Abstract
α-Galactosylceramide (GalCer) is a glycolipid widely known as an activator of Natural killer T (NKT) cells, constituting a promising adjuvant against cancer, including melanoma. However, limited clinical outcomes have been obtained so far. This study evaluated the synergy between GalCer and major histocompatibility complex (MHC) class I and MHC class II melanoma-associated peptide antigens and the Toll-Like Receptor (TLR) ligands CpG and monophosphoryl lipid A (MPLA), which we intended to maximize following their co-delivery by a nanoparticle (NP). This is expected to improve GalCer capture by dendritic cells (DCs) and subsequent presentation to NKT cells, simultaneously inducing an anti-tumor specific T-cell mediated immunity.
The combination of GalCer with melanoma peptides and TLR ligands successfully restrained tumor growth. The tumor volume in these animals was 5-fold lower than the ones presented by mice immunized with NPs not containing GalCer. However, tumor growth was controlled at similar levels by GalCer entrapped or in its soluble form, when mixed with antigens and TLR ligands. Those two groups showed an improved infiltration of T lymphocytes into the tumor, but only GalCer-loaded nano-vaccine induced a prominent and enhanced infiltration of NKT and NK cells. In addition, splenocytes of these animals secreted levels of IFN-γ and IL-4 at least 1.5-fold and 2-fold higher, respectively, than those treated with the mixture of antigens and adjuvants in solution. Overall, the combined delivery of the NKT agonist with TLR ligands and melanoma antigens via this multivalent nano-vaccine displayed a synergistic anti-tumor immune-mediated efficacy in B16F10 melanoma mouse model.
Keywords: α-Galactosylceramide, Natural killer T cells, Nanoparticles, Melanoma, Toll-like receptor ligands
1. Introduction
α-Galactosylceramide (GalCer) is a glycosphingolipid known as an activator of Natural killer T (NKT) cells [1]. NKT cells are divided in two different populations, type I and II [2,3]. The type I population is known as invariant NKT cells with a semi-invariant T-cell receptor (TCR) (Vα14Jα18 in mice and Vα24Jα18 in humans) [4–6] while the type II population comprises variant NKT cells and bear variable TCR [3,6]. The type I NKT cells (hereinafter referred as NKT cells) have received increased interest over the past decade. Although conventional T cells recognize peptides bound to classical major histocompatibility complex (MHC) class I (MHCI) and II (MHCII) molecules, these NKT cells recognize self and foreign lipids presented by dendritic cells (DCs) via the MHC class I-like molecule CD1 (CD1-TCR ligation) [3,7]. Thus, by bridging the adaptive and innate immune responses, NKT cells will impact on DC maturation and downstream activation of Natural Killer (NK), B, CD4+ T and CD8+ T cells [8–10].
GalCer has emerged as a potential adjuvant in cancer immunotherapy, including melanoma [5,11–15]. Upon the presentation of GalCer via CD1 to NKT cells, these cells will secrete IFN-γ and IL-4, and up-regulate CD40L (CD40L-CD40 ligation) promoting DC maturation and subsequent up-regulation of co-stimulatory markers (e.g. CD80/CD86) and production of IL-12/CCL17 [16]. The chemokine CCL17 attracts CCR4+ CD8+ T cells that increase the DC ability to attract effector cells [16,17]. Therefore, NKT cells can act as helpers in the activation of CD8+ T cells [17,18]. However, limited clinical outcomes have emerged so far following the systemic administration of GalCer. It has been shown that, the combined delivery of GalCer with tumor-associated antigens (TAA), such as Trp2 and gp100, to the same antigen-presenting cell (APC) was required to attain the effective presentation of this lipid antigen by DCs, as well as a robust antigen-specific CD8+ T cell response against co-presented cancer peptides [11,13,19]. In addition, the insolubility of this glycolipid in aqueous media and its systemic administration have been considered as the major causes for GalCer impaired efficacy on NKT cell stimulation [5,18,20].
Nanoparticles (NPs) comprise important advantages over the systemic administration of antigens and adjuvants by improving their biodistribution to lymphoid organs and subsequent capture by APCs, where a controlled release of the antigens together with adjuvants will promote stronger specific immune responses [11,20,21].
NP fabrication processes and physicochemical properties can be optimized to advance their efficacy as vaccines [22]. NPs presenting a diameter lower than 200 nm are able to reach the lymph nodes (LNs) by direct diffusion through the cellular junctions present in the lymphatic vessel endothelium, and there be captured by DCs and further induce the activation of naïve T cells [23]. The conjugation of poly(ethylene glycol) (PEG) at the NP surface has been used to reduce the recognition and clearance of these carriers by cells of the reticuloendothelial system (RES) [24,25].
The major aim of this study was to evaluate the impact on host anti-tumor immune response of the combined delivery of GalCer, melanoma-associated antigens and the immune regulators Toll-Like Receptor (TLR) ligands, in solution or co-entrapped in a PEGylated lipid-poly(lactic-co-glycolic) acid (PLGA) NP. The peptide antigens, Melan-A (26–35(27L)) (hereafter Melan-A:26 or M), and gp100 (44–59) (hereafter gp100:44 or G), were selected due to their high immunogenicity [26]. The TLR ligands, synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG ODN) (ligand for TLR9) and monophosphoryl lipid A (MPLA) (ligand for TLR4) were used as vaccine adjuvants to enhance DC activation and maturation [27,28]. The intratumoral injections of TLR ligands have recently been shown to enable tumor elimination in animals and humans by increasing the frequency of activated APCs and reducing the infiltration of myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment [29–31]. MPLA adjuvant is already approved as a component of two prophylactic cancer vaccines, Cervarix®and Fendrix®, used to prevent the cervical cancer or the hepatocellular carcinoma, respectively [32].
The lipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1,2-dimyristoyl-sn-glycero-3-phospho-rac-glycerol, sodium salt (DMPG) were added to the PLGA matrix during NP fabrication to improve the loading of those multiple bioactive molecules inside this single carrier, increase the stability of the NPs and to potentiate NP interaction with the surface of DCs [33,34].
Herein, we show that the combination of the NKT agonist GalCer, melanoma antigens and TLR ligands, and their co-delivery by NPs showed a synergistic effect on the induction of strong antitumor immune responses able to delay tumor growth in B16F10 melanoma model. Importantly, this synergistic effect was maximized by our nano-vaccine as this was the only one able to induce an extensive infiltration of NK and NKT cells, while triggering the secretion of the highest levels of IFN-γ and IL-4 cytokines. It also upregulated CD40L (CD40L-CD40 ligation) that promotes DC maturation and consequently leads to the up-regulation of co-stimulatory markers (e.g. CD80/CD86) and the production of IL-12/CCL17 [16]. The chemokine CCL17 attracts CCR4+ CD8+ T cells that increase the DC ability to attract effector cells [16,17]. Therefore, NKT cells can act as helpers in the activation of CD8+ T cells [17,18].
2. Materials and methods
2.1. Materials
PLGA Resomer®RG 755 s (lactide:glycolide molar ratio 75:25) was purchased from Boehringer Ingelheim GmbH (Ingelheim, Germany). Cyanine 5.5 carboxylic acid-grafted PLGA (PLGA-Cy5.5) was synthesized by esterification based on Freichels et al. [35].
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1,2-dimyristoyl-sn-glycero-3-phospho-rac-glycerol (DMPG) were obtained by Lipoid (Steinhausen, Switzerland). Poly(vinyl alcohol) (PVA) (Mw 13000–23000 Da); dichloromethane (DCM); poly(ethylene glycol) methyl ether-block-poly(lactide-co-glycolide) (PLGA-PEG) (PEG average Mn 2,000, PLGA average Mn 11,500); Lipid A, monophosphoryl from Salmonella enterica serotype minnesota Re 595 (Re mutant) (MPLA); fluorescamine and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) human were supplied by Sigma-Aldrich (St. Louis, MO, USA).
Aqueous MTS reagent powder was purchased from Promega (Madison, Wisconsin, USA).
Roswell Park Memorial Institute (RPMI) 1640 Medium GlutaMAX™ Supplement; Dulbecco’s Modified Eagle Medium (DMEM), high glucose; fetal bovine serum (FBS); sodium pyruvate 100 mM; penicillin streptomycin; 4-(2-hydroxyethyl)-1-piperazi neethanesulfonic acid (HEPES) solution buffer 1 M; 2-mercaptoethanol 50 mM; Quant-iT Oligreen ssDNA assay Kit; Pierce™ LAL Chromogenic Endotoxin Quantitation Kit; Ammonium-Chloride-Potassium (ACK) lysing buffer; CD4 and CD8 antibodies for immunohistochemistry and eosin were all purchased from ThermoFisher Scientific (Waltham, Massachusetts, USA). Hematoxylin was purchased from Bio-optica (Milan, Italy).
Melan-A (26–35(27L))-sequence ELAGIGILTV (hereafter Melan-A:26 or M) and gp100 (44–59)-sequence WNRQLYPEWTEAQRLD (hereafter gp100:44 or G) were synthesized by Genecust (Ellange, Luxembourg). CpG ODN 1826 was synthesized by Mycrosynth (Balgach, Switzerland). α-Galactosylceramide (GalCer) was obtained from Abcam (Cambridge, UK).
B16F10 cell line (ATCC® CRL-6475) was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA).
The antibodies CD3-APC, CD45-PB, CD4-FITC, CD8-PE, CD107–APC-Cy7, NK1.1-PECy7, CD11b-APC-Vio770 and CD11c-APC were purchased to Miltenyi Biotec (Bergisch-Gladbach, Germany). The immPRESSTM Reagent Kit α-Rat Ig was supplied by Vector Laboratories (Burlingame, California, USA).
