Abstract
Atypical antipsychotics (AAPs) are increasingly used for the treatment of psychotic disorders but are known to be associated with metabolic abnormalities. This study is a systematic review and meta-analysis of randomized controlled trials (RCTs) studying the effectiveness of melatonin for the amelioration of AAP-induced metabolic syndrome. The MEDLINE (accessed via PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials, PsycINFO, LILACS, CINAHL, and OpenGrey databases were searched for RCTs without language restrictions. Inclusion criteria were randomized, double-blind clinical trials comparing melatonin or melatonin agonists with placebo for the amelioration of AAP-induced effects at any age with selected components of metabolic syndrome as outcome measures. Two reviewers independently selected articles and assessed quality using Cochrane risk of bias and concealment tools. Of 53 records, five RCTs were eligible for the systematic review and three for the meta-analysis. The meta-analyses showed no statistically significant difference in any anthropometric or metabolic variable considered. Analysis according to psychiatric diagnosis from one RCT showed significant decreases in diastolic blood pressure (5.5 vs. −5.7 mmHg for the placebo and melatonin groups, respectively; p=0.001), fat mass (2.7 vs. 0.2 kg, respectively; p=0.032), and triglycerides (D) (50.1 vs. −20 mg/dl, respectively; p=0.08) in the bipolar group but not the schizophrenia group. Although limited to five RCTs with small sample sizes, evidence from RCT indicates that melatonin improves AAP-induced metabolic syndrome. This beneficial effect seems more significant in patients with bipolar disorder than those with schizophrenia. Further RCTs are needed to definitively establish the potential ameliorative effect of melatonin and to justify its efficacy as an add-on therapy to curtail AAP-induced metabolic syndrome.
Keywords: Melatonin, Atypical anti-psychotics, Metabolic syndrome, Systematic review, Meta-analysis
INTRODUCTION
The second-generation antipsychotics (SGAs), also known as atypical antipsychotics (AAPs), are increasingly used to treat psychiatric disorders. They are rapidly becoming a favorite first choice for this indication, mainly due to their low propensity to cause extrapyramidal adverse effects and favorable efficacy in ameliorating both positive and negative symptoms.1) Some studies have, however, shown that these novel compounds are associated with metabolic abnormalities characterized by hyperglycemia, dyslipidemia, and an increased risk of diabetes mellitus.2,3) Metabolic syndrome is characterized by the symptoms of central obesity, insulin resistance, atherogenic dyslipidemia, hypertension, ischemic heart disease (IHD), and overall mortality.4,5) Raised triglyceride (TG) levels, decreased high-density lipoproteins (HDL), elevated blood pressure (BP), and diabetes mellitus constitute the important criteria for metabolic syndrome.6)
According to criteria established by the National Heart, Lung, and Blood Institute and the American Heart Association,7) an individual is defined as having metabolic syndrome if he/she has at least three of the following: waist circumference >102 cm in males and >88 cm in females, plasma TGs >150 mg/dl, plasma HDL <40 mg/dl, BP >130/85 mmHg, and fasting glucose >110 mg/dl. Metabolic side effects seen with long-term intake of AAPs include increases in weight, dyslipidemia, hyperglycemia, insulin resistance, and an increased risk of IHD.8,9) Among the commonly prescribed AAPs, olanzapine and clozapine have been noted to frequently induce profound weight gain.10,11)
Several management strategies have recently been proposed to reduce the metabolic burden of AAPs. Drugs that have been used to prevent AAP-associated metabolic disturbances include metformin, nizatidine, topiramate, and fluoxetine.12) However, results on the efficacy of such strategies are contradictory since they are also known to produce intolerable side effects, which has necessitated a search for alternatives. A potential approach for preventing AAP-induced metabolic syndrome without adverse effects is the use of the endogenous hormone melatonin.13)
Melatonin is synthesized in the pineal gland, from where it is released into the circulation in response to photic light/dark information transmitted from the retina to the suprachiasmatic nucleus of the hypothalamus.14) Its biological activities include scavenging free radicals, modulating sleep, and regulating energy metabolism and fat distribution.15) Melatonin has been shown to be effective in improving metabolic syndrome through its anti-hyperlipidemic action, anti-inflammatory action, modulatory action on insulin’s synthesis and release, and its anti-oxidant actions.16) Melatonin exerts many physiological actions by acting through membrane bound (types 1 and 2 melatonin receptors [MT1 and MT2]), although its free-radical scavenging actions do not require any mediation of melatonin receptors. Some of the actions mediated by melatonin receptors are MT1 melatonin receptors mediated vasoconstriction, whereas MT2 melatonin receptors mediate melatonin’s vasodilator effects. Melatonin receptors have been identified in various peripheral tissues concerned with regulation of energy and metabolism, and cardiovascular system.6)
Studies have been performed examining the effects of melatonin on AAP-induced metabolic syndrome, but definitive guidelines on its use in this setting are lacking.
The objective of this review was to identify and systematically evaluate the evidence on the effectiveness of melatonin for ameliorating the metabolic side effects of AAPs.
