Abstract
Objective
Baclofen is a promising treatment for alcohol use disorders (AUD), although its clinical response in humans is mixed. The present study aimed at investigating the impact of baclofen treatment on cue-induced brain activation pattern and its relationship with relapse outcomes.
Methods
Twenty-three inpatients with AUD underwent a functional magnetic resonance imaging cue-reactivity task before beginning medication with baclofen and 2 weeks later. Twelve additional inpatients with AUD, who did not receive any anticraving medications, formed the control group. All subjects were prospectively followed up for 90 days post-discharge or until lapse to first alcohol use.
Results
Whole-brain linear mixed effects analysis revealed a significant group-by-time interaction with greater activation of the bilateral dorsolateral pre-frontal cortex and right anterior cingulate cortex (ACC) following baclofen treatment in comparison with the control group. Further, cox regression analysis revealed that increased activation of ACC and deactivation of insular cortex (IC) was associated with longer time to first alcohol use only in the baclofen treatment group but not in the control group.
Conclusion
This study provides preliminary evidence for the neural predictors of baclofen treatment response in AUD. Baclofen treatment in AUD was associated with changes in cue-reactivity at critical brain regions within the incentive-salience network. Importantly, baclofen treatment-related specific activation of regions involved in cognitive control (ACC) and deactivation of regions involved in reward anticipation (IC) prolonged the time to first alcohol drink.
Keywords: Baclofen, Functional magnetic resonance imaging, Cue-reactivity, Relapse prediction
INTRODUCTION
Alcohol use disorders (AUD) are chronic relapsing medical conditions1) that rank high among the preventable causes of morbidity and mortality.2) Although non-pharmacological interventions have dominated long-term treatment strategies for relapse prevention in AUD, there is growing evidence for the effectiveness of pharmacological “anti-craving” agents in this area. A judicious combination of both these approaches provides optimal treatment, without which at least 40% to 70% patients relapse within one year.3) Although several drugs have been tested for treatment of AUDs, till date only three pharmacological agents have been approved by the US Food and Drug Administration (FDA).4) Of these, disulfiram, which produces unpleasant hypersensitivity to alcohol by blocking its oxidation at acetaldehyde stage, comes under the category of deterrent drugs. The remaining two, naltrexone (a μ-opioid antagonist) and acamprosate (a putative glutamate modulator), come under the purview of “anti-craving drugs”. Nalmefene, an opioid with μ-antagonism and partial κ-agonism, was recently approved for use by the European Medicines Agency to reduce alcohol consumption.5)
Baclofen, a presynaptic gamma amino butyric acid-B (GABA-B) receptor agonist,6) was investigated as a potential anti-craving agent after it was observed to have bidirectional effects on the mesolimbic dopamine system.7) However, the existing data on its clinical usefulness in humans is equivocal, with three positive8–10) and three negative11–13) randomized controlled trials. Several factors have been examined to explain the inconsistency noted in the response to baclofen. These include baclofen dosing,14,15) AUD typology and severity,16) subjective responses to alcohol,17) presence of alcohol withdrawal18) and comorbid anxiety19) symptoms. However, changes in neural cue reactivity (CR) as a potential predictor of treatment response to baclofen have not been explored.
Human laboratory models of addiction have examined brain CR elicited by a wide array of cues, including visual,20,21) olfactory22–24) and gustatory25,26) stimuli. Additionally patterns of brain CR that relate to alcohol craving,26) relapse,27) duration and severity of AUD28) have also been explored. However, changes in neural CR during the course of treatment with pharmacotherapeutic agents and its relationship with relapse outcomes have not been adequately explored.29) The present study aimed to explore the effects of baclofen treatment on alcohol-related visual CR and its relationship with relapse outcomes in subjects with AUD using a controlled before- and after functional magnetic resonance imaging (fMRI) study design. Based on the literature,30,31) areas of the brain associated with decision-making (bilateral dorsolateral prefrontal [DLPFC], anterior cingulate [ACC], and orbitofrontal cortices [OFC]) and reward processing (insula and ventral striatum [VS]) were chosen as primary regions of interest (ROI). We hypothesised that a differential response to baclofen in these areas will be predictive of the time to relapse.
METHODS
The study was conducted at the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India. The institutional ethics review board approved the study protocol (NIMH/DO/ESC-26/2015/BS/2.03) and all participants signed a written informed consent form.