2.2. Nano-vaccine synthesis
NPs were prepared following a double emulsion solvent evaporation method previously developed, with specific modifications [23]. Antigens in the aqueous phase (Melan-A:26 and gp100:44) were entrapped within a single NP (250 μg from each antigen; NP[M+G]). NPs entrapping only Melan-A:26 (250 μg) (NP[M]) were also formulated. Adjuvants, CpG and MPLA, were incorporated in all developed NPs. MPLA (150–240 μg/mL) was added to the organic phase, while CpG (360–650 μg/mL) was dissolved in the aqueous phase. GalCer adjuvant (20 ng) was added to the organic phase in Melan-A:26/gp100:44 NPs (NP[M+G+GalCer]). Briefly, PLGA, PLGA-PEG (10% (w/w)) and the lipids POPC/DMPG (7.5:2.5 mol) (20% (w/w)) were dissolved in the organic solvent and emulsified with a 15% (w/v) PVA solution (aqueous phase) using a sonicator (Branson S-250D, 50/60 Hz, 20 kHz) for 15 s at 20% amplitude. Fluorescent NPs were formulated by replacing 0.5% (w/w) of the NP matrix mass by PLGA-Cy5.5.
The second emulsion was formed after the addition of 2.5% (w/v) PVA solution and subsequent dispersion using the referred conditions. The double emulsion was added dropwise to 0.25% (w/v) PVA solution and stirred for 1 h at room temperature (RT), allowing for solvent evaporation. NPs were retrieved from the suspension by centrifugation at 17,500 rpm, 4°C for 45 min (Beckman Coulter Avanti J-E Centrifuge, JA-20 rotor). NPs were washed with water and the pellet was suspended in PBS.
2.3. Physicochemical characterization of NPs
NP hydrodynamic mean diameter (Z-ave) and polydispersity index (PdI) were determined by Dynamic Light Scattering (DLS) (Zetasizer® Nano ZS, Malvern Instruments, UK). Zeta potential (ZP) was measured by Laser Doppler Electrophoresis (Malvern Instruments, Worcestershire, UK). All the measurements were determined at 25°C in triplicate.
NP entrapment efficiency (EE) and loading capacity (LC) were determined by the indirect method, analyzing the amount of antigens and adjuvants in the supernatants, where agent0 is the initial amount of agent and agentsup is the amount of agent quantified in the supernatant.
Antigen and CpG amounts were evaluated using fluorescamine and Oligreen assay® kit, respectively. The fluorescence intensity was measured at 360 nm excitation and 460 nm emission wavelengths (for antigen), and at 480 nm excitation and 520 nm emission wavelengths (for CpG) using a microplate reader (POLARstar OPTIMA, BMG Labtech, Durham, NY, USA).
MPLA amount was inferred by the LAL Chromogenic Endotoxin Quantitation Kit®. The absorbance was measured at 405 nm using the microplate reader (POLARstar OPTIMA, BMG Labtech, Durham, NY, USA).
The stability of the NPs (41.7 mg/mL) was evaluated at two different storage conditions, 4°C and 25°C, in PBS (pH 7.4). NP Z-ave, PdI and ZP were followed for 88 days.
2.4. NP surface morphology evaluation
NP size, shape and surface morphology were evaluated by Atomic Force Microscopy (AFM), using a Nanoscope IIIa Multimode AFM (Digital Instruments, Veeco), as previously described [36]. A suspension of NPs (10 mg/mL) was added to cleaved mica at RT and dried with N2. AFM analyses were performed at a scan rate of 1.6 Hz, using tapping mode in air at RT with etched silicon tips (ca. 300 kHz), obtaining topography and phase images.
2.5. DC isolation from the bone marrow
Bone marrow suspension was obtained from femurs and tibias of male C57BL/6 mice (8–10 weeks old; Charles River; Wilmington, MA, USA) [36]. The suspension was filtered through a 70 lm cell strainer, centrifuged and resuspended in ACK lysing buffer to lyse erythrocytes. The cells were washed with complete RPMI medium supplemented with 20 ng/mL of murine GM-CSF and plated in Petri dishes (107 cells/plate). On the seventh day, clusters of DCs were released and their purity was tested by flow cytometry (gating CD11c+MHCII+) using an LSRFortessa II cell analyzer (BD Biosciences). Untreated cells were used as controls and the results were analyzed with FlowJo software version 9.8 (TreeStar, San Carlos, CA).
2.6. NP impact on bone marrow-derived DC (BMDC) cell viability
Cell viability of BMDCs in the presence of increased concentrations of NPs (250, 500 and 1000 μg/mL) was inferred using MTS® Assay. The cells (10,000 cells/well) were incubated for 48 h at 37°C and 5% CO2. After this period, the culture medium and NPs were removed and replaced with 100 μL of fresh culture medium. Afterwards, MTS reagent was added in an amount of 20% (v/v) of the total volume and the plates were read after 3 h of incubation (37°C and 5% CO2) with the reagent. The absorbance was measured at 490 nm using a microplate reader (Biotek, ELx800, USA). PBS and 0.5% (v/v) Triton X-100 were used as positive and negative controls, respectively.
2.7. In vitro NP internalization by BMDCs
The cells (50,000 cells/well) were seeded and incubated overnight at 37°C and 5% CO2. Cy5.5-NPs were added to cells (0.5 mg/ mL) and incubated during 15, 48 and 72 h. Cells were then washed with PBS, harvested by centrifugation (1000 rpm, 5 min, 4°C) and resuspended with flow cytometry buffer (PBS buffer +2% (v/v) FBS). The fluorescence was analyzed using an LSRFortessa II cell analyzer (BD Biosciences). Untreated cells were used as control and the results were analyzed with FlowJo software version 9.8 (TreeStar, San Carlos, CA).
2.8. Mice
Male C57BL/6 mice (6–8 weeks old) were obtained from Charles River (Wilmington, MA, USA). The animal handling protocols were approved by the competent authority for animal protection, Direcção Geral de Alimentação e Veterinária, Lisbon, Portugal.
2.9. In vivo study of NP uptake by APCs and activation markers and co-stimulatory molecules in DCs in draining lymph nodes
Mice (n = 3/group) were injected with fluorescent Cy5.5-labeled NP[M+G] (20 mg/mL) or Cy5.5-labeled NP[M+G+GalCer] (20 mg/mL) into both flanks by subcutaneous hock immunization. After 17 h post-immunization, inguinal LNs were harvested and homogenized in a single cell suspension. Cells were stained with fluorescent-labeled anti-mouse antibodies against CD11b, CD11c, MHCII (I-Ab), (MHCI (H-2Db), CD80 and CD86, for 10 min at 4°C protected from light. The fluorescence was obtained using a LSRFortessa II cell analyzer (BD Biosciences) and results were analyzed with FlowJo software version 9.8 (TreeStar, San Carlos, CA).
2.10. B16F10 and spleen cell culture
Murine melanoma B16F10 cells (ATCC#CRL-6475) were cultured in DMEM medium with 10% (v/v) inactivated FBS and 1% (v/v) penicillin/streptomycin solution.
Spleen cells were cultured in RPMI medium with GlutaMAX with 10% (v/v) FBS, 1% (v/v) sodium pyruvate, 1% (v/v) penicillin streptomycin, 1% (v/v) HEPES buffer and 0.1% (v/v) 2-mercaptoethanol.
2.11. Nano-vaccine anti-tumor efficacy
Mice were randomized into five groups (n = 6): (1) PBS; (2) M+G+GalCer control mixture (Melan-A:26, gp100:44, CpG, MPLA and GalCer); (3) NP[M] (NP with Melan-A:26, CpG and MPLA); (4) NP [M+G] (NP with Melan-A:26/gp100:44, CpG and MPLA) and (5) NP[M+G+GalCer] (NP with Melan-A:26/gp100:44, CpG, MPLA and GalCer). Depending on the groups of treatment, mice received 200 μL of NP suspension containing 100 μg of antigen (MelanA:26 or Melan-A:26/gp100:44), 20 μg of CpG, 20 μg of MPLA and/or 20 ng of GalCer.
The M+G+GalCer control mixture was prepared by mixing stock solutions previously prepared for each of the components: 12.5 mg/mL of antigens (Melan-A:26 and gp100:44) in PBS, 3.2 mg/mL of MPLA in DMSO, 8 mg/mL CpG in PBS and 4 103 ng/mL GalCer in DMSO/PBS (50:50).
B16F10 cells (105) were subcutaneously inoculated in the right flank of each mouse. The first immunization occurred three days after tumor inoculation, and a booster dose was given ten days after, subcutaneously in the inguinal region at both mice flanks. The animals were sacrificed 18 days after tumor inoculation. During the assay, mice weight and tumor volume were monitored. At the endpoint, tumors were excised, weighted, homogenized and analyzed by flow cytometry.
2.12. T lymphocyte population and NKT cell characterization within tumor microenvironment
At the endpoint, a single cell suspension was prepared from tumors (106 cells in 200 μL of flow cytometry buffer/well) obtained from each group of animals and analyzed by flow cytometry using a LSRFortessa II cell analyzer (BD Biosciences), for the expression of CD3-APC, CD45-PB, CD4-FITC, CD8-PE, CD107–APC-Cy7 and NK1.1-PECy7. The single-cell flow cytometry analysis was carried out with FlowJo X (TreeStar, San Carlos, CA).
2.13. Tumor histology
Tumors collected were placed in 10% neutral buffered formalin for hematoxylin and eosin (H&E) and immunohistochemistry analysis. Deparaffinization in xylene and rehydratation by ethanol/ water solutions were performed. Primary antibodies, CD4 and CD8 (1:100) were incubated with the samples for 60 min at RT. After the washing steps, slides were incubated with the secondary antibody, immPRESS™ Reagent Kit α-Rat Ig during 30 min at RT. Slides were digitalized by NanoZoomer SQ slide scanner (Hamamatsu Photonics, Hamamatsu City, Japan), 20x magnification. Melanoma sections from each tumor stained for CD4 and CD8 were analyzed by ImageJ software 1.51 m9 (Wayne Rasband, National Institutes of Health, USA).
2.14. Cytokine secretion from spleens of immunized animals
At the endpoint, the spleens of treated animals were collected and homogenized on ice. The cell suspension was filtered using 70 mm strainers and erythrocytes were lysed by ACK lysing buffer. Cells were cultured in T-flasks and incubated with 20 μg/mL of Melan-A:26 (for mice immunized with NP[M]) or with a solution of 20 μg/mL of Melan-A:26 and gp100:44 (for the remaining groups) for five days at 37°C, 5% CO2. After this period of time, the supernatants were collected and the cytokines were measured by Quantikine ELISA® (Mouse interleukin (IL)-4, interferon (IFN)-γ and tumor necrosis factor (TNF)-α Immunoassays) following the manufacturer’s protocol.
