Abstract
Objective:
To assess the safety and efficacy of extracorporeal shockwave myocardial revascularization (ESMR) therapy in treating patients with refractory angina pectoris.
Patients and Methods:
A single-arm multicenter prospective trial to assess safety and efficacy of the ESMR therapy in patients with refractory angina (class III/IV angina) was performed. Screening exercise treadmill tests and pharmacological single-photon emission computed tomography (SPECT) were performed for all patients to assess exercise capacity and ischemic burden. Patients were treated with 9 sessions of ESMR to ischemic areas over 9 weeks. Efficacy end points were exercise capacity by using treadmill test as well as ischemic burden on pharmacological SPECT at 4 months after the last ESMR treatment. Safety measures included electrocardiography, echocardiography, troponin, creatine kinase, and brain natriuretic peptide testing, and pain questionnaires.
Results:
Fifteen patients with medically refractory angina and no revascularization options were enrolled. There was a statistically significant mean increase of 122.3±156.9 seconds (38% increase compared with baseline; P=.01) in exercise treadmill time from baseline (319.8±157.2 seconds) to last follow-up after the ESMR treatment (422.1±183.3 seconds). There was no improvement in the summed stress perfusion scores after pharmacologically induced stress SPECT at 4 months after the last ESMR treatment in comparison to that at screening; however, SPECT summed stress score revealed that untreated areas had greater progression in ischemic burden vs treated areas (3.69±6.2 vs 0.31±4.5; P=.03). There was no significant change in the mean summed echo score from baseline to posttreatment (0.4±5.1; P=.70). The ESMR therapy was performed safely without any adverse events in electrocardiography, echocardiography, troponins, creatine kinase, or brain natriuretic peptide. Pain during the ESMR treatment was minimal (a score of 0.5±1.2 to 1.1±1.2 out of 10).
Conclusion:
In this multicenter feasibility study, ESMR seems to be a safe and efficacious treatment for patients with refractory angina pectoris. However, larger sham-controlled trials will be required to confirm these findings.
Major advances in medical therapy as well as improved revascularization techniques with coronary artery bypass surgery or percutaneous intervention have markedly improved life expectancy and quality of life in patients with coronary artery disease (CAD) over the past 3 decades.1 Despite these therapies, 9 million people are estimated to have angina in the United States. Of these, approximately 7% of the patients (≈ 60,000 new patients per year in the United States) have considerable CAD burden with ischemia and intractable angina, which is not amenable to further traditional revascularization options2,3 Refractory angina, defined as persistent (>3 months) chest pain due to CAD in patients on optimal medical therapy and for whom revascularization is not feasible,4 is a major challenge to cardiologists because treatment options are limited. New treatment options including ranolazine,5 ivabradine,6 enhanced external counterpulsation,7 and spinal cord stimulation8 have been reported to improve symptoms in patients with refractory angina. Despite these new therapies, patients may continue to be limited with angina or dyspnea at low work thresholds, compromising quality of life. Strategies to enhance myocardial neovascularization are under extensive investigation. Transmyocardial laser revascularization9 has been studied extensively over the past decade but has never been fully translated to clinical use owing to its invasive nature and owing to large studies indicating no improvement in clinical symptoms. Intracoronary or myocardial stem cell,10-12 gene,13 and protein therapy,14 which have exhibited promising results but are invasive in nature, are under intensive investigation.
A new therapy, extracorporeal shockwave myocardial revascularization (ESMR), has been developed in which the noninvasive application of low-intensity shock waves is used to stimulate angiogenesis through the induction of growth factors, such as vascular endothelial growth factor15 and nitric oxide synthase,16 as well as the recruitment of endothelial progenitor cells.17 Preliminary studies on animal models have found safety and efficacy of ESMR in pigs with ischemia and post—myocardial infarction.15,18 We thus performed a pilot study to evaluate the safety and efficacy of the ESMR treatment in patients with refractory angina.
PATIENTS AND METHODS
Study Design, Study Population, and Data Collection
We designed a prospective, single-arm, multicenter pilot study to assess the safety and efficacy of the ESMR therapy in patients with at least class III angina. Investigational device exemption for Cardiospec (Medispec Ltd) was granted by the Food and Drug Administration for this study, and an approval was obtained from the institutional review board at all sites. Fifteen patients were recruited as per protocol at 3 centers in the United States: University of California, San Diego (n=7); Albert Einstein Medical Center, Philadelphia (n=5); and Mayo Clinic, Rochester (n=3).
