 individuals/trial) to generate a mean LT50 value. During the experiment, limpets were housed in 500mL containers (10 individuals/container) filled with natural aerated seawater to simulate aquatic conditions or dampened with seawater to simulate aerial exposure [44]. Temperatures within the containers were controlled by submerging them in a Grant programmable water bath (GP 200, Grant, Germany). A Fluke 54II thermometer (Fluke cooperation, USA) fitted with a T-type thermocouple (Fluke cooperation and Cromega) was used to measure temperature at the bottom of the containers, which were recorded with a PowerLab recording system.
A wide range of heating rates (10°C/min—1°C/3.5 days) have been used to determine thermal limits in past studies [45]. Similar studies on various intertidal organisms, including limpets, have generally used heating rates between 0.1°C.min-1 and 0.3°C.min-1 [35, 36, 46, 47]. In this study, temperature was programmed to increase at 0.2–0.4°C.min-1 for the LT50 measurements (S1 Table) using a water bath, following the ramping protocol described in S3 Fig.
To avoid influencing the results through repeated thermal shock [48, 49] a new batch of specimens was used for each temperature interval. After each test run, limpet mortality was determined after a 24-hour recovery period in the holding tank (S3 Fig) and limpet shell lengths were measured to the nearest 0.02mm using Vernier callipers. Mortality was assessed by probing the limpets for tactile responsiveness using a blunt probe. Limpets were classified as dead if they showed no response to having the foot muscle or the edge of the mantle probed.
Heart rate measurements
Heart rate measurements were carried out on 20 individuals per species in each medium, housed in a total of 56 500mL containers with 3 individuals/container in 48 of the containers, and 2 individuals/container in the rest. To simulate aquatic conditions, the containers were filled with natural seawater aerated using air stones. To determine thermal limits in air, the containers were dampened with seawater to maintain relatively high levels of humidity during heat exposure [44].
Heart rate was recorded using non-invasive plethysmography [50] by attaching optoelectronic (infrared) sensors (Vishay semiconductors, V69 CNY70 732/735, Germany) to the shells of each limpet near the heart using Pattex super glue (Henkel (Pty) Ltd, South Africa). These sensors produced signals which were amplified by a custom-built preamplifier, after which Triangular-Bartlett smoothing was used to produce an additional smooth trace on a separate channel. The signal was then filtered before being recorded as beats per minute on a computerised recording system (PowerLab/4SP and 430, Chart version 5 and 7, ADInstruments, Australia). The amplitude ranged between 40 and 100mV at a sampling rate of 40Hz. The specimens were exposed to a temperature increase of 30°C from 20–50°C at a rate of 0.25°C.min-1, using the Grant programmable water bath heating system whilst simultaneously recording heart rate. Prior to heat exposure, the animals were allowed to settle at 20°C in the water bath for 30 minutes. Limpet mortality and shell lengths were determined after each treatment, as described for the LT50 measurements.
Data and statistical analyses
One caveat here is that individuals in the same container during pre-treatment could be considered to be pseudoreplicates. However, species were interspersed during pre-treatments and monitoring of the water bath temperature showed no significant or systematic variation. Similarly, there was no evidence that the presence of conspecifics influenced individual thermal physiology.
The LT50 values for each trial were generated from limpet mortality at each temperature interval using probit analysis [51, 52]. Thereafter, mean LT50 values were compared between media and respiratory modes using a nested ANOVA with species nested in respiratory mode.
Cardiac thermal response curves were plotted and checked manually to control for artefacts and anomalous trends, for example due to movement of the animals. This meant that the final ABT and heart rate analyses were carried out on fewer than 40 individuals from each species (S2 Table). The temperature ranges used to determine ABT were 25–45°C for all species in air (except Scutellastra granularis; 25–40°C) and 25–40°C for all species in water (except C. capensis; 25–45°C). Outside of these temperature ranges the data points were distributed haphazardly.
Arrhenius plots were then generated from these thermal response curves using Eq 1 to analyse the effect of temperature on heart rate:
| (1) |
In Eq 1, HR represents the heart rate (bpm), a is the normalization constant, Ea is the activation energy (J.mol-1), R is the ideal gas constant (J.K-1.mol-1) and T is the absolute temperature (K). Piecewise linear regression was used to calculate the breakpoints using transformed heart rate (Ln (HR)) and temperature (1/T) data. These breakpoints were then converted back into degrees Celsius, to present them as ABTs (°C) before conducting further analyses. The ABT values were then compared using a nested ANOVA as described for the LT50 values above.
Once the ABT values were determined, the increase in heart rate as a function of temperature was compared among treatments, by considering only the data points below the ABTs. The slopes (Ea/R) from the resultant, linear Arrhenius plots were compared using a nested ANOVA as described for the LT50 and ABT analyses above.
Tukey HSD analyses were used post hoc to determine where significant differences lay for all the ANOVA models.All statistical analyses were performed with Statistica 13.