METHODS
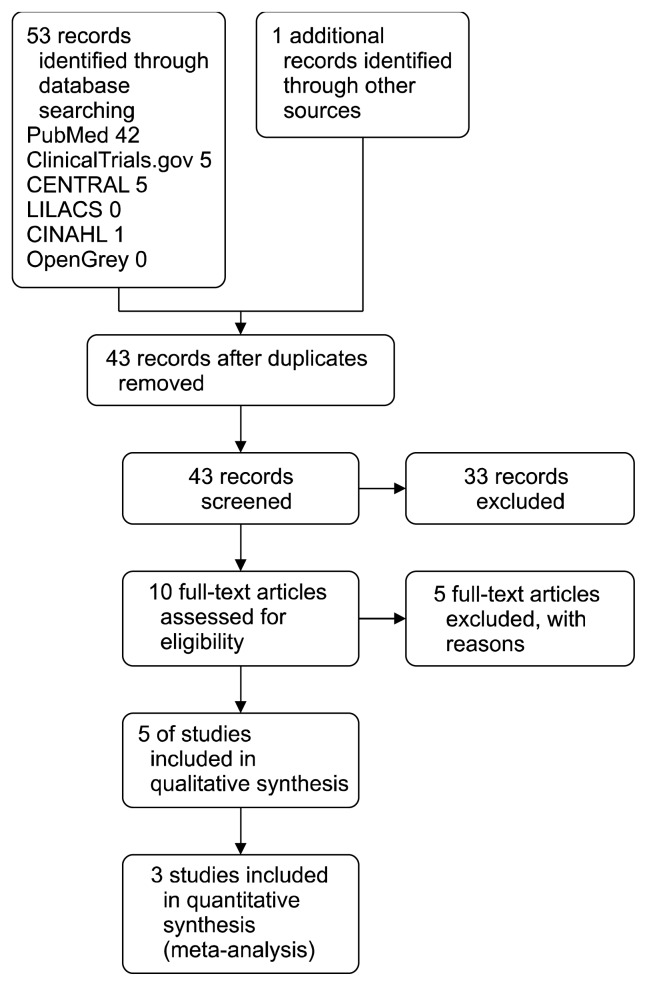
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines were adopted.17) The flow of studies is summarized in Figure 1.
Fig. 1.
PRISMA study flow diagram.
Inclusion Criteria for Studies Included in This Review
Blinded or unblinded randomized controlled trials (RCTs) of subjects treated with AAPs were included regardless of age, sex, ethnicity, and diagnosis and includes children and adults with disabilities. Uncontrolled and non-randomized trials were excluded. Trials in which exogenous melatonin or melatonin agonist (added to any AAP) was compared with placebo (added to any AAP) to evaluate the effect on metabolic side effects of AAPs were considered.
Outcomes
Mean change from baseline to endpoint in the following metabolic variables:
Body weight
Body mass index (BMI)
Waist circumference
Hip circumference
Systolic blood pressure (SBP)
Diastolic blood pressure (DBP)
Total cholesterol (TC)
LDL cholesterol (LDL-C)
HDL cholesterol (HDL-C)
Triglycerides (TG)
Fasting blood glucose (FBG)
Glycosylated hemoglobin (HbA1c)
Heart rate
Outcomes were kept in their natural units.
Search Methods for Study Identification
Electronic searches
A comprehensive review of the literature in computerized databases and searches to find unpublished trials were performed to minimize publication bias. The following electronic databases and data sources were searched through June 2017:
- MEDLINE (accessed by PubMed)
- Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 12, The Cochrane Library, December 2014)
- ClinicalTrials.gov (available at: https://clinicaltrials.gov/ct2/home)
- LILACS (available at: http://lilacs.bvsalud.org/en/)
- CINAHL
- OpenGrey (available at: http://www.opengrey.eu/)
Searching other resources
Supplementary manual searches of the reference lists of included trials were also conducted. The pharmaceutical company Nathura (a melatonin manufacturer; Reggio Emilia, Italy) was contacted directly to identify unpublished trials or data missing from articles. There were no language restrictions.
Data Collection and Analysis
Study selection
Following initial searches, all paper titles and abstracts were examined and assessed for relevance and appropriateness of the main question under review. Full texts of potentially relevant papers were obtained. Paper authors were contacted where necessary.
Quality assessment
Trials were scrutinized, and the methodological quality of all included studies was evaluated. Two reviewers (SCI and FB) independently evaluated RCT quality and any discrepancies were resolved by discussion. Quality assessment included the following methodological aspects: study design, definition and clinical relevance of outcomes, type of control, method of allocation concealment, total study duration, completeness of follow-up, intention-to-treat analysis, data concerning adverse effects, risk of bias, and conflict of interests. The randomized trials were judged on the reported method of allocation concealment and on the risk of bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011].18) Author disclosure, conflicts of interest, and sponsorship by pharmaceutical companies were evaluated.
Data extraction
The participant characteristics (total number, age, and sex of participants for each treatment group), intervention description (type, length of session, frequency), control group description, duration of follow-up, number of patients randomized at baseline and number at follow-up, and effect measures (pre- and post-mean and standard deviations in intervention and control arms, mean change scores and standard deviations if reported) were extracted from each eligible study. Data were independently extracted and checked for accuracy and completeness by SCI and FB. Any discrepancies were resolved by discussion.