The study protocol consisted of 5 major phases. Phase I involved screening, evaluating demographic characteristics and medical history, physical examination, pharmacological single-photon emission computed tomography (SPECT), and exercise treadmill test (ETT). Inclusion and exclusion criteria are summarized in Table 1. Patients had 2 consecutive ETTs less than 2 weeks apart (but >1 day apart), with the average taken as the baseline ETT time.
TABLE 1.
Inclusion and Exclusion Criteria
| I. Inclusion criteria |
| 1. Age ≥ 18 y |
| 2. Refractory angina |
| a. With ≥3 mo grade III or IV angina |
| b. Despite optimal medical therapy (at least 2 of β-blockers, calcium channel antagonists, and nitrates for a minimum of 6 wk) |
| c. Deemed not amenable to further revascularization by an interventional cardiologist and a cardiac surgeon |
| d. With documented reversible ischemia on pharmacological (adenosine, adenosine-analog, or dipyridamole) stress single-photon emission computed tomography |
| e. With exercise tolerance time of <10 min on the modified Bruce protocol |
| II. Exclusion criteria |
| 1. Life expectancy of <12 mo |
| 2. Refused revascularization |
| 3. Active endocarditis, myocarditis, or pericarditis |
| 4. Moderately severe or severe valvular heart disease |
| 5. Intraventricular thrombus |
| 6. Severe chronic lung disease |
| 7. Active or nonactive implantable devices (pacemakers, defibrillators, and abandoned leads) |
| 8. Malignant disease in the treatment area |
| 9. Participating in other drug/device studies or previous transmyocardial revascularization |
| 10. Unable to cooperate or terminated the screening exercise test for symptoms other than angina pectoris or equivalent |
| 11. Inadequate echocardiographic acoustic window for the extracorporeal shockwave myocardial revascularization therapy |
During phase II, participants underwent baseline evaluation blood testing for brain natriuretic peptide (BNP), creatine kinase (CK), and troponin I as well as resting wall motion analysis with 16-segment model echocardiography to locate adequate acoustic windows for the ESMR therapy.
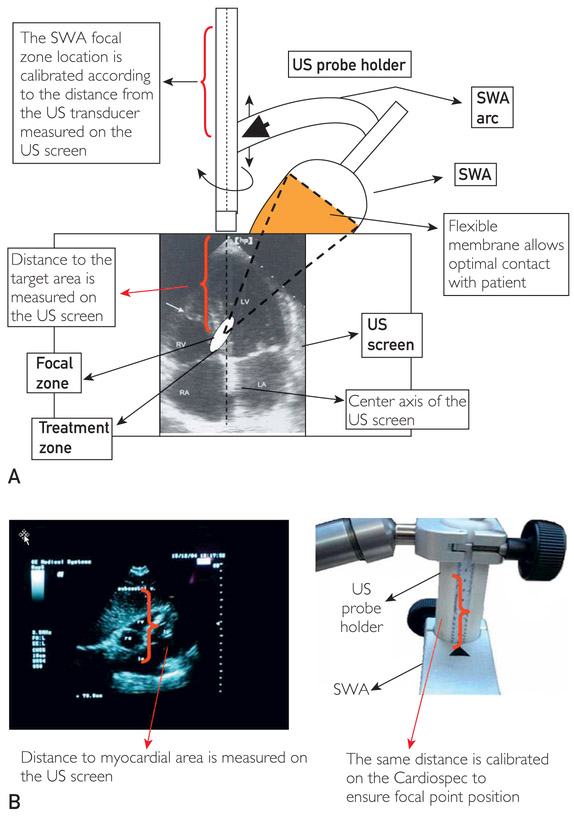
Phase II involved ESMR treatment with Cardiospec (for details, see the Supplemental Online Material) according to the standard protocol. The patient was positioned, connected with the electrocardiogram (ECG) monitor, and a shock wave applicator (SWA) membrane and an ultrasound probe were used to identify the target area. The SWA was connected with the ultrasound transducer and placed with the membrane in contact with the skin, and the “treatment zone” was positioned in the center of the ultrasound screen (Figure 1, A). To focus the shock waves, calibration of the SWA location according to the distance from the target zone on the ultrasound screen was performed (Figure 1, B). Shock waves were applied with ultrasound imaging in myocardial locations in flux densities of 0.09 mJ/mm2 at 14 kV with uniform intensity. Treatment was given in the synchronized mode so that shock waves were delivered to the patient only according to their heart rate (R-wave trigger).
