Abstract
Type 2 diabetes is a debilitating disease that impacts the life expectancy, quality of life, and health of an individual. Cardiovascular disease (CVD) is a common diabetes-associated complication and a principal cause for death in diabetic patients. This review aims to investigate and summarize the effect of Type 2 diabetes mellitus (T2DM) medications on CVD issues. A comprehensive literature review mainly from level 1 evidence was performed. Thirty-seven articles were extracted from Google Scholar, ScienceDirect, ProQuest, and PubMed Database using a combination of keywords. The findings suggest that different glucose-lowering agents have been tested for their efficacy and safety in T2DM with CVD. Some of the recent trials such as the “United Kingdom Prospective Diabetes Study,” “Empagliflozin (EMPA) Cardiovascular (CV) Outcome Event Trial in T2DM Patients-Removing Excess Glucose” (EMPA-REG OUTCOME), “Liraglutide Effect and Action in Diabetes: Evaluation of CV Outcome Results,” and “Trial to Evaluate CV and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes” (SUSTAIN6) have shed important light on this vital clinical concern, thus demonstrating a convincing effect of liraglutide, semaglutide, and EMPA on CVD outcomes, while metformin is thought to be the first-line optimal oral agent to manage Type 2 diabetics. Some classes of drugs demonstrate CV protection, some of them may be a result of a class effect, and some differences might be based on the population enrolled individually. Most of the trials failed to show a significant benefit with regard to mortality and morbidity in spite of intensive glycemic control. This study, therefore, enabled us to develop a guide of potential antidiabetic medication that can influence or promote CV health. Health professionals in future should weigh the CV risk against possible advantages while prescribing antidiabetic medications.
Keywords: Antidiabetic drugs, cardiovascular impact, cardiovascular risk, diabetes medications, type 2 diabetes
Introduction
Individuals with type 2 diabetes mellitus (T2DM) face significantly higher risk of developing cardiovascular diseases (CVD) compared to the general population. Diabetes mellitus and CVD are common chronic progressive disorders and major public health issue in Saudi Arabia.[1] The World Health Organization stated that Saudi Arabia ranks 7th globally in terms of the rates of diabetes.[2] One of the major impacts of T2DM is its high incidence of premature deaths and morbidity that primarily results from macrovascular and microvascular complications.[3-5] In the previous years, the condition of diabetes has demonstrated a 10-fold increase among Saudi population.[6] It has been estimated that 2 of 5 diabetic adults are undiagnosed in the Middle East.[7] The resultant shift in the lifestyle and modernization to a more sedentary life with consumption of high-fat diets and obesity are the primary cause of increased diabetes mellitus prevalence within the Saudi community.
According to the Centers for Disease Control and Prevention (CDC),[8] CVD tends to be the principal cause of morbidity and mortality in individuals suffering from T2DM. The medication used in the treatment of T2DM has potential cardiovascular (CV) effect (harmful, beneficial, or neutral). Thus, the Food and Drug Administration (FDA) in 2008/2010 and the “European Medicines Agency” indicated that novel compounds that are mainly being developed for T2DM need to go through different clinical trials to guarantee safety from CVD.[9,10] Moreover, for majority of the antidiabetic drugs, the effects of CV are not as yet clarified.[11] The FDA[9] published a report, namely “Guidance for Industry: Diabetes Mellitus - Evaluating CV Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes,” now demands novel drugs being licensed for diabetes to show that no adverse CV effects are caused during the trials.
Limited evidence is available from the randomized trials with regard to how well T2DM can be treated in the CVD population. The “European Association for the Study of Diabetes and American Diabetes Association” have shown a consensus algorithm for the purpose of managing T2DM, wherein metformin was known to be the initial pharmacologic agent of choice that can be merged with other drugs in triple and double therapy.[12] The study will be of substantial assistance for all medical physicians, who are treating the patients of diabetes such as diabetologists, endocrinologists, internal medicine doctors, primary care physicians, and experienced personnel including young doctors. The study will further help in prioritizing add-on drug choices for managing diabetes. Hence, this article seeks to establish why people suffering from diabetes are at a greater risk of developing CVD and then considers to summarize the impact of type 2 diabetes drugs on CV outcomes.
METHODS
Garrad[13] suggested that an intensive and wide literature on the topic must be in place before conducting any informative literature review. Therefore, a search strategy was performed using the following key words: “CV impact, CV risk, diabetes medications, and antidiabetic drugs” to retrieve relevant evidence from the electronic database selected such as “Google Scholar, ScienceDirect, ProQuest, and PubMed.” Furthermore, Boolean operators such as OR, AND, and NOT were also used for combining both the set of search terms, thus instructing the databases to retrieve relevant material. Primarily, English articles were incorporated. Cross-reference lists and regulatory guidelines were also included in the review.
Consistent with the trend toward “Evidence-Based Medicine,” the level of evidence is also considered to assess the quality of the research articles selected. The studies are primarily assessed based on two dimensions: Study quality and level of evidence. It is an establishment of a hierarchy of the study design based on the ability of the design to protect against bias. Randomized controlled trials (RCTs) are least susceptible to bias and are considered the gold standard for evaluating efficacy in clinical research [Figure 1].
Figure 1.
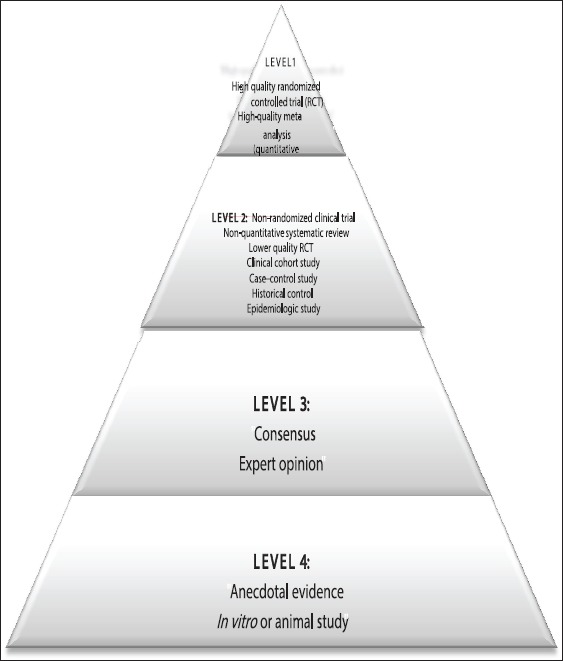
Evidence hierarchy[14]
RESULTS AND DISCUSSION
In the present review, we encountered a range of clinical trials related to the T2DM and CVD-associated risk. Diabetes is a primary risk factor for CVD and its development, such as stroke, hypertension, heart, and vascular, or CV, diseases. Our study retrieved 37 trials summarizing the outcome of T2DM medications on CVD-related outcomes. In total, 37 clinical trials were included (7 trials including thiazolidinediones [TZDs] [with sub-classes pioglitazone and rosiglitazone],[15-21] 2 trials: Biguanides [metformin],[22,23] 4 trials: Second-generation sulfonylureas [glimepiride, glipizide, and glyburide],[24-26] 2 trials: Meglitinides (nateglinide and repaglinide),[27,28] 2 trials: Alpha-glucosidase inhibitors [AGIs] [acarbose, and miglitol],[29,30] 7 trials: Glucagon-like peptide-1 [GLP-1] receptor agonist [exenatide, liraglutide, lixisenatide, albiglutide, dulaglutide, and semaglutide],[31-37] 1 trial: Amylin analogs [pramlintide][38] trial, 5 trials: Dipeptidyl peptidase-4 inhibitors [DPP-IV] [saxagliptin, sitagliptin, linagliptin, and alogliptin],[39-43] 2 trials: Insulins [glargine],[44,45] and 5 trials: Sodium-glucose cotransporter 2 [SGLT2] [canagliflozin, dapagliflozin, and empagliflozin (EMPA)][46-50]).
