Abstract
Objectives:
Lactoferrin (LF) and lactoperoxidase (LPO) are present in human saliva. LF has been demonstrated to show antibacterial and antiviral activities. In saliva, LPO catalyzes the hydrogen peroxide-dependent oxidation of thiocyanate to hypothiocyanite that exhibits antimicrobial and antiviral properties. A randomized, open-label, parallel-group clinical trial was conducted to examine the effectiveness of sucking tablets containing LF and LPO (LF+LPO) in alleviating symptoms of the common cold and/or influenza infection.
Methods:
A total of 407 subjects were randomized into two groups, treatment and non-treatment groups, and each group was further classified into subgroups habitually wearing a face mask, washing their hands, or gargling. The common cold, influenza, and gastrointestinal symptoms were used to evaluate the effectiveness, and the incidence and duration of symptoms were statistically analyzed.
Results:
The incidence and duration of common cold, gastrointestinal symptoms, and influenza infection were not statistically different between treatment and non-treatment groups. LF+LPO tablets were moderately effective in reducing the incidence and duration of common cold symptoms in the subgroup that did not gargle and especially to shorten significantly the duration of fever higher than 38°C in the subgroup that did not wear a face mask.
Conclusion:
The results suggested that the effect of ingestion of the tablet is not obvious in alleviating common cold symptoms but may be helpful when the subjects do not follow precautionary measures such as gargling and the use of a protective face mask.
Keywords: Common cold syndromes, face mask, gargling, lactoferrin, lactoperoxidase
Introduction
Common cold syndrome is the acute inflammatory diseases of the upper respiratory tracts such as the nasal cavity, tonsils, pharynx, and larynx and includes rhinitis, tonsillitis, pharyngitis, and laryngitis.[1,2] Influenza and sinusitis are sometimes characterized as common cold syndromes. The symptoms are sniffling, nasal obstruction, sneezing, sore throat, cough, sputum, headache, fever, general malaise, muscle pains, and arthralgia. Nausea, vomiting, diarrhea, and abdominal pain are also often seen. In influenza infection, these subjective symptoms are acutely severe. Common cold syndrome is mainly caused by viral infection of the upper respiratory tracts, although changes of temperature and non-infectious factors such as allergy may be the cause. In winter and spring, virus infections caused by influenza virus, rhinovirus, respiratory syncytial virus, parainfluenza virus, etc., are commonly prevalent. These viruses infect the upper respiratory tracts and cause acute inflammatory diseases.
Saliva coats the surface of upper respiratory tracts and is an important body fluid to interfere with virus infection of the tracts. Lactoferrin (LF) and lactoperoxidase (LPO) are glycoproteins and are components of saliva as well as milk and tears.[3-6] LF has been demonstrated to show antibacterial activity against periodontopathic bacteria in vitro.[7-9] In periodontitis patients, oral administration of bovine LF has been shown to reduce the number of periodontopathic bacteria in the subgingival plaques.[10] LPO catalyzes the hydrogen peroxide-dependent oxidation of thiocyanate to hypothiocyanite and exhibits antimicrobial properties.[5] The viability of oral bacteria has been shown to be reduced by the LPO thiocyanate-hydrogen peroxide system.[11,12] The studies indicating the formation of hypothiocyanite (a potent antimicrobial agent) in saliva by the enzymatic action of LPO and rapid reduction of the infectivity of influenza virus by hypothiocyanite have also been reported.[13,14] The efficacy of LF and LPO in maintaining oral hygiene by reducing periodontal pathogens and suppressing oral malodor has been demonstrated by clinical trials, in which foods containing LF and LPO have been eaten.[15] Exogenous LF and LPO have been shown to be effective in preventing common viral infections containing influenza virus infection.[16,17] Thus, it is possible that LF and LPO in saliva are effective in reducing virus infection and alleviating the symptoms.
In this study, we conducted an exploratory clinical trial to examine the effectiveness of sucking a tablet containing LF and LPO in alleviating the symptoms of a common cold or influenza virus infection.
Methods
A randomized, open-label, parallel-group trial was conducted from January to March, 2015, in accordance with the Helsinki Declaration of 1975 and as revised in 2013. This study including the protocol, amendments, and informed consent documents was approved by the Ethical Committee of Kyushu University of Health and Welfare (No. 14-034 on January 14, 2015) and performed at Kyushu University of Health and Welfare.