Data Analysis Plan
We sought data on the number of participants in the treatment groups and with each outcome. Clinically appropriate and methodologically heterogeneous results were summarized in a meta-analysis. Continuous data (mean change from baseline to endpoint in metabolic variables) were analyzed by calculating the mean difference for each trial, with the uncertainty in each study being expressed using 95% confidence intervals (CI). Homogeneity in study results was evaluated using the chi-squared test combined with the I2 statistic, and the hypothesis of homogeneity was rejected if the p value was less than 0.10. The interpretation of I2 for heterogeneity was as follows: 0–25% represented low heterogeneity, 25–50% moderate heterogeneity, 50–75% substantial heterogeneity, and 75–100% high heterogeneity.19) Trial outcomes were combined to obtain a summary estimate of effect (and the corresponding CI) using a random-effect model.
Statistical analyses were undertaken using Review Manager Software (ver. 5.3; The Cochrane Collaboration, Copenhagen, Denmark).
RESULTS
Search Results
The search strategy yielded 53 results (42 MEDLINE, 5 CENTRAL, 5 ClinicalTrials.gov, 0 LILACS, 1 CINAHL, 0 OpenGrey, and 1 additional record identified in reference lists). Nathura was contacted (August 2015), but no additional unpublished trials were found. After excluding duplicate studies (11) and reading the abstracts, 10 studies were provisionally selected according to the inclusion and exclusion criteria. After reading the full texts a further 5 studies were excluded. Hence, 5 studies (Fig. 1) were finally included.13,20–23)
STUDY CHARACTERISTICS
The main features of the included studies have been summarized in Table 1.
Table 1.
Features of studies included in the systematic review
| Study | Characteristics of participants | Intervention (active group) | Intervention (control group) | Duration of FU (wk) | Study outcome |
|---|---|---|---|---|---|
| Borba et al., 201120) | 19 patients, 18–65 yr, either schizophrenia or schizoaffective disorder | Olanzapine, clozapine, quetiapine or risperidone, ramelteon 8 mg/day | Olanzapine, clozapine, quetiapine or risperidone, placebo | 8 | Decrease in total cholesterol level and a reduction in fat in the abdominal and trunk areas in ramelteon group |
| Modabbernia et al., 201413) | 34 males and females adults, 18–65 yr, first episode schizophrenia | Olanzapine 25 mg/day and clonazepam 2 mg melatonin 3 mg | Olanzapine 25 mg/day and clonazepam 2 mg placebo | 8 | Less weight gain, BMI, waist circumference & triglyceride level in melatonin group |
| Mostafavi et al., 201421) | 48 adolescents, 11–17 yr, first time diagnosis of bipolar mood disorder | Melatonin (3 mg/day), lithium carbonate (3–4 mg/day) and olanzapine (5–10 mg/day) | Placebo, lithium carbonate (3–4 mg/day) and olanzapine (5–10 mg/day) | 12 | Melatonin attenuates increase of systolic BP and cholesterol level |
| Romo-Nava et al., 201422) | 44 adults, 18–45 yr, schizophrenia or bipolar disorder type I | Olanzapine, clozapine, risperidone, or quetiapine 5-mg slow-release melatonin | Olanzapine, clozapine, risperidone, or quetiapine, placebo | 8 | Melatonin attenuates the increase of diastolic BP, fat mass, and triglyceride level In the bipolar disorder group |
| Mostafavi et al., 201723) | 38 (19 patients per arm) bipolar I disorder patients; within the age of 11–17 yr | Olanzapine, lithium carbonate and melatonin 3 mg/day | Olanzapine, lithium carbonate and placebo | 12 | Melatonin attenuates the sharp weight gain side effect of these drugs to near significance |
FU, follow-up; BMI, body mass index; BP, blood pressure.
Borba et al., 201120)
This was a double-blind, placebo-controlled, 8-week pilot trial to study the effect of ramelteon on obesity and metabolic disturbances among subjects with schizophrenia. Vital signs, anthropometric measurements, including height, weight, waist circumference, and body fat were assessed and laboratory assays were tracked to monitor changes in metabolic markers. Twenty-five subjects were randomly assigned to treatment with study drug or placebo and twenty subjects were included in the final analysis. Ramelteon did not improve anthropometric measurements, glucose metabolism and inflammatory markers. There was, however, a significant decrease in TC and cholesterol to HDL ratio in the ramelteon group. While the standard anthropometric measures did not show significant change, the DEXA scan showed a trend toward reduction in fat in the abdominal and trunk areas with a moderate effect size.
Modabbernia et al., 201413)
This was a single-center, eight-week, randomized, double-blind, placebo-controlled, and parallel-group study conducted in 48 adults aged 18 to 65 years with the diagnosis of schizophrenia based on the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) criteria. Participants were randomly allocated to receive either olanzapine 25 mg/day and melatonin 3 mg (active group) or olanzapine 25 mg/day and placebo (control group). In each group, olanzapine was started at 5 mg/day and was titrated up to 25 mg/day (increasing the dose by 5 mg every 3 to 7 days based on the response to treatment and patient’s tolerance). All patients received clonazepam 2 mg at night for sleep enhancement. No other medication was allowed. Six patients in each group dropped out prior to first post-baseline visit, and one further patient in each group was lost to follow-up after the fourth week. At week eight, melatonin was associated with significantly less weight gain (mean difference [MD] 3.2 kg, p=0.023), increase in waist circumference (MD 2.83 cm, p=0.041) and TG concentration (MD 62 mg/dl, p=0.090 [nearly significant]) than the placebo. Changes in cholesterol, insulin, and blood sugar concentrations did not differ significantly between the two groups. Patients in the melatonin group experienced significantly more reduction in their PANSS scores (MD 12.9 points, p=0.014) than the placebo group.