Results
Differences in thermal limits between species and/or media
LT50
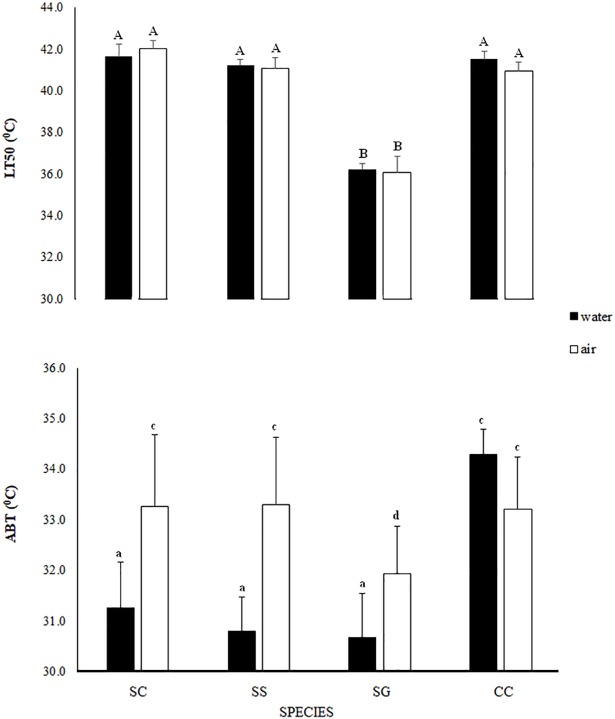
The nested ANOVA comparing LT50 values (Fig 1; S3 Table), indicated significant effects of Respiratory Mode and of Species nested in Respiratory Mode (p < 0.0001 in both cases), with no effect of Medium or its interaction with Respiratory Mode. In both media, Scutellastra granularis had significantly lower values than the other three species, whose LT50 values were the same, and there were no significant differences between LT50 values in air and water for any species.
Fig 1. Comparisons of mean (+ S.D) LT50 (°C) and ABT (°C) values made among the limpet species in both media, and between media for each species.
Limpet species and corresponding respiratory mode are listed as: SC–Siphonaria capensis, Lungs; SG–Scutellastra granularis, Gills; CC–Cellana capensis, Gills; SS–Siphonaria serrata, Lungs. Homogenous groups are shown in upper case for LT50 and lower case for ABT.
The significant effect of Respiratory Mode was not expected on the basis of the raw data and presumably reflects the low LT50 of Scutellastra granularis, which decreases the mean value for the patellids. This effect is probably exacerbated by the small sample size (n = 3) used in the LT50 analysis, particularly compared to the ABT (n ~ 10) analysis.
ABT
The nested ANOVA of ABT data (S4 Table) indicated significant effects of Medium, Species nested in Respiratory Mode and the interaction between Medium and Respiratory Mode (p < 0.0001 in all cases). Other than C. capensis, the limpets had significantly higher ABT values in air than water (Fig 1). Although the effect of Respiratory mode was non-significant in the nested Analysis (p = 0.34), there was a significant effect of Species. Scutellastra granularis exhibited a significantly lower ABT in air than the other species, while C. capensis had a significantly higher ABT in water than the others (p < 0.05 in both cases).
Relationship between heart rate and temperature
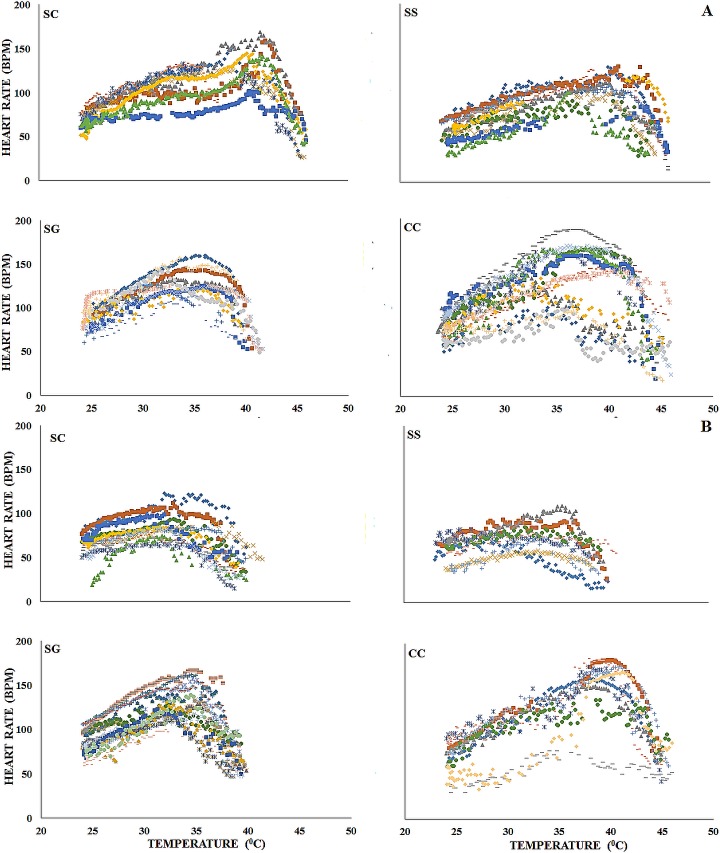
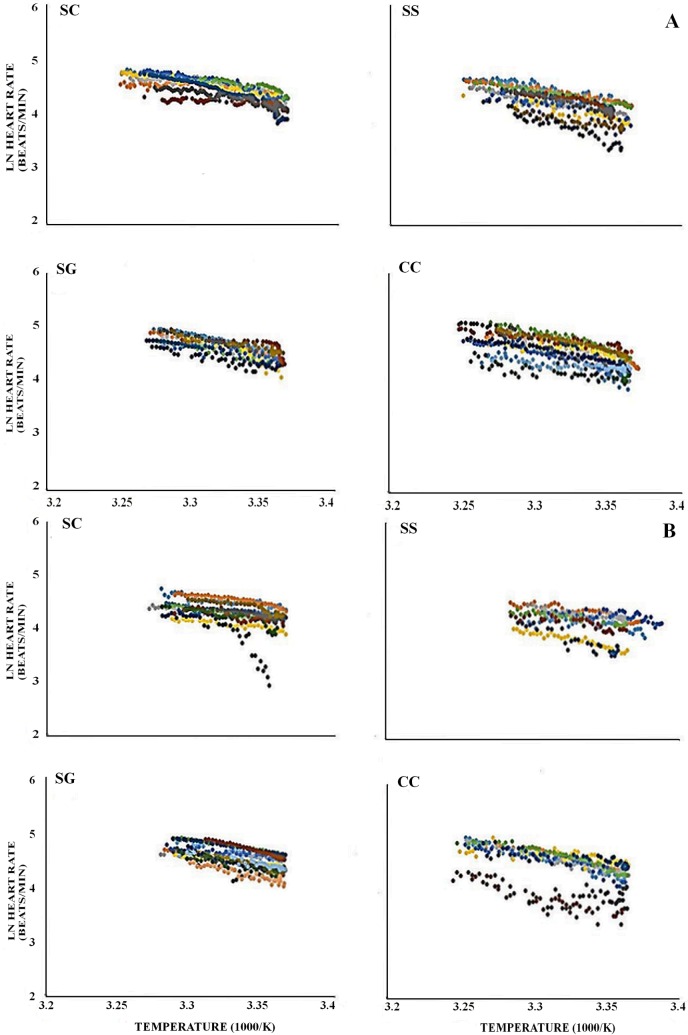
The cardiac thermal response curves (Fig 2) and Arrhenius plots (Fig 3) displayed a high degree of inter-individual variability within each species in both media.
Fig 2.
The cardiac thermal response curves of the study species in air (A) and water (B). SC–Siphonaria capensis; SG–Scutellastra granularis; SS–Siphonaria serrata; CC—Cellana capensis. Each colour represents a different individual.
Fig 3.
Arrhenius plots of the study species in water (A) and air (B). SC–Siphonaria capensis; SG–Scutellastra granularis; SS–Siphonaria serrata; CC—Cellana capensis. Each colour represents a different individual.