Participants in the trial were healthy adult volunteers chosen from students and faculty members of Kyushu University of Health and Welfare who were 20–65 years old. The trial schedule is shown in Figure 1. The aim and schedule of the study were explained, and written informed consent was obtained from the subjects. A background survey of subject characteristics was performed. In the survey, the main exclusion criteria were milk allergy; previous or present clinically significant diseases of digestive, endocrine, circulatory, or metabolic organs including the liver, kidney, heart, or lung; or judged ineligible by this research manager. Of the 414 volunteers, seven were excluded based on the exclusion criteria. Finally, 407 subjects were randomized into treatment (203 subjects) and non-treatment (204 subjects) groups, as shown in Figure 2.
Figure 1.
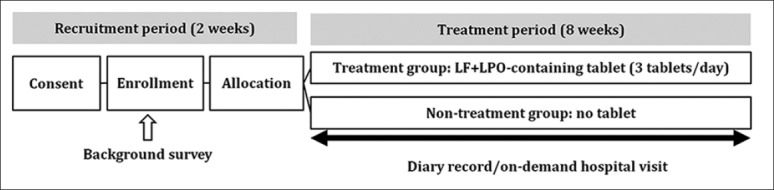
Trial schedule. During recruitment period, informed consent was obtained, a background survey was performed, and subjects were randomized into two groups. During treatment period, subjects in the treatment group received tablets containing lactoferrin+lactoperoxidase and the non-treatment group did not receive any tablets
Figure 2.
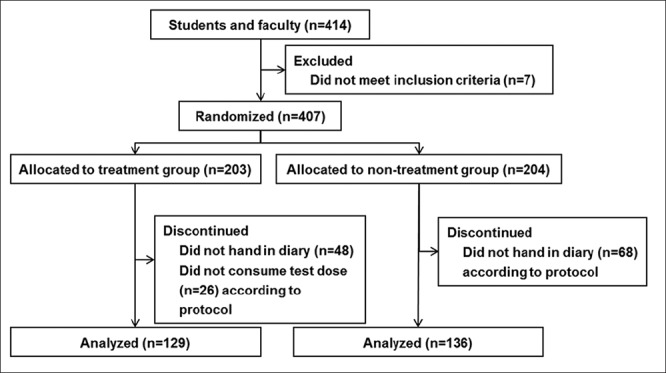
Participant flowchart. Of the 414 volunteers, seven were excluded based on the exclusion criteria. Finally, 407 subjects were randomized into treatment and non-treatment groups. In each group, subjects who did not hand in a diary according to protocol and/or did not consume the test tablets at an uptake rate more than 67% were not included in the analysis. The data obtained from 129 in the treatment group and 136 in the non-treatment group were statistically analyzed
Subjects in the treatment group received tablets containing LF+LPO (20 mg LF, 2.6 mg LPO, and 2.6 mg glucose oxidase per tablet, Morinaga Milk Industry) for 8 weeks at a dose of 3 tablets per day for sucking, preferably during their commute, ambulation, or travel [Figure 1], because this dose was effective in the previous clinical studies investigated oral periodontal bacteria and microbiota.[18,19] The non-treatment group did not receive any tablets [Figure 1].
During the 8 weeks, the subjects in both groups were asked to record the presence or absence of common cold symptoms such as fever (body temperature), sore throat, cough, nasal secretion, sniffles, sputum, headache, joint pain, and muscle ache in their diaries. Daily records were summed up to calculate the total numbers of common cold symptoms. Subjects were also asked to record the diagnosis of influenza infection and the presence or absence of the gastrointestinal symptoms such as diarrhea, nausea, and vomiting. To evaluate the safety, the subjects were asked to record subjective symptoms other than those of the common cold and gastroenteritis.
Symptoms of the common cold, influenza virus infection, and gastrointestinal symptoms were used as items of the effectiveness evaluation. In principle, as analysis sets, we used all cases in which the study was completed without plan violations, defined as failing to obtain informed consent, meet eligibility criteria, provide data after randomization, and an uptake rate <67%. As the analysis sets of safety evaluation, all cases of non-treatment and treatment groups with at least one intake of a LF+LPO-containing tablet were used.