Participants and Procedures
Thirty-five male subjects seeking in-patient treatment for alcohol dependence, aged between 23 and 50 years, participated in this naturalistic, observational study. They had a current diagnosis of alcohol dependence according to 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10).32) All subjects were screened for (1) the presence of significant medical, neurological, or psychiatric conditions (axis I diagnoses other than alcohol or nicotine dependence); (2) contraindications to MRI; (3) alcohol withdrawal complicated by seizures or delirium; (4) ongoing treatment with psychotropic drugs that could affect study outcome (i.e., stimulants, sedatives, anticonvulsants, anti-depressants, antipsychotics, or other AUD relapse prevention drugs such as naltrexone, acamprosate, or disulfiram); and (5) left or mixed handedness as per Edinburgh handedness inventory.33)
All subjects received thiamine supplementation and individualized detoxification with either diazepam or lorazepam, with dosage and duration titrated for severity of withdrawal symptoms and other clinical factors. They also underwent thrice weekly relapse prevention group sessions conducted by trained psychiatric social workers. After completion of detoxification, 23 inpatients that were prescribed baclofen as an anti-craving medication and 12 inpatients that were not prescribed any anti-craving medication were invited to participate in the study. All the consenting subjects first underwent a benzodiazepine-wash-out period of five days. They were considered withdrawal-free if they had scores <3 on Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar)34) and a negative urine screen for benzodiazepines. For sample-description purposes, self-report questionnaires were filled out to assess demographic and clinical information. During the course of their inpatient stay, all subjects participated in 2 sessions of fMRI, the baseline study (Time-0 [T0]) and the 2nd-week study (Time-1 [T1]) fMRI scans. Patients in the medication group were initiated on baclofen after the baseline study and the dose was titrated up to 60 mg/day. Subjective alcohol craving was assessed using the Penn Alcohol Craving Scale (PACS)35) on the days of both T0 and T1 study. After completion of the T1 study, all subjects were discharged. Patients in the medication group were advised to continue baclofen at the same dose while those in the control group were started on anti-craving medications if they reported craving. All patients were prospectively followed-up with in-person interviews with the patient and their family members conducted at 7, 14, 30, 60, and 90 days post-discharge. Time to relapse was defined as the number of days since discharge to the first day of any alcohol use.
fMRI Paradigm
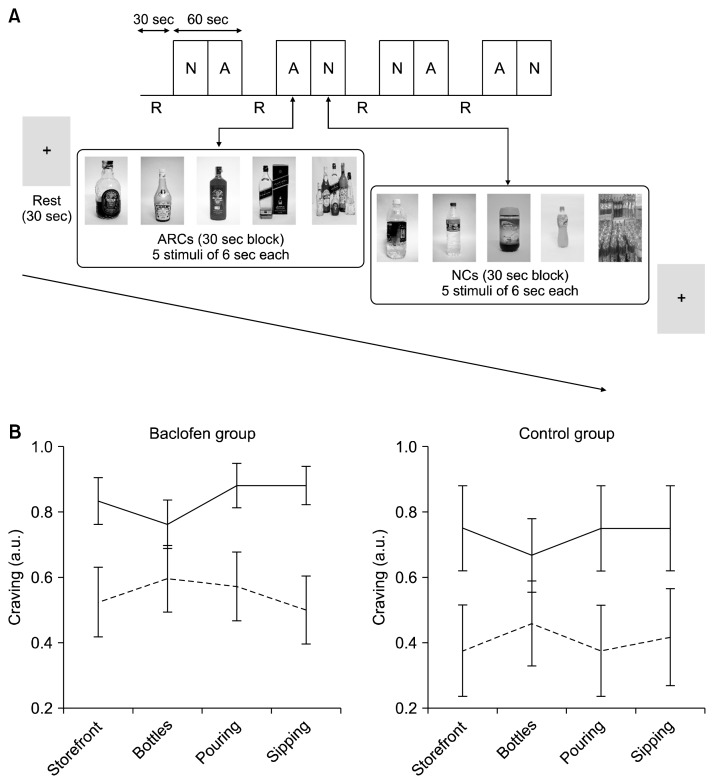
The visual image-induced craving for ethanol (VICE)36) is a culturally validated tool for craving induction in controlled settings. This paradigm was presented in a block design, consisting of four 90-second epochs. Each epoch started with a 30-second resting control block (fixation cross-hair), followed by two active blocks consisting of 30 seconds of alcohol-related cues (ARC; 5 images for 6 seconds each) and 30 seconds of non-alcohol-related neutral cues (NC; 5 images for 6 seconds each). The content and order in which these blocks were presented was fully matched and counterbalanced (Fig. 1).37) The ARC blocks consisted of image scenes related to alcohol consumption (e.g., liquor storefronts, alcohol bottles, pouring, and sipping alcohol). The NC blocks matched the content of ARC images with non-alcoholic beverages like milk, coffee, tea, or water. During the active blocks, participants were instructed to indicate by means of button presses whether they experienced craving for alcohol or not for each visual cue and during the resting control block, they had to stay still without engaging in any mental activity. All subjects participated in two runs of this 6-minute VICE paradigm in each T0 and T1 studies.
Fig. 1.
(A) The visual image induced craving for ethanol (VICE) functional magnetic resonance imaging paradigm was presented in a block design, consisting of four 90-second epochs. The active blocks, alcohol-related cues (ARC; block ‘A’) and neutral cues (NC; block ‘N’), had 5 images which stayed on screen for 6 seconds. The resting control block (R) was a blank screen with fixation cross-hair. (B) Changes in the in-scanner craving responses (mean and standard error) averaged across subjects for different ARC exposure blocks for the baseline (solid lines) and 2nd week (dashed lines) scans shown for baclofen and control groups separately. For illustration each ARC block (liquor storefront, alcohol bottles, pouring and sipping alcohol) was scored 1 if the subjects experienced craving for alcohol (indicated by means of button presses) for at least two of the five cues in the block.