The findings of the trials demonstrate that T2DM patients have an increased and inherent risk for CVD. CV benefits with metformin are promising with evidence demonstrating reduction to some extent in diabetes-associated deaths, diabetes-associated end points, along with all-cause mortality.[22] Studies for the sulfonylureas (SUs) therapy and its safety are still conflicting; however, when compared with metformin, SU use is concomitant to an increased risk for causing heart failure (HF), explicitly at a higher dose.[25,26] Pioglitazone is also known to reduce the composite of stroke and non-fatal myocardial infarction (MI) besides all-cause mortality in T2DM patients who are at a high risk for macrovascular event.[15] While, use of TZDs predominantly rosiglitazone is contra-indicated in HF patients as it is well known to intensify HF-related risk.[18,20] On the other hand, DPP-4 and GLP-1 agonist are known to potentially show positive effects on the CV system.[31-37,39-43] Exenatide, a GLP-1 analog, is linked with a substantial decline in the CVD-related risk and hospitalization among individuals with T2DM.[32] Although “SGLT2 inhibitors” are unique glucose-lowering oral agents, the potential for CVD-related benefits from the SGLT2 inhibitors remains to be established.[46,47] A large body of evidence specifies a different multifactorial approach to the care of diabetes that mainly targets glycemic control besides treatment of dyslipidemia as well as hypertension that will considerably reduce the CVD-related risk. Hence, the findings obtained from the ongoing clinical trials will further provide indications regarding CV safety in addition to diabetic drug efficacy. Such trials will most likely assist to further refine the therapeutic guideline in the near future. Tables 1-10 summarize key diabetes and CV trials. The results of each selected trials are discussed in detail in the sections below.
Table 1.
Cardiovascular outcome trials for antidiabetics (TZDs) in type 2 diabetes
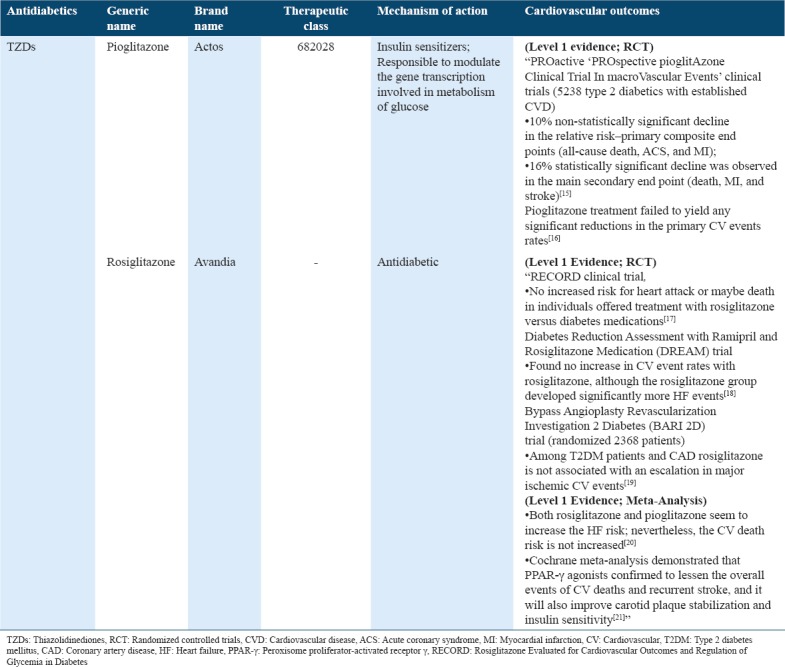
Table 2.
Cardiovascular outcome trials for antidiabetics (biguanides) in type 2 diabetes
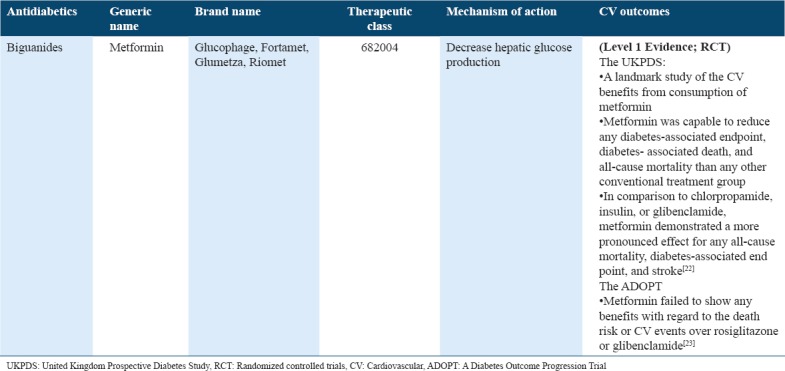
Table 3.
Cardiovascular outcome trials for antidiabetics (sulfonylureas - second generation) in type 2 diabetes
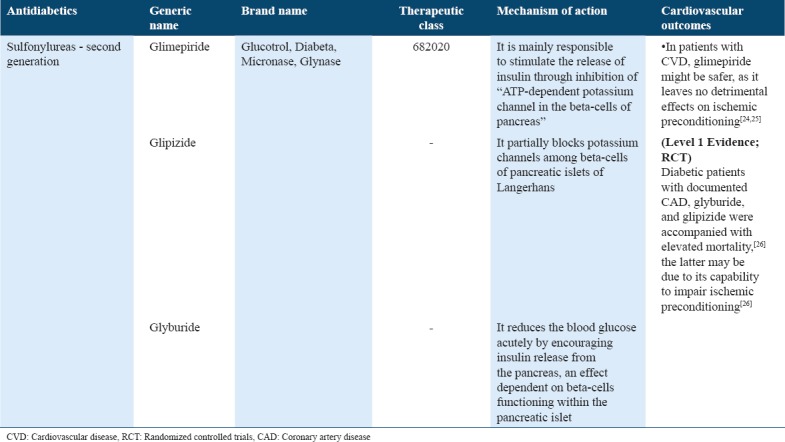
Table 4.