The incidence and duration (number of days) of symptoms were analyzed by Fisher’s exact test and Wilcoxon ranked sum test, respectively. Subgroups were classified based on the background characteristics obtained from the subjects including habits of wearing a face mask, handwashing, and gargling. Data collected from these subgroups were also analyzed by Student’s t-test. For the safety evaluation, the number of subjects with adverse events and the frequency in each group were analyzed by Fisher’s exact test. JMP ver. 9.0.2 (SAS Institute) was used for analyses.
Results
Of the 407 volunteers enrolled, 203 and 204 were allocated to treatment and non-treatment groups, respectively. In each group, subjects who did not hand in a diary according to protocol (48 in treatment group and 68 in non-treatment group) and/or did not consume the test tablets at an uptake rate more than 67% (26 in treatment group) were not included in the analysis, as shown in Figure 2. Finally, 265 subjects completed the study, and the data obtained from 129 in the treatment group and 136 in the non-treatment group were statistically analyzed [Figure 2]. The proportion of loss to follow up was 34.9%. Testing periods in treatment and non-treatment groups were 55.6 ± 1.9 days and 55.8 ± 1.2 days, respectively, and no significant difference was found. The intake rate of LF+LPO-containing tablets was 91.5 ± 8.0% (67.3 – 100.0%) in the treatment group.
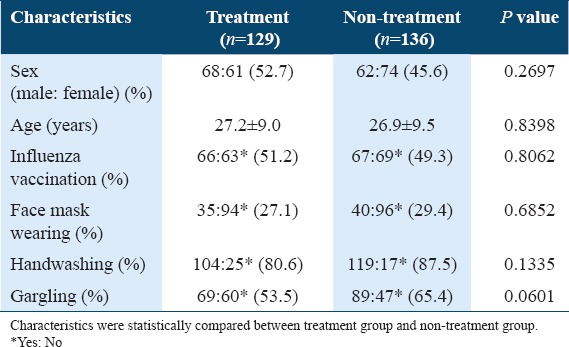
In the background survey, subjects were classified into subgroups based on characteristics of sex, age, influenza vaccination, and habits of wearing a face mask, handwashing, and gargling. As shown in Table 1, a slight imbalance in the ratio of subjects with the habit of gargling was detected between treatment (69:60, 53.5%) and non-treatment (89:47, 65.4%) groups [P = 0601, Table 1]. The other characteristics of subjects were statistically balanced between treatment and non-treatment groups.
Table 1.
Characteristics of subjects
For the safety evaluation, recorded diaries of 313 subjects were analyzed (data not shown). No serious adverse event was observed in this study. One subject discontinued the test. In this case, stomach ache disappeared after cessation of the intake of LF+LPO-containing tablets. There was no statistical difference between the incidence of adverse events in treatment and non-treatment groups.
As the primary end point of the study, the incidence and duration of common cold, gastrointestinal symptoms, and influenza infection were analyzed [Tables 2 and 3]. No statistically significant difference was found in the incidence and duration of common cold, gastrointestinal symptoms, and influenza infection between treatment and non-treatment groups.
Table 2.
Incidence of common cold, gastrointestinal symptoms, and influenza infection
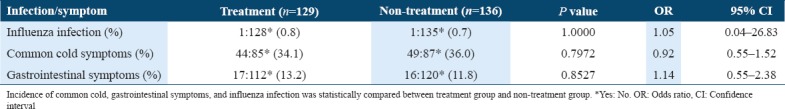
Table 3.
