Abstract
Objectives:
The aim of the study was to compare the levels of oxidative stress biomarkers and antioxidants in acute myocardial infarction (AMI) patients with healthy individuals and to investigate the effectiveness of these parameters as risk or illness indicators.
Methodology:
This study was conducted on AMI patients admitted to Intensive Care Unit of Al-Salam Hospital and Ibn-Sina Hospital in Mosul, Iraq. Considering inclusion and exclusion criteria, a total of 161 patients and 156 healthy individuals in the age group of 30–80 years were selected for the study. The study groups were screened by estimating cardiac markers and electrocardiography (ECG).
Results:
The results indicated a significant increase in the level of serum malondialdehyde, peroxynitrite, and uric acid (P< 0.001). A minor increase in the serum ceruloplasmin level was observed in patients with AMI as compared to healthy individuals. The study also observed a significant decrease in the level of glutathione, Vitamin E, and Vitamin C (P< 0.001), with no significant difference in the level of Vitamin A in patients with AMI.
Conclusion:
The imbalance in the oxidative status and antioxidant activity in AMI patients reflects the importance of measuring the level of serum oxidative stress biomarkers and antioxidants as a diagnostic and prognostic tool for the medical management of AMI. Oxidative stress biomarkers and antioxidants might be good predictors or indicators for the risk of AMI. Oxidative stress markers contribute in the pathogenesis of AMI and excess of reactive oxygen species overwhelm the stability of the antioxidants.
Keywords: Antioxidants, myocardial infarction, oxidative stress, reactive oxygen species
Introduction
Acute myocardial infarction (AMI) is one of the most critical vascular diseases which results in the consequence of myocardial ischemia due to the occlusion of coronary arteries. Oxidative stress has a significant role in the development of cardiovascular diseases (CVDs).[1] The incidence of death due to AMI is increasing worldwide and the risk of death is very high within the first few hours of the onset of disease. A biomarker is defined as a “characteristic which is measured and evaluated as an indicator of biological processes or pharmacologic responses to a therapeutic intervention.” The analysis and identification of various biomarkers may increase the existing diagnostic procedures for the evaluation of AMI patients.[2] Auto-oxidation of lipids exposed to oxygen is responsible for the in vivo tissue damage causing inflammatory diseases and atherosclerosis. Studies have demonstrated that the excessive activation of lipid peroxidation has a key role in the development of many diseases such as angina and AMI. This is because the lipid peroxidation is a chain of reactions providing a continuous supply of free radicals that increase further peroxidation.[3,4] The best evidence of lipid peroxidation is the increased formation of malondialdehyde (MDA) which is one of the principal breakdown products by the action of endoperoxidase, and hence the determination of MDA has been widely used in human studies to prove the involvement of lipid peroxidation in various diseases. A number of studies reported the elevated level of serum MDA in heart diseases, indicating a link between oxidative stress and AMI.[5,6]
Reactive oxygen species (ROS) with an impairment of endogenous antioxidant mechanisms play an important role in the pathogenesis of myocardial infarction. Atherosclerosis is the main cause of AMI.[7] These ROS contribute in mitochondrial dysfunction related to many human diseases and aging. ROS level is regulated by intracellular antioxidants such as superoxide dismutase, catalase (CAT), glutathione (GSH), and glutathione peroxidase along with antioxidant vitamins. In pathological conditions, excess of ROS overwhelms the stability of the antioxidants.[7-9] This inefficiency of antioxidants is more severe in mitochondria which lack CAT and the excessive production of H2O2 damages lipids, protein, and DNA, causing destruction and death of the cell by necrosis. Mitochondrial oxidative stress has been implicated in heart diseases.[8,10]
The aim of the study was to compare the levels of oxidative stress biomarkers and antioxidants in AMI patients with healthy individuals and to investigate the effectiveness of these parameters as risk or illness indicators. The vast diversity of oxidative stress in AMI among Iraqi population leads to study the status of oxidative stress markers, antioxidants, and its association with AMI.