Cardiovascular outcome trials for antidiabetics (meglitinides) in type 2 diabetes
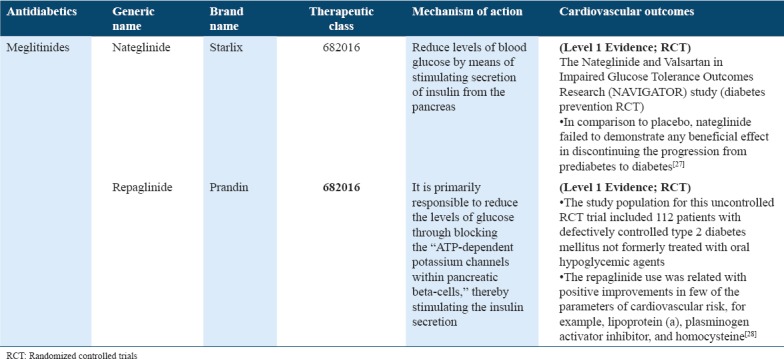
Table 5.
Cardiovascular outcome trials for antidiabetics (A - glucosidase inhibitors) in type 2 diabetes
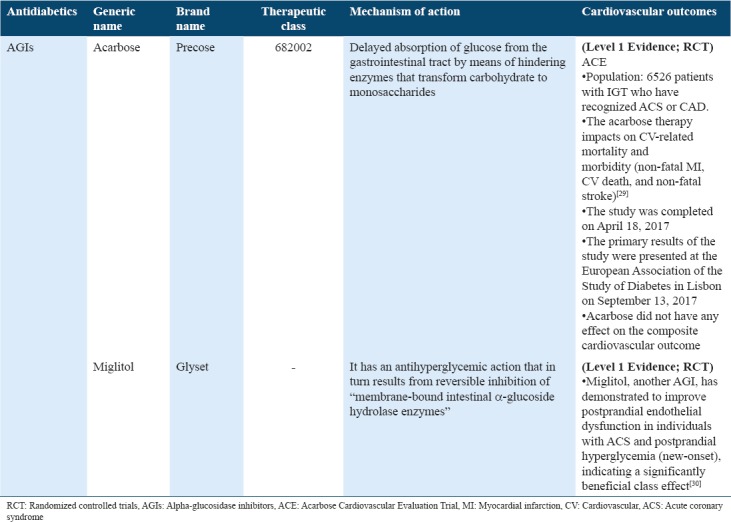
Table 6.
Cardiovascular outcome trials for antidiabetics (glucagon-like peptide-1 receptor agonist) in type 2 diabetes
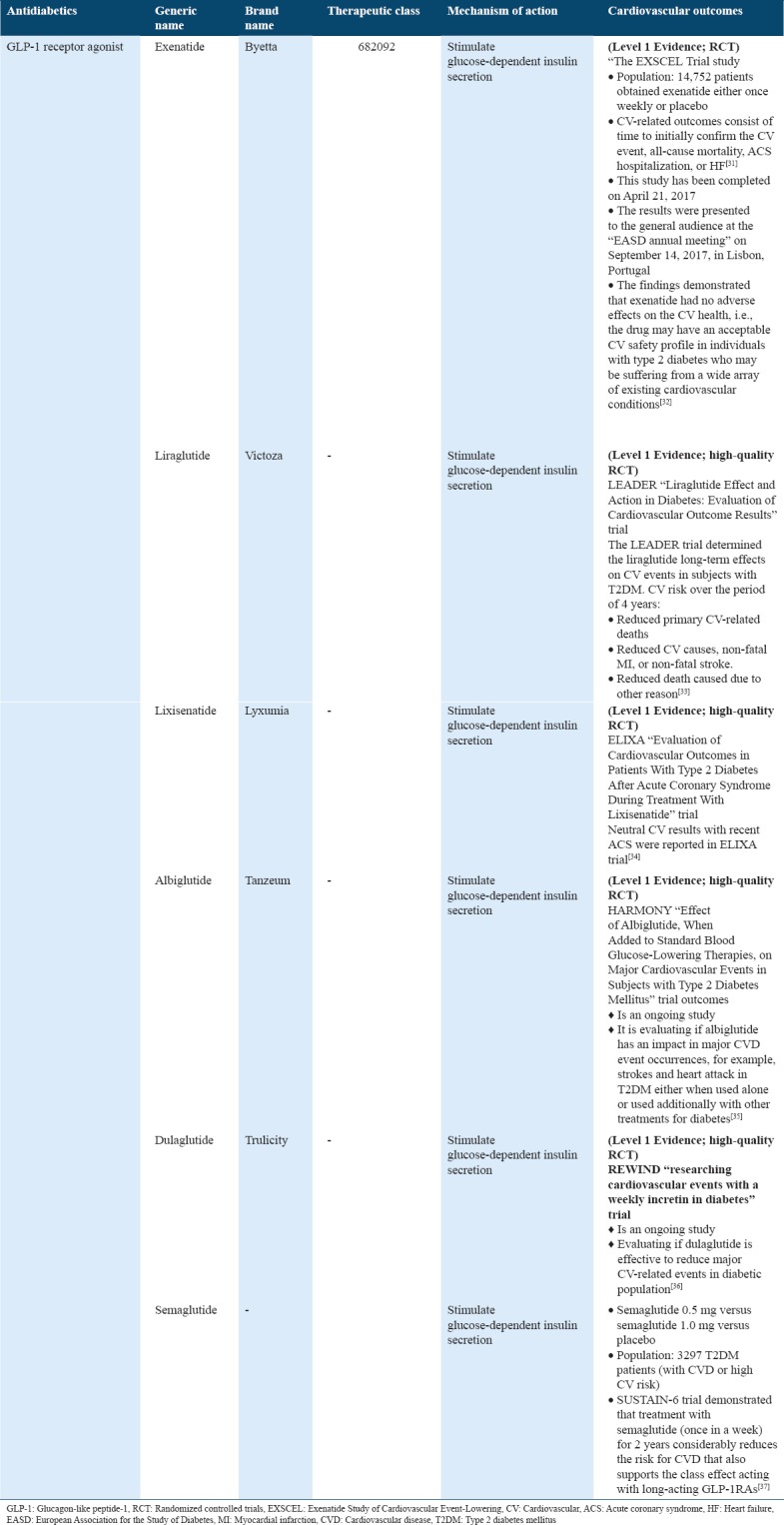
Table 7.
Cardiovascular outcome trials for antidiabetics (amylin analogs) in type 2 diabetes
Table 8.