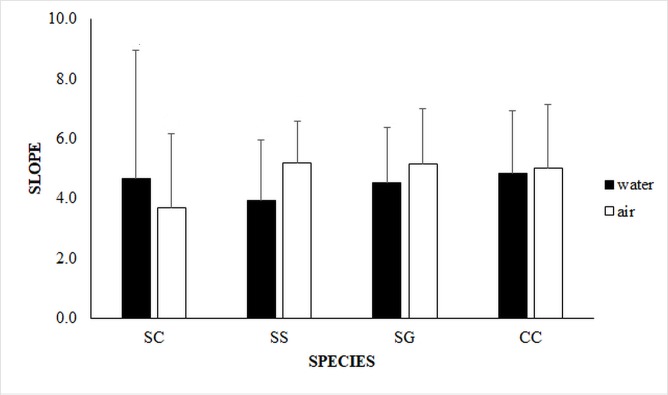
The nested ANOVA comparing slopes (S5 Table) revealed insignificant effects for all factors (Fig 4).
Fig 4. Comparisons of mean (+ S.D) slope values made among the limpet species in both media, and between media for each species.
Limpet species and corresponding respiratory mode are listed as: SC–Siphonaria capensis, Lungs; SG–Scutellastra granularis, Gills; CC–Cellana capensis, Gills; SS–Siphonaria serrata, Lungs.
Discussion
We hypothesised that differences in the respiratory morphology of the two limpet groups would be reflected in differences in their responses to increasing temperatures. While the nested analysis for LT50 did show a significant effect of respiratory mode, the results from both the LT50 and ABT analyses suggested species-specific effects, rather than an over-riding influence of respiratory morphology. A lower aerial temperature tolerance and aquatic LT50 was measured for Scutellastra granularis compared to the other three species (which did not differ). Similarly, C. capensis had a higher ABT in water than the other three species whose aquatic ABTs were similar. In addition, there was no obvious effect of medium on species-specific LT50 values, while ABT values were generally higher in air except in the case of C. capensis. There were no significant effects of either respiratory mode or medium on the slopes of the Arrhenius plots.
Aerial exposure
All experiments performed in air showed important differences between species. Scutellastra granularis had the lowest ABT and LT50. Conversely, the response of the other patellogastropod was not significantly different to those of the two pulmonates.
The lungs of the Siphonariid limpets were expected to give them a better breathing capability than patellids in air [17, 22, 53, 54, 55], making it easier for them to meet their mitochondrial O2 demands. This in turn should allow for the efficient use of energy stores, a delay in the onset of anaerobic metabolism and relatively high upper thermal limits [25, 26, 56, 57].
However, our results indicate that some patellogastropods, like C. capensis, can survive similar levels of thermal stress to pulmonate limpets during low tide. This may be explained by the fact that some high shore “gill-bearing” limpets can respire efficiently in air despite not having a lung [27, 58–60]. This helps reduce the accumulation of anaerobic by products and water loss, allowing for an increase in aerial thermal tolerance [25, 61, 62]. For example, unexpectedly high thermal limits in air have been measured for the high shore species Cellana toreuma (LT50 = 41.29–43.36°C) [63] and C. grata (ABT = 47°C) [35].