2.15. Statistics
The statistical analysis was performed with One-way ANOVA followed by Tukey Post-Hoc test (p < 0,05) and independent samples t-test (p < 0,05) using SPSS version 24 (IBM, NY, USA).
3. Results
3.1. NP reproducible synthesis, and physicochemical properties suitable for antitumor vaccination
NPs were prepared by a modified double emulsion solvent evaporation method [23], having the antigens (Melan-A:26 and gp100:44) and the adjuvant CpG dissolved in the internal aqueous phase, while the polymers (PLGA, PLGA-PEG), the lipids POPC/ DMPG and the adjuvants MPLA and/or GalCer were dissolved in the organic phase (Fig. 1). According to the procedure used for NP synthesis, we expect to have the peptides and adjuvants dispersed within the NP matrix, which surface is coated by PEG chains.
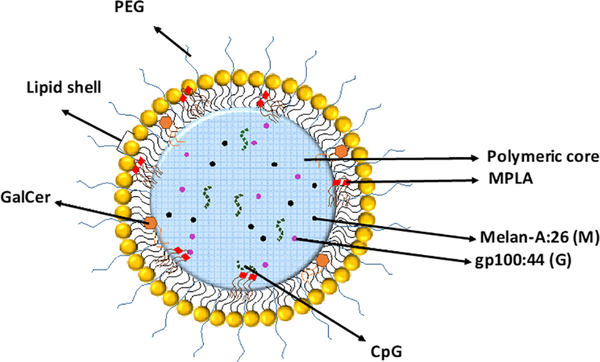
Fig. 1.
Schematic representation of the nano-vaccine containing entrapped melanoma-associated antigens (Melan-A (26–35(27L)) (Melan-A:26; M) and gp100 (44:59) (gp100:44 or G), α-Galactosylceramide (GalCer) and the Toll-Like Receptor (TLR) ligands CpG and monophosphoryl lipid A (MPLA). Poly(lactic-co-glycolic) acid (PLGA) is the major component of PEGylated-coated nanoparticles (NPs). The lipid shell is composed by 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1,2-dimyristoyl-sn-glycero-3-phospho-rac-glycerol, sodium salt (DMPG).
The different batches of NPs differed on the entrapped antigens (Melan-A:26 or Melan-A:26 plus gp100:44) and in the presence or absence of the NKT cell agonist, GalCer (Table 1).
Table 1.
Summary of the composition of the different developed formulations.
| Formulations | Polymers | Lipids | TAAa | TLRb ligands | GalCerc |
|---|---|---|---|---|---|
| M+G+GalCer control mixture | – | – | Melan-A:26d gp100:44e | CpGh & MPLAi | Yes |
| NP[Md] | 90% (w/w) PLGA + 10% (w/w) PLGA-PEG | POPCf/DMPGg | Melan-A:26d | No | |
| NP[M+Ge] | Melan-A:26d gp100:44e |
No | |||
| NP[M+G+GalCer ] | Melan-A:26d gp100:44e |
Yes |
Tumor-associated antigens.
Toll-like receptor.
α-Galactosylceramide.
Melan-A (26–35(27L), sequence ELAGIGILTV).
gp100 (44–59), sequence WNRQLYPEWTEAQRLD.
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine.
1,2-Pimyristoyl-sn-glycero-3-phospho-rac-glycerol.
Synthetic unmethylated CpG oligodeoxynucleotides (CpG ODN).
Monophosphoryl lipid A.
The developed fabrication procedure led to reproducible formulations. All NPs showed similar physicochemical properties, despite the distinct combinations of antigens and adjuvants entrapped within carrier matrix (Table 2). NPs showed a Z-ave close to 130 nm, a monodispersed population (0.048 ≤ PdI ≤ 0.160) and a neutral surface charge (Table 2).
Table 2.
Physicochemical properties of NP formulations. Mean ± SD; N = 3, n = 3, where N denotes the number of independent experiments and n denotes the number of measurements per experiment.
| Formulations | Z-Aved (nm) | PdIe | ZPf (mV) | EEg (%) | LCh(¼g/mg) (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Antigens | CpG | MPLAi | Antigens | CpG | MPLAi | ||||
| NP[Ma] | 134 ± 6.1 | 0.067 ± 0.024 | −6.30 ± 0.80 | 57.8 ± 7.12 | 64.67 ± 3.67 | 99.97 | 14.42 ± 3.43 | 2.33 ± 0.13 | 3.00 |
| NP[M+Gb] | 138 ± 4.7 | 0.072 ± 0.021 | −4.57 ± 0.65 | 53.1 ± 10.08 | 83.49 ± 0.41 | 99.96 | 26.56 ± 5.04 | 5.43 ± 0.03 | 4.80 |
| NP[M+G+GalCerc] | 138 ± 6.1 | 0.159 ± 0.038 | −4.17 ± 1.20 | 51.98 ± 0.78 | 78.71 ± 4.90 | 99.94 | 25.99 ± 0.39 | 5.12 ± 0.32 | 4.80 |
Melan-A (26–35(27L), sequence ELAGIGILTV).
gp100 (44–59), sequence WNRQLYPEWTEAQRLD.
α-Galactosylceramide.
Hydrodynamic mean diameter (Z-ave).
Polydispersity index (PdI).
Zeta potential (ZP).
Entrapment efficiency (EE).
Loading capacity (LC).
MPLA content determined by LAL Chromogenic Endotoxin Quantitation Kit® (SD is zero due to high loading values and kit sensitivity).
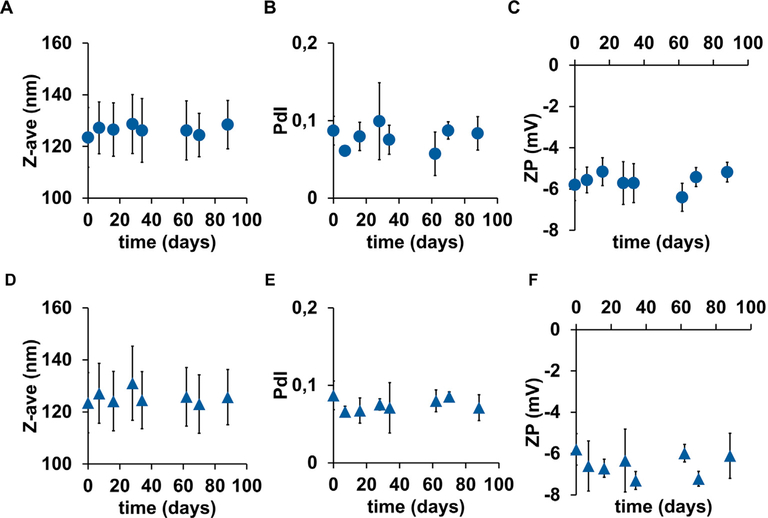
The variation on Z-ave, PdI and ZP of our NP suspended (41.7 mg/mL) in PBS (pH 7.4) was followed for 88 days, at 4°C and 25°C. During this period, we did not observe any significant change on Z-Ave, PdI and ZP of all batches of NPs at both storage conditions (Fig. 2).
Fig. 2.
NP stability. Circles are for the formulations stored at 4°C and triangles are for the formulations stored at 25°C. (A) and (D) hydrodynamic mean diameter (Zave), (B) and (E) polydispersity index (PdI), and (C) and (F) Zeta potential (ZP). Mean ± SD; N = 3, n = 3, where N stands for the number of independent experiments and n stands for the number of measurements per experiment.
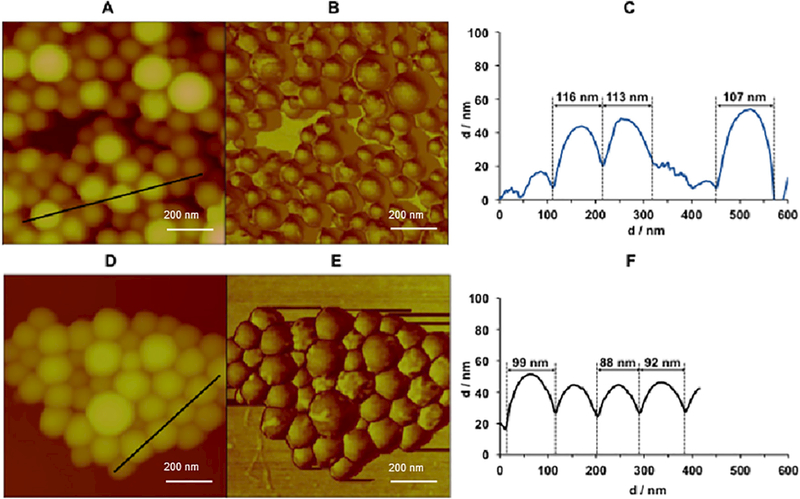
AFM was used to analyze NP shape, size and surface morphology [37]. NPs presented a spherical shape (Fig. 3A and B), smooth surface (Fig. 3B), fairly non-homogenous size distribution and hydrodynamic diameter close to that obtained by DLS (AFM: 98 ± 20 nm, DLS: 129 ± 4 nm). The NP size decreased and the roughness disappeared (Fig. 3D–F) when lipids were used in NP synthesis (non-PEGylated NP), in addition to PLGA co-polymers. NPs (Fig. 3B and C) differ from the non-PEGylated NP (Fig. 3D–F) exclusively on the presence of PLGA-PEG within NP matrix.
Fig. 3.
NP (A, B and C) and non-PEGylated NP (D, E and F) surface morphology by atomic force microscopy (AFM) (0.715 × 0.715 μm2). Topography (A and D), Phase (B and E) and NP diameter (C and F). Mean diameters were calculated from 50 individual NP from section analysis (black lines A (Z = 600 nm) and D (Z = 414 nm)) of three different areas.
The EE of melanoma antigens was close to 50%, isolated or in combination, within a single NP (Table 2). NP[M] presented a lower LC for the Melan-A:26 isolated, than when entrapped in combination with gp100:44 in NP[M+G] and NP[M+G+GalCer]. The concentration of the Melan-A:26 peptide solution used during the formulation process of NP[M] was half of that used during the development of NP[M+G] or NP[M+G+GalCer] due to the high viscosity of the Melan-A:26 solution. These differences between the internal phase of NP[M] and that used for the preparation of NP [M+G] or NP[M+G+GalCer] explain the variations in the values shown for LC in Table 2.