Image Acquisition
Blood-oxygen-level dependent fMRI scans covering the whole brain was acquired with a 3 Tesla SIEMENS Magnetom Skyra scanner (Erlangen, Germany) using a 32 channel head coil. Functional data consisted of 360 whole-brain gradient-echo echo-planar images obtained from two runs of the VICE paradigm. The scan parameters were as follows: repetition time (TR)=2,000 ms; echo time (TE)=30 ms; flip angle=78°; slice order=descending; number of slices=37; gap=25%; matrix=64×64, field of view (FOV)=192 mm2, voxel size=3.0×3.0×3.75 mm3. After obtaining the fMRI images, a three-dimensional, high-resolution T1-weighted-MPRAGE (magnetization-prepared rapid acquisition with gradient echo) imaging was performed with TR=1,900 ms, TE=2.43, TI=900 ms, FOV=240×240 mm2 yielding 192 sagittal slices and a voxel size of 1×1×1 mm3 for anatomical coregistration and segmentation.
Image Analysis
The functional and structural MRI pre-processing was performed using statistical parametric mapping (SPM12; Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). The first three functional images of each time series (each run of VICE) were discarded to allow for signal equilibration and to provide time for habituation of the individual in the scanner. Each of the remaining images was first registered to a mean image and then realigned to correct for head movements within the scan. After realignment, the functional images were slice-time corrected to the middle slice of each set and co-registered to the corresponding MPRAGE image of the individual. After co-registration, MPRAGE images were segmented (into gray matter, white matter, and cerebrospinal fluid) and deformation fields were calculated. The deformation fields were used for bias correction of magnetization inhomogeneity, and to transform the functional images of each individual to Montreal Neurological Institute (MNI) standard space. Finally, the data was smoothed using a Gaussian kernel at 8 mm full width half maximum.
In order to examine head motion during the scanner, a frame-wise displacement (FD) scalar was computed for each patient.38) Two subject sessions with mean FD >0.5 mm were excluded (one T0 and one T1 session of two different patients in Baclofen group). In addition, motion correction was carried out using Friston 24-parameter model regression (3 translations, 3 rotations, 3 translations and 3 rotations shifted 1 volume before, and the 12 corresponding quadratic terms) to control for both linear and non-linear influences.39) The first level design specification and estimation of brain CR, defined by the contrast ARC minus NC, was then carried out for each subject. Second level linear mixed effects (LME) group analysis was implemented in Analysis of Functional NeuroImages (AFNI) using 3dLME program (http://afni.nimh.nih.gov). Group was modelled as a between-subject factor with 2 levels (baclofen and control), time (coded as T0=0 and T1=number of days since T0) was centered across group and modelled as within-subject continuous variable and subjects as random factors. The resulting whole-brain map was corrected for multiple comparisons using AFNI’s 3dClustSim program (version 17.0.09) with 10,000 iterations, a whole-brain mask, and mixed model smoothness estimated using 3dFWHMx with the spatial autocorrelation function option.40) Based on the Monte Carlo simulations, cluster-level p<0.05 family-wise error was given by a cluster forming voxel-wise threshold of p=0.001 and κ>53 voxels. In order to examine the relationship between baclofen’s effects on brain CR and subsequent time to the first relapse, a cox proportional hazard (PH) regression was carried out on five independent apriori defined ROIs. Cox regression was implemented using MATLAB function ‘coxphfit’. This analysis provided a way to test whether variables relate to the likelihood of an event (e.g., relapse to alcohol use) when not all participants have experienced the event. If a patient did not relapse for 90 days post-discharge, the observation was censored. The anatomical ROI masks for ACC, DLPFC, OFC, IC and VS were downloaded from the Nielsen and Hansen database.41) These probabilistic ROIs were binarized with a threshold of ≥0.7. The MarsBar toolbox42) was used to extract beta estimates (β) of the ROIs from T0 and T1 scans and a unweighted mean difference of beta estimates were computed (Δβ=β [T1]−β [T0]). All Δβ scores were z-transformed before analysis so that the exponent of model coefficients would equal the hazard ratio that a change in one standard deviation would result in relapse/survival. Preliminary cox PH regression analyses indicated that none of the clinical variables (e.g., years of AUD, baseline level of alcohol use before inpatient treatment, baseline alcohol craving and days between the two fMRI sessions) were predictive of relapse except for the treatment status (Supplementary Table 1). Thus, cox PH regression models of blood-oxygen-level dependent (BOLD) signal change predicting relapse were implemented for the two groups separately. Further, to examine the predictive value of significant ROIs, receiver operating characteristic curves were calculated.
Cox regression was implemented using MATLAB function ‘coxphfit’.