Immersion
Increasing water temperature affected the limpet species differently. The two pulmonates had similar ABT and LT50 values, while the two patellogastropods reacted differently. Scutellastra granularis had lower thermal limits compared to Cellana capensis, which had an exceptionally high ABT.
Pulmonate limpet accessory gills evolved secondarily after loss of the ctenidium, and are not primarily adapted to aquatic respiration, in contrast to the pallial gills of patellogastropod limpets [22, 28]. Despite this, the pulmonate limpets had surprisingly high thermal limits in water, probably due to the efficient use of their accessory gills as shown previously by Koopman et al. [64]. These authors found that, when submerged the freshwater pulmonate limpets Physa fontinalis and P. acuta had higher upper thermal limits (CTmax) than the gill-bearing caenogastropods Bithynia tentaculata and Potamopyrgus antipodarum.
Air vs water
When comparing thermal performance in air and water, there were strong similarities between the two limpet groups. For both groups, there was no significant effect of medium when performance was measured as LT50. In the case of the pulmonates, both species showed significantly higher ABT values in air. Among the patellogastropods, the same was true for Scutellastra granularis, but not C. capensis. Regarding the slopes from the Arrhenius plots, the non-significant effects were probably related to the high inter-individual variability (Fig 4) in the species sensitivity to increasing temperature. The slope of the heart rate response represents thermal metabolic sensitivity [17, 35, 37, 55, 65], and this has previously been shown to be highly variable inter-individually [66].
Past studies have generally focused on the influence of geographic [37, 67] or vertical distribution [35, 36, 68] on the thermal tolerances of intertidal organisms. Most such studies examined thermal tolerance in submersed animals, with only a few examining aerial thermal tolerances or comparing tolerances in different media [36, 62, 69]. Even fewer studies have compared the aerial thermal limits of air and water breathing gastropod molluscs [but see 26, 70, 71].
Aquatic animals adapted to aerial respiration (such as pulmonate limpets) should be able to respire more efficiently in air where oxygen concentrations are higher, but the emersion period is also characterized by extreme temperature and desiccation stress involving important energetic costs [25, 72–75]. Dye [76] found that Siphonaria capensis and S. concinna had higher respiration rates in air than water, indicating that it would be easier to avoid anaerobic metabolism, which should translate into higher aerial thermal limits [77–79].
Because patellogastropods respire primarily through their pallial gills, they were expected to have lower thermal limits in air. In the case of LT50, there was no difference between media for either Scutellastra granularis or Cellana capensis, while the ABT for S. granularis was unexpectedly higher in air. This has also been shown for the mid-to-high shore patellogastropod limpet Lottia digitalis, which had a higher thermal limit (final cardiac breakpoint temperature) in air [80], and in both cases, this presumably reflects the efficiency of O2 uptake by the highly vascularised mantle cavity of many high shore patellogastropod limpets.
Conclusions
Although the results differed slightly between LT50 and ABT, they provide no clear indication that respiratory morphology is important in determining either aerial or aquatic thermal limits in the study species, indicating that other factors play important roles. Within species, reproductive and nutritional state can influence susceptibility to high temperatures, and probably contributed to the high degree of individual variability we observed in the relationship between the limpet heart rates and temperature [81–85]. Recent thermal history, including the influence of microhabitat use, is also likely to influence thermal limits [16, 30, 74, 86–88]. In this regard, body temperature estimates generated from the range of microhabitats occupied by each species would have benefited this study. Nevertheless, our overall conclusion is that the data do not support the hypothesis that the respiratory morphology of these species has an overriding influence on the interaction of thermal tolerance and respiratory medium.
Supporting information
SC–Siphonaria capensis; SG–Scutellastra granularis; SS–Siphonaria serrata; CC—Cellana capensis.
(JPG)
Vertical zonation patterns were derived from Allanson [38], Branch [39, 41] and, Chambers and McQuaid [42]. Limpet species and intertidal zones are listed as: SC–Siphonaria capensis; SG–Scutellastra granularis; SS–Siphonaria serrata; CC—Cellana capensis. LZ–Littorina Zone; HMZ–High Mid-Shore Zone; LMZ–Low Mid-Shore Zone; SFZ–Subtidal Fringe Zone.
(PNG)
Mortality (nr of individuals) was determined at the 5 and 29 hour marks.
(TIF)
(DOCX)
SC–Siphonaria capensis; SG–Scutellastra granularis; SS–Siphonaria serrata; CC—Cellana capensis.
(DOCX)
Medium, Respiration mode (R. Mode) and Species nested in Respiration mode (R. Mode) were considered as fixed factors.
(DOCX)
Medium, Respiration mode (R. Mode) and Species nested in Respiration mode (R. Mode) were considered as fixed factors.
(DOCX)
Medium, Respiration mode (R. Mode) and Species nested in Respiration mode (R. Mode) were considered as fixed factors.