Mostafavi et al., 201421)
This was a 12-week, parallel-group, randomized, double-blind, placebo-controlled trial. The study was conducted in 38 adolescents aged 11 to 17 years with a first-time diagnosis of bipolar mood disorder (made according to DSM-IV criteria) and of normal weight. No patients received psychiatric medications or had concomitant medical disorders. Participants were randomized into two groups: the active group received melatonin (3 mg/day), lithium carbonate (3–4 mg/day) and olanzapine (5–10 mg/day), whereas the control group received placebo with lithium carbonate and olanzapine at the same dosage as the active group. No subject in the placebo group but 2 in the melatonin group were lost to follow-up. Five patients in the placebo group and 3 in the melatonin group discontinued the intervention because of weight gain. Before treatment initiation and at sixth and twelfth weeks after treatment, lipid profile, fasting blood sugar (FBS), SBP and DBP were measured. FBS and TG (especially in boys) demonstrated greater increase in the placebo group compared to the melatonin group but the differences were not statistically significant. Melatonin significantly inhibited the rise in TC levels compared to placebo (p=0.032). Mean SBP rose more slowly in the melatonin group (1.05 mmHg) compared to placebo (6.36 mmHg) (p=0.023).
Romo-Nava et al., 201422)
This was an eight-week, randomized, double blind, parallel-group, placebo-controlled trial of 50 adults aged between 18 and 45 years diagnosed with schizophrenia or bipolar disorder type I according to DSM-IV criteria. Patients had no concomitant medical or neurological illness, and they had initiated continuous treatment with SGAs (clozapine, olanzapine, quetiapine, or risperidone) for a period no greater than the last three months prior to their inclusion in the study. Participants were allocated either 5 mg slow-release melatonin or placebo. One patient in the placebo group and 5 in the melatonin group were lost to follow-up. Results were analyzed according to the psychiatric diagnosis (bipolar disorder vs. schizophrenia). Furthermore, the authors analyzed changes in anthropometric and metabolic variables according to the risk of antipsychotics inducing metabolic disturbances: quetiapine and risperidone were classified as medium risk, whereas olanzapine and clozapine were classified as high risk.
The melatonin group showed a decrease in DBP (5.1 vs. 1.1 mmHg for placebo, p=0.003) and attenuated weight gain (1.5 vs. 2.2 kg for placebo, F=4.512, p= 0.040) compared to the placebo group. The strong beneficial metabolic effects of melatonin in comparison to placebo on fat mass (0.2 vs. 2.7 kg, respectively, p=0.032) and DBP (5.7 vs. 5.5 mmHg, respectively, p=0.001) were observed in the bipolar disorder and not in the schizophrenia group. No adverse events were reported.
Mostafavi et al., 201723)
In this study, the effectiveness of melatonin in weight gain reduction following olanzapine use in adolescent with bipolar disorder was evaluated. Twenty-four patients were allocated to receive olanzapine, lithium carbonate, and melatonin, and 24 patients were allocated to receive olanzapine, lithium carbonate, and placebo by simple randomization. The Young Mania Rating Scale (YMRS) was performed at baseline. The weight, height, and BMI were measured before treatment and after 6 and 12 weeks of treatment.
Mean rise in BMI in the melatonin group compared with placebo (2.45 vs. 3.25 kg/m2 respectively) was marginally significant (t=1.936; df=36; p=0.061). ANOVA with repeated measure also showed a marginally significant difference (F=3.74; df=1; p=0.061) between groups and across time in regard to BMI. Mean body weight rise in the melatonin group compared with the placebo group (5.8 vs. 8.2 kg respectively) was marginally significant (t=1.923; df=28; p=0.065). ANOVA with repeated measure also showed a marginally significant difference (F=3.73; df=1.1; p=0.056) between groups and across time for body weight.
RISK OF BIAS
Details on risk of bias for each study are reported in detail in Appendices 1 to 7.
In four studies, a computer random number generator was used to generate random sequences (low risk of selection bias).13,21–23) Allocation concealment was adequate in all trials, except in one,20) which did not report enough information to judge this methodological issue. In the study of Romo-Nava et al. (2014),22) allocation was concealed by first generating random numbers for identical bottles labeled with this number. Numbers corresponded to randomly assigned placebo or melatonin groups in a 1:1 ratio, with information kept by a co-investigator that did not participate in patient recruitment or evaluation. Codes were opened once the study was finished (unpublished information provided by the lead author). Not all studies reported in detail how participants and study personnel were blinded. Specifically, the Mostafavi et al.’s study (2014)21) did not specify whether similar-looking comparison drugs were used. One study used identical kits of placebo and melatonin.23) However, the outcomes chosen in all studies are not likely to be influenced by a lack of blinding. As a consequence, all studies were deemed to have a low risk of detection bias.
Due to the limited number of studies included, it was impossible to systematically evaluate the risk of publication bias.
INTERVENTION EFFECTS
Not all the included studies reported details on all the outcomes of interest we initially planned to consider. Furthermore, results are reported according to the AAPs given to patients.