Cardiovascular outcome trials for antidiabetics (DPP-IV inhibitors) in type 2 diabetes
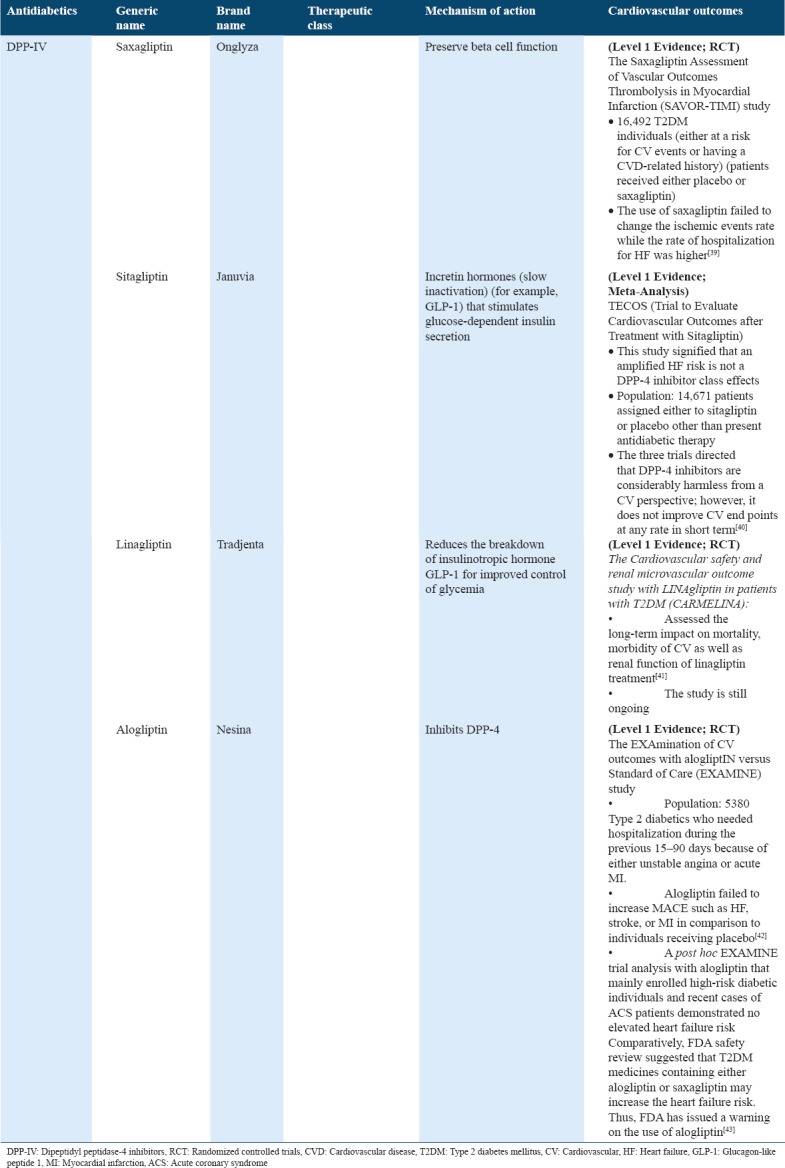
Table 9.
Cardiovascular outcome trials for insulins in type 2 diabetes
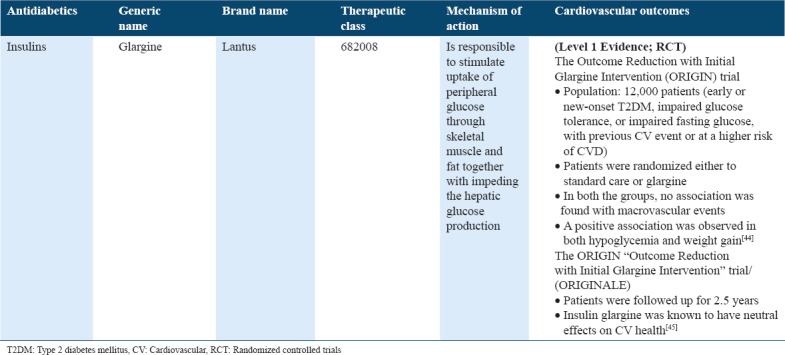
Table 10.
Cardiovascular outcome trials for antidiabetics (sodium-glucose cotransporter 2) in type 2 diabetes
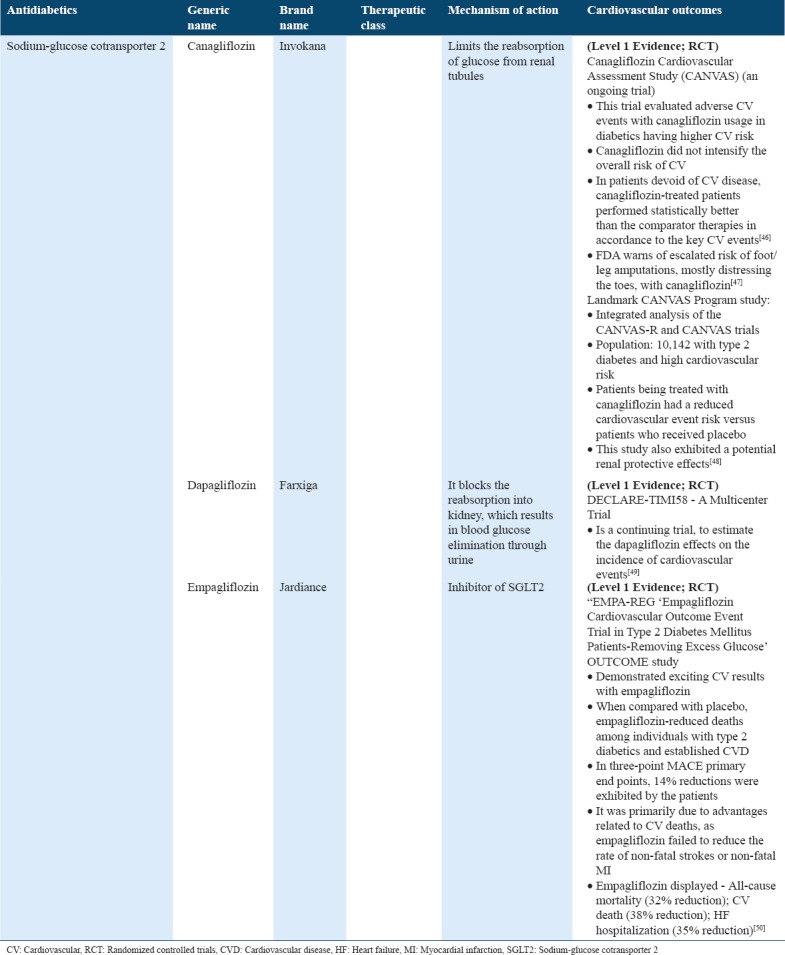
TZDs
TZDs are known to act as insulin sensitizers and ligands of the “transcription factor peroxisome proliferator-activated receptor γ (PPAR-γ). The ‘Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication’ trial found no increase in the rates of CV event with rosiglitazone, while the rosiglitazone group significantly developed supplementary HF events.[18] On the other hand, ‘Rosiglitazone Evaluated for CV Outcomes and Regulation of Glycemia in Diabetes’ (RECORD) trial confirmed that rosiglitazone fails to increase the risk of overall mortality and morbidity, whereby confirming an elevated HF risk.[51] Issues with regard to data integrity and design led FDA to call for an independent reevaluation of data RECORD, which reported comparable results.[17] Another meta-analysis revealed that rosiglitazone is linked with a significant increase in the MI risk as well as deaths caused due to CV.[52] RECORD clinical trial exhibited no increased risk of heart attack or death in individuals who were provided rosiglitazone versus diabetes medications as a treatment[17] [Table 1].”