Duration of common cold, gastrointestinal symptoms, and influenza infection
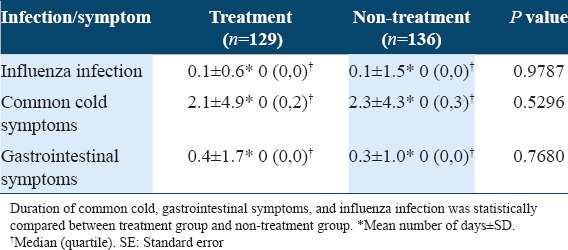
As the secondary analysis [Table 4], in subgroups with and without the face mask-wearing habit or handwashing habit, there were no statistical differences between the incidences of common cold symptoms of treatment and non-treatment groups. In the subgroup with the gargling habit (n = 158), no significant difference was found between the incidences of common cold symptoms of treatment (23:46, 33.3%) and non-treatment groups (26:65 27.0%), either. However, in the subgroup without the gargling habit (n = 107), the incidence of common cold symptoms in the treatment group (21:39, 35.0%) was slightly lower than that in the non-treatment group (25:22, 53.2%) [P = 0.0771, Table 4]. As shown in Figure 3 and Table 5, also, in the subgroup without gargling habit (n = 107), the duration of common cold symptoms in the treatment group (n = 60) was 2.4 ± 5.0 days and slightly shorter than that in the non-treatment group (3.3 ± 5.0 days, n = 47) [P = 0.0612, Figure 3 and Table 5]. In subgroups with and without face mask-wearing habit or handwashing habit or the subgroup with gargling habit, there were no statistical differences between treatment and non-treatment groups. Thus, LF+LPO-containing tablets were suggested to be beneficial in reducing the incidence and duration of common cold symptoms in the subgroup without gargling habit.
Table 4.
Subgroup analysis of the incidence of common cold symptoms
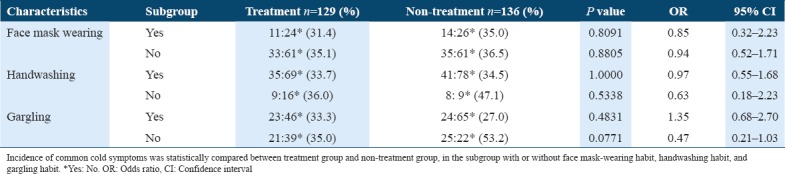
Figure 3.

Subgroup analysis of the duration of common cold symptoms. Duration of common cold symptoms was statistically compared between treatment group and non-treatment group, in the subgroup with or without face mask-wearing habit, handwashing habit, and gargling habit
Table 5.
Subgroup analysis of duration of common cold symptoms (corresponding to Figure 3)
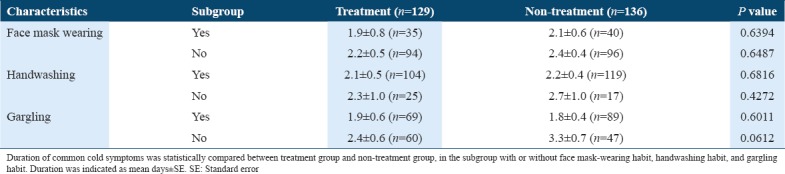
When the incidences of common cold symptoms in non-treatment groups [n = 136, Table 4] were compared between subgroups with and without habitual gargling, the incidence in the subgroup with the gargling habit (24:65, 27.0%) was significantly lower than that in subgroup without the gargling habit (25:22, 53.2%) [P = 0.0045, Table 4]. Furthermore, the duration of common cold symptoms of the non-treatment group in the subgroup that gargled was 1.8 ± 4.0 days (n = 89) and significantly shorter than that in subgroup that did not gargle (3.3 ± 4.7 days, n = 47) [P = 0.0051, Figure 3 and Table 5]. The gargling habit, itself, was effective in reducing the incidence and duration of common cold symptoms.
In the subgroup that did not gargle (n = 107), total numbers of common cold symptoms and duration of sniffles in treatment groups were 3.3 ± 6.8 and 0.1 ± 0.3 days, respectively, and lower than those in the non-treatment group (5.1 ± 7.9 and 0.5 ± 1.5 days, respectively), although the difference was not statistically significant. As shown in Figure 4 and Table 6, the duration of fever (body temperature ≥38°C) in the subgroup not wearing a face mask (n = 190) was significantly shorter in the treatment group (0.0 ± 0.0 days, n = 94) than in the non-treatment group (0.1 ± 0.4 days, n = 96) (P = 0.0466). In the subgroup of males (n = 130), the duration of fever higher than 38°C in the treatment group (0.0 ± 0.0 days) seemed to be shorter than that in the non-treatment group (0.1 ± 0.5 days). In the subgroup older than 23 years (n = 141), the duration of fever higher than 38°C in the treatment group (0.000 ± 0.000 days) seemed to be shorter than that in non-treatment group (0.073 ± 0.040 days). In the subgroup of females (n = 135), the duration of sniffles in the treatment group (0.1 ± 0.3 days) seemed to be shorter than that in the non-treatment group (0.4 ± 1.2 days). It was possible that tablets containing LF+LPO contributed to the alleviation of common cold symptoms.