FIGURE 1.
Alignment during the administration of extracorporeal shockwave therapy. LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle; SWA = shock wave applicator; US = ultrasound.
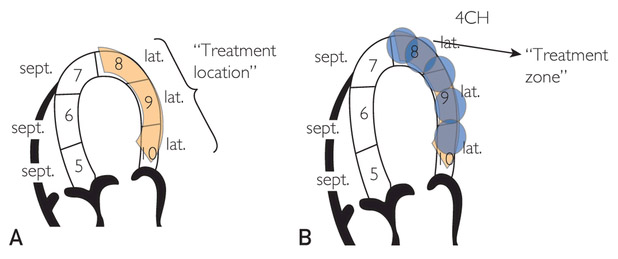
Each patient was treated at 3 different “treatment locations” on the basis of the ischemic areas identified on pharmacological SPECT.Each ischemic area to be treated was anatomically divided into a treatment location consisting of 3 segments that included the ischemic area and its borders. Each treatment location was then divided into 5 treatment zones measuring 1×1×1 cm3, which corresponded to the therapeutic area (Figure 2). A dose of up to 100 shocks was delivered to each treatment zone. Nine 20-minute treatment sessions total over 9 weeks were given to each patient according to the protocol19:
Week 1: Three treatment sessions on alternate days ±1 day delivered to treatment location 1
Week 5: Three treatment sessions on alternate days ±1 day delivered to treatment location 2
Week 9: Three treatment sessions on alternate days ±1 day delivered to treatment location 3
FIGURE 2.
A, Illustration of extracorporeal shockwave myocardial revascularization treatment locations and treatment zones. Each treatment location consists of 3 segments, which in turn consist of 5 treatment zones each measuring 1×1×1 cm3. Thus, if, for example, segment 9 is ischemic, the treatment location would include ischemic segment 9 and the borders of the ischemic segment (segments 8 and 10). B, Extracorporeal shockwave myocardial revascularization therapy focuses on the border of the ischemic area inward to potentially induce revascularization from the healthy area. 4CH = 4 chamber view; lat. = lateral; sept. = septal.
Physical examination was performed before and after each treatment session. Patients were monitored for pain, oxygen saturation, ECG abnormalities, or adverse events. Creatine kinase and troponin I were measured after treatments 3 and 9. Wall motion analysis by 16-segment echocardiography was performed after the last (ninth) treatment session to ensure no worsening had occurred.
Follow-up visits were performed at 2 months (phase IV) and 4 months (phase V) after the last ESMR treatment (±14 days) and consisted of physical examination, ETT, measurement of BNP, and documentation of pain or adverse events. Last follow-up included pharmacologically induced stress SPECT. No changes in medication treatment were made over the period of treatment and follow-up.
Exercise, Echocardiogram, and SPECT Protocols
Exercise Treadmill Test.
The ECG was monitored continuously, and a 12-lead ECG was obtained at every stage of exercise and during recovery. Vital signs were recorded at least every 3 minutes. The exercise treadmill test was discontinued if the patient developed symptoms of chest pain, lightheadedness, or cyanosis, or if the ECG revealed changes. The modified Bruce protocol or the Bruce protocol was used.20,21 Follow-up ETT was performed with the same protocol as at baseline.
Vasodilator-SPECT.
Dual-isotope (201Thallium and 99mTc-sestamibi) imaging SPECT was performed with a 180° circular orbit for 64 projections at 20 seconds per projection.22 Resting thallium injection (2.5-4 mCi) and subsequent scan, followed by pharmacological stress with adenosine infusion (140 μg/kg per minute for 6 minutes), or an adenosine analog or dipyridamole with an injection of 24 to 36 mCi 99mTc-sestamibi during stress was performed. Semiquantitative visual interpretation was performed with short-axis and vertical long-axis myocardial tomograms divided into 17 segments. Segments were scored at rest and stress by using a 6-point scoring system (0 = normal perfusion; 1 = minimal perfusion defect; 2 = mild perfusion defect; 3 = moderate perfusion defect; 4 = severe perfusion defect; 5 = no perfusion). A summed rest score (SRS) and a summed stress score (SSS) were obtained by adding the scores of the 17 segments of the rest and stress sestamibi images, respectively. The sum of the differences between each of the 17 segments on the stress and rest images is the summed difference score (SDS).