Materials and Methods
The experiment was designed carefully to ensure the random selection of patient’s sample that will reflect the actual status of AMI among the citizens of Mosul and its suburbs. Samples were collected from patients admitted to Al-Salam Hospital and Ibn-Sina Hospital in Mosul, Iraq. Ethical approval was taken from Institutional Ethics Committee and informed consent was obtained from individual participants or close relatives of the individuals included in the study. Blood samples were collected through venipuncture. The blood was immediately transferred to a polystyrene tube, incubated for 10 min at 37°C and centrifuged at 3000 rpm for 10 min. The separated serum was kept in a freezer for further analysis.[11] A total of 161 patients (35 females and 126 males) were screened with age ranging from 30 to 80 years. The control group was selected carefully from people with no history of heart diseases. A total of 156 healthy individuals with age ranging from 30 to 60 years (83 males and 73 females) were selected to participate in the study.
Inclusion criteria
Newly diagnosed AMI patients admitted in Intensive Care Unit (both genders) with elevated ST-segment of at least 2 mm in two or more consecutive leads of electrocardiography were included.
Exclusion criteria
Patients with a previous history of chronic angina or AMI, family history of heart and cerebrovascular diseases, on thrombolytic therapy, and unable to obtain informed consent were excluded.
Serum MDA was estimated by thiobarbituric acid (TBA) method in which MDA reacts with TBA to form a red-colored product which is useful in the determination of lipid peroxidation.[12] Serum peroxynitrite level was estimated by the spectrophotometric method which involves peroxynitrite-mediated nitration of phenol.[13] Ceruloplasmin concentration was estimated by quantitative immuno-turbid metric method.[14] Serum uric acid level was estimated by uricase enzymatic method using the kit manufactured by Biomerieux, France. Serum GSH was estimated by DTNB/GR recycling method.[15] Vitamin A was estimated based on optical density differences between irradiated and non-irradiated serum extracts.[16] Determination of Vitamin E in serum was based on an oxidation–reduction reaction following a specific elution technique.[17] Serum level of Vitamin C was determined photometrically by 2,4-dinitrophenyl hydrazine method.[18]
Statistical analysis
Results were expressed as mean ± standard error of the mean and statistical analysis was carried out using the STATISTICA 12.5 Package of Stat soft Inc., 2014 (Sandton, South Africa). Student’s t-test was used to compare between the groups. For all statistical evaluations, P < 0.05 was considered statistically significant. A Visual Fortran program was also used to analyze the sensitivity of different parameters.
Results
Table 1 shows the concentration of oxidative stress biomarkers and antioxidants in controls and patients with AMI. A significant increase in serum MDA concentrations was observed in AMI patients as compared to the control group with P < 0.001. The study shows a significant increase (at P < 0.001) in the concentration of peroxynitrite (ONOO−) in sera of AMI patient as compared with healthy controls. The results showed a significant decrease (P < 0.001) in GSH concentration in AMI patients when compared with the controls. The study also observed a minor increase in the concentration of ceruloplasmin in AMI patients’ serum when compared with the controls. A significant increase (P < 0.001) in uric acid concentration was observed in patients with AMI when compared with control group. Table 2 shows the concentration of oxidative antioxidant vitamins in controls and patients with AMI. There was no significant difference in Vitamin A concentration in serum of patients with AMI when compared with control group. The results showed a significant decrease in Vitamin E concentration in sera of patients with AMI when compared with control group at (P < 0.001). The results also showed a significant decrease in Vitamin C concentration in sera of patients with AMI as compared with the control group (P < 0.01). Table 3 shows the clinical sensitivity, specificity, and efficiency for oxidative stress biomarkers and antioxidants related to AMI patients. The uric acid shows the best characteristics followed by Vitamin C and Vitamin E. Table 4 shows some specific characteristics of the study cases including living locality and smoking habits. Among the patients, 78.3% were male (53.9 ± 10.9 years) and 21.7% were female (57.3 ± 10.4 years). Table 5 summarizes age-wise distribution of AMI patients.
Table 1.