As expected due to the hydrophobic nature of NP matrix, MPLA EE was close to 100% (Table 2) despite formulation composition. The LC for CpG was higher for NPs co-entrapping the combination of the melanoma-associated antigens M and G, with or without GalCer.
3.2. NPs did not affect BMDC cell viability
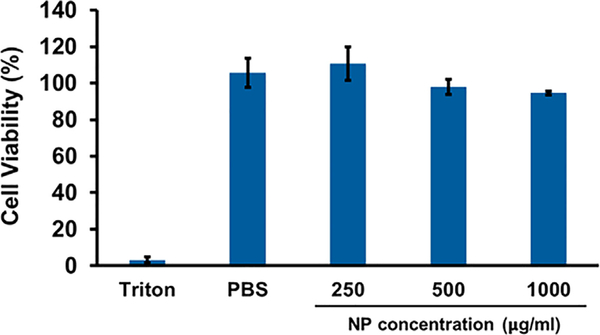
A MTS assay was performed to evaluate the impact of NPs on BMDC viability. The cells were incubated with increasing concentrations of the NPs (250, 500 and 1000 mg/mL) for 48 h. Fig. 4 shows that our nano-vaccine did not have a negative impact on BMDC viability, which remained higher than 94%, even for concentrations as high as 1 mg/mL.
Fig. 4.
Cell viability of BMDCs determined by MTS® assay after 48 h of incubation in the presence of the NPs. Mean ± SD; N = 3, n = 3, where N stands for number of independent experiments and n stands for number of measurements per experiment. One-way ANOVA and Tukey’s Post Hoc test were used to compare cell viability in PBS and the three different NP concentrations.
3.3. NPs were highly internalized by BMDCs
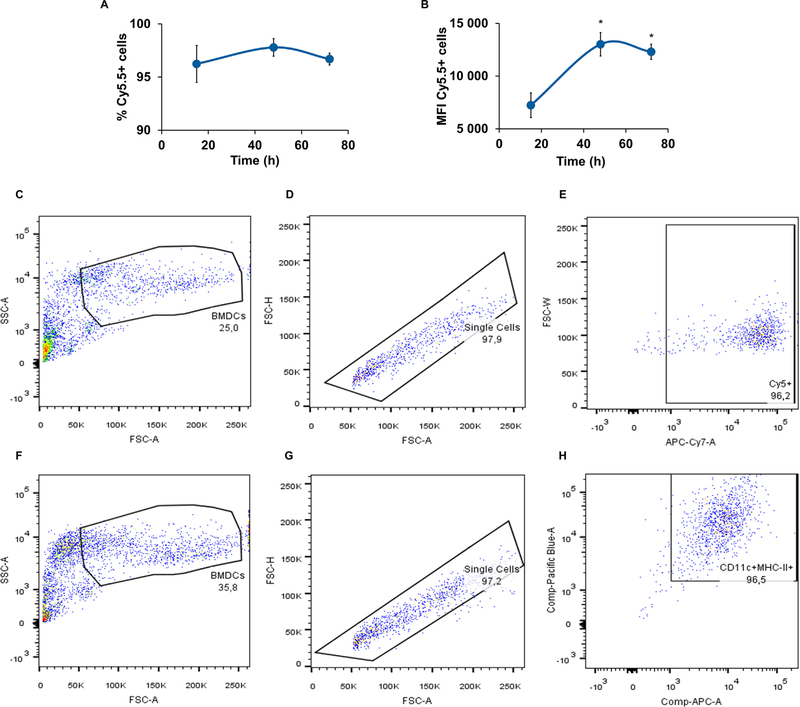
DCs are the main targets of our NPs. BMDC capacity to internalize NPs was evaluated at different time points (15, 48 and 72 h) by flow cytometry. The percentage of positive cells for Cy5.5 was close to 96% and there were no differences between those values obtained for the three time points (Fig. 5A).
Fig. 5.
Uptake of NPs after 15 h, 48 h and 72 h of incubation with BMDCs, expressed as percentage of positive cells (A) and mean fluorescence intensity (MFI) in the sorted population by flow cytometry (B). Gating strategy of the uptake study (C, D and E) and evaluation of BMDC purity (F, G and H). Mean ± SD; N = 3, n = 3, where N stands for the number of independent experiments and n stands for the number of measurements per experiment. One-way ANOVA and Tukey’s Post Hoc test were used to compare internalization levels at any time point up to 72 h incubation: *p < 0.01.
However, the internalization extent expressed as median fluorescence intensity (MFI) was time-dependent up to 72 h (Fig. 5B), occurring a slight decrease after 48 h of incubation. However, the difference between the MFI at 48 h and 72 h was not statistically significant.
3.4. NPs were efficiently internalized by BMDCs and induced the maturation of these cells in the draining lymph nodes of immunized animals
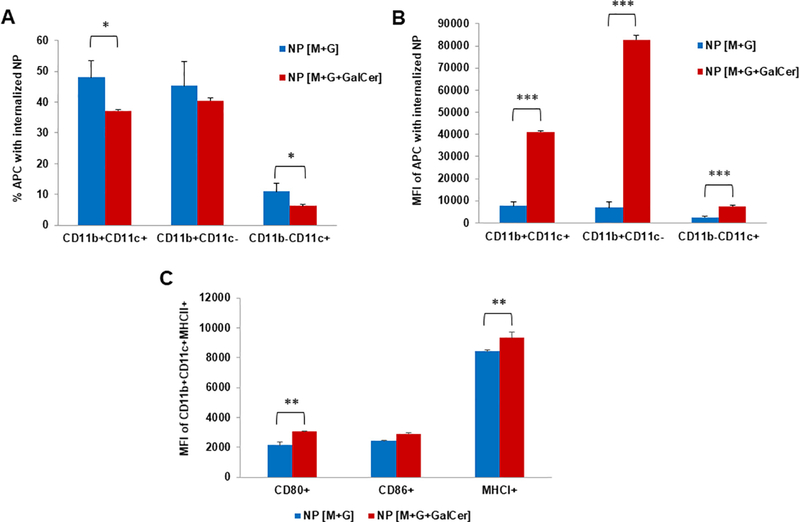
The in vitro experiments showed that NPs were efficiently internalized by BMDCs in culture (Fig. 5). To test if this also holds for a physiological setting, we immunized C57BL/6 mice into the flanks. Cy5.5-labeled NPs allowed the distinction and comparison of subpopulations of DCs with and without internalized NPs. First, we verified that a higher percentage of CD11b+CD11c+ and CD11b-CD11c+ cell populations was able to internalize the NP [M+G], compared with those able to capture NP [M+G+GalCer] (p < 0.05) (Fig. 6A). However, having in consideration those cells that effectively internalized NPs, NP [M+G+GalCer] were taken up to a greater extent by all of the studied APC populations (CD11b+CD11c+, CD11b+CD11cand CD11b-CD11c+) (p < 0.001 compared to NP [M+G]) (Fig. 6B).
Fig. 6.
In vivo NP internalization and activation of DCs in immunized C57BL/6 mice, 17 h upon immunization. (A) Percentages and (B) median florescence intensity (MFI) of NP internalization 17 h after immunization with NP [M+G] and NP [M+G+GalCer]. (C) Expression of surface activation markers on DCs that internalized NPs, present in the LN, 17 h after immunization with NP [M+G] and NP [M+G+GalCer]. Mean ± SD; N = 3, n = 3, where N denotes the number of independent experiments and n denotes the number of measurements per experiment. Statistics: Independent samples T-test: *p < 0.05, **p < 0.01 and ***p < 0.001.
In addition, we analyzed the expression of MHCI, MHCII, and the co-stimulatory molecules CD80 and CD86 in DCs (sorted as CD11b+CD11c+MHCII+). As shown in Fig. 6C, the LNs collected from mice immunized with NP[M+G+GalCer] displayed a significant increase in the expression of both CD80+ and MHCI+ in CD11b+-CD11c+MHCII+cells (p < 0.01 compared to NP [M+G]). These results indicate an increased expression of these co-stimulatory molecules on the cell surface, confirming the immunostimulant potential of the NP [M+G+GalCer].
3.5. GalCer, melanoma-associated antigens and TLR ligands had a synergistic anti-tumor immunity against melanoma
Mice were randomized into five groups: (1) PBS; (2) M+G+GalCer control mixture; (3) NP[M]; (4) NP[M+G] and (5) NP[M+G+GalCer], and immunized according to schedule in Fig. 7A. We investigated the impact on anti-tumor immunity of the subcutaneous administration of a combination of GalCer with TLR ligands and the two melanoma associated antigens (Melan-A:26 restricted MHCI peptide and gp100:44 restricted MHCII peptide) dispersed within the matrix of NPs to reach the same APCs.
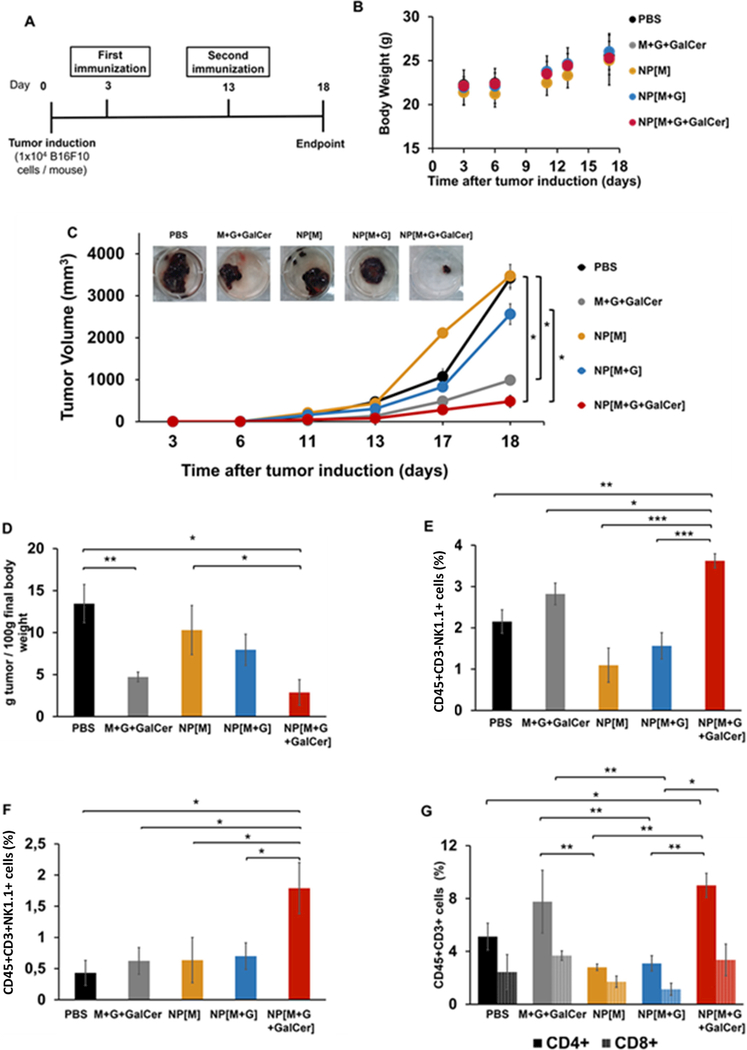
Fig. 7.