Rest Echocardiography.
A 2.5-, 3.5-, or 5.0- MHz transducer was used, and 15 beat cycles for each view were obtained. The 16-segment model was used.23 Wall motion was graded according to the 5-point system (1 = normal; 2 = hypokinesis; 3 = akinesis; 4 = dyskinesis; 5 = aneurysmal).
Central core laboratories were used to read and analyze the exercise stress ECGs (Medifacts International), echocardiography results (Med-star Health Research Institute), and SPECT images (Imagepace).
Study End Points
The primary safety objective of this pilot study was to report the incidence of serious adverse events/complications from baseline to 4 months after the last ESMR treatment, defined as acute myocardial infarction, new ECG ischemic changes, angina pectoris or its equivalent, elevated troponin I or CK, heart failure (acute or deterioration), severe arrhythmia (>25 ventricular premature contractions/h, ventricular tachycardia or fibrillation), complete heart block, deterioration of wall motion (change of at least 2 classes on a 5-point scale) or new wall motion abnormalities in the treated areas upon echocardiography, and all-cause death. Other reportable serious adverse events included coronary revascularization, cerebrovascular accident, acute renal failure, pulmonary edema, hemoptysis, and acute hepatic failure. Additional nonserious adverse events were treatment pain, transient local hematoma or purpura at treatment area, transient local edema, palpitations, and arrhythmia with no malignant potential, fatigue, dizziness, and syncope.
The primary efficacy end point was the maximal ETT time to angina (or angina equivalent) at 4 months after the last ESMR treatment (as compared with baseline). The prespecified end point for success was a prolonged exercise time of at least 90 seconds at 4 months after the treatment as compared with at baseline. The secondary efficacy end point was a change in SSS by pharmacologically induced stress SPECT at 4 months after the treatment as compared with at baseline.
Statistical Analyses
Categorical variables are described as absolute number (percentage) and continuous variables as mean ± SD if normally distributed or median (interquartile range) if not normally distributed. The paired t test or nonparametric signed-rank test was applied for testing the statistical significance of the changes. All tests applied were 2-tailed, and a P value of .05 or less was considered statistically significant. The data were analyzed using the SAS version 9.1 for Windows (SAS Institute).
RESULTS
The demographic characteristics, baseline characteristics, risk factors, and cardiac history of the 15 patients who were enrolled are summarized in Table 2.
TABLE 2.
| Age (y) | 65.0±12.1 |
| Sex | |
| Male | 13 (86.7) |
| Female | 2 (13.3) |
| BMI | |
| Obesity | 5 (33.3) |
| Race | |
| White | 11 (73.3) |
| Black | 3 (20.0) |
| Asian or Pacific Islander | 1 (6.7) |
| Smoking | |
| Never smoked | 7 (46.7) |
| Quit smoking | 8 (53.3) |
| Hypercholesterolemia | 12 (80.0) |
| Hypertension | 15 (100) |
| Family history of CAD | 5 (33.3) |
| Diabetes | 6 (40.0) |
| Bradyarrhythmias or complete heart block | 0 (0) |
| Atrial tachyarrhythmia | 1 (6.7) |
| Ventricular tachyarrhythmia or fibrillation | 0 (0) |
| Peripheral vascular disease | 2 (13.3.) |
| Chronic obstructive pulmonary disease | 2 (13.3.) |
| Congestive heart failure | 3 (20.0) |
| Uncontrolled hypertension | 2 (13.3) |
| CABG | 10 (66.7) |
| PTCA | 12 (80.0) |
| Coronary stent | 10 (66.7) |
| Rotational atherectomy | 1 (6.7) |
| Angiojet thrombolysis catheter | 1 (6.7) |
| Spinal cord stimulator | 0 (0) |
| External counter pulsation | 3 (20.0) |
| Medications | |
| Aspirin | 14 (100) |
| Clopidogrel | 7 (50.0) |
| Warfarin | 1 (7.1) |
| Statin | 14 (100) |
| β-Blocker | 11 (78.6) |
| Calcium antagonist | 6 (42.9) |
| Nitrate | 11 (78.6) |
| Ranolazine | 5 (35.7) |
| ACE-I/ARB | 7 (50.0) |
ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; CABG=coronary artery bypass graft; CAD = coronary artery disease; PTCA = percutaneous transluminal coronary angioplasty.