RESULTS
Sample Characteristics
As evident in Table 1, there were no significant baseline differences in demographics, alcohol use or craving parameters between the subjects in the baclofen and the control groups. Because the duration between the scans was significantly lesser for the control group, it was controlled for in all subsequent between-group analyses. The PACS alcohol craving scores at T1 reached trend level significance, after accounting for baseline craving and duration between the scans. The mean number of days to first alcohol drink was significantly longer (log-rank test χ2=10.54, p=0.001) in the baclofen group (60 days) compared to control group (25 days). The follow-up time-to-event data of the control subjects (n=3, 25%) who subsequently received an anti-craving medication (due to persistent craving) before their first alcohol relapse, were censored.
Table 1.
Demographics and clinical characteristics of participants
| Characteristic | Baclofen (n=23) | Control (n=12) | p value |
|---|---|---|---|
| Age (yr) | 35.2±7 | 38.2±7.7 | 0.27 |
| Education (yr) | 11.8±1.4 | 10.8±3.2 | 0.30 |
| AUD (yr) | 10.9±7 | 11.1±7.4 | 0.93 |
| Average daily consumption (in alcohol units*) in prior 3 months | 18.6±5.6 | 19.5±10.2 | 0.69† |
| Cigarette smokers | 10 (83.3) | 17 (73.9) | 0.53‡ |
| Baclofen dose (mg/day) | 57.6±8.9 | - | - |
| PACS score | |||
| T0 | 16.6±6.2 | 19.3±4.5 | 0.21 |
| T1 | 5.4±2.2 | 7.6±3.4 | 0.07§ |
| Interval between fMRI scans (day) | 14.7±2.6 | 9.2±3.8 | <0.01 |
| Period to first alcohol relapse (day) | 60.3±6.2 | 25.3±7.1¶ | 0.00|| |
Values are presented as mean±standard deviation or number (%) except the period to first alcohol relapse which is mean±standard error.
AUD, alcohol use disorder; PACS, Penn Alcohol Craving Scale; T0, Time 0/Pre-treatment; T1, Time 1/Post-treatment; fMRI, functional magnetic resonance imaging.
1 Alcohol unit=1 standard drink=10 g of pure alcohol.
Mann-Whitney U test,
chi-square test,
analysis of covariance accounting for baseline PACS score and number of days between the fMRI scans;
Log-rank test.
Three (25%) subjects in the control group who were prescribed an anti-craving medication in the immediate post-discharge period were censored.
fMRI Results
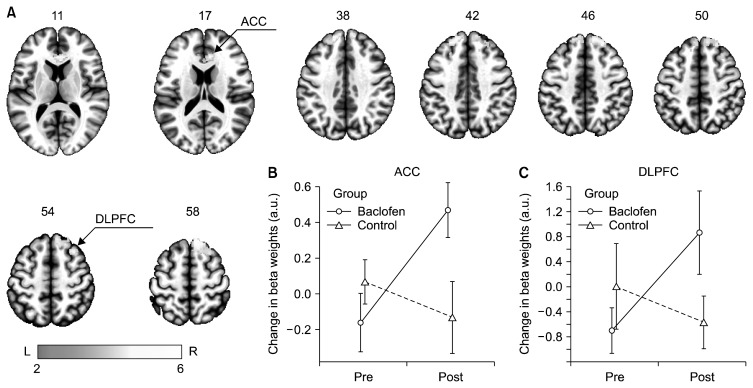
Whole brain LME analysis indicated a significant group-by-time interaction in bilateral DLPFC and right ACC for the ARC vs. NC contrast. Specifically, whereas the subjects in the baclofen group showed significantly greater activation of bilateral DLPFC and right ACC after baclofen treatment relative to baseline, the subjects in the control group showed no such activation (Table 2 and Fig. 2). Additionally, there was reduction of right insular cortex activity after baclofen treatment relative to baseline (uncorrected p<0.005), whereas the subjects in the control group showed no such reduction (Supplementary Fig. 1).
Table 2.
Brain regions that showed significant group-by-time interaction with significantly greater activation in baclofen group relative to control group for the contrast alcohol-neutral cues
| Brain region | Lat | BA | X | Y | Z | z score | Volume (mm3) |
|---|---|---|---|---|---|---|---|
| DLPFC | R | 8,9 | 10 | 36 | 60 | 5.23 | 4,280 |
| L | 9 | −16 | 46 | 42 | 4.28 | 2,264 | |
| ACC | R | 32 | 10 | 36 | 14 | 5.76 | 1,528 |
Lat, laterality; R, right; L, left; BA, brodmann area; X, Y, Z, MNI co-ordinates; DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex.
All regions are significant at cluster p family-wise error <0.05, whole-brain corrected.
Fig. 2.
Brain regions depicting significant group-by-time interactions for alcohol vs. neutral cues thresholded at cluster p<0.05 family-wise error, whole-brain corrected. The results indicate significant activation of bilateral dorsolateral prefrontal cortex (DLPFC) and right anterior cingulate cortex (ACC) following baclofen treatment compared to the control group. Color-bar is indicative of z-values. For illustration of the group-by-time effects, mean (95% confidence inverval) beta weights from baclofen (circle, solid line) and control (triangle, dashed lines) groups were extracted using a 10 mm-radius sphere centered around the peak voxel at (B) ACC and (C) DLPFC and the change was plotted using an interaction plot. L, left; R, right.