Anti-tumor therapeutic efficacy of nano-vaccines. (A) Therapeutic assay schedule, (B) Body weight over time, (C) Tumor growth curve and representative tumor images of each treated group on day 18, (D) Tumor mass per 100 g of final body weight on day 18, Mean percentage of (E) NK (CD45+CD3-NK1.1+) cells, (F) NKT (CD45+CD3+NK1.1+) cells and (G) CD4+ (CD45+CD3+CD4+) and CD8+ (CD45+CD3+CD8+) T cells at day 18 obtained by flow cytometry. Mean ± SD; n = 6, where n stands for number of measurements per experiment. Statistics: Independent samples t-test; *p < 0.05, **p < 0.01 and ***p < 0.001.
The safety of the formulations was evaluated by measuring the body weight over time (Fig. 7B). All animals increased their body weight, despite the administered treatment. Additionally, B16F10-bearing mice did not present weight loss, pain and distress throughout this study.
Fig. 7C shows that the slowest tumor growth rate was achieved in the group NP[M+G+GalCer] (p = 0.017 relative to NP[M]; p = 0.033 relative to NP[M+G] and p = 0.039 relative to PBS), which correlates with the representative images of the tumors collected. The lowest tumor masses per 100 g of final body weight were observed in the groups NP[M+G+GalCer] (p = 0.010 relative to PBS and p = 0.048 relative to NP[M]) and free M+G+GalCer (p = 0.008 relative to PBS) (Fig. 7D).
3.6. Prominent and enhanced recruitment of tumor-infiltrating lymphocytes (TIL) to tumor site
Following the promising results obtained with the combination of GalCer with Melan-A peptides and TLR ligands against melanoma growth, we were interested in characterizing the frequency of NK (CD45+CD3-NK1.1+), NKT (CD45+CD3+NK1.1+) and CD4 (CD45+CD3+CD4+) and CD8 (CD45+CD3+CD8+) T cells within tumor site of treated animals by flow cytometry (Fig. 7E–G).
The group that showed the lowest tumor volume, NP[M+G+GalCer], was the one presenting the highest number of NK (CD45+CD3-NK1.1+) cells in the tumor mass (p = 0.004 relative to PBS; p = 0.034 relative to free M+G+GalCer) (Fig. 7E).
NKT (CD45+CD3+NK1.1+) cells activated by GalCer can activate CD4+ (CD45+CD3+CD4+) T and CD8+ (CD45+CD3+CD8+) T cells [10]. Therefore, we assessed the levels of NKT (CD45+CD3+NK1.1+) cells within tumors and observed that the group NP[M+G+GalCer] presented NKT (CD45+CD3+NK1.1+) infiltration levels at least 2-fold higher those quantified in all the other groups (NP[M+G] (p = 0.015), NP[M] (p = 0.021), M+G+GalCer control mixture (p = 0.036) and PBS (p = 0.021) (Fig. 7F). Animals treated with formulations containing GalCer presented higher infiltration of both CD4+ (CD45+CD3+CD4+) and CD8+ (CD45+CD3+CD8+) T cells within the tumor microenvironment (Fig. 7G). NP[M+G+GalCer] also induced an extensive infiltration of CD4+ (CD45+CD3+CD4+) T cells into tumors (p = 0.024 relative to PBS). Mice immunized with NP[M +G+GalCer] or M+G+GalCer control mixture showed 3-fold higher infiltration of CD4+ (CD45+CD3+CD4+) T cells than mice immunized with NP not incorporating GalCer (NP[M] and NP[M+G]) (p = 0.001 NP[M+G+GalCer] relative to NP[M]; p = 0.001 NP[M+G+GalCer] relative to NP[M+G]; p = 0.005 M+G+GalCer control mixture relative to NP[M]; p = 0.007 M+G+GalCer control mixture relative to NP [M+G]).
Fig. 7G shows that the percentage of CD8+ (CD45+CD3+CD8+) T cells was 3-fold higher in animals treated with NP[M+G+GalCer] and M+G+GalCer control mixture, compared to the group NP[M +G] (p = 0.041 NP[M+G+GalCer] relative to NP[M+G] and p = 0.002 M+G+GalCer control mixture relative to NP[M+G]).
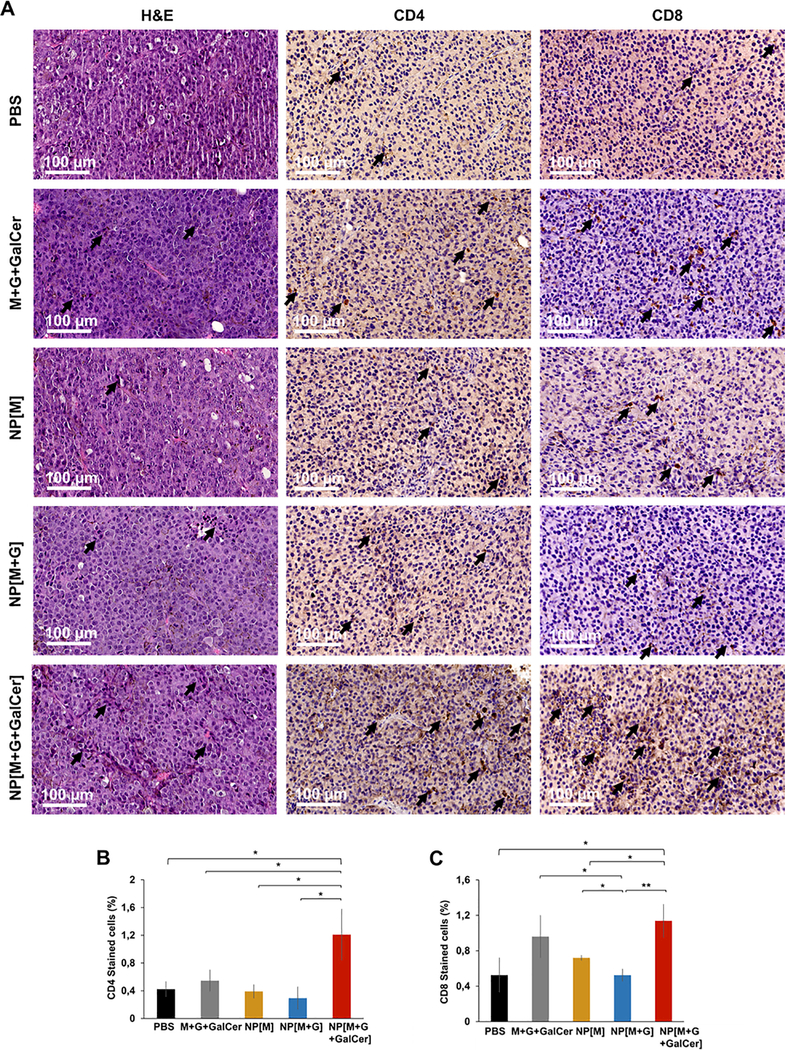
Concerning the CD4+ (CD45+CD3+CD4+) stromal T cell populations, the highest percentage was obtained within the tumors of mice immunized with NP[M+G+GalCer] relative to PBS (p = 0.024), M+G+GalCer control mixture (p = 0.045), NP[M] (p = 0.020) and NP[M+G] (p = 0.017) (Fig. 8A and B).
Fig. 8.
TILs in melanoma sections. Images representative of B16F10 melanoma histological sections in the therapeutic study with H&E staining (A) and immunohistochemistry staining for CD4 and CD8 (B and C, respectively). Scale bar = 100 μm. Black arrows indicate TILs. Mean percentage of CD4 (B) and CD8 (C) stained cells in tumors from the therapeutic assay by immunohistochemistry. Mean ± SD; N = 3, n = 3, where N stands for the number of independent experiments and n stands for the number of measurements per experiment. Statistics: Independent samples Ttest; *p < 0.05 and **p < 0.01.
The highest percentage of the CD8+ (CD45+CD3+CD8+) stromal T cell populations was achieved on tumors collected from groups NP [M+G+GalCer] and M+G+GalCer control mixture (Fig. 8A and C). The results were statistically significant for NP[M+G+GalCer] group relative to PBS (p = 0.017), NP[M] (p = 0.018) and NP[M+G] (p = 0.006), and for M+G+GalCer control mixture group relative to NP[M+G] (p = 0.038).