Figure 4.
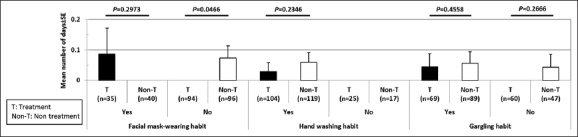
Subgroup analysis of the duration of fever (body temperature ≥38°C). Duration of fever (body temperature ≥38°C) was statistically compared between treatment group and non-treatment group, in the subgroup with or without face mask-wearing habit, handwashing habit, and gargling habit
Table 6.
Subgroup analysis of duration of fever (body temperature≥38°C) (corresponding to Figure 4)

Discussion
We studied the effects of orally administered tablets containing LF and LPO on symptoms of the common cold. The incidence and duration of common cold, gastrointestinal symptoms, and influenza infection were not statistically different between treatment and non-treatment groups [Tables 2 and 3]. The observed limitation of the effects of LF and LPO tablets may have come from (1) a small number of subjects, (2) the type of subjects, who were young healthy individuals, (3) difference in the ratio of number of subjects with or without gargling habit between the treatment group and non-treatment group, and (4) overall low incidence of common cold, gastrointestinal symptoms, and influenza infection.
However, LF and LPO tablets were moderately effective in reducing the incidence and duration of common cold symptoms in the subgroup that did not gargle habitually [Table 4 and 5 Figure 3 respectively], and especially, in shortening the duration of fever higher than 38°C in that subgroup that did not wear a face mask habitually [Figure 4 and Table 6]. It was possible that LF and LPO tablets play a protective role against the common cold and influenza. LF and the catalytic product of LPO system and hypothiocyanite exhibit antiviral activities against influenza virus and viruses causing common cold.[13,17] The LF and LPO contained in saliva act on not only the oral cavity but also the upper respiratory tracts. Most viruses that cause the common cold infect upper respiratory tracts and cause acute inflammatory diseases. LF also enhances the expression of antiviral cytokines and interferon α/β/λ, in the intestinal cells.[20,21] LPO was reported to exhibit anti-inflammatory effects in mice suffering from influenza virus-induced pneumonia and dextran sulfate sodium-induced colitis.[16,22] Thus, LF and LPO might be useful to alleviate common cold symptoms.
Preventive effects of wearing a face mask, handwashing, and gargling on seasonal respiratory illnesses have been reported in previous clinical trials.[23-25] In this study, we took account of their preventive effects and compared the incidences of common cold symptoms of subgroups with and without habitual face mask-wearing, handwashing, or gargling, as shown in Table 4. In the non-treatment group of the current study, the incidence and duration of common cold symptoms were significantly lower in the subgroup that habitually gargled than in the subgroup that did not habitually gargle [Table 4]. This was consistent with previous reports,[25] although there was no significant difference between non-treatment subgroups with and without face mask-wearing habit or handwashing habit. Habitual gargling affected the incidence of common cold symptoms much more than face mask-wearing and handwashing in this study.
LF- and LPO-containing tablets were beneficial to the reduction of the incidence and duration of common cold symptoms in the subgroup that did not habitually gargle [Figure 3 and Tables 4 and 5]. LF and LPO are primary components of saliva, and their oral administration probably increases their concentration in the saliva. Without gargling, the higher concentration can be maintained in the saliva longer than in the subgroup that did gargle. Therefore, the effect of LF and LPO might be remarkable in the subgroup without the gargling habit.
The duration of fever as a common cold symptom was significantly shortened by the administration of LF and LPO in the subgroup that did not habitually wear a face mask [Figure 3 and Table 5]. As shown in Table 2, the incidences of influenza infection, common cold symptoms, and gastrointestinal symptoms were similar between treatment and non-treatment groups. Thus, the shortening of fever duration by the administration of LF and LPO suggested that the treatment was significantly effective in alleviating common cold symptoms.