“PROspective pioglitAzone Clinical Trial In macroVascular Events” trials recruited 5238 type 2 diabetics with established CVD. Results demonstrated a non-statistically significant 10% relative risk (RR) reduction within primary composite end-points (MI, acute coronary syndrome [ACS], and all-cause death); and 16% statistically significant decline was observed for the core secondary end point (MI, death, and plus stroke).[15] Moreover, treatment with pioglitazone failed to produce any substantial reductions in the primary CV event rate.[16] On the contrary, both rosiglitazone and pioglitazone seem to increase the HF risk; nevertheless, the risk of death caused due to CV is not increased.[20] A Cochrane meta-analysis considerably demonstrated that PPAR-γ agonists tend to lessen the overall events of CV deaths and recurrent stroke. However, it considerably improved carotid plaque stabilization and insulin sensitivity[21] [Table 1].
Biguanides
One of the biguanides, metformin is widely consumed in treating diabetes and is one of the first-line treatments for majority of the patients. If it is administered at a lower dose, gently titrated, metformin can be well tolerated. It has been potentially associated with losing weight. With respect to the United Kingdom Prospective Diabetes Study (UKPDS) results, it is a well-known cardioprotective drug. Individuals being treated with metformin often have a reduced risk of diabetes-related deaths, any diabetes-related clinical end point, in addition to all-cause mortality that was considered to be primary outcome measures.[8] Consequently, some of the individuals in the metformin-treated arm of the work (just over 300) led to a review of additional studies, where this drug was used for determining that whether being cardioprotective is justified.[53] The study supported the metformin use for its cardioprotective properties over and above its role as an oral hypoglycemic agent. Hence, this drug makes it an ideal first choice for population with type 2 diabetes, explicitly holding a less purchase cost. On the other hand, “A Diabetes Outcome Progression Trial” trial failed to show any benefit with regard to the CV events and the risk of deaths over rosiglitazone or glibenclamide.[9] Glimepiride, however, might be safe in CVD patients as it has no detrimental effects on ischemic preconditioning as demonstrated in Table 2.[24,25]
Second-generation sulfonylureas
If strengthening of treatment is required for improving glycemic control along with getting HbA1c to target, several physicians offer SUs as a second-line treatment in accordance to the National Institute for Health and Care Excellence guidelines.[4] SUs are associated to hypoglycemia and weight gain, due to which it might not be an ideal choice, exclusively as several other drug classes have become available. Frier et al.[54] suggested that hypoglycemia may lead to an amplified risk of CV events. It is, therefore, argued that medications with a higher risk of triggering hypoglycemia need to be avoided. With regard to the SUs effect on CV health, limited clarity is available. Few of the studies demonstrated no detrimental effects on ischemic preconditioning by glimepiride.[24,25] Others offer a more reassuring picture with an increase in CVD events, possibly associated with an increased hypoglycemia risk[28] [Table 3].
Meglitinides
In comparison to SUs, the meglitinides are structurally different as they apply their effects through sulfonylurea receptor-1. It acts likewise through regulating ATP-dependent potassium channels in pancreatic beta-cells, thus increasing insulin secretion. Repaglinide fails to be concomitant to elevated mortality and risk of CVD as compared to metformin in one of the large cohort of diabetics either without or with MI.[55] One uncontrolled randomized study involved 112 patients with insufficiently controlled type 2 diabetes that were not treated formerly with oral hypoglycemic agents. The repaglinide use was likely associated with positive outcomes in most of the CVD risk parameters, for example, homocysteine, lipoprotein (a), and plasminogen activator inhibitor.[56] Another “The Nateglinide and Valsartan in Impaired Glucose Tolerance (IGT) Outcomes Research” trial showed that nateglinide failed to demonstrate any beneficial result in discontinuing the progression from prediabetes to diabetes compared to placebo [Table 4].[27]
AGIs
AGIs are a unique class of drugs that tend to inhibit the carbohydrate absorption from gut and might be utilized in treating T2DM patients or people suffering from IGT. AGIs are drugs that prevent absorption of carbohydrates from the gut and it might be utilized in the management and handling of individuals with type 2 diabetes or IGT. Acarbose CV Evaluation Trial (ACE) trial reported that acarbose can prevent individuals with IGT and coronary heart disease (CHD) from dying or experiencing additional strokes or heart attacks. This trial further reported if acarbose reduces blood glucose following a meal can either delay or prevent people progressing from IGT to type 2 diabetes.[29] The results of the trial failed to show superiority for the primary end point, without reductions observed with acarbose in the risk of major adverse CV events. Conversely, it demonstrated a significant 18% reduction in the new-onset incidence of diabetes cases with acarbose than placebo.[56] Thus, ACE offers reassurance that acarbose can be safely consumed for improving the glucose levels in individuals with impaired glucose regulation and CHD without impacting the rates of HF or CV complications. Thus, a reduced incidence of diabetes observed in this trial may, however, help in reducing the risk for CV conditions in future by delaying the diabetes onset among high-risk population under study.[56] These findings extended the knowledge of the acarbose safety as well as efficacy for delaying the diabetes onset in a population with both CHD and IGT. Conversely, miglitol, another AGI, showed improved postprandial endothelial dysfunction in ACS patient other than new-onset postprandial hyperglycemia, thus referring to a potential use of class effect[30] [Table 5].