3.7. Cytokine secretion by splenic T helper cells
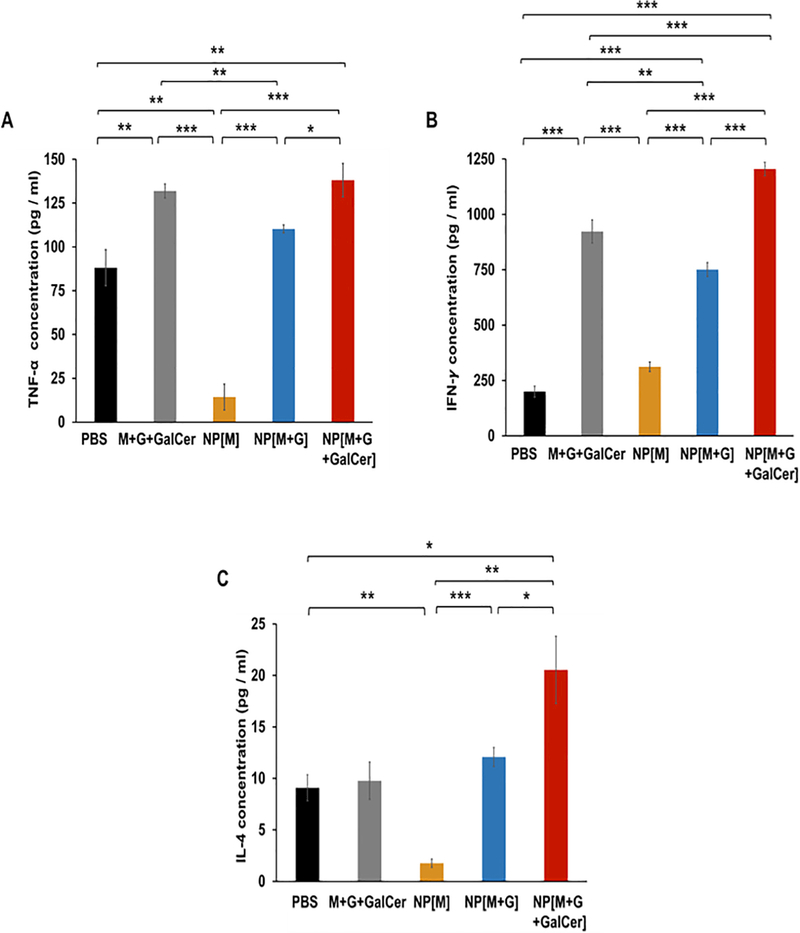
The cytokine levels present in the supernatants of cultured splenocytes reactivated with Melan-A:26 and/or gp100 (44–59) melanoma antigens contained on vaccine formulations used to treat melanoma-bearing mice can be observed in Fig. 9. Splenocytes from the group NP[M] were restimulated with Melan-A:26, while all the other splenocytes were re-stimulated with the combination of Melan-A:26 and gp100:44 antigens, as both were present on the formulations administered to animals.
Fig. 9.
Mean cytokine levels in the supernatants of spleens collected from mice in the therapeutic study. At day 18, mice were sacrificed and splenocytes were restimulated with 20 μg/mL of Melan-A:26 isolated or combined with gp100:44 antigens for five days. The levels of the cytokines (A) TNF-α, (B) IFN-γ and (C) IL-4, were measured in culture supernatants by Quantikine®ELISA. Mean ± SD; n = 3, where n stands for the number of measurements per experiment. Statistics: Independent samples T-test; *p < 0.05,**p < 0.01 and ***p < 0.001.
Regarding TNF-α (Th1 cytokine), the highest levels were obtained for the NP[M+G+GalCer] and M+G+GalCer groups (Fig. 9A). Concerning IFN-γ (Th1 cytokine) and IL-4 (Th2 cytokine), the levels were the highest for the NP[M+G+GalCer] (Fig. 9B and C).
4. Discussion
The major aim of this study was to combine GalCer, a NKT cell agonist, with melanoma-associated antigens presented by MHCI (Melan-A:26) and MHCII (gp100:44) molecules, and TLR ligands (MPLA and CpG), into a NP matrix (Fig. 1) to induce a prominent anti-tumor immune response able to restrict melanoma growth.
By presenting a mean average size close to 130 nm (Table 2), our NPs may enter the lymphatic system, reach the LNs and be internalized by LN-resident DCs, that have a key role on the presentation of antigens to naïve T cells [38]. The ideal size of NP to be captured by DC is not fully understood. However, Foged et al. [39] showed that NP with Z-ave lower than 500 nm were efficiently taken up by DCs. The neutral surface charge presented by NP will contribute for a lower recognition by RES, thus improving NP circulation time and consequent internalization by DCs, and subsequent trafficking to LNs [40].
The lipids POPC and DMPG were added to PLGA/PLGA-PEG matrix to improve the loading of the multiple bioactive molecules within nanocarrier and increase the stability of the NPs [33,34]. Lipids have emulsifying properties reducing the surface energy needed to obtain NPs, thus yielding NPs with mean diameters lower than those based only on the polymeric PLGA matrix [41]. Liu et al. [41] prepared lipid-polymer NPs coating the PLGA core with 1,2-dilauroylphosphatidylcholine by a single solvent evaporation method, and showed that the Z-ave of those lipid-polymer NP decreased when higher amounts of lipids were added to the formulation.
Additionally, by AFM analysis (Fig. 3), these NPs presented a smoother surface and lower mean average size than those previously developed composed solely by PLGA [42]. The presence of PEG at NP surface may be responsible for a smoother surface. In fact, PLGA-PEG NPs and PLGA/Polycaprolactone (PCL)-PEG NPs, previously developed also using a double emulsion solvent evaporation method, but in the absence of lipids, showed spherical morphology with a smooth surface by AFM due to the presence of PEG at the surface of the NPs [36,43].
The EE of CpG was higher than 60% (Table 2). Similar EE values for CpG and antigens were reported by Silva et al. [23], using PEGylated PLGA/PCL NPs. Moreover, the amount of antigens and adjuvants used in the NP formulation was adjusted according to the doses needed for the in vivo proof of concept studies. MPLA loading in NPs was high, with EE values close to 100%. These observations are in line with previous works, which have shown that the LC of MPLA is usually high in PLGA NPs [44].
The obtained data demonstrate the biocompatibility of our NPs (Fig. 4), suggesting the successful removal of organic solvent used during the formulation process, as well as the efficacious washing process to discard the excess of surfactant. Our previous studies evidenced that the amount of DCM that remained in polymeric microspheres prepared by a similar formulation procedure was lower than 0.04% (w/w) [45]. In addition, having in consideration the increasing concerns regarding the presence of considerable high amounts of surfactant on final product, we have also demonstrated in previous works that this washing procedure ensured the removal at least 96% of PVA [43] and 100% of sodium cholate [36] used during the double emulsion solvent evaporation process. However, the residual PVA that remained in the formulations was enough to create a stable network on the NP surface [46,47], maintaining the NP suspension stability besides its neutral charge, as observed in our previous study with PLGA NPs [42].
Developing a NP that avoids nonspecific uptake is important for an efficient internalization by APCs, such as DCs. Our NPs were efficiently internalized by BMDCs after 48 h of incubation (Fig. 5). According to Hu et al. [48], PEG-lipid-polymeric NPs composed by PLGA and the lipids 1,2-dioleoyl-3-trimethylammonium-pro pane, DSPE-PEG(2000) and cholesterol, were efficiently taken up by JAWSII (ATCC CRL-11904™) immature DCs, after 90 min of incubation. Moreover, higher amounts were internalized compared to those NPs in the absence of DSPE-PEG. In addition, higher cholesterol concentrations led to higher JAWSII cellular uptake and this was related to the higher NP stability in the presence of cholesterol [48]. A similar internalization profile expressed as percentage was observed by Silva et al. [23] for PEGylated PLGA/PCL NPs, and by Zupančič et al. [43] for PEGylated PLGA NPs when internalized by those primary APCs.
In addition, physiological internalization of NPs by APCs and activation of DCs was examined upon an in vivo immunization with NP injection. DCs with internalized NPs expressed significantly higher levels of the co-stimulatory molecules CD86 and the activation marker MHCI after the immunization with the NP [M+G+GalCer] (Fig. 6). NP [M+G+GalCer] preferentially led to the up-regulation of MHCI on the DCs, driving the antigen processing and presentation pathway towards the activation of a cytotoxic phenotype of CD8+ T cells, which is essential for the control of tumor and infectious diseases [43]. The presence of GalCer was shown to be crucial in the NPs, since it acts as a stimulus for the full maturation of DCs in mice, as previously described by Fujii et al. [49].
The main goal of the anti-tumor therapeutic assay was to assess if the developed GalCer-loaded nano-vaccine was able to deliver antigens and adjuvants to DCs and further improve the infiltration of antigen-specific CD4+ (CD45+CD3+CD4+) and CD8+ (CD45+CD3+CD8+) T cells, as well as NKT (CD45+CD3+NK1.1+) cells, in melanoma microenvironment.
Tumor growth rates suggest that the presence of both antigens Melan-A:26/gp100:44 and GalCer, in addition to the TLR ligands was crucial for the induction of a cytotoxic effect and consequently potentiated the regression of tumor size, as observed for NP[M+G +GalCer] (Fig. 7).
Additionally, the entrapment of Melan-A:26/gp100:44, TLR ligands and GalCer in NPs was more efficient in attracting NK (CD45+CD3-NK1.1+) and NKT (CD45+CD3+NK1.1+) cells into tumor site than the free form of these components. The mixture GalCer, Melan-A:26/gp100:44, CpG and MPLA, constitutes a control mixture, as the amount of MPLA necessary for the adjuvant effect is above its critical aggregate concentration (57 μM > 5 μM) [50]. Lipid A can indeed form complex aggregates, inverted hexagonal structures, where the lipid head groups are on the inner side and the fluid hydrocarbon chains on the outer side [51]. Therefore, GalCer most probably is not in its soluble form, being rather present within that expected MPLA micellar structure. According to Dölen et al., [11] mixtures of PLGA NP with entrapped ovalbumin (OVA) and free GalCer were ineffective in achieving an immune response against B16.OVA mouse melanoma model. Both components needed to be entrapped within a single NP to obtain effective primary antigen-specific in vivo killing responses. This formulation was able to confer protection against melanoma development and decreased the tumor growth in a therapeutic setting.
Moreover, Dölen et al. [11] showed advantages in using GalCer instead of TLR agonists entrapped concomitantly with OVA in the same NP. However, significant lower levels of antigen specific target cell killing and antigen specific CD8+T cells were induced when short peptides were used instead of the full length OVA protein. This fact was not related to the presence of MHCII peptides present in full length protein, but the authors suggested tolerogenicity or less activation by the non-professional APCs. In addition, the authors explained the differences in the release profile of short peptides and full length OVA, which could be related to those observed differences [11].
In our study, we used short peptide melanoma antigens, and showed the advantage of co-entrapping GalCer with both TLR agonists and antigens within a single NP. The superior anti-tumor efficacy obtained for NP[M+G+GalCer] could have been further confirmed by evaluating tumor volume after day 18. However, since we were interested in analyzing the infiltration of CD4+, CD8+, NKT and NK cells within the tumor microenvironment, the tumors were collected at day 18 to compare these infiltration values obtained in large tumors and in those which growth was controlled by our nano-vaccine.