Comparisons between levels of oxidative stress biomarkers and antioxidants in serum of patients with AMI and controls
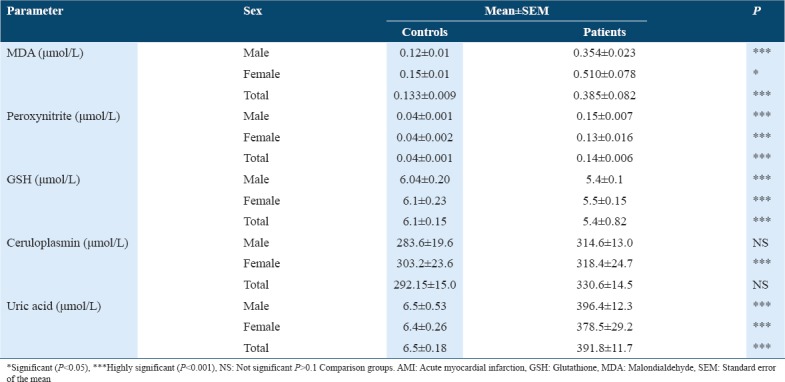
Table 2.
Comparisons between concentration of Vitamin A, E, and C in serum of patients with AMI patients and controls
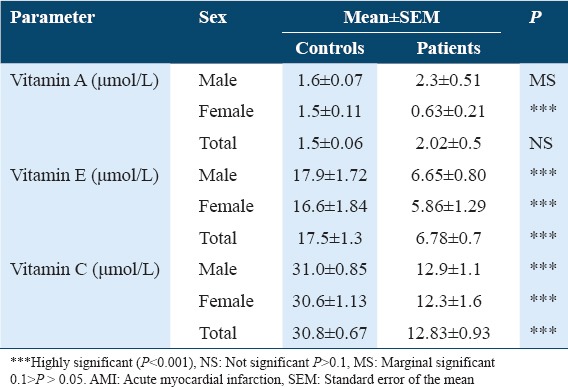
Table 3.
Clinical sensitivity, specificity, and efficiency for selected oxidative stress biomarkers and antioxidants related to patients with AMI
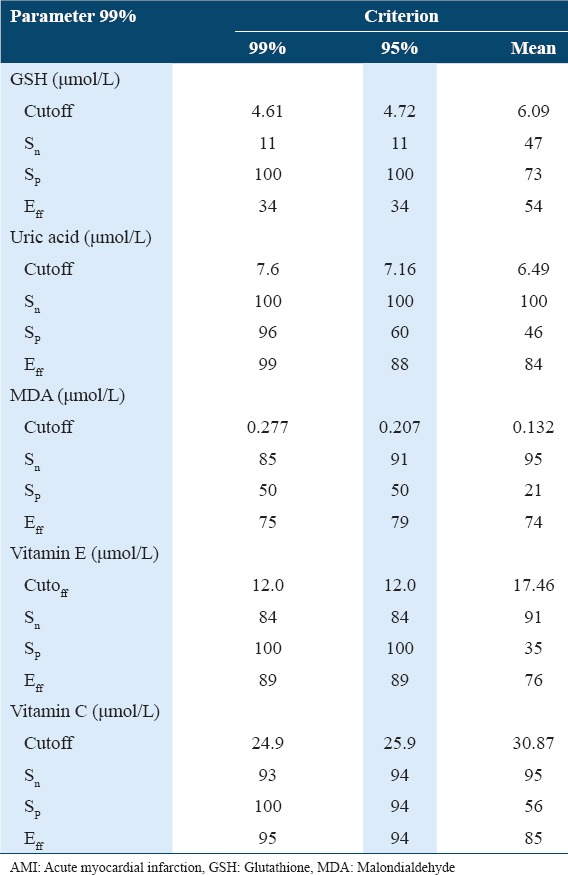
Table 4.
Some specific characteristics of the AMI patients
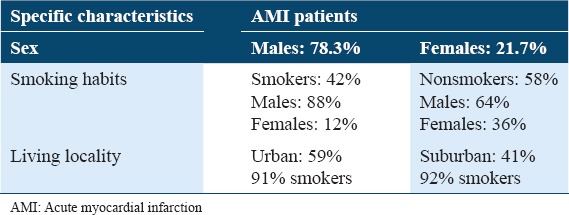
Table 5.