(DOCX)
Acknowledgments
We would like to thank Prof Anthony Sullivan for his help making the heart rate probes. Also, thank you to Aldwyn Ndhlovu, Zolile Maseko and Jaqueline Trassierra for their help in the field and laboratory. This work is based upon research supported by the National Research Foundation of South Africa (Grant number 64801).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is based upon research supported by the Rhodes University Research Committee Grant to Dr Morgana Tagliarolo: https://www.ru.ac.za/staffdevelopment/funding/researchcommitteegrant/; and the National Research Foundation of South Africa (grant number 64801 to SK): http://www.nrf.ac.za/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.IPCC, 2012: Changes in climate extremes and their impacts on the natural physical environment. Managing the risks of extreme events and disasters to advance climate change adaptation. [DOI] [PubMed]
- 2.Cossins AR, Bowler K. Temperature Biology of Animals London: Chapman and Hall; 1987. [Google Scholar]
- 3.Schmidt-Nielsen K. Animal physiology: adaptation and environment Cambridge: Cambridge University Press; 1997. [Google Scholar]
- 4.Perry AL, Low PJ, Ellis JR, Reynolds JD. Climate change and distribution shifts in marine fishes. Science. 2005; 308: 1912–1915. 10.1126/science.1111322 [DOI] [PubMed] [Google Scholar]
- 5.Grebmeier JM, Overland JE, Moore SE, Farley EV, Carmack EC, Cooper LW, et al. A major ecosystem shift in the northern Bering Sea. Science. 2006; 311(5766): 1461–1464. 10.1126/science.1121365 [DOI] [PubMed] [Google Scholar]
- 6.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006; 37: 637–669. [Google Scholar]
- 7.Pörtner HO, Farrell AP. Ecology: Physiology and climate change. Science. 2008; 322: 690–692. 10.1126/science.1163156 [DOI] [PubMed] [Google Scholar]
- 8.Helmuth B, Harley CDG, Halpin PM, O’Donnell M, Hofmann GE, Blanchette CA. Climate change and latitudinal patterns of intertidal thermal stress. Science. 2002; 298: 1015–1017. 10.1126/science.1076814 [DOI] [PubMed] [Google Scholar]
- 9.Helmuth B, Broitman BR, Blanchette CA, Gilman S, Halpin P, Harley CDG, et al. Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol Monograph. 2006; 76: 461–476. [Google Scholar]
- 10.Harley CDG. Climate change, keystone predation and biodiversity loss. Science. 2011; 334(6059): 1124–1127. 10.1126/science.1210199 [DOI] [PubMed] [Google Scholar]
- 11.Somero GN. The physiology of climate change: how potential for acclimatization and genetic adaptation will determine “winners” and “losers.” The J Exp Biol. 2010; 213: 912–920. 10.1242/jeb.037473 [DOI] [PubMed] [Google Scholar]
- 12.Tomanek L, Somero GN. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snail (Genus Tugela) from different thermal habitats: Implications for limits of thermotolerance and biogeography. J Exp Biol. 1999; 202: 2925–2936. [DOI] [PubMed] [Google Scholar]
- 13.Harley CDG, Helmuth BST. Local- and regional-scale effects of wave exposure, thermal stress, and absolute versus effective shore level on patterns of intertidal zonation. Limnol Oceanogr. 2003; 48: 1498–1508. [Google Scholar]
- 14.Little C, Williams GA, Trowbridge CD. The biology of rocky shores New York: Oxford University Press; 2009. [Google Scholar]
- 15.Judge ML, Botton ML, Hamilton MG. Physiological consequences of the supralittoral fringe: microhabitat temperature profiles and stress protein levels in the tropical periwinkle Cenchritis muricatus (Linneaus, 1758). Hydrobiologia. 2011; 675: 143–156. [Google Scholar]
- 16.Cartwright SR, Williams GA. Seasonal variation in utilization of biogenic microhabitats by littorinid snails on the tropical rocky shores. Mar Biol. 2012; 159: 2323–2332. 10.1007/s00227-012-2017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall DJ, McQuaid CD. Seasonal and diel variation of in situ heart rate of the intertidal limpet Siphonaria oculus Kr. (Pulmonata). J Exp Mar Bio Ecol. 1994; 179: 1–9. [Google Scholar]
- 18.Santini G, De Pirro M, Chelazzi G. In situ and laboratory assessment of heart rate in Mediterranean limpet using a non-invasive technique. Physiol Biochem Zool. 1999; 72: 198–204. 10.1086/316656 [DOI] [PubMed] [Google Scholar]
- 19.Branch GM. The biology of limpets: Physical factors, energy flow, and ecological interactions. Oceanogr Mar Biol Ann Rev. 1981; 19: 235–380. [Google Scholar]
- 20.Hawkins SJ, Hartnoll RG. Grazing of intertidal algae by marine invertebrates. Oceanogr Mar Biol Ann Rev. 1983; 21: 195–282. [Google Scholar]
- 21.Branch GM. Limpets: Their role in littoral and sublittoral community dynamics In: Moore PG, Seed R, editors. The Ecology of Rocky Coasts. London: Hodder and Stoughton; 1985. pp. 97–116. [Google Scholar]