Melatonin (3–5 mg/day) vs. placebo in adolescents or adults with bipolar disorders or schizophrenia (concomitant antipsychotic treatment: olanzapine or clozapine)
Data for this comparison was derived from three studies.13,22,23) Results from these three studies were pooled for meta-analysis. In one study,13) participants were randomly allocated to receive either olanzapine 25 mg/day and melatonin 3 mg (active group) or olanzapine 25 mg/day and placebo (control group). In the second study,22) patients who had initiated continuous treatment with antipsychotics (clozapine, olanzapine, quetiapine, or risperidone) for a period no greater than three months prior to their inclusion in the study were allocated either 5 mg slow-release melatonin or placebo; results were then analyzed according to the metabolic risk of the anti-psychotics. To reduce methodological and clinical heterogeneity, we only considered data from patients receiving antipsychotics at high risk of metabolic disturbances (i.e., olanzapine and clozapine) when including results from this study in the meta-analysis. The third study23) was conducted in 38 adolescent patients allocated to olanzapine, lithium carbonate, and 3 mg melatonin (19 patients), and to olanzapine, lithium carbonate, and placebo (19 patients); in both arms, olanzapine was administered at 5 to 10 mg/day and lithium carbonate was begun from 3–4 mg/kg/day and increased to 15–50 mg/kg/day.
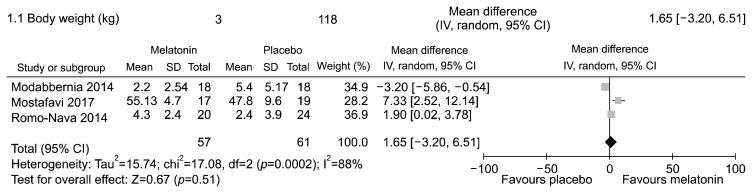
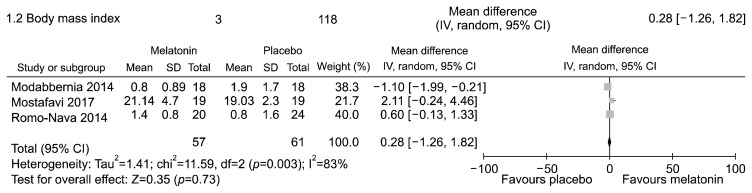
The three trials were conducted in adolescents23) or adults13,22) affected by schizophrenia or bipolar disorders treated with olanzapine or clozapine. In these studies, participants had no concomitant medical or neurological illnesses and no known risk factors for metabolic disturbances. Furthermore, study participants were comparable in terms of gender and age. The only outcomes provided by all three studies to enable meta-analyses were body weight and mass index.
The meta-analyses showed no statistically significant difference in body weight (Fig. 2, Appendix 8) and mass index (Fig. 3, Appendix 9). Significant statistical heterogeneity between trials was found.
Fig. 2.
Melatonin (any dosage) vs. placebo in adolescents or adults with bipolar disorder or schizophrenia receiving olanzapine or other atypical antipsychotic with high risk of metabolic disturbances. Outcome: mean change in body weight (kg) from baseline to endpoint.
SD, standard deviation; IV, inverse variance method; CI, confidence interval; df, degree of freedom.
Fig. 3.
Melatonin (any dosage) vs. placebo in adolescents or adults with bipolar disorder or schizophrenia receiving olanzapine or other atypical antipsychotic with high risk of metabolic disturbances. Outcome: mean change in body mass index from baseline to endpoint.
SD, standard deviation; IV, inverse variance method; CI, confidence interval; df, degree of freedom.
SUBGROUP ANALYSES ACCORDING TO AGE
Adolescents
Melatonin (3 mg/day) vs. placebo in adolescents with schizophrenia or bipolar disorder (concomitant antipsychotic treatment: lithium carbonate 3–4 mg/day and olanzapine 5–10 mg/day)
One study was conducted in adolescents with schizophrenia.21) An analysis using two-factor repeated measure analysis of variance (ANOVA) was performed to assess the effect of each treatment over time. No statistically significant differences were found between the placebo and melatonin groups for FBS, mean TGs, and DBP. However, significant differences between groups and across time were found in mean cholesterol and mean SBP, which were higher in the placebo group (mean cholesterol: 24.26 vs. 6.84 mg/dl, p=0.032; mean SBP: 6.36 vs. 1.05 mmHg; p=0.018).
One study was conducted in adolescents with bipolar disorder.23) An analysis repeated measure analysis of variance (ANOVA) was performed to assess the effect of each treatment over time. No difference was found either in body weight rise or in the BMI between the melatonin and the placebo group.
Adults
Melatonin (3–5 mg/day) vs. placebo in adults with bipolar disorders or schizophrenia (concomitant antipsychotic treatment: quetiapine and risperidone)
Data for this comparison was derived from the study by Romo-Nava et al. (2014).22) Patients treated with quetiapine or risperidone (antipsychotics with medium metabolic risk) allocated to melatonin showed a significant difference in mean change in DBP values compared with those allocated placebo (1.8 vs. −4.6 mmHg, respectively; p=0.008).
Data for this comparison was derived from the studies by Modabbernia et al. (2014)13) and Romo-Nava et al. (2014).22) Results from these two studies were pooled for meta-analysis. In one study,13) participants were randomly allocated to receive either olanzapine 25 mg/day and melatonin 3 mg (active group) or olanzapine 25 mg/day and placebo (control group). In the second study,22) patients who had initiated continuous treatment with anti-psychotics (clozapine, olanzapine, quetiapine, or risperidone) for a period no greater than three months prior to their inclusion in the study were allocated either 5 mg slow-release melatonin or placebo; results were then analyzed according to the metabolic risk of the antipsychotics. To reduce methodological and clinical heterogeneity, we only considered data from patients receiving antipsychotics at high risk of metabolic disturbances (i.e., olanzapine and clozapine) when including results from this study in the meta-analysis.