Data are presented as mean ± SD or as No. (percentage).
N = 15; n = 14 for medications.
Safety Results
ESMR was well tolerated without major complications in all patients. However, 2 patients (13.3%) died during the study but the deaths were deemed not related to the ESMR treatment. One patient who had bipolar disorder committed suicide. The autopsy of this patient revealed large amounts of ranolazine tablets in the stomach and detected ranolazine in the blood. The second death was of a patient 2 months after his last ESMR therapy who was admitted with chest pain. He had cardiac catheterization that documented the previously known severe coronary disease, but no intervention was felt possible. A noncontrast computed tomography scan revealed a descending thoracic aorta dissection with a compromise of the celiac and renal vessels that was complicated by renal failure, peritonitis, sepsis, and death. Patients rated pain associated with ESMR after every treatment by using the visual analog pain scale, with scores ranging from 0 (no pain) to 10 (worst pain possible). The mean pain/discomfort for ESMR treatments ranged between 0.5±1.2 and 1.1±1.2, indicating only minimal discomfort (if any) during the treatment.
There was no clinically significant elevation noted for BNP, CK, and troponin I levels in individual patients during the trial. The mean levels of BNP at screening and 2- and 4-month follow-up were 172.8±286.9, 147.9±303.8, and 173.7±299.1 pg/mL, respectively (to convert to mmol/L, multiply by 0.003247; P=.64). There were no significant changes in ECGs from baseline to follow-up.
Echocardiography was performed to detect significant deterioration in wall motion (increase of at least 2 points in the wall motion score in any treated segment): this was not observed in any patient. The mean change from baseline to week 9 (after the last ESMR treatment) in the mean summed echo score was 0.4±5.1 (P=.70), and the change from baseline to week 9 (after the last ESMR treatment) of the treated segments and untreated segments was 0.0±3.4 (P>.99) and 0.4±2.7 (P=.68), respectively.
Efficacy Results
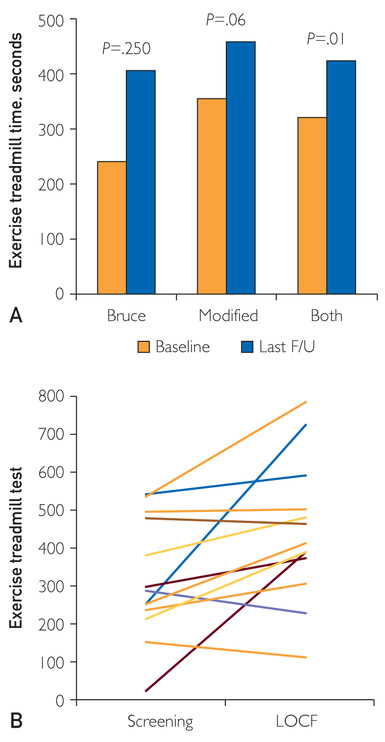
The primary effectiveness end point was a mean change of at least 90 seconds in the ETT time on the modified Bruce protocol. Some (n=4) patients underwent the Bruce protocol instead of the modified Bruce protocol at baseline: these patients underwent the same Bruce protocol at follow-up so that the results from screening and follow-ups would be comparable. Follow-up ETT times were performed at 2 and 4 months after the last ESMR treatment. For all patients (n=13), there was a statistically significant mean increase of 122.3±156.9 seconds (38% increase compared with baseline; P=.01) in ETT time from baseline (319.8±157.2 seconds) to last follow-up after the ESMR treatment (422.1± 183.3 seconds) (Figure 3). In 12 patients who had follow-up at 4 months after the last ESMR treatment, there was a statistically significant increase of 118.2±190.6 seconds in the total ETT time (P=.021). Four patients underwent the Bruce protocol, and their improvement in exercise time at the last follow-up from the ESMR treatment was 165.0±216.7 seconds more than that at baseline; however, this was not statistically significant (P=.25). Nine patients underwent the modified Bruce protocol, with no statistical improvement in exercise time at the last follow-up from the ESMR treatment (103.3±134.2 seconds vs that at baseline; P=.06). At baseline, more patients discontinued the ETT for cardiac reasons (angina or angina equivalent; 80% and 71% on the 2 screening tests) than at follow-up (at 2 months, 53.8%; at 4 months, 54.5%), at which more patients had noncardiac reasons for discontinuing (eg, dyspnea, fatigue, and leg cramps).