- 22.Little C. Factors governing patterns of foraging activity in littoral marine herbivorous molluscs. J Molluscan Stud. 1989; 55: 273–284. [Google Scholar]
- 23.Hodgson AN. The biology of Siphonariid limpets (Gastropoda Pulmonata). Oceanogr Mar Biol Ann Rev. 1999; 37: 245–314. [Google Scholar]
- 24.O’Mahoney PM, Full RJ. Respiration of crabs in air and water. Comp Biochem Physiol A. 1984; 79(2): 275–82. [Google Scholar]
- 25.McMahon RF. Respiratory response to periodic emergence in intertidal molluscs. Am Zool. 1988; 28: 99–114. [Google Scholar]
- 26.Marshall DJ, McQuaid CD. Comparative aerial metabolism and water relations of the intertidal limpets Patella granularis L. (Mollusca: Prosobranchia) and Siphonaria oculus Kr. (Mollusca: Pulmonate). Physiol Zool. 1992; 65: 1040–1056. [Google Scholar]
- 27.Innes AJ, Marsden ID, Wong PPS. Bimodal respiration of intertidal pulmonates. Comp Biochem Physiol A Physiol. 1984; 77(3): 441–445. [Google Scholar]
- 28.Houlihan DF. Respiration in air and water of three mangrove snails. J Exp Mar Bio Ecol. 1979; 41: 143–161. [Google Scholar]
- 29.Yonge CM. The pallial organs in the Aspidobranch Gastropoda and their evolution throughout the Mollusca. Philos Trans R Soc Lon B Biol Sci. 1947; 232: 443–518. [DOI] [PubMed] [Google Scholar]
- 30.Evans RG. The lethal temperatures of some common British littoral molluscs. J Anim Ecol. 1948; 17: 165–173. [Google Scholar]
- 31.McMahon RF. Thermal tolerance, evaporative water loss, air-oxygen consumption and zonation of intertidal prosobranchs: a new synthesis. Hydrobiologia. 1990; 193: 241–260. [Google Scholar]
- 32.Davenport J, Davenport JL. Effect of shore height, wave exposure and geographical distance on thermal niche width of intertidal fauna. Mar Ecol Prog Ser. 2005; 292: 41–50. [Google Scholar]
- 33.Compton TJ, Rijkenberg MJA, Drent J, Piersma T. Thermal tolerances range and climate variability: a comparison between bivalves from different climates. J Exp Mar Bio Ecol. 2007; 352: 200–211. [Google Scholar]
- 34.Stillman JH, Somero GN. Adaptation to temperature stress and aerial exposure in congeneric species of intertidal porcelain crabs (genus Petrolisthes): correlation of physiology, biochemistry and morphology with vertical distribution. The J Exp Biol. 1996; 199: 1845–1855. [DOI] [PubMed] [Google Scholar]
- 35.Dong YW, Williams GA. Variation in cardiac performance and heat shock protein expression to thermal stress in two differently zoned limpets on a tropical shore. Mar Biol. 2011; 158: 1223–1232. [Google Scholar]
- 36.Tagliarolo M, McQuaid CD. Sub-lethal and sub-specific temperature effects are better predictors of mussel distribution than thermal tolerance. Mar Ecol Prog Ser. 2015; 535: 145–159. [Google Scholar]
- 37.Xing Q, Li Y, Guo H, Yu Q, Huang X, Wang S, et al. Cardiac performance: a thermal tolerance indicator in scallops. Mar Biol. 2016; 163(12): 244. [Google Scholar]
- 38.Allanson BR. On the systematics and distribution of the molluscan genus Siphonaria in South Africa. Hydrobiologia. 1958; 12(2–3): 149–80. [Google Scholar]
- 39.Branch GM. The ecology of Patella Linnaeus from the Cape Peninsula, South Africa. I. Zonation, movements and feeding. Afr Zool. 1972; 6(1): 1–38. [Google Scholar]
- 40.Branch GM, Cherry MI. Activity rhythms of the pulmonate limpet Siphonaria capensis as an adaptation to osmotic stress, predation and wave action. J Exp Mar Biol Ecol. 1985; 87(2): 153–68. [Google Scholar]
- 41.Branch GM. Mechanisms of reducing competition in limpets: migration, differentiation and territorial behaviour. J Anim Ecol. 1975; 44: 575–600. [Google Scholar]
- 42.Chambers RJ, McQuaid CD. A review of larval development in the intertidal limpet genus Siphonaria (Gastropoda: Pulmonata). J Molluscan Stud. 1994; 60: 415–423. [Google Scholar]
- 43.Clarke AP, Mill PJ, Grahame J. Biodiversity in Littorina species (Mollusca: Gastropoda): a physiological approach using heat-coma. Mar Biol. 2000; 137: 559–565. [Google Scholar]
- 44.Denny MW, Miller LP, Harley CD. Thermal stress on intertidal limpets: long-term hindcasts and lethal limits. J Exp Biol. 2006; 209(13): 2420–2431. [DOI] [PubMed] [Google Scholar]
- 45.Lutterschmidt WI, Hutchison VH. The critical thermal maximum: history and critique. Can J Zool. 1997; 75: 1561–1574. [Google Scholar]
- 46.Fangue NA, Osborne EJ, Todgham AE, Schulte PM. The onset temperature of the heat-shock response and whole-organism thermal tolerance are tightly correlated in both laboratory-acclimated and field-acclimatized tidepool sculpins (Oligocottus maculosus). Physiol Biochem Zool. 2011; 84: 341–352. 10.1086/660113 [DOI] [PubMed] [Google Scholar]