GLP-1 receptor agonist (GLP-1RA)
The GLP-1 actions are mediated through GLP-1R mainly expressed in peripheral tissues in addition to pancreatic islet cells. Consequently, this wide distribution of GLP-1R and GLP-1 tends to exert extrapancreatic actions which have beneficial effects on CNS, gastrointestinal, and CV systems.[57] At present, the GLP-1 RA that is accepted for type 2 diabetes treatments includes extended-release exenatide, dulaglutide, exenatide, lixisenatide, liraglutide and albiglutide.[58] The Exenatide Study of CV Event-Lowering Trial is a phase 3 randomized placebo-controlled trial, which determines the outcomes of CV after exenatide use. An estimated 14,752 individuals were randomized and obtained exenatide either on one occasion weekly or placebo. CV-related outcomes entail time to initially confirm CV-related event, all-cause mortality, ACS hospitalization, or HF.[31] The results show Type 2 diabetes, without or with prior CVD conditions, and the incidence of major adversative CV events did not significantly vary between individuals who received exenatide compared to those receiving placebo.[32] The “Liraglutide Effect and Action in Diabetes: Evaluation of CV Outcome Results” (LEADER) trial demonstrated the long-term liraglutide effect on CV events in T2DM patients. CV risk over the period of 4 years dropped significantly.[3] The “Evaluation of CV Outcomes in Patients With Type 2 Diabetes After ACS During Treatment With Lixisenatide” (ELIXA) trial demonstrated counting lixisenatide to conventional therapies in diabetic patients with a recent ACS holding neutral effect on CV-related outcomes.[34] Conversely, “Effect of Albiglutide, When Added to Standard Blood Glucose-Lowering Therapies, on Major CV Events in Subjects with T2DM” (HARMONY) trial outcomes and “Researching CV Events With a Weekly INcretin in Diabetes” trials are still ongoing.[35,36] Marso et al.[37] addressed a lower CV event rate among T2DM patients who received semaglutide (GLP-1 analog) than individuals receiving placebo. The results of this trial were consistent with the LEADER trial that examined the effects of liraglutide [Table 6].
Amylin analogs
Amylin analogs are injectable medication which are being consumed for treating diabetics and are considerably taken before meals and work correspondingly to the amylin hormone. CV safety evaluation with pramlintide was performed using accepted regulatory medical AE definitions in five of the previous randomized controlled phase and four trials of 16–52 weeks duration in type 2 diabetes adults. Mealtime pramlintide with insulin demonstrated no increase in the risk for CV AEs type 2 diabetics using insulin[38] [Table 7].
DPP-IV
The DPP-IV inhibitors belong to the antihyperglycemic agent class that is shown to improve the glycemic control in type 2 diabetics. Initially, there were no concerns with reference to the risk of CVD in individuals taking DPP4 inhibitors. Currently published data about HF admissions among individuals using DPP4 inhibitors revealed that such a risk should be considered before initiating individuals on treatment with gliptin. Other studies also showed that inherent difference in drugs is observed with the DPP4 inhibitor class, which may be influenced, if the risk of HF exists. “CV and Renal Microvascular Outcome Study with Linagliptin in Patients with T2DM” (CARMELINA) study was designed to assess the long-term CV influence on CV mortality, morbidity, in addition to the renal function of treatment with linagliptin.[41] Alternatively, “Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus Thrombolysis in MI” study found an increased number of HF admissions of individuals on saxagliptin, while “Trial Evaluating CV Outcomes with Sitagliptin”[40] study reported that sitagliptin fails to have a similar effect.[40] The impact of alogliptin on patient with high CVD risk was assessed in the “Examination of CV Outcomes with Alogliptin versus Standard of Care” (EXAMINE) study. The study found evidence of CV harm while no increase in HF was stated. This study also failed to demonstrate any benefit. Conversely, a recent FDA safety review suggested that T2DM medicines containing either alogliptin or saxagliptin may increase the HF risk. Thus, FDA has issued a warning on the use of alogliptin and saxagliptin mainly in patients who already have either a kidney or heart disease[59] [Table 8].
Insulins
The insulin resistance in Type 2 diabetics is accompanied with higher insulin levels, which has also been associated with the risk of CVD. It has been demonstrated that if insulin is associated with higher CVD risk, then exogenous insulin might do the same.[60] While only limited evidence exists to determine if it is true. Another evidence associates hypoglycemic events to CVD though, indicating that the use of insulin is linked with hypoglycemia risk. Hence, it is believed that individuals being offered insulin treatment may be at an elevated CVD risk.[54] The Outcome Reduction with Initial Glargine Intervention trial together with long-acting insulin glargine randomized 12,000 patients (to standard care or glargine) with early or new T2DM, IGT, or impaired fasting glucose, with higher CVD risk and prior event of CVD. No association was demonstrated with macrovascular events in both the groups. A positive association was found in both hypoglycemia and weight gain[45] [Table 9].
SGLT2
SGLT2 inhibitors are one of the latest class of oral hypoglycemic agents which were introduced in the diabetes market. SGLT2 inhibitors tend to inhibit the glucose reabsorption through kidneys so that it can be excreted in the urine. Such a glucose loss in the urine reduces the levels of blood glucose and consequently results in the loss of weight.[61] SGLT2 inhibitors, as the latest market additions, had to meet the FDA standards and demonstrated the evidence of CVD risk[62] [Table 10]. EMPA-REG reported the long term EMPA effects versus placebo on the mortality and morbidity in about 7000 patients with type 2 diabetes together with higher CVD events. Results indicated that EMPA limited the RR for 3-point MACE (non-fatal stroke, non-fatal MI, and time to initial incidence of CVD death) by 14%. It was also known to reduce the RR of hospitalization for HF by 35%, survival improvements by reducing RR of all-cause mortality by 32%, and reduced CV death RR by 38%[50] [Table 10]. It was likely that this study tends to raise the SGLT2 inhibitor consumptions, specifically in groups at higher risk following the diabetic ketoacidosis warning among individuals on SGLT2 inhibitors that came last year.[63] Overall, additional experience and research with SGLT2 inhibitors are warranted in future.