Both trials were conducted in adults affected by schizophrenia or bipolar disorders treated with olanzapine or clozapine. In both studies, participants had no concomitant medical or neurological illnesses and no known risk factors for metabolic disturbances. Furthermore, study participants were comparable in terms of gender (p=0.137) and age (p=0.389).
Results of meta-analytic comparisons are reported in Table 2 and Appendix 10. The meta-analyses showed no statistically significant difference in any anthropometric (body weight and BMI, hip and waist circumference) or metabolic (total, LDL and HDL cholesterol, TGs, and fasting glucose levels) variable considered. Significant statistical heterogeneity between trials was found in 4 meta-analytic comparisons (those assessing body weight, BMI, waist circumference, and TC).
Table 2.
Melatonin (3–5 mg/day) vs. placebo in adults with bipolar disorders or schizophrenia (concomitant antipsychotic treatment: quetiapine and risperidone)
| Outcome or subgroup | Study (n) | Participant (n) | Effect estimate |
|---|---|---|---|
| 1.1 Body weight | 2 | 80 | −0.56 [−5.55, 4.44] |
| 1.2 Body mass index | 2 | 80 | −0.23 [−1.90, 1.43] |
| 1.3 Waist circumference | 2 | 80 | 0.15 [−5.44, 5.73] |
| 1.4 Hip circumference | 2 | 80 | −0.79 [−2.86, 1.28] |
| 1.5 Total cholesterol | 2 | 80 | −4.23 [−38.96, 30.50] |
| 1.6 LDL cholesterol | 2 | 80 | 5.30 [−22.18, 32.78] |
| 1.7 HDL cholesterol | 2 | 80 | 1.72 [−1.78, 5.21] |
| 1.8 Trigylcerides | 2 | 80 | −30.09 [−67.92, 7.73] |
| 1.9 Fasting glucose | 2 | 80 | 4.70 [−3.83, 13.23] |
Statistical method: mean difference (inverse variance method, random, 95% confidence interval).
LDL, low-density lipoprotein; HDL, high-density lipoprotein.
SUBGROUP ANALYSES ACCORDING TO PSYCHIATRIC DIAGNOSIS
Bipolar Disorder
Melatonin (3–5 mg/day) vs. placebo in adolescents or adults with bipolar disorder (concomitant antipsychotic treatment: quetiapine or risperidone or clozapine or olanzapine)
Two studies provided data on this outcome.22,23) In the study by Romo-Nava et al. (2014),22) conducted in adults, patients allocated to melatonin had a significant decrease in DBP (5.5 vs. −5.7 mmHg for the placebo and melatonin groups, respectively; p=0.001), fat mass (2.7 vs. 0.2 kg, respectively; p=0.032), and TGs (D) (50.1 vs. −20 mg/dl, respectively; p=0.08) compared to controls.
In the study by Mostafavi et al.23) conducted in adolescents, no difference was found either in body weight rise or in the BMI between the melatonin and the placebo group.
Schizophrenia
Melatonin (3–5 mg/day) vs. placebo in adults with schizophrenia (concomitant antipsychotic treatment: olanzapine or clozapine)
Data for this comparison were derived from the studies by Modabbernia et al.13) and Romo-Nava et al.22) Results from these two studies were pooled for meta-analysis. Data from the study conducted by Romo-Nava et al.22) were kindly provided by study’s lead author.
Results of meta-analytic comparisons are reported in Table 3 and Appendix 11. The meta-analyses showed no statistically significant difference in any anthropometric (body weight and BMI, hip and waist circumference) or metabolic (total, LDL and HDL cholesterol, TGs, and fasting glucose levels) variable considered. Significant statistical heterogeneity among trials was found in 3 meta-analytic comparisons (those assessing body weight, BMI, waist circumference, and TC).
Table 3.
Melatonin (3–5 mg/day) vs. placebo in adults with schizophrenia (concomitant antipsychotic treatment: olanzapine or clozapine)
| Outcome or subgroup | Study (n) | Participant (n) | Effect estimate |
|---|---|---|---|
| 2.1 Body weight | 2 | 43 | 1.16 [−7.58, 9.90] |
| 2.2 Body mass index | 2 | 43 | 0.43 [−2.68, 3.55] |
| 2.3 Waist circumference | 2 | 43 | 0.44 [−6.32, 7.20] |
| 2.4 Hip circumference | 2 | 43 | −0.39 [−5.02, 4.25] |
| 2.5 Total cholesterol | 2 | 43 | 0.30 [−50.46, 51.06] |
| 2.6 LDL cholesterol | 2 | 62 | 4.79 [−29.04, 38.62] |
| 2.7 HDL cholesterol | 2 | 43 | 0.79 [−5.33, 6.90] |
| 2.8 Trigylceride | 2 | 43 | −49.32 [−111.42, 12.78] |
| 2.9 Fasting glucose | 2 | 43 | 3.07 [−4.28, 10.41] |
Statistical method: mean difference (inverse variance method, random, 95% confidence interval).
LDL, low-density lipoprotein; HDL, high-density lipoprotein.