- 47.Logan CA, Kost LE, Somero GN. Latitudinal differences in Mytilus californianus thermal physiology. Mar Ecol Prog Ser. 2012; 450: 93−105. [Google Scholar]
- 48.Clarke AP, Mill PJ, Grahame J. The nature of heat coma in Littorina littorea (Mollusca: Gastropoda). Mar Biol. 2000; 137: 447–451. [Google Scholar]
- 49.Jones SJ, Mieszkowska N, Wethey DS. Linking thermal tolerances and biogeography: Mytilus edulis (L.) at its southern limit on the east coast of the United States. Biol Bull. 2009; 217: 73–85. 10.1086/BBLv217n1p73 [DOI] [PubMed] [Google Scholar]
- 50.Depledge MH, Andersen BB. A computer-aided physiological monitoring system for continuous, long-term recording of cardiac activity in selected invertebrates. Comp Biochem Physiol A. 1990; 96: 473–477. [Google Scholar]
- 51.Finney DJ, Stevens WL. 1948. A table for the calculation of working probits and weights in probit analysis. Biometrika. 1948; 35(1–2): 191–201. [PubMed] [Google Scholar]
- 52.Finney DJ. Probit Analysis Cambridge: Cambridge University Press; 1952. [Google Scholar]
- 53.Purchon RD. The biology of the Mollusca Weinheim: Pergamon Press; 1977. [Google Scholar]
- 54.Fretter V, Peake J. Pulmonates: Functional Anatomy and Physiology London: Academic Press; 1975. [Google Scholar]
- 55.Marshall DJ, McQuaid CD. Relationship between heart rate and oxygen consumption in the intertidal limpets Patella granularis and Siphonaria oculus. Comp Biochem Physiol A. 1992; 103: 297–300. [Google Scholar]
- 56.Pörtner HO, Zielinski S. Environmental constraints and the physiology of performance in squids. Afr J Mar Sci. 1998; 20: 207–221. [Google Scholar]
- 57.Peck LS, Pörtner HO, Hardewig I. Metabolic demand, oxygen supply, and critical temperatures in the Antarctic bivalve Laternula elliptica. Physiol Biochem Zool. 2002; 75: 123–133. 10.1086/340990 [DOI] [PubMed] [Google Scholar]
- 58.McMahon RF, Russell-Hunter WD. Temperature relations of aerial and aquatic respiration in six littoral snails in relation to their vertical zonation. Biol Bull. 1977; 152: 182–198. 10.2307/1540558 [DOI] [PubMed] [Google Scholar]
- 59.Branch GM, Newell RC. A comparative study of metabolic energy expenditure in the limpets Patella cochlear, P. oculus and P. granularis. Mar Biol. 1978; 49: 351–361. [Google Scholar]
- 60.Branch GM. Respiratory adaptations in the limpet Patella granatina: A comparison with other limpets. Comp Biochem Physiol A. 1979; 62: 641–647. [Google Scholar]
- 61.Deshpande RD. Observations on the anatomy and ecology of British trochids, electronic, scholarly article, PhD. Thesis, University of Reading. 1957. Available from: https://rdg.ent.sirsidynix.net.uk/client/en_GB/library/search/detailnonmodal/ent:$002f$002fSD_ILS$002f0$002fSD_ILS:834545/ada?qu=deshpande&lm=EXCL_LR2&rt=false%7C%7C%7CAUTHOR%7C%7C%7CAuthor
- 62.Huang X, Wang T, Ye Z, Han G, Dong Y. Temperature relations of aerial and aquatic physiological performance in a mid-intertidal limpet Cellana toreuma: Adaptation to rapid changes in thermal stress during emersion. Integr Zool. 2015; 10(1): 159–170. 10.1111/1749-4877.12107 [DOI] [PubMed] [Google Scholar]
- 63.Dong YW, Han GD, Ganmanee M, Wang J. Latitudinal variability of physiological responses to heat stress of the intertidal limpet Cellana toreuma along the Asian coast. Mar Ecol Prog Ser. 2015; 529: 107–119. [Google Scholar]
- 64.Koopman KR, Collas FP, Van der Velde G, Verberk WC. Oxygen can limit heat tolerance in freshwater gastropods: differences between gill and lung breathers. Hydrobiologia. 2016; 763: 301–312. [Google Scholar]
- 65.Widdows J. Effect of temperature and food on the heartbeat, ventilation rate and oxygen uptake of Mytilus edulis. Mar Biol. 1973; 20: 269–276. [Google Scholar]
- 66.Angilletta MA. Looking for answers to questions about heat stress: researchers are getting warmer. Func Ecol. 2009; 23: 231–232. [Google Scholar]
- 67.Matumba TG. Genetics and thermal biology of littorinid snails of the genera Afrolittorina, Echinolittorina and Littorina (Gastropoda: Littorinidae) from temperate, subtropical and tropical Regions, electronic, scholarly journal. PhD. Thesis. Rhodes University. 2013. Available from: vital.seals.ac.za:8080/vital/access/manager/Repository/vital:5588
- 68.Stenseng E, Braby CE, Somero GN. Evolutionary and acclimation induced variation in the thermal limits of heart function in congeneric marine snails (Genus Tugela): implications for vertical zonation. Biol Bull. 2005; 208: 138–144. 10.2307/3593122 [DOI] [PubMed] [Google Scholar]
- 69.Tagliarolo M, Grall J, Chauvaud L, Clavier J. Aerial and underwater metabolism of Patella vulgata L.: comparison of three intertidal levels. Hydrobiologia. 2013; 702: 241–253. [Google Scholar]