Age-wise distribution of AMI patients

Discussion
Evidence of oxidative stress in acute myocardial disease due to reperfusion, an imbalance between antioxidants and pro-oxidants, has been reported.[19,20] As an antioxidant, GSH plays a significant role in the autoxidation of oxygen-free radicals involved in atherosclerosis.[20,21] The findings suggest that decreased levels of GSH may be interconnected with increased protective mechanisms and oxidative stress in AMI. It is also suggested that oxygen-free radicals are produced in the early stage of AMI, and GSH is associated with the reduction of hydrogen peroxide radicals, which results in a decline in the level of GSH during that period.[19-21] On the other hand, the increased production of reactive oxygen radicals could be a feature of CVDs such as AMI, and cells can respond to mild oxidative stress by upregulating antioxidant defense in terms of increased production of GSH.[22,23] Other results indicate that low concentration of GSH in the circulation of AMI patients may be due to increased utilization to scavenge lipid peroxides.[23,24]
GSH protects the myocardium against injury caused by oxygen-free radicals. A reduction in cellular GSH content would impair recovery after a short period of myocardial ischemia. An increase in the level of GSH disulfide and a decrease in the level of GSH occur in myocardium throughout coronary occlusion.[25,26] The level of vascular GSH is decreased in atherosclerosis and diabetes mellitus (DM), which results in an increased incidence of cardiovascular complication in these patients.[27] The present study confirms the importance of the existence of counterbalance between the oxidative and protective mechanisms in AMI patients. Uric acid is the final breakdown product of the nucleic acid and purine catabolism in humans. Uric acid formation occurs only in tissues that contain the enzyme xanthine oxidase. Previous studies showed an increased production of uric acid in patients on diuretic drug which may explain the high levels of uric acid in patients in this study.[28,29] Several studies have found hyperuricemia to be independently associated with increased mortality in patients with CVD. The results of previous studies showed no significant effect of sex on the concentration of uric acid in AMI patients.[28,29]
MDA levels are measured to indicate the level of damage to polyunsaturated fatty acids. Thus MDA, which is the characteristic end product of lipid peroxidation, is commonly used as one of the important parameters to judge the oxidative damage taking place in the body of AMI patients.[30] The data suggested that free radical production and subsequently the reaction of lipid peroxidation increased in patients as compared to healthy persons. Higher level of MDA is associated with reduction of antioxidants and thereby different forms of scavengers.[4,6] The management of myocardial ischemia might be possible by therapeutic antioxidants and scavengers such as GSH, Vitamin E, and Vitamin C. It can be suggested that high serum levels of MDA might be a biochemical marker for coronary artery disease as well as a high-risk factor for AMI.[31] Nitric oxide is highly reactive that can interact with other free radicals such as superoxide anion and cause the production of peroxynitrite. Thus, inflammation and ischemia may lead to the production of a large amount of peroxynitrite that result in the modification of low-density lipoprotein (LDL) and finally the formation of fat-filled cells that gradually gather in the atherosclerotic lesions. Nitric oxide is produced by endothelium and has a protective effect against atherogenesis.[7,32] Accordingly, it may be suggested that oxidative stress and nitric oxide might have multiple effects on the initiation and also the progression of atherogenesis. Peroxynitrite has a high affinity to sulfhydryl groups and thereby inactivate sulfhydryl-bearing enzymes. This effect of peroxynitrite is controlled by GSH content in the cells since GSH is the main intracellular sulfhydryl-bearing peptide.[26,33] The vasodilator action of nitric oxide in vascular smooth muscle is mediated by its activation of soluble guanylate cyclase and increased cyclic GMP synthesis by guanylate cyclase that results in the relaxation of smooth muscle. Peroxynitrite in solution at physiological pH is a powerful oxidant which cleaves DNA molecule and oxidizing thiols.[26,33]