A canagliflozin CV assessment study (CANVAS) program and landmark study currently published in the New England Journal of Medicine[48] combined data from two of the trials CANVAS-R and CANVAS, which involved recruiting of a total of 10,142 diabetic and cardiac patients. The CANVAS patients were randomized 1:1:1 to canagliflozin 300 mg or 100 mg or placebo, while CANVAS-R patients were randomized to placebo or 100 mg (having an option to elevate to 300 mg after 13 weeks). The results demonstrated that INVOKANA® (canagliflozin) considerably reduces the combined risk of non-fatal stroke, MI, and CV death versus placebo in diabetic patients either with a CV disease history or at risk. Thus, treatment with canagliflozin is often associated with a reduced hospitalization from HF risk and also showed protective renal effects. However, FDA issued warning of escalated risk of foot/leg amputations mostly distressing the toes, with canagliflozin.[59]
CONCLUSION
This study highlighted issues related to CVD risk and diabetes and has also identified possible concern areas while prescribing blood glucose-lowering therapies with respect to its impact on the risk of CVD. Diabetes is thought to be a multifaceted illness that considerably carries a significant burden with regard to the complications of CVD. Even though, medication and lifestyle interventions aimed at treating blood pressure and lipids are of great significance during diabetes management, considerably glycemia control is also required for reducing such a risk. There are a number of classes of drugs available for treating high blood glucose levels. Most of the trials failed to demonstrate a significant benefit with regard to mortality and morbidity in spite of intensive glycemic control. Some of the recent trials such as EMPA-REG OUTCOME, UKPDS, SUSTAIN6, and LEADER have shed important light on this vital clinical concern, thus demonstrating a convincing effect of liraglutide, semaglutide, and EMPA on CVD outcomes. Trials are ongoing for CVD safety in novel classes of drugs. Drugs within classes often provide different levels of risk. Personalized care should take account of tailored treatment plans for every patient. Hence, physicians need to be aware of the benefits and risk of such therapies with respect to the CV outcomes and impact. Since the findings obtained from trials are uncertain or contradictory. Therefore, it is essential to tailor all therapies to every individual case by case, and physicians should be vigilant of the new findings from different clinical trials as they become available. As diabetic patients are at an increased risk for CV mortality and morbidity, considering the CV safety of such drugs unquestionably constitutes knowledge of vital importance for all clinicians dealing with this condition in the future.
References
- 1.Bahijri SM, Jambi HA, Al Raddadi RM, Ferns G, Tuomilehto J. The prevalence of diabetes and prediabetes in the adult population of Jeddah, Saudi Arabia–a community-based survey. PLoS One. 2016;11:e0152559. doi: 10.1371/journal.pone.0152559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert AA, Al Dawish MA, Braham R, Musallam MA, Al Hayek AA, Al Kahtany NH, et al. Type 2 diabetes mellitus in saudi arabia:Major challenges and possible solutions. Curr Diabetes Rev. 2017;13:59–64. doi: 10.2174/1573399812666160126142605. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Dept. of Noncommunicable Disease Surveillance:WHO. 1999. [[Last accessed on 2017 Jul 30]]. Available from: http://www.apps.who.int/iris/bitstream/10665/66040/1/WHO_NCD_NCS_99.2.pdf.ucd4r4 .
- 4.National Institute for Health and Care Excellence. Type 2 diabetes in Adults:Management of type 2 Diabetes in Adults. 2015. [[Last accessed 0n 2017 Jul 30]]. Available from: http://www.tinyurl.com/jucd4r4 .
- 5.British Cardiac Society, British Hypertension Society, Diabetes UK, HEART UK, Primary Care Cardiovascular Society, Stroke Association. et al. JBS 2:Joint british societies'guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(Suppl 5):1–52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Dawish MA, Robert AA, Braham R, Al Hayek AA, Al Saeed A, Ahmed RA, et al. Diabetes mellitus in Saudi Arabia:A Review of the recent literature. Curr Diabetes Rev. 2016;12:359–68. doi: 10.2174/1573399811666150724095130. [DOI] [PubMed] [Google Scholar]
- 7.Lindström J, Peltonen M, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinänen-Kiukaanniemi S, et al. Improved lifestyle and decreased diabetes risk over 13 years:Long-term follow-up of the randomised finnish diabetes prevention study (DPS) Diabetologia. 2013;56:284–93. doi: 10.1007/s00125-012-2752-5. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention, National diabetes Fact Sheet:National Estimates and General Information on Diabetes and Prediabetes in the United States. 2011 [Google Scholar]
- 9.Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Guidance for Industry:Diabetes Mellitus-Evaluating Cardiovascular Risk in New Antidiabetic Therapies To Treat Type 2 Diabetes. 2008. [[Last accessed on 2017 Jul 30]]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf .
- 10.European Medicine Agency, Committee for Medicinal Products for Human Use, Guideline on Clinical Investigation of Medicinal Products in the Treatment of Diabetes Mellitus. 2010. [[Last accessed on 2017 Jul 30]]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/02/WC500073570.pdf .
- 11.Paneni F 2013 ESC/EASD guidelines on the management of diabetes and cardiovascular disease:Established knowledge and evidence gaps. Diab Vasc Dis Res. 2014;11:5–10. doi: 10.1177/1479164113512859. [DOI] [PubMed] [Google Scholar]
- 12.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015:A patient-centered approach:Update to a position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2015;38:140–9. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 13.Garrard J. Health Sciences Literature Review Made Easy. United States: Jones and Bartlett Publishers; 2016. [Google Scholar]
- 14.Siwek J, Gourlay ML, Slawson DC, Shaughnessy AF. How to write an evidence-based clinical review article. Am Fam Physician. 2002;65:251–8. [PubMed] [Google Scholar]
- 15.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macroVascular events):A randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 16.Yoshii H, Onuma T, Yamazaki T, Watada H, Matsuhisa M, Matsumoto M, et al. Effects of pioglitazone on macrovascular events in patients with type 2 diabetes mellitus at high risk of stroke:The PROFIT-J study. J Atheroscler Thromb. 2014;21:563–73. [PubMed] [Google Scholar]
- 17.Mahaffey KW, Hafley G, Dickerson S, Burns S, Tourt-Uhlig S, White J, et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J. 2013;166:240–90. doi: 10.1016/j.ahj.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Punthakee Z, Alméras N, Després JP, Dagenais GR, Anand SS, Hunt DL, et al. Impact of rosiglitazone on body composition, hepatic fat, fatty acids, adipokines and glucose in persons with impaired fasting glucose or impaired glucose tolerance:A sub-study of the DREAM trial. Diabet Med. 2014;31:1086–92. doi: 10.1111/dme.12512. [DOI] [PubMed] [Google Scholar]
- 19.Bach RG, Brooks MM, Lombardero M, Genuth S, Donner TW, Garber A, et al. Rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the bypass angioplasty revascularization investigation 2 diabetes (BARI 2D) trial. Circulation. 2013;128:785–94. doi: 10.1161/CIRCULATIONAHA.112.000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones:A meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–36. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Wang LN. Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack. Cochrane Db Syst Rev. 2014;1:CD010693. doi: 10.1002/14651858.CD010693.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Effect of Intensive Blood-Glucose Control with Metformin on Complications in Overweight Patients with Type 2 Diabetes (UKPDS 34). UK prospective diabetes study (UKPDS) group. Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 23.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 24.Basit A, Riaz M, Fawwad A. Glimepiride:Evidence-based facts, trends, and observations (GIFTS). [corrected] Vasc Health Risk Manag. 2012;8:463–72. doi: 10.2147/HIV.S33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klepzig H, Kober G, Matter C, Luus H, Schneider H, Boedeker KH, et al. Sulfonylureas and ischaemic preconditioning;a double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J. 1999;20:439–46. doi: 10.1053/euhj.1998.1242. [DOI] [PubMed] [Google Scholar]
- 26.Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Jain A, et al. Increase in overall mortality risk in patients with type 2 diabetes receiving glipizide, glyburide or glimepiride monotherapy versus metformin:A retrospective analysis. Diabetes Obes Metab. 2012;14:803–9. doi: 10.1111/j.1463-1326.2012.01604.x. [DOI] [PubMed] [Google Scholar]
- 27.NAVIGATOR Study Group. Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463–76. doi: 10.1056/NEJMoa1001122. [DOI] [PubMed] [Google Scholar]
- 28.Schramm TK, Gislason GH, Vaag A, Rasmussen JN, Folke F, Hansen ML, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction:A nationwide study. Eur Heart J. 2011;32:1900–8. doi: 10.1093/eurheartj/ehr077. [DOI] [PubMed] [Google Scholar]
- 29.ACE Acarbose Cardiovascular Evaluation Trial. [[Last accessed on 2017 Jul 30]]. Available from: http//:www.ClinicalTrials.govidentifierNCT00829660 . http://www.clinicaltrials.gov/ct2/show/NCT00829660?term=ACE+trial&rank=2 .