DISCUSSION
This systematic review and meta-analysis examined the effect of melatonin on metabolic syndrome induced by AAPs. Five studies were included in the review, but only three studies13,22,23) were eventually included in the quantitative synthesis (meta-analysis). The study by Borba et al.20) was conducted using a melatonin analogue, rameltron, hence it was excluded from the meta-analysis to avoid unacceptably high methodological heterogeneity. A systematic review (without meta-analysis) has earlier been published on this topic.24) However, our study has included a recent RCT13) and we also obtained additional unpublished information from the lead author of one of the RCTs included in the previous systematic review.22) We have also organized homogeneous data to conduct a meta-analysis.
The study conducted by Mostafavi et al.21) was excluded in the meta-analysis because, unlike the other studies that were conducted in adults, it was conducted in adolescents (clinical heterogeneity). In a cross-sectional analysis of 10,206 Norwegians aged 20 to 89 years,25) the prevalence of metabolic syndrome was highly age dependent, especially in women, with a seven-fold increase in prevalence from those aged 20 to 29 years to those aged 80 to 89 years. Furthermore, subjects in the Mostafavi et al.’s study (2014)21) not only received olanzapine but also lithium carbonate (risk of methodological heterogeneity). There are contradictory reports on the relationship between lithium use and the diagnosis of metabolic syndrome.26,27) Hence, the contribution of lithium to the eventual development of metabolic syndrome in the subjects in Mostafavi et al.’s study (2014)21) is not clear.
The results of the quantitative synthesis should be regarded with caution, mainly because of the considerable statistical heterogeneity observed in four out of nine meta-analyses. This might be indicative of inconsistency in the results of the included studies. The term “statistical heterogeneity” describes the degree of variation in the effect estimates from a set of studies and indicates the presence of variability among studies beyond the amount expected due solely to chance.
Due to the limited number of studies available (only 3), it was impossible to formally address the possible reasons for the significant statistical heterogeneity observed. Possible sources of clinical heterogeneity should, however, be taken into account. The included trials were conducted in adults affected by schizophrenia or bipolar disorders treated with olanzapine or clozapine. In the included studies, participants had no concomitant medical or neurological illness and no known risk factors for metabolic disturbances. Furthermore, participants in these three studies were comparable in terms of gender and age. However, the Modabbernia et al.’s study13) included previously untreated patients, whereas the Romo-Nava et al.’s study22) was conducted in patients who had already initiated continuous treatment with antipsychotics, although for a period no greater than the three months prior to their inclusion in the study. In their meta-analysis of prevalence rates and moderators of metabolic syndrome and metabolic abnormalities in bipolar disorder, Vancampfort et al.28) reported that metabolic syndrome was significantly more prevalent in patients currently treated with antipsychotics. Hence, participants from Romo-Nava et al.22) were exposed to the metabolic effects of antipsychotics for longer than those in the study by Modabbernia et al.13) Furthermore, in the Romo-Nava et al. study,22) patients were allowed to take concomitant treatment, and a statistically significant difference in the percentage of patients receiving mood stabilizers (lithium, valproate, carbamazepine, and lamotrigine) was present (65% in the melatonin group vs. 33.3% in the placebo group; p=0.03). In this trial, stratified randomization with the assumption of concomitant drug use was not taken into account, and post-hoc statistical analyses were not carried out to adjust for this potentially relevant confounder. Of note, mood stabilizers are known to be associated with a higher risk of metabolic disturbances, and the fact that a higher percentage of patients allocated melatonin received mood stabilizers, might have obscured a beneficial metabolic effect of melatonin in these subjects.
The study by Modabbernia et al.13) used melatonin 3 mg/day and in both the melatonin and placebo groups; 2 mg clonazepam was given at night for sleep enhancement. In contrast, in the study by Romo-Nava et al.,22) the dose of melatonin was 5 mg (slow-release) and benzodia-zepines were also given in 5 out of 24 patients (20%) allocated to placebo and 8 out of 20 (40%) patients allocated to melatonin.
Finally, the study by Modabbernia et al.13) only included patients diagnosed with first-episode schizophrenia, whereas the study by Romo-Nava et al.22) included patients with schizophrenia (58%) and bipolar disorder (44%) with an illness duration of several years (23.0±8.9 years in the melatonin group vs. 28.6±9 in the placebo group). A particularly beneficial metabolic effect of melatonin in a subset of patients with a specific psychiatric diagnosis might have occurred. Of note, the study by Romo-Nava et al.22) showed a significant reduction in mean changes between baseline and study endpoint in DBP, fat mass and TGs only in patients with bipolar disorders allocated to melatonin. Although these findings may simply reflect spurious results (i.e., false positives) from multiple comparisons, a selective metabolic behavior of melatonin according to psychiatric diagnosis cannot be excluded. For example, it is known that patients with bipolar disorder or schizophrenia have a disrupted circadian cycle.29) It has also been hypothesized that melatonin may be more effective in restoring a disrupted circadian rhythm in bipolar disorder than in schizophrenia.22)
CONCLUSION
The few available articles, small sample size, and considerable statistical heterogeneity limit this analysis. Nevertheless, we found some evidence in one study that melatonin may improves AAP-induced metabolic syndrome including decreased DBP, fat mass, and TGs. These beneficial effects appear to be more significant in patients with bipolar disorder than those with schizophrenia. However, in the meta-analysis all comparisons related to fat mass and TGs did not yield statistically significant results. Further RCTs with larger sample size are needed to definitely establish the potential beneficial effect of melatonin and to justify its efficacy as an add-on therapy to curtail AAP-induced metabolic syndrome.