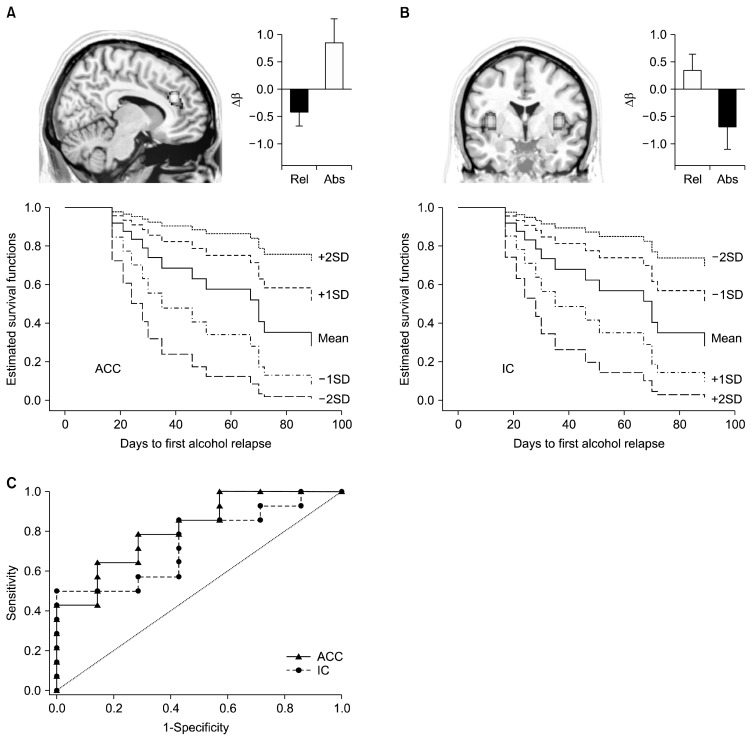
Cox PH regression models of BOLD signal change that predicted time to first alcohol use was carried out for the two groups separately, using the five apriori chosen independent ROIs (ACC, DLPFC, OFC, IC, and VS). We found that baclofen-treatment related effects at ACC and IC significantly predicted time to relapse. Specifically, after treatment with baclofen, the greater activation of ACC during the ARC vs. NC condition reduced the likelihood of early lapse by half and persistent activation of IC during the ARC vs. NC condition increased the likelihood of early lapse by 1.9 times (Table 3 and Fig. 3). As shown in Fig. 3C, the treatment related change in ACC (mean±standard error [SE] of the area under the curve [AUC]=0.82±0.11, p=0.03) and IC (mean±SE of AUC=0.79±0.11, p=0.04) cue-reactivity produced significant and accurate classification of relapsers vs. abstainers at 90 days post-discharge. The BOLD signal change at none of the ROIs for the control group predicted relapse risk (Supplementary Table 2).
Table 3.
Cox proportional hazard regression models of baclofen treatment-related BOLD signal change predicting time to relapse
| ROI | z score | p value | HR (95% CI) |
|---|---|---|---|
| ACC | −2.14 | 0.03* | 0.51 (0.23–0.94) |
| DLPFC | −0.88 | 0.37 | 0.78 (0.44–1.36) |
| OFC | 0.68 | 0.49 | 1.19 (0.73–1.94) |
| IC | 1.99 | 0.04* | 1.92 (1.01–3.65) |
| VS | 0.86 | 0.38 | 1.26 (0.75–2.12) |
BOLD, blood-oxygen-level dependent; ROI, region of interest; HR, hazard ration; CI, confidence interval; ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex; IC, insular cortex; VS, ventral striatum.
BOLD signal change at a ROI represents unweighted mean difference of beta estimates computed (β=β [T1]−β [T0]) for ‘alcohol minus neutral’ cues contrast.
Significant results.
Results indicate that greater activation of ACC and deactivation of insula following baclofen treatment, predicted longer time to first alcohol relapse.
Fig. 3.
Estimated survival functions of baclofen treatment effects, Δβ=β (T1)−β (T0), predicting time to first alcohol relapse shown at mean Δβ value with 1 and 2 standard deviation (SD) above/below the mean. Greater activation in the (A) anterior cingulate cortex (ACC) and greater deactivation in the (B) insular cortex (IC) was significantly (p< 0.05) associated with longer time to relapse. Bar plots depict the Δβ values for relapsers (Rel) and abstainers (Abs) at the end of 90 days post discharge. (C) Accuracy of relapse prediction as computed by the receiver operating characteristic analysis curves. The area under the curve (AUC) indexed the accuracy at which treatment related effects at ACC (AUC=0.82, p=0.03) and IC (AUC=0.79, p=0.04) regions predicted relapse.
DISCUSSION
This study investigated the brain CR changes associated with baclofen treatment in AUD individuals and its relationship with relapse outcomes. Baclofen treatment was associated with significantly greater activity in bilateral DLPFC and right ACC, critical regions that are involved in decision-making and executive control. More importantly, greater activation of ACC and deactivation of insula following treatment with baclofen predicted longer time to first alcohol relapse.