Regarding the presence of CD8+ T cells in tumors, the non-statistically significant difference between NP[M+G+GalCer]/M+G +GalCer and PBS groups may suggest exhausted CD8+T cells in the latter group of tumors (Fig. 8). Having in consideration the strong suppression of tumor growth obtained for groups treated with formulation containing GalCer in addition to melanoma antigens and TLR ligands, we can anticipate that these CD8+ TILs may have been effector cells that contributed to tumor size regression. The achievement of antigen-specific effector CD8+ T cells is indeed a major goal while developing an efficient cancer vaccine [52]. These cells are crucial for anti-tumor immune responses. It was shown that in a phase I clinical trial, the adoptive transfer of autologous Melan-A/gp100-specific CD8+ T cells was effective in the elimination of melanoma cells and in the regression of metastases in patients with metastatic melanoma [53].
The H&E (Fig. 8A) and immunohistochemistry results (Fig. 8B and C) corroborate flow cytometry data (Fig. 7G). These results thus support TIL infiltration within tumor microenvironment assessed at tumor histological sections. The treatment groups presenting the highest recruitment of TIL also showed the most prominent values for TNF-α, IFN-γ and IL-4. These data demonstrate that the delivery of GalCer, Melan-A:26/gp100:44 peptide antigens, in addition to CpG and MPLA to the same APC (NP[M+G +GalCer] group) was fundamental for inducing balanced Th1 and Th2 immune responses against melanoma and to enhance the trafficking of NK/NKT and T cells into tumors.
The low levels of TNF-α, IFN-γ and IL-4 quantified in the supernatant of splenocytes collected from animals treated with NPs entrapping only the peptide Melan-A:26 (Fig. 9), together with the high tumor volume presented by those animals (Fig. 7C and D) suggest the induction of limited Melan-A-specific T cell responses in the group NP[M]. Thus, the low number of TIL present in this group was expected.
Tan et al. [54] used PEG-lipid-polymeric NPs to deliver a single peptide (TRP2) or multiple peptides (TRP2 plus hgp100 or TRP2 plus p15E or TRP2 plus hgp100 and p15E) to a single animal, but in a prophylactic setting against the B16F10 murine melanoma model, with three weekly immunizations. The TRP2-loaded NPs showed stronger specific peptide T cell responses, measured by IFN-γ levels, compared to multiple peptide NPs, with peptides entrapped in different NPs. The authors suggested that this fact probably resulted from the competition between peptides for antigen-presenting mechanisms. Moreover, it is not fully understood if peptides entrapped in different NPs are able to activate the same APCs. Therefore, the competition between peptides for MHCI and MHCII restricted antigen processing and presentation could still be expected for our vaccine, since we ensured the delivery of different antigens to the same APCs.
Even though, we observed a prominent immune-mediated antitumor effect obtained following the use of both of our melanoma peptides in a single NP, compared to this NP loaded with a single peptide. In fact, having in consideration the TNF-α and IL-4 levels quantified on the supernatant of splenocytes collected from animals treated with NP[M+G] or NP[M], this possible peptide competition regarding their binding to MHC molecules did not impair the broad immune modulatory effect of our multiple peptide-loaded NPs. Tan et al. [54] also showed that despite the higher antigen-specific T cell responses, single peptide NPs (TRP2 or p15E or hgp100) were not able to lead to statistically significant changes in tumor growth compared to control, whereas multiple peptide NPs reduced significantly the tumor growth. The discrepancy obtained by Tan et al. [54] regarding the higher peptide-specific T cell responses measured by splenic IFN-c levels and unexpected higher tumor growth observed for animals treated with single peptide PEG-lipid-polymeric NPs compared to those injected with multiple peptide PEG-lipid-polymeric NPs was not observed in our study. Here, neither NP[M] nor NP[M+G] groups were able to reduce tumor growth without GalCer. Our study indeed shows that using multiple antigens plus GalCer is a strategy to improve anti-tumor immunity against melanoma by bridging both innate and adaptive immune responses. The highest specific peptide T cell responses measured by splenic Th1 and Th2 cytokines and the highest infiltration levels of NK, NKT and T cells found in tumors of animals treated with formulations containing GalCer, in addition to the two peptide antigens and TLR ligands, are in accordance with overall tumor growth.
5. Conclusions
The development of a therapeutic cancer vaccine to treat established tumors is still challenging. In this study, we developed stable and monodispersed biodegradable NPs with the size close to 130 nm and neutral surface charge. The NPs were remarkably able to co-entrap peptides and glycolipid antigens, CpG oligonucleotides and Lipid A. NPs were efficiently taken up by DCs in vitro without affecting their viability. The in vivo study in mice revealed that the NPs containing both antigens (Melan-A:26 and gp100:44), TLR ligands (MPLA and CpG) and the NKT cell agonist, GalCer, are potential candidates to treat melanoma.
To the best of our knowledge, this is the first study to report the positive impact on the induction of an immune-mediated antitumor effect by combining a NKT cell agonist with TLR ligands and two melanoma-associated antigens presented by MHCI (Melan-A:26) and MHCII (gp100:44) molecules, free or entrapped within the matrix of our novel NPs.
NPs enabled the delivery of all the entrapped molecules to the same APCs, which improved the infiltration of CD4+, CD8+, NKT and NK cells into tumor microenvironment. Additionally, the levels of splenic cytokines released by Th1 and Th2 subsets were also improved by our nano-vaccine entrapping antigens, TLR ligands and GalCer. Therefore, the delivery of all these compounds to the same APC was crucial for inducing specific anti-tumor immune responses and subsequent tumor size regression.
Taking into consideration the wide versatility of our NPs and the exciting data obtained in our study, this multivalent nanovaccine formulation constitutes a promising tool to overcome tumor growth by inducing an effective and broad anti-tumor immunity bridging both innate and adaptive immune responses.
Statement of Significance.
Combination of α-galactosylceramide (GalCer), a Natural Killer T (NKT) cell agonist, with melanoma-associated antigens presented by MHC class I (Melan-A:26) and MHC class II (gp100:44) molecules, and Toll-like Receptor (TLR) ligands (MPLA and CpG), within nanoparticle matrix induced a prominent anti-tumor immune response able to restrict melanoma growth. An enhanced infiltration of NKT and NK cells into tumor site was only achieved when the combination GalCer, antigens and TLR ligands were co-delivered by the nanovaccine.
Acknowledgements
The authors thank to Fundação para a Ciência e a Tecnologia-Ministério da Ciência, Tecnologia e Ensino Superior (FCT-MCTES), Portugal for PhD Grant SFRH/BD/87869/2012 (VS), SFRH/ BPD/94111/2013 (LM), SFRH/BD/87591/2012 (CP) and PD/ BD/113959/2015 (AIM); research projects (UTAP-ICDT/DTP-FTO/ 0016/2014 (HF, NP)) and iMed.ULisboa grant UID/DTP/04138/ 2013. This project has received funding from European Structural & Investment Funds through the COMPETE Programme and from National Funds through FCT under the Programme grant SAICTPAC/0019/2015 (HF). This work was supported by The Israeli Ministry of Health (3–13620) and FCT-MCTES (ENMed/0051/2016) under the frame of EuroNanoMed-II (HF, RS-F). The University of Hertfordshire is acknowledged for providing support to this project.
Footnotes
Disclosure
The authors declare no conflicts of interest underlying the content of this manuscript.