Conclusion
The incidence and duration of common cold, gastrointestinal symptoms, and influenza infection were not statistically different between treatment and non-treatment groups. However, orally administered LF and LPO were effective in reducing the incidence and duration of common cold symptoms and shortening the duration of fever in the subgroups that did not habitually gargle or wear a face mask, respectively. These results suggest the possibility of a complementary protective role by the ingestion of food-containing LF+LPO on the upper respiratory tract, especially in the absence of the precautionary measures such as gargling or wearing a face mask.
Acknowledgment
We thank Ms. Yukiko Shimoda for her excellent technical assistance and Ms. Katherine Ono for her editorial assistance.
Conflicts of Interest
This work was supported by research grants from Morinaga Milk Industry Co., Ltd. Four of the authors, K. Shin, H. Wakabayashi, K. Yamauchi, and F. Abe, are employees of Morinaga Milk Industry Co., Ltd. The other authors, C. Sugita, H. Yoshida, K. Sato, T. Sonoda, and M. Kurokawa, declare no potential conflicts of interest with respect to the authorship and/or publication of this manuscript.
References
- 1.Pappas DE, Hendley JO, Hayden FG, Winther B. Symptom profile of common colds in school-aged children. Pediatr Infect Dis J. 2008;27:8–11. doi: 10.1097/INF.0b013e31814847d9. [DOI] [PubMed] [Google Scholar]
- 2.Allan GM, Arroll B. Prevention and treatment of the common cold:Making sense of the evidence. Can Med Assoc J. 2014;186:190–9. doi: 10.1503/cmaj.121442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakabayashi H, Yamauchi K, Takase M. Lactoferrin research, technology and applications. Int Dairy J. 2006;16:1241–51. [Google Scholar]
- 4.Wakabayashi H, Kondo I, Kobayashi T, Yamauchi K, Toida T, Iwatsuki K, et al. Periodontitis, periodontophathic bacteria and lactoferrin. Biometals. 2010;23:419–24. doi: 10.1007/s10534-010-9304-6. [DOI] [PubMed] [Google Scholar]
- 5.Thomas EL, Bozeman PM, Learn DB. Lactoperoxidase:Structure and catalytic properties. In: Everse J, Everse KE, Grisham MB, editors. Peroxidases in Chemistry and Biology. Vol. 1. Boca Raton, Florida: CRC Press; 1991. pp. 123–42. [Google Scholar]
- 6.Sharma S, Singh AK, Kaushik S, Sinha M, Singh RP, Sharma P, et al. Lactoperoxidase:Structural insights into the function ligand binding and inhibition. Int J Biochem Mol Biol. 2013;4:108–28. [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilera O, Andrés MT, Heath J, Fierro JF, Douglas CW. Evaluation of the antimicrobial effect of lactoferrin onPorphyromonas gingivalis Prevotella intermedia and Prevotella nigrescens . FEMS Immunol Med Microbiol. 1998;21:29–36. doi: 10.1111/j.1574-695X.1998.tb01146.x. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Kong W, Nakayama K. Human lactoferrin binds and removes the hemoglobinreceptor protein of the periodontopathogenPorphyromonas gingivalis. J Biol Chem. 2000;275:30002–8. doi: 10.1074/jbc.M001518200. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi H, Yamauchi K, Kobayashi T, Yaeshima T, Iwatsuki K, Yoshie H. Inhibitory effects of lactoferrin on growth and biofilm formation ofPorphyromonas gingivalisandPrevotella intermedia. Antimicrob Agents Chemother. 2009;53:3308–16. doi: 10.1128/AAC.01688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondo I, Kobayashi T, Wakabayashi H, Yamauchi K, Iwatsuki K, Yoshie H. Effects of oral administration of bovine lactoferrin on periodontitis patients. Jpn J Conserv Dent. 2008;51:281–91. [Google Scholar]
- 11.Thomas EL, Milligan TW, Joyner RE, Jefferson MM. Antibacterial activity of hydrogen peroxide and the lactoperoxidase-hydrogen peroxide-thiocyanate system against oral streptococci. Infect Immun. 1994;62:529–35. doi: 10.1128/iai.62.2.529-535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ihalin R, Loimaranta V, Lenander-Lumikari M, Tenovuo J. The sensitivity ofPorphyromonas gingivalisandFusobacterium nucleatumto different (pseudo)halide-peroxidase combinations compared with mutant streptococci. J Med Microbiol. 2001;50:42–8. doi: 10.1099/0022-1317-50-1-42. [DOI] [PubMed] [Google Scholar]