Several meta-analyses have attempted to build upon traditional concepts of drug dependence which stress on mesolimbic response to pleasure and reward, to a more complex meso-cortical syndrome of impaired response inhibition and salience attribution (iRISA).31,43) Moreover, the consistent finding among human neuroimaging studies has been the correlation of cue-elicited craving, attentional bias and impulsivity with activity in the OFC, ventromedial prefrontal cortex, ACC, and insula.30,44,45) As per iRISA model, the incentive-salience network comprising of the ACC-IC system, when faced with a salient stimulus (ARC), switches the brain from a default mode to an executive mode through its connections to prefrontal regions.46) This assists decision-making in favour of the stimulus in spite of the risks and long-term negative consequences of such behaviours. Here, the IC is postulated to store pleasurable interoceptive effects of previous alcohol use, and anticipate these rewarding effects in future44) and the ACC is believed to be involved in control and inhibition of prepotent maladaptive responses47) including craving. Thus, upon exposure to such salient stimulus, AUD individuals, who undergo treatment and intend to stay abstinent, may be able to mobilize coping skills, including attempts to inhibit craving.48,49) Given this background, the present study finding of baclofen-treatment related reversal of the iRISA abnormalities being predictive of a better relapse outcome, is in agreement with this theoretical construct. Further, narrative accounts of individuals with AUDs50,51) that report of not experiencing the characteristic ‘loss of control’ after a lapse or a ‘slip’ (i.e., single drink) along with complete ‘indifference’ to presence of alcohol while on high doses of baclofen is also consistent with reversal of iRISA abnormalities noted in the current study. While two resting state perfusion fMRI based studies in humans have revealed that both acute and chronic administration of baclofen52,53) blunts the cerebral blood flow to ventral anterior insula, it would be further interesting to examine in future studies if higher doses of baclofen can induce more profound deactivation of insula.
The current study results are largely consistent with studies that have evaluated the impact of other pharmacological treatment protocols on brain CR, albeit with some key differences. For example, fMRI CR studies conducted on non-treatment seeking AUD subjects have noted a reduction in VS activity with drugs such as naltrexone, ondansetron,20) and aripiprazole54) compared to a placebo arm. However, another fMRI study in treatment seeking AUD subjects that evaluated the effects of long-acting naltrexone on brain CR22) found no difference in subcortical activity. This discrepancy is explained by a series of studies that have demonstrated that even direct intravenous alcohol infusion induces robust activation of the VS only in healthy social drinkers,55,56) and not in heavy drinking AUD subjects.57) This indicates that the ability of alcohol to activate the mesolimbic reward circuitry reduces on continued heavy exposure. This could explain the lack of VS CR in the heavy-drinking, treatment-seeking AUD patients in the present study. Further, there are also some differences noted in the prefrontal CR changes with treatment. For example, the fMRI study that examined long-acting naltrexone on brain CR22) found reduced activity in orbital gyri, inferior and middle frontal gyrus, and cingulate gyrus for visual cues. Whereas in contrast, an fMRI study that examined the effects of a GABA-ergic combination (gabapentin/flumazenil) on CR58) found greater activation of ACC and DLPFC, consistent with our findings. Also an fMRI study with acamprosate found no significant difference in brain CR compared with placebo.59) Therefore, these subtle differences in fMRI pharmaco-responses across studies could in fact be a function of both patient characteristics and medications that are being examined. Therefore, the baclofen treatment-related reversal of iRISA abnormalities at critical brain regions (ACC-IC) within the incentive-salience network, that predicted longer time to first alcohol use, might be central to its therapeutic “anti-craving” clinical effects.
Although longitudinal fMRI studies are crucial to model the dynamic changes in brain CR over the treatment course in AUDs, very few studies have employed such design22,59) and none have examined the predictive value of treatment related CR change for relapse outcomes. However, studies that either have utilized a pre- or post-treatment fMRI CR have reported interesting results congruent to the present study using baclofen. For example, subject with higher baseline VS CR responded better to naltrexone with longer time to relapse.60) In another study, subjects with increased ACC CR following treatment with a GABA-ergic combination (gabapentin/flumazenil) had longer time to relapse compared to placebo.58) Given this background, present study results need further replication along with other studies that utilize such design for different medications for it to be considered useful in personalizing addiction treatment.