Supplementary Information
Acknowledgments
Special thanks to Marianna Altieri (Nathura) for providing information on unpublished studies on this topic. The authors also thank the Nextgenediting Global Initiative (www.nextgenediting.com) for editorial help. No specific grant from any funding agency was received and no conflict of interest to declare.
Footnotes
Appendices 1–11 are available at https://doi.org/10.9758/cpn.2018.16.3.235.
REFERENCES
- 1.Leo RJ, Regno PD. Atypical antipsychotic use in the treatment of psychosis in primary care. Prim Care Companion J Clin Psychiatry. 2000;2:194–204. doi: 10.4088/PCC.v02n0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA. Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19:101–109. doi: 10.1089/cap.2008.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko YK, Soh MA, Kang SH, Lee JI. The prevalence of metabolic syndrome in schizophrenic patients using antipsychotics. Clin Psychopharmacol Neurosci. 2013;11:80–88. doi: 10.9758/cpn.2013.11.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deedwania PC, Gupta R. Management issues in the metabolic syndrome. J Assoc Physicians India. 2006;54:797–810. [PubMed] [Google Scholar]
- 5.Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshminarayanan M, Casey DE, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101:277–288. doi: 10.1016/S0165-1781(01)00234-7. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan V, Ohta Y, Espino J, Pariente JA, Rodriguez AB, Mohamed M, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7:11–25. doi: 10.2174/187221413804660953. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 8.Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds GP. Pharmacogenetic aspects of antipsychotic drug-induced weight gain: a critical review. Clin Psychopharmacol Neurosci. 2012;10:71–77. doi: 10.9758/cpn.2012.10.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 12.Baptista T, Kin NM, Beaulieu S, de Baptista EA. Obesity and related metabolic abnormalities during antipsychotic drug administration: mechanisms, management and research perspectives. Pharmacopsychiatry. 2002;35:205–219. doi: 10.1055/s-2002-36391. [DOI] [PubMed] [Google Scholar]
- 13.Modabbernia A, Heidari P, Soleimani R, Sobhani A, Roshan ZA, Taslimi S, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133–140. doi: 10.1016/j.jpsychires.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris. 2011;105:170–182. doi: 10.1016/j.jphysparis.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Raskind MA, Burke BL, Crites NJ, Tapp AM, Rasmussen DD. Olanzapine-induced weight gain and increased visceral adiposity is blocked by melatonin replacement therapy in rats. Neuropsychopharmacology. 2007;32:284–288. doi: 10.1038/sj.npp.1301093. [DOI] [PubMed] [Google Scholar]
- 16.Espino J, Pariente JA, Rodríguez AB. Role of melatonin on diabetes-related metabolic disorders. World J Diabetes. 2011;2:82–91. doi: 10.4239/wjd.v2.i6.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Altman DG, Sterne JAC. Cochrane Collaboration, editor. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011) Oxford: Cochrane Collaboration; 2011. [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borba CP, Fan X, Copeland PM, Paiva A, Freudenreich O, Henderson DC. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31:653–658. doi: 10.1097/JCP.0b013e31822bb573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mostafavi A, Solhi M, Mohammadi MR, Hamedi M, Keshavarzi M, Akhondzadeh S. Melatonin decreases olanzapine induced metabolic side-effects in adolescents with bipolar disorder: a randomized double-blind placebo-controlled trial. Acta Med Iran. 2014;52:734–739. [PubMed] [Google Scholar]
- 22.Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, Saracco Alvarez R, Becerra-Palars C, Moreno J, et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16:410–421. doi: 10.1111/bdi.12196. [DOI] [PubMed] [Google Scholar]
- 23.Mostafavi SA, Solhi M, Mohammadi MR, Akhondzadeh S. Melatonin for reducing weight gain following administration of atypical antipsychotic olanzapine for adolescents with bipolar disorder: a randomized, double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2017;27:440–444. doi: 10.1089/cap.2016.0046. [DOI] [PubMed] [Google Scholar]
- 24.Wang HR, Woo YS, Bahk WM. The role of melatonin and melatonin agonists in counteracting antipsychotic-induced metabolic side effects: a systematic review. Int Clin Psychopharmacol. 2016;31:301–306. doi: 10.1097/YIC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 25.Hildrum B, Mykletun A, Hole T, Midthjell K, Dahl AA. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC Public Health. 2007;7:220. doi: 10.1186/1471-2458-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teixeira PJ, Rocha FL. The prevalence of metabolic syndrome among psychiatric inpatients in Brazil. Rev Bras Psiquiatr. 2007;29:330–336. doi: 10.1590/S1516-44462007000400007. [DOI] [PubMed] [Google Scholar]
- 27.Genc A, Kalelioglu T, Tasdemir A, Genc ES, Ozver I, Yesilbas D, et al. The prevalence of metabolic syndrome parameters among bipolar disorder outpatients on lithium monotherapy. Klinik Psikofarmakol Bülteni. 2012;22:320–324. doi: 10.5455/bcp.20120412021518. [DOI] [Google Scholar]
- 28.Vancampfort D, Vansteelandt K, Correll CU, Mitchell AJ, De Herdt A, Sienaert P, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013;170:265–274. doi: 10.1176/appi.ajp.2012.12050620. [DOI] [PubMed] [Google Scholar]
- 29.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.