References
- [1].Birkholz AM, Howell AR, Kronenberg M, The alpha and omega of galactosylceramides in T cell immune function, J. Biol. Chem 290 (2015) 15365–15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kumar V, Delovitch TL, Different subsets of natural killer T cells may vary in their roles in health and disease, Immunology 142 (2014) 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arrenberg P, Halder R, Kumar V, Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens, J. Cell. Physiol 218 (2009) 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Taniguchi M, Harada M, Dashtsoodol N, Satoshi K, Discovery of NKT cells and development of NKT cell-targeted anti-tumor immunotherapy, Proc. Jpn. Acad. Ser. B 91 (2015) 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bontkes HJ, Moreno M, Hangalapura B, Lindenberg JJ, de Groot J, Lougheed S, Attenuation of invariant natural Killer T-cell anergy induction through intradermal delivery of α-galactosylceramide, Clin. Immunol 136 (2010) 364–374. [DOI] [PubMed] [Google Scholar]
- [6].Gebremeskel S, Slauenwhite D, Johnston B, Reconstitution models to evaluate natural killer T cell function in tumor control, Immunol. Cell Biol 94 (2016) 90–100. [DOI] [PubMed] [Google Scholar]
- [7].Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, CD1d-restricted and TCR-mediated activation of Va14 NKT cells by glycosylceramides, Science 278 (1997) 1626–1629. [DOI] [PubMed] [Google Scholar]
- [8].Bendelac A, Savage PB, Teyton L, The biology of NKT cells, Ann. Rev. Immunol 25 (2007) 297–336. [DOI] [PubMed] [Google Scholar]
- [9].Faveeuw C, Trottein F, Optimization of natural killer T cell-mediated immunotherapy in cancer using cell-based and nanovector vaccines, Cancer Res. 74 (2014) 1632–1638. [DOI] [PubMed] [Google Scholar]
- [10].Carreño LJ, Kharkwal SS, Porcelli SA, Optimizing NKT cell ligands as vaccine adjuvants, Immunotherapy 6 (2014) 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dölen Y, Kreutz M, Gileadi U, Tel J, Vasaturo A, van Dinther EA, van Hout-Kuijer MA, Cerundolo V, Figdor CG, Co-delivery of PLGA encapsulated invariant NKT cell agonist with antigenic protein induce strong T cellmediated antitumor immune responses, Oncoimmunology 5 (2016) e1068493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Macho-Fernandez E, Cruz LJ, Ghinnagow R, Fontaine J, Bialecki E, Frisch B, Trottein F, Faveeuwet C, Targeted delivery of alpha-galactosylceramide to CD8 alpha+ dendritic cells optimizes type I NKT cell-based antitumor responses, J. Immunol 193 (2014) 961–969. [DOI] [PubMed] [Google Scholar]
- [13].Gehrmann U, Hiltbrunner S, Georgoudaki A-M, Karlsson MC, Näslund TI, Gabrielsson S, Synergistic induction of adaptive antitumor immunity by codelivery of antigen with α-galactosylceramide on exosomes, Cancer Res. 73 (2013) 3865–3876. [DOI] [PubMed] [Google Scholar]
- [14].Neumann S, Young K, Compton B, Anderson R, Painter G, Hook S, Synthetic TRP2 long-peptide and α-galactosylceramide formulated into cationic liposomes elicit CD8+ T-cell responses and prevent tumour progression, Vaccine 33 (2015) 5838–5844. [DOI] [PubMed] [Google Scholar]
- [15].Schmieg J, Yang G, Franck RW, Tsuji M, Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-galactosylceramide, J. Exp. Med 198 (2003) 1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gottschalk C, Mettke E, Kurts C, The role of invariant natural killer T cells in dendritic cell licensing, cross-priming, and memory CD8+ T cell generation, Front. Immunol 6 (2015) 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bousso P, Albert ML, Signal 0 for guided priming of CTLs: NKT cells do it too: the cross-priming of antigen-specific [CD8. sup.+] T cells requires help. The mechanism by which natural killer T cells provide such help is now characterized, Nat. Immunol 11 (2010) 284–287. [DOI] [PubMed] [Google Scholar]
- [18].Speir M, Hermans IF, Weinkove R, Engaging natural killer T cells as ‘Universal Helpers’ for vaccination, Drugs 77 (2017) 1–15. [DOI] [PubMed] [Google Scholar]
- [19].Ghinnagow R, De Meester J, Cruz LJ, Aspord C, Corgnac S, Macho-Fernandez E, Soulard D, Fontaine J, Chaperot L, Charles J, Soncin F, Mami-Chouaib F, Plumas J, Faveeuw C, Trottein F, Co-delivery of the NKT agonist α-galactosylceramide and tumor antigens to cross-priming dendritic cells breaks tolerance to self-antigens and promotes antitumor responses, OncoImmunology (2017). :00-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thapa P, Zhang G, Xia C, Gelbard A, Overwijk WW, Liu C, Hwu P, Chang DZ, Courtney A, Sastry JK, Wang PG, Li C, Zhou D, Nanoparticle formulated alpha-galactosylceramide activates NKT cells without inducing anergy, Vaccine 27 (2009) 3484–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nakamura T, Kuroi M, Harashima H, Influence of endosomal escape and degradation of α-galactosylceramide loaded liposomes on CD1d antigen presentation, Mol. Pharm 12 (2015) 2791–2799. [DOI] [PubMed] [Google Scholar]
- [22].Bachmann MF, Jennings GT, Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns, Nat. Rev. Immunol 10 (2010) 787–796. [DOI] [PubMed] [Google Scholar]
- [23].Silva JM, Zupancic E, Vandermeulen G, Oliveira VG, Salgado A, Videira M, Gaspar M, Graca L, Préat V, Florindo HF, In vivo delivery of peptides and Toll-like receptor ligands by mannose-functionalized polymeric nanoparticles induces prophylactic and therapeutic anti-tumor immune responses in a melanoma model, J. Control. Release 198 (2015) 91–103. [DOI] [PubMed] [Google Scholar]
- [24].Kulkarni SA, Feng S-S, Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery, Pharm. Res 30 (2013) 2512–2522. [DOI] [PubMed] [Google Scholar]
- [25].Jokerst JV, Lobovkina T, Zare RN, Gambhir SS, Nanoparticle PEGylation for imaging and therapy, Nanomedicine 6 (2011) 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kawakami Y, Rosenberg SA, Immunobiology of human melanoma antigens MART-1 and gp100 and their use for immuno-gene therapy, Int. Rev. Immunol 14 (1997) 173–192. [DOI] [PubMed] [Google Scholar]
- [27].Fang H, Ang B, Xu X, Huang X, Wu Y, Sun Y, Wang W, Li N, Cao X, Wan T, TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells, Cell. Mol. Immunol 11 (2014) 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Krieg AM, Toll-like receptor 9 (TLR9) agonists in the treatment of cancer, Oncogene 27 (2008) 161–167. [DOI] [PubMed] [Google Scholar]
- [29].Davis MB, Vasquez-Dunddel D, Fu J, Albesiano E, Pardoll D, Kim YJ, Intratumoral administration of TLR4 agonist absorbed into a cellular vector improves antitumor responses, Clin. Cancer Res 17 (2011) 3984–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shirota Y, Shirota H, Klinman DM, Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells, J. Immunol 188 (2012) 1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mehta NK, Moynihan KD, Irvine DJ, Engineering new approaches to cancer vaccines, Cancer Immunol. Res 3 (2015) 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Casella CR, Mitchell TC, Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant, Cell. Mol. Life Sci 65 (2008) 3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Krishnamurthy S, Vaiyapuri R, Zhang L, Chan JM, Lipid-coated polymeric nanoparticles for cancer drug delivery, Biomater. Sci 3 (2015) 923–936. [DOI] [PubMed] [Google Scholar]
- [34].Zhang L, Chan JM, Gu FX, Rhee J-W, Wang AZ, Radovic-Moreno AF, Alexis F, Langer R, Farokhzad OC, Self-assembled lipid-polymer hybrid nanoparticles: a robust drug delivery platform, ACS Nano 2 (2008) 1696–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Freichels H, Danhier F, Préat V, Lecomte P, Jérôme C, Fluorescent labeling of degradable poly (lactide-co-glycolide) for cellular nanoparticles tracking in living cells, Int. J. Artif. Organs 34 (2011) 152–160. [DOI] [PubMed] [Google Scholar]
- [36].Silva JM, Vandermeulen G, Oliveira VG, Pinto SN, Rodrigues C, Salgado A, Afonso CA, Viana AS, Jérôme C, Silva LC, Graca L, Préat V, Florindo HF, Development of functionalized nanoparticles for vaccine delivery to dendritic cells: a mechanistic approach, Nanomedicine 9 (2014) 2639–2656. [DOI] [PubMed] [Google Scholar]
- [37].Chithrani BD, Chan WC, Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes, Nano Lett. 7 (2007) 1542–1550. [DOI] [PubMed] [Google Scholar]
- [38].Silva JM, Videira M, Gaspar R, Préat V, Florindo HF, Immune system targeting by biodegradable nanoparticles for cancer vaccines, J. Control. Release 168 (2013) 179–199. [DOI] [PubMed] [Google Scholar]
- [39].Foged C, Brodin B, Frokjaer S, Sundblad A, Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model, Int. J. Pharm. 298 (2005) 315–322. [DOI] [PubMed] [Google Scholar]
- [40].Fröhlich E, The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles, Int. J. Nanomed 7 (2012) 5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu Y, Pan J, Feng S-S, Nanoparticles of lipid monolayer shell and biodegradable polymer core for controlled release of paclitaxel: effects of surfactants on particles size, characteristics and in vitro performance, Int. J. Pharm 395 (2010) 243–250. [DOI] [PubMed] [Google Scholar]
- [42].Sainz V, Peres C, Ciman T, Rodrigues C, Viana A, Afonso C, Barata T, Brocchini S, Zloh M, Gaspar RS, Florindo HF, Lopes JA, Optimization of protein loaded PLGA nanoparticle manufacturing parameters following a quality-by-design approach, RSC Adv. 6 (2016) 104502–104512. [Google Scholar]
- [43].Zupancˇicˇ E, Curato C, Paisana M, Rodrigues C, Porat Z, Viana AS, Afonso CAM, Pinto J, Gaspar R, Moreira JN, Satchi-Fainaro R, Jung S, Florindo HF, Rational design of nanoparticles towards targeting antigen-presenting cells and improved T cell priming, J. Control. Release (2017). [DOI] [PubMed] [Google Scholar]
- [44].Sarti F, Perera G, Hintzen F, Kotti K, Karageorgiou V, Kammona O, Kiparissides C, Bernkop-Schnürch A, In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A, Biomaterials 32 (2011) 4052–4057. [DOI] [PubMed] [Google Scholar]
- [45].Florindo H, Pandit S, Gonçalves L, Alpar H, Almeida A, Streptococcus equi antigens adsorbed onto surface modified poly-e-caprolactone microspheres induce humoral and cellular specific immune responses, Vaccine 26 (2008) 4168–4177. [DOI] [PubMed] [Google Scholar]
- [46].Sahoo SK, Panyam J, Prabha S, Labhasetwar V, Residual polyvinyl alcohol associated with poly (D, L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake, J. Controll. Rel 82 (2002) 105–114. [DOI] [PubMed] [Google Scholar]
- [47].Nafee N, Taetz S, Schneider M, Schaefer UF, Lehr C-M, Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of the formulation parameters on complexation and transfection of antisense oligonucleotides, Nanomed. Nanotechnol. Biol. Med 3 (2007) 173–183. [DOI] [PubMed] [Google Scholar]
- [48].Hu Y, Hoerle R, Ehrich M, Zhang C, Engineering the lipid layer of lipid–PLGA hybrid nanoparticles for enhanced in vitro cellular uptake and improved stability, Acta Biomater. 28 (2015) 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fujii S.-i., Shimizu K, Smith C, Bonifaz L, Steinman RM, Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein, J. Exp. Med 198 (2003) 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Aurell CA, Wistrom AO, Critical aggregation concentrations of gram-negative bacterial lipopolysaccharides (LPS), Biochem. Biophys. Res. Commun 253 (1998) 119–123. [DOI] [PubMed] [Google Scholar]
- [51].Jeannin J-F, Lipid A in Cancer Therapy, Springer Science & Business Media, 2010. [Google Scholar]
- [52].Henderson RA, Mossman S, Nairn N, Cheever MA, Cancer vaccines and immunotherapies: emerging perspectives, Vaccine 23 (2005) 2359–2362. [DOI] [PubMed] [Google Scholar]
- [53].Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD, Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells, Proc. Natl. Acad. Sci 99 (2002) 16168–16173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tan S, Sasada T, Bershteyn A, Yang K, Ioji T, Zhang Z, Combinational delivery of lipid-enveloped polymeric nanoparticles carrying different peptides for anti-tumor immunotherapy, Nanomedicine 9 (2013) 635–647. [DOI] [PubMed] [Google Scholar]