This study represents one of the first attempts to examine baclofen treatment-related fMRI cue-reactivity in AUD individuals, but has certain limitations that need to be considered when interpreting the results. While this study may have the limitations of a non-randomized trial, several non-treatment related confounding factors (like baseline characteristics and treatment setting) were controlled. Despite the group difference in the time interval between the fMRI scans, we employed mixed effects modelling that provides flexibility in allowing for unequal time intervals, missing values, and imbalanced data. The present study did not specifically control for placebo-effect; however, true effects related to baclofen are still likely because both groups received standard treatment protocols that included comparable detoxification regimes with benzodiazepines, thrice a week group therapy for relapse prevention, oral thiamine supplementation, with only the addition of baclofen in the baclofen-treatment arm. The encouraging observations of the present study, make a case for further, randomized double-blind, placebo controlled longitudinal fMRI studies of baclofen treatment, preferably following longer periods of administration of baclofen. While we explored the relationship of ACC-IC cue-reactivity with relapse outcomes, further examination with neuro-cognitive tests of response inhibition and reward functions are warranted. Also, the study results may be biased as we included only male participants, but that reflects the strong preponderance of males in treatment centres and indeed the extreme male preponderance of drinking prevalence in India.61,62) Lastly, a two-week treatment period may have been too short for assessing the efficacy of baclofen in reducing craving for alcohol; however, we demonstrated that neurobiological effects can be seen even at this early stage and that these could be predictive of short-term relapse outcomes.
Despite these limitations, this study provides both novel and valuable preliminary neurobiological evidence for baclofen response in AUD and its relationship with relapse outcomes. Baclofen appears produce its clinical effects in AUD through modulation of critical brain regions within the incentive-salience network, with activation of regions involved in cognitive control like ACC; and deactivation of regions involved in reward anticipation like insula.
Supplementary Information
Acknowledgments
This study was supported by the Centre for Addiction Medicine, Department of Psychiatry, NIMHANS. BH was supported by Wellcome Trust/DBT India Alliance research training grant (WTDBT-IA/R/14/1/1002).
REFERENCES
- 1.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global status report on alcohol and health 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.Swift RM. Drug therapy for alcohol dependence. N Engl J Med. 1999;340:1482–1490. doi: 10.1056/NEJM199905133401907. [DOI] [PubMed] [Google Scholar]
- 4.Zindel LR, Kranzler HR. Pharmacotherapy of alcohol use disorders: seventy-five years of progress. J Stud Alcohol Drugs Suppl. 2014;75(Suppl 17):79–88. doi: 10.15288/jsads.2014.75.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soyka M. Nalmefene for the treatment of alcohol dependence: a current update. Int J Neuropsychopharmacol. 2014;17:675–684. doi: 10.1017/S1461145713001284. [DOI] [PubMed] [Google Scholar]
- 6.Addolorato G, Leggio L, Agabio R, Colombo G, Gasbarrini G. Baclofen: a new drug for the treatment of alcohol dependence. Int J Clin Pract. 2006;60:1003–1008. doi: 10.1111/j.1742-1241.2006.01065.x. [DOI] [PubMed] [Google Scholar]
- 7.Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- 8.Müller CA, Geisel O, Pelz P, Higl V, Krüger J, Stickel A, et al. High-dose baclofen for the treatment of alcohol dependence (BACLAD study): a randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2015;25:1167–1177. doi: 10.1016/j.euroneuro.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 10.Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- 11.Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2010;34:1849–1857. doi: 10.1111/j.1530-0277.2010.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponizovsky AM, Rosca P, Aronovich E, Weizman A, Grinshpoon A. Baclofen as add-on to standard psychosocial treatment for alcohol dependence: a randomized, double-blind, placebo-controlled trial with 1 year follow-up. J Subst Abuse Treat. 2015;52:24–30. doi: 10.1016/j.jsat.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Beraha EM, Salemink E, Goudriaan AE, Bakker A, de Jong D, Smits N, et al. Efficacy and safety of high-dose baclofen for the treatment of alcohol dependence: A multicentre, randomised, double-blind controlled trial. Eur Neuropsychopharmacol. 2016;26:1950–1959. doi: 10.1016/j.euroneuro.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Addolorato G, Leggio L, Ferrulli A, Cardone S, Bedogni G, Caputo F, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46:312–317. doi: 10.1093/alcalc/agr017. [DOI] [PubMed] [Google Scholar]
- 15.Shukla L, Shukla T, Bokka S, Kandasamy A, Benegal V, Murthy P, et al. Correlates of baclofen effectiveness in alcohol dependence. Indian J Psychol Med. 2015;37:370–373. doi: 10.4103/0253-7176.162913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leggio L, Garbutt JC, Addolorato G. Effectiveness and safety of baclofen in the treatment of alcohol dependent patients. CNS Neurol Disord Drug Targets. 2010;9:33–44. doi: 10.2174/187152710790966614. [DOI] [PubMed] [Google Scholar]