Studies suggest that there is a correlation between ceruloplasmin, a copper-carrying protein, and CVD.[34] Elevated serum ceruloplasmin levels have been found in patients with a multitude of CVD and these correlations may partly be due to the involvement of ceruloplasmin as an acute-phase reactant. Ceruloplasmin or copper may form an independent risk factor for CVD.[35] Studies showed that an elevated LDL level in blood is correlated with serum copper level with accelerated atherosclerosis.[36] Studies on Vitamin A suggest a weak protective effect of Vitamin A on the risk of AMI.[37,38] Our study showed no significant difference in Vitamin A concentration in serum of AMI patients as compared with controls. No differences were observed between male and female patients as well. The decrease in Vitamin E concentration observed in this study might be either due to the metabolic or nutritional origin. It is not clear whether the decrease in Vitamin E concentration is a result of the disease or its further aggravation. However, a strong interconnection is observed between low Vitamin E levels and high risk of coronary heart disease (CHD) events and hence Vitamin E inadequacy could be a risk factor for coronary disease.[38]
The protective effect of Vitamin E against atherosclerosis may be caused by its role in the inhibition of LDL oxidation. Oxidized LDLs are atherogenic through their increased uptake by macrophage scavenger receptors, immunogenicity, or their induction of cytotoxic, chemotactic, and growth factors. In addition, Vitamin E has a potential role to prevent other injurious effects involved in the pathogenesis of atherosclerosis. Vitamin E also decreases the secretion of interleukin-1 and endothelial adhesion of monocytes, platelet adhesion, platelet aggregation, and proliferation of smooth muscle. Adequate Vitamin E intake through diet or supplements may prevent CHD.[38,39] Vitamin C is considered to be the most effective aqueous-phase antioxidant in humans. Free radicals formed in the body fluid in patients with AMI are detoxified by antioxidants such as Vitamin C, which explain the depletion in Vitamin C concentration in the present study. Vitamin C inhibits the oxidation of LDL in vitro. Ascorbate is an important physiological antioxidant that helps to reduce tocopheroxyl radical to tocopherol. Vitamin C participates in different biological activities concerning electron transport reaction and scavenging ROS, thereby preventing oxidative damage to DNA, lipids, and proteins or regeneration of Vitamin E from α-tocopheroxyl radical.[38,40,41] Thus, Vitamin C protects the cell membrane from external oxidants and cannot be transported efficiently into blood cells. The existence of dihydroxy ascorbic acid has long been considered an evidence of ascorbate oxidation. Depleted serum Vitamin C levels were shown in several conditions related to oxidative stress such as DM, cancer, and CHD.[38,41]
A measured parameter can be used to put in a framework that enables its use as a “risk factor,” to indicate the probability level of developing (AMI) or a “marker,” to indicate the presence and severity of AMI. Researchers have developed the concepts of sensitivity, specificity, and efficiency of tests to assess the effectiveness of a parameter as a diagnostic tool.[42] Sensitivity of a test defines its capability to diagnose a patient and is given by,
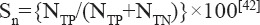
where NTP is the number of successfully diagnosed patients and NTN is the number of successfully identified healthy persons in any sample.
The specificity is a measure of the capability of the test to isolate healthy persons, it is defined as:
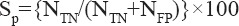
where NFP is the number of healthy persons with the positive test in the sample.
Finally, the efficiency of any test is defined as:
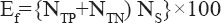
where NS is the total sample size.
Successful implementation of the above measures to any marker requires an accurate estimation of the “cutoff value” or “reference interval” of the marker.[43] It is defined as “the usual value of a healthy population.” Cutoff values can either be identified as the 95th or 99th percentiles of the control group values depending on the accuracy of the test. It could also be chosen to optimize the sensitivity and specificity according to the receiver operator characteristics method.[44]
Conclusion
The imbalance in the oxidative status and antioxidant activity in AMI patients reflects the importance of measuring the level of serum oxidative stress biomarkers and antioxidants as a diagnostic and prognostic tool for medical management of AMI. Estimation of non-enzymatic compounds such as serum uric acid and ceruloplasmin can rule the status of antioxidant defense system which may help in the treatment, prognosis, and prevention of AMI. The study reflects the ability of oxidative stress markers and antioxidants to integrate these parameters in the development of CVD. Therefore, monitoring of serum oxidative stress biomarkers and antioxidants in AMI patients is necessary for the effective management of the disease which may help in the treatment. The grouping of parameters gave tools for use as a risk factor and/or diagnostic markers with efficiency over individual parameters.