- 13.Cegolon L, Salata C, Piccoli E, Juarez V, Palu' G, Mastrangelo G, et al. In vitroantiviral activity of hypothiocyanite against A/H1N1/2009 pandemic influenza virus. Int J Hyg Environ Health. 2014;217:17–22. doi: 10.1016/j.ijheh.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Gingerich A, Pang L, Hanson J, Dlugolenski D, Streich R, Lafontaine ER, et al. Hypothiocyanite produced by human and rat respiratory epithelial cells inactivates extracellular H1N2 influenza A virus. Inflamm Res. 2016;65:71–80. doi: 10.1007/s00011-015-0892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin K, Yaegaki T, Murata T, Ii H, Tanaka T, Aoyama I, et al. Effects of a comparison containing lactoferrin and lactoperoxidase on oral malodor and salivary bacteria:A randomized, double-blind, crossover, placebo-controlled clinical trial. Clin Oral Investig. 2010;15:1–9. doi: 10.1007/s00784-010-0422-x. [DOI] [PubMed] [Google Scholar]
- 16.Shin K, Wakabayashi H, Yamauchi K, Teraguchi S, Tamura Y, Kurokawa M, et al. Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection in mice. J Med Microbiol. 2005;54:717–23. doi: 10.1099/jmm.0.46018-0. [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi H, Oda H, Yamauchi K, Abe F. Lactoferrin for prevention of common viral infections. J Infect Chemother. 2014;20:666–71. doi: 10.1016/j.jiac.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Morita Y, Ishikawa K, Nakano M, Wakabayashi H, Yamauchi K, Abe F, et al. Effects of lactoferrin and lactoperoxidase-containing food on the oral hygiene status of older individuals:A randomized, double blinded, placebo-controlled clinical trial. Geriatr Gerontol Int. 2017;17:714–21. doi: 10.1111/ggi.12776. [DOI] [PubMed] [Google Scholar]
- 19.Nakano M, Wakabayashi H, Sugahara H, Odamaki T, Yamauchi K, Abe F, et al. Effects of lactoferrin and lactoperoxidase-containing food on the oral microbiota of older individuals. Microbiol Immunol. 2017;61:416–26. doi: 10.1111/1348-0421.12537. [DOI] [PubMed] [Google Scholar]
- 20.Wakabayashi H, Takakura N, Yamauchi K, Tamura Y. Modulation of immunity-related gene expression in small intestines of mice by oral administration of lactoferrin. Clin Vaccine Immunol. 2006;13:239–45. doi: 10.1128/CVI.13.2.239-245.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin K, Oda H, Wakabayashi H, Yamauchi K, Abe F. Effects of lactoferrin on the production of interferon-? By the human intestinal epithelial cell line HT-29. Biochem Cell Biol. 2017;95:53–6. doi: 10.1139/bcb-2016-0031. [DOI] [PubMed] [Google Scholar]
- 22.Shin K, Horigome A, Yamauchi K, Takase M, Yaeshima T, Iwatsuki K. Effects of orally administered bovine lactoperoxidase on dextran sulfate sodium-induced colitis in mice. Biosci Biotechnol Biochem. 2008;72:1932–5. doi: 10.1271/bbb.70636. [DOI] [PubMed] [Google Scholar]
- 23.Sim SW, Moey KS, Tan NC. The use of facemasks to prevent respiratory infection:A literature review in the context of the health belief model. Singapore Med J. 2014;55:160–7. doi: 10.11622/smedj.2014037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merk H, Kühlmann-Berenzon S, Linde A, Nyrén O. Associations of hand-washing frequency with incidence of acute respiratory tract infection and influenza-like illness in adults:A population-based study in Sweden. BMC Infect Dis. 2014;14:509. doi: 10.1186/1471-2334-14-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satomura K, Kitamura T, Kawamura T, Shimbo T, Watanabe M, Kamei M, et al. Great cold investigators-I. Prevention of upper respiratory tract infections by gargling:A randomized trial. Am J Prev Med. 2005;29:302–7. doi: 10.1016/j.amepre.2005.06.013. [DOI] [PubMed] [Google Scholar]