- 17.Leggio L, Zywiak WH, McGeary JE, Edwards S, Fricchione SR, Shoaff JR, et al. A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacol Biochem Behav. 2013;103:784–791. doi: 10.1016/j.pbb.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Wang L. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2011;(1):CD008502. doi: 10.1002/14651858.CD008502.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Morley KC, Baillie A, Leung S, Addolorato G, Leggio L, Haber PS. Baclofen for the treatment of alcohol dependence and possible role of comorbid anxiety. Alcohol Alcohol. 2014;49:654–660. doi: 10.1093/alcalc/agu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braus DF, Wrase J, Grüsser S, Hermann D, Ruf M, Flor H, et al. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm (Vienna) 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- 22.Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, et al. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. Neuroimage. 2013;78:176–185. doi: 10.1016/j.neuroimage.2013.03.055. [DOI] [PubMed] [Google Scholar]
- 23.Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, et al. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Clin Exp Res. 2004;28:550–557. doi: 10.1097/01.ALC.0000122764.60626.AF. [DOI] [PubMed] [Google Scholar]
- 24.Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- 25.George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- 26.Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 27.Beck A, Wüstenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, et al. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69:842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- 28.Sjoerds Z, van den Brink W, Beekman AT, Penninx BW, Veltman DJ. Cue reactivity is associated with duration and severity of alcohol dependence: an FMRI study. PLoS One. 2014;9:e84560. doi: 10.1371/journal.pone.0084560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Courtney KE, Schacht JP, Hutchison K, Roche DJ, Ray LA. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol. 2016;21:3–22. doi: 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. p. 362. [Google Scholar]
- 33.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 35.Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23:1289–1295. doi: 10.1111/j.1530-0277.1999.tb04349.x. [DOI] [PubMed] [Google Scholar]
- 36.Holla B, Viswanath B, Agarwal SM, Kalmady SV, Maroky AS, Jayarajan D, et al. Visual image-induced craving for ethanol (VICE): development, validation, and a pilot fMRI study. Indian J Psychol Med. 2014;36:164–169. doi: 10.4103/0253-7176.130984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groefsema M, Engels R, Luijten M. The role of social stimuli content in neuroimaging studies investigating alcohol cue-reactivity. Addict Behav. 2016;58:123–128. doi: 10.1016/j.addbeh.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 38.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 40.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7:152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen FÅ, Hansen LK. Automatic anatomical labeling of Talairach coordinates and generation of volumes of interest via the BrainMap database. Neuroimage. 2002;16(2 Suppl 1):1126–1128. [Google Scholar]
- 42.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16(2 Suppl 1):S497. [Google Scholar]
- 43.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- 46.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- 48.Ekhtiari H, Nasseri P, Yavari F, Mokri A, Monterosso J. Neuroscience of drug craving for addiction medicine: From circuits to therapies. Prog Brain Res. 2016;223:115–141. doi: 10.1016/bs.pbr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Monti PM, Rohsenow DJ, Hutchison KE. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction. 2000;95( Suppl 2):S229–S236. doi: 10.1046/j.1360-0443.95.8s2.11.x. [DOI] [PubMed] [Google Scholar]
- 50.de Beaurepaire R. Suppression of alcohol dependence using baclofen: a 2-year observational study of 100 patients. Front Psychiatry. 2012;3:103. doi: 10.3389/fpsyt.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ameisen O. Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: a self-case report of a physician. Alcohol Alcohol. 2005;40:147–150. doi: 10.1093/alcalc/agh130. [DOI] [PubMed] [Google Scholar]
- 52.Franklin TR, Shin J, Jagannathan K, Suh JJ, Detre JA, O’Brien CP, et al. Acute baclofen diminishes resting baseline blood flow to limbic structures: a perfusion fMRI study. Drug Alcohol Depend. 2012;125:60–66. doi: 10.1016/j.drugalcdep.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franklin TR, Wang Z, Sciortino N, Harper D, Li Y, Hakun J, et al. Modulation of resting brain cerebral blood flow by the GABA B agonist, baclofen: a longitudinal perfusion fMRI study. Drug Alcohol Depend. 2011;117:176–183. doi: 10.1016/j.drugalcdep.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myrick H, Li X, Randall PK, Henderson S, Voronin K, Anton RF. The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J Clin Psychopharmacol. 2010;30:365–372. doi: 10.1097/JCP.0b013e3181e75cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37:467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spagnolo PA, Ramchandani VA, Schwandt ML, Zhang L, Blaine SK, Usala JM, et al. Effects of naltrexone on neural and subjective response to alcohol in treatment-seeking alcohol-dependent patients. Alcohol Clin Exp Res. 2014;38:3024–3032. doi: 10.1111/acer.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacology (Berl) 2013;227:627–637. doi: 10.1007/s00213-013-2996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langosch JM, Spiegelhalder K, Jahnke K, Feige B, Regen W, Kiemen A, et al. The impact of acamprosate on cue reactivity in alcohol dependent individuals: a functional magnetic resonance imaging study. J Clin Psychopharmacol. 2012;32:661–665. doi: 10.1097/JCP.0b013e318267b586. [DOI] [PubMed] [Google Scholar]
- 60.Mann K, Vollstädt-Klein S, Reinhard I, Leménager T, Fauth-Bühler M, Hermann D, et al. Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res. 2014;38:2754–2762. doi: 10.1111/acer.12546. [DOI] [PubMed] [Google Scholar]
- 61.Benegal V. India: alcohol and public health. Addiction. 2005;100:1051–1056. doi: 10.1111/j.1360-0443.2005.01176.x. [DOI] [PubMed] [Google Scholar]
- 62.Gururaj G, Murthy P, Girish N, Benegal V. Alcohol related harm: implications for public health and policy in India. Bangalore: NIMHANS; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.