References
- 1.Nikolic-Heitzler V, Rabuzin F, Tatzber F, Vrkic N, Bulj N, Borovic S, et al. Persistent oxidative stress after myocardial infarction treated by percutaneous coronary intervention. Tohoku J Exp Med. 2006;210:247–55. doi: 10.1620/tjem.210.247. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad MI, Sharma N. Biomarkers in acute myocardial infarction. J Clin Exp Cardiol. 2012;3:222. [Google Scholar]
- 3.Lien PH, Hua H, Chuong PH. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 4.Rodwell VW, Bender DA, Botham KM, Kennelly PJ, Weil PA. Harper's Illustrated Biochemistry. 30th ed. New York, NY: McGraw-Hill Education; 2015. pp. 111–21. [Google Scholar]
- 5.Subhakumari KN, Reshmy GS, Sajitha KP. Evaluation of antioxidant status in myocardial infarction in diabetic and non-diabetic subjects:A comparative study. Adv Diabetes Metab. 2015;3:1–6. [Google Scholar]
- 6.Muslih RK, AlNimer MS, Yasser OM. The level of malondialdehyde after activation with (H2O2 and CuSO4) and inhibition by desferoxamine and molsidomine in the serum of patients with AMI. Nat J Med. 2002;5:139–48. [Google Scholar]
- 7.Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic Biol Med. 2009;47:1673–706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–92. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta RK, Patel AK, Shah N, Chaudhary AK, Jha UK, Yadav UC, et al. Oxidative stress and antioxidants in disease and cancer:A review. Asian Pac J Cancer Prev. 2014;15:4405–9. doi: 10.7314/apjcp.2014.15.11.4405. [DOI] [PubMed] [Google Scholar]
- 10.He F, Zuo L. Redox roles of reactive oxygen species in cardiovascular diseases. Int J Mol Sci. 2015;16:27770–80. doi: 10.3390/ijms161126059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burtis CA, Ashwood ER, David EB. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th ed. St Louis: Saunders/Elsevier; 2013. pp. 1240–55. [Google Scholar]
- 12.Khoubnasabjafari M, Ansarin K, Jouyban A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts. 2015;5:123–7. doi: 10.15171/bi.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffee PA, Jones ME. A Subsidiary of Harcourt Brace. Vol. 51. New York: Jovanovich Publisher; 1978. Methods in Enzymology; p. 302. [Google Scholar]
- 14.Menden EE, Boiano HM, Murthy L, Petering HG. Modification of phenylene diamine oxidase method to permit non-automated ceruloplasmin determination in batches of rat serum of plasma microsamples. Analytical. 1977;10:197–204. [Google Scholar]
- 15.Shaik IH, Reza M. Rapid determination of reduced and oxidized glutathione levels using a new thiol-masking reagent and the enzymatic recycling method. Anal Bioanal Chem. 2006;1:105–13. doi: 10.1007/s00216-006-0375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wotton LD. Microanalysis in Medical Biochemistry. 5th ed. Edinburgh: Churchill Livingstone; 1974. Estimation of proteins by biuret methods; pp. 156–8. [Google Scholar]
- 17.Baker H, Frank O. Determination of serum tocopherol. Varley's Practical Clinical Biochemistry. 6th ed. Vol. 35. Oxford: Heinemann Medical Books; 1988. p. 902. [Google Scholar]
- 18.Castelli A, Martorana GE, Frasca AM, Meucci E. Colorimetric determination of plasma vitamin C:Comparison between 2,4-dinitrophenylhydrazine and phosphotungstic acid methods (author's transl) Acta Vitaminol Enzymol. 1981;3:103–10. [PubMed] [Google Scholar]
- 19.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease:From molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957–97. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang T, Yuan G, Zhang Z, Zou Z, Li D. Cardiovascular pathogenesis in hyperhomocysteinemia. Asia Pac J Clin Nutr. 2008;17:8–16. [PubMed] [Google Scholar]