- 30.Kitano D, Chiku M, Li Y, Okumura Y, Fukamachi D, Takayama T, et al. Miglitol improves postprandial endothelial dysfunction in patients with acute coronary syndrome and new-onset postprandial hyperglycemia. Cardiovasc Diabetol. 2013;12:92. doi: 10.1186/1475-2840-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EXSCEL Exenatide Study of Cardiovascular Event Lowering Trial:A Trial to Evaluate Cardiovascular Outcomes After Treatment with Exenatide Once Weekly in Patients with Type 2 Diabetes Mellitus. Clinical Trials.Gov Identifiernct. [[Last accessed on 2017 Jul 30]]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01144338 .
- 32.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 35.ClinicalTrials.gov. Effect of Albiglutide, When Added to Standard Blood Glucose Lowering Therapies, on Major Cardiovascular Events in Subjects with Type 2 Diabetes. 2016. [[Last accessed on 2017 Aug 9]]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02465515?term =albiglutide+cardiovascular&rank=1 .
- 36.ClinicalTrials.gov. Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) 2016. [[Last accessed on 2017 Aug 9]]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01394952?term =dulaglutide+cardiovascular&rank=1 .
- 37.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann K, Zhou M, Wang A, de Bruin TWA. Cardiovascular safety assessment of pramlintide in type 2 diabetes:Results from a pooled analysis of five clinical trials. Clin Diabetes Endocrinol. 2016;2:12. doi: 10.1186/s40842-016-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 40.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov, Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus (CARMELINA). NCT01897532. 2013. [[Last accessed on 2017 Aug 9]]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01897532?term=CARMELINA&rank=1 .
- 42.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 43.FDA. FDA Drug Safety Communication:FDA Adds Warnings About Heart Failure Risk to Labels of Type 2 Diabetes Medicines Containing Saxagliptin and Alogliptin, U.S. Food and Drug Administration. 2017. [[Last accessed on 2017 Aug 9]]. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm4↡6.htm .
- 44.ORIGIN Trial Investigators. Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 45.ORIGIN Trial Investigators. Cardiovascular and other outcomes postintervention with insulin glargine and omega-3 fatty acids (ORIGINALE) Diabetes Care. 2016;39:709–16. doi: 10.2337/dc15-1676. [DOI] [PubMed] [Google Scholar]
- 46.Haas B, Eckstein N, Pfeifer V, Mayer P, Hass MD. Efficacy, safety and regulatory status of SGLT2 inhibitors:Focus on canagliflozin. Nutr Diabetes. 2014;4:e143. doi: 10.1038/nutd.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FDA. Canagliflozin (Invokana®, Invokanamet®):Drug Safety Communication–Clinical Trial Results Find Increased risk Of Leg and Foot Amputations. [[Last accessed on 2017 Jul 30]]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm500965.htm .
- 48.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 49.Bailey CJ. Interpreting adverse signals in diabetes drug development programs. Diabetes Care. 2013;36:2098–106. doi: 10.2337/dc13-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 51.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD):A multicentre, randomised, open-label trial. Lancet. 2009;373:2125–35. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 52.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 53.Batchuluun B, Sonoda N, Takayanagi R, Inoguchi T. The cardiovascular effects of metformin:Conventional and new insights. J Endocrinol Diabetes Obes. 2014;2:1035. [Google Scholar]
- 54.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34(Suppl 2):S132–7. doi: 10.2337/dc11-s220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derosa G, Mugellini A, Ciccarelli L, Crescenzi G, Fogari R. Comparison of glycaemic control and cardiovascular risk profile inpatients with type2 diabetes during treatment with either repaglinide or metformin. Diabetes Res Clin Pract. 2003;60:161–9. doi: 10.1016/s0168-8227(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 56.Holman RR, Coleman RL, Chan JCN, Chiasson JL, Feng H, Ge J, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE):A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:877–86. doi: 10.1016/S2213-8587(17)30309-1. [DOI] [PubMed] [Google Scholar]
- 57.Seufert J, Gallwitz B. The extra-pancreatic effects of GLP-1 receptor agonists:A focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes Metab. 2014;16:673–88. doi: 10.1111/dom.12251. [DOI] [PubMed] [Google Scholar]
- 58.Ryan D, Acosta A. GLP-1 receptor agonists:Nonglycemic clinical effects in weight loss and beyond. Obesity (Silver Spring) 2015;23:1119–29. doi: 10.1002/oby.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. [[Last accessed on 2017 Jul 30]];FDA. FDA Drug Safety Communication:FDA Adds Warnings About Heart Failure Risk to Labels of Type 2 Diabetes Medicines Containing Saxagliptin and Alogliptin, U.S. Food and Drug Administration. 2017 Available from: https://www.fda.gov/Drugs/DrugSafety/ucm4↡6.htm . [Google Scholar]
- 60.Muis MJ, Bots ML, Grobbee DE, Stolk RP. Insulin treatment and cardiovascular disease;friend or foe?A point of view. Diabet Med. 2005;22:118–26. doi: 10.1111/j.1464-5491.2004.01416.x. [DOI] [PubMed] [Google Scholar]
- 61.Valentine V. The Role of the kidney and sodium-glucose cotransporter-2 inhibition in diabetes management. Clin Diabetes. 2012;30:151–5. [Google Scholar]
- 62.Food and Drug Administration. Guidance for Industry:Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. 2008. [[Last accessed on 2017 Jul 30]]. Available from: http://www.tinyurl.com/z9yyoj5 .
- 63.European Medicines Agency. SGLT2 inhibitors. 2016. [[Last accessed on 2017 Jul 30]]. Available from: http://www.tinyurl.com/hr2rq82 .