- 70.Davenport J. Comparisons of the biology of the intertidal sub Antarctic limpets Nacella concinna and Kerguelenella lateralis. J Molluscan Stud. 1997; 63(1): 39–48. [Google Scholar]
- 71.Harley CDG, Denny MW, Mach KJ, Miller LP. Thermal stress and morphological adaptations in limpets. Funct Ecol. 2009; 23: 292–301. [Google Scholar]
- 72.Boyden CR. The behaviour, survival and respiration of the cockles Cerastoderma edule and C. glaucum in air. J Mar Biol Assoc U.K. 1972; 52(03): 661–680. [Google Scholar]
- 73.Coleman N. The oxygen consumption of Mytilus edulis in air. Comp Biochem Physiol A. 1973; 45: 393–402. [Google Scholar]
- 74.Bayne BL, Bayne CJ, Carefoot TC, Thompson RJ. The physiological ecology of Mytilus californianus Conrad. Oecol. 1976; 22: 229–250. [DOI] [PubMed] [Google Scholar]
- 75.Denny MW, Dowd WW, Bilir L, Mach KJ. Spreading the risk: Small-scale body temperature variations among intertidal organisms and its implications for species persistence. J Exp Mar Bio Ecol. 2011; 400: 175–190. [Google Scholar]
- 76.Dye AH. Aerial and aquatic oxygen consumption in two siphonariid limpets (Pulmonata: Siphonariidae). Comp Biochem Physiol. 1987; 87(3): 695–698. [Google Scholar]
- 77.Frederich M, Pörtner HO. Oxygen of thermal tolerance defined by cardiac and ventilator performance in spider crab, Maja squinado. Am J Physiol Regul Integr Comp Physiol. 2000; 279: R1531–R1538. 10.1152/ajpregu.2000.279.5.R1531 [DOI] [PubMed] [Google Scholar]
- 78.Pörtner HO. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate related stressor effects in marine ecosystems. J Exp Biol. 2010; 213: 881–893. 10.1242/jeb.037523 [DOI] [PubMed] [Google Scholar]
- 79.Pörtner HO. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Sci Nat. 2001; 88: 137–146. [DOI] [PubMed] [Google Scholar]
- 80.Bjelde BE, Todgham AE. Thermal physiology of the fingered limpet Lottia digitalis under emersion and immersion. J Exp Biol. 2013; 216: 2858–2869. 10.1242/jeb.084178 [DOI] [PubMed] [Google Scholar]
- 81.Tully O, O’Donovan V, Fletcher D. Metabolic rate and lipofuscin accumulation in juvenile European lobster (Homarus gammarus) in relation to stimulated seasonal changes in temperature. Mar Biol. 2000; 137: 1031–1040. [Google Scholar]
- 82.Huxham M, Maitland D, Mocogni M. Respiration rates in Littorina littorea infected with three species of digenean parasite. J Mar Biol Assoc U.K. 2001; 81: 351–352. [Google Scholar]
- 83.Baeza JA, Fernández M. Active brooding Cancer setosus (Crustacea: Decapoda): the relationship between female behaviour, embryo oxygen consumption and the cost of brooding. Funct Ecol. 2002; 16: 241–251. [Google Scholar]
- 84.Cook SJ. Sex-specific differences in cardiovascular performance of a centrarchid fish are only evident during the reproductive period. Funct Ecol. 2004; 18: 398–403. [Google Scholar]
- 85.Bates AE, Leiterer F, Wiedeback ML, Poulin R. Parasitized snails take the heat: a case of host manipulation? Oecol. 2011; 167: 613–621. [DOI] [PubMed] [Google Scholar]
- 86.Britton JC. The relationship between position on shore and shell ornamentation in two size-dependent morphotypes of Littorina striata, with an estimate of evaporative water loss in these morphotypes and in Melarhaphe neritoides. Hydrobiologia. 1995; 309: 129–142. [Google Scholar]
- 87.Chapperon C, Seuront L. Behavioural thermoregulation in tropical gastropod: links to climate change scenarios. Glob Change Biol. 2011; 17: 1740–1749. [Google Scholar]
- 88.Chapperon C, Seuront L. Space-time variability in environmental thermal properties and snail thermoregulatory behaviour. Funct Ecol. 2011; 25: 1040–1050. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SC–Siphonaria capensis; SG–Scutellastra granularis; SS–Siphonaria serrata; CC—Cellana capensis.
(JPG)
Vertical zonation patterns were derived from Allanson [38], Branch [39, 41] and, Chambers and McQuaid [42]. Limpet species and intertidal zones are listed as: SC–Siphonaria capensis; SG–Scutellastra granularis; SS–Siphonaria serrata; CC—Cellana capensis. LZ–Littorina Zone; HMZ–High Mid-Shore Zone; LMZ–Low Mid-Shore Zone; SFZ–Subtidal Fringe Zone.
(PNG)
Mortality (nr of individuals) was determined at the 5 and 29 hour marks.
(TIF)
(DOCX)
SC–Siphonaria capensis; SG–Scutellastra granularis; SS–Siphonaria serrata; CC—Cellana capensis.
(DOCX)
Medium, Respiration mode (R. Mode) and Species nested in Respiration mode (R. Mode) were considered as fixed factors.
(DOCX)
Medium, Respiration mode (R. Mode) and Species nested in Respiration mode (R. Mode) were considered as fixed factors.
(DOCX)
Medium, Respiration mode (R. Mode) and Species nested in Respiration mode (R. Mode) were considered as fixed factors.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.