- 21.Tsimikas S. In vivomarkers of oxidative stress and therapeutic interventions. Am J Cardiol. 2008;101:34D–42D. doi: 10.1016/j.amjcard.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Esra B, Umit MS, Cansin S. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;51:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamkani WA, Jafar NS, Narayanan SR, Rajappan AK. Acute myocardial infarction in a young lady due to vitamin B12 deficiency induced hyperhomocysteinemia. Heart Views. 2015;16:25–9. doi: 10.4103/1995-705X.152998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senthil S, Verrappan RM, Ramakrishna RM, Pugalendi KV. Oxidative stress and antioxidants in patients with cardiogenic shock complication acute myocardial infarction. Clin Chim Acta. 2004;348:131–7. doi: 10.1016/j.cccn.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, et al. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–97. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katzung BG. Basic and Clinical Pharmacology. 12th ed. New York: MC-Hill Medical Publishing Division; 2012. pp. 326–32. [Google Scholar]
- 27.Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bita O, Fazlolah A, Mohammad A. The prognostic role of serum uric acid level in patients with acute ST elevation myocardial infarction. J Saudi Heart Assoc. 2012;24:73–8. doi: 10.1016/j.jsha.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadkar MY, Jain VI. Serum uric acid in acute myocardial infarction. J Assoc Physicians. 2008;56:759–62. [PubMed] [Google Scholar]
- 30.Madole MB, Bachewar NP, Aiyar CM. Study of oxidants and antioxidants in patients of acute myocardial infarction. Adv Biomed Res. 2015;4:241. doi: 10.4103/2277-9175.168608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venardos KM, Perkins A, Headrick J, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium:A review. Curr Med Chem. 2007;14:1539–49. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- 32.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–90. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 33.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Wilson T, Yuping W, Jaana H. Clinical and Genetic Association of Serum Ceruloplasmin with Cardiovascular Risk. Arterioscler Thromb Vasc Biol. 2012;32:516–22. doi: 10.1161/ATVBAHA.111.237040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panichi V, Taccola D, Rizza GM. Ceruloplasmin and acute phase protein levels are associated with cardiovascular disease in chronic dialysis patients. J Nephrol. 2004;17:715–20. [PubMed] [Google Scholar]
- 36.Dadu RT, Dodge R, Nambi V, Virani SS, Hoogeveen RC, Smith NL, et al. Ceruloplasmin and heart failure in the atherosclerosis risk in communities study. Circ Heart Fail. 2013;6:936–43. doi: 10.1161/CIRCHEARTFAILURE.113.000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX study:A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–42. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 38.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. Arandomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women:Results from the women's antioxidant cardiovascular study. Arch Intern Med. 2007;167:1610–8. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM. Vitamin e in the primary prevention of cardiovascular disease and cancer:The women's health study:A randomized controlled trial. J Am Med Ass. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro DA, Buttros JB, Oshima CT. Ascorbic acid prevents acute myocardial infarction induced by isoproterenol in rats:Role of inducible nitric oxide synthase production. J Mol Histol. 2009;40:99–105. doi: 10.1007/s10735-009-9218-1. [DOI] [PubMed] [Google Scholar]
- 41.Osganian SK, Stampfer MJ, Rimm E, Spiegelman D, Hu FB. Vitamin c and risk of coronary heart disease in women. J Am Coll Card. 2003;42:246–52. doi: 10.1016/s0735-1097(03)00575-8. [DOI] [PubMed] [Google Scholar]
- 42.Mythili S, Malathi N. Diagnostic markers of acute myocardial infarction. Biomed Rep. 2015;3:743–8. doi: 10.3892/br.2015.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pezzilli R, D'Eril G, Morselli A. Serum amyloid A, procalcitonin and c-reactive protein in early assessment of severity of acuter pancreatitis. Digest Dis Sci. 2000;6:1072–8. doi: 10.1023/a:1005525329939. [DOI] [PubMed] [Google Scholar]
- 44.Bishop M, Duben J, Fody E. Clinical Chemistry:Techniques, Principles and Correlations. 6th ed. Philadelphia, USA: Lipincott Williams and Wilkins; 2010. pp. 541–55. [Google Scholar]