FIGURE 3.
A, Exercise treadmill time at baseline and follow up. B, Parallel plot describing individual exercise time at baseline and at last follow-up; excludes 2 patients who died. F/U = follow-up; LOCF = last observation carried forward.
Vasodilator-SPECT.
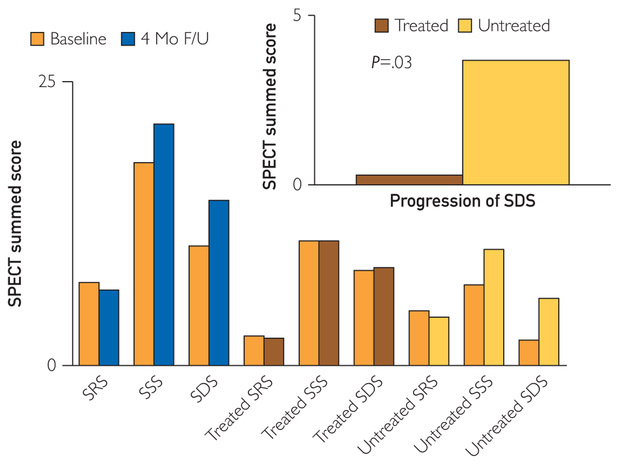
The secondary effectiveness end point was defined as an improvement in the summed stress perfusion score upon pharmacologically induced stress SPECT, 17-segment model, at 4 months after the last ESMR treatment in comparison to the stress SPECT at screening. The mean SSS did not change at 4 months after the last ESMR treatment. There was no statistical difference in mean SRS (−0.7±5.3 points; P=.687) or mean SDS (SSS minus SRS, 4.0±9.4; P=.122) (Figure 4).
FIGURE 4.
Summed scores of vasodilator single-photon emission computed tomography stress testing. F/U = follow-up; SDS = summed difference score; SPECT = single-photon emission computed tomography; SRS = summed rest score; SSS = summed stress score.
When comparing segments that were treated with ESMR and those that were not treated with ESMR, we found that after 4-month follow-up, the progression in ischemic burden (SDS at 4-month follow-up vs SDS at baseline) of the untreated segments was significantly greater compared with that of the treated segments (3.69±6.2 vs 0.31±4.5, respectively; P=.03; Figure 4, top right). The mean SSS, SRS, and SDS values of the treated segments did not improve significantly at 4 months after the last ESMR treatment: mean SSS 0.15±4.6 (P=.94), mean SRS −0.15±2.1 (P=.87), and mean SDS 0.31±4.5 (P=.59). The mean SSS, SRS, and SDS values of the untreated segments did not improve significantly at 4 months after the last ESMR treatment: mean SSS 3.15±6.6 (P=.14), mean SRS −0.54±3.9 (P=.53), and mean SDS 3.69±6.2 (P=.04).
DISCUSSION
This comprehensive multicenter pilot study suggests that ESMR therapy is a safe, noninvasive, and efficacious treatment for patients with refractory angina pectoris who are on optimal medical therapy and have no revascularization options.
The major advantages of ESMR are its non-invasive nature, its ease of application, and its apparent safety profile while achieving improvement in exercise time results similar to those achieved by using more invasive methods. Shock waves are sonic pulses (acoustic energy) characterized by high peak pressure, a short life cycle (10 ms), fast pressure rise (<10 ns), and a wide frequency spectrum (16-20 MHz). The therapy is based on extracorporeal shock wave lithotripsy, which is used successfully for the treatment of kidney stones.24,25 The energy density used for cardiac therapy (0.09 mJ/mm2 at 14 kV) is 10 times lower than that used for lithotripsy. The Cardiospec uses an electrohydraulic or “spark gap” method to create a shock wave that passes through a conducting medium (patient) to the intended treatment area. The shock wave releases jet streams that can lead to changes in the myocardial microenvironment with shear stress on cell membranes26 with hyperpolarization and activation of Ras27 and formation of free radicals, which in turn result in the up-regulation of vascular endothelial growth factor and its receptor Flt-115,28 and in the enhanced expression of stromal-derived factor-1 with an increase in the synthesis of nitric oxide.16 Thus, the potential mechanism of action of shock wave therapy follows 2 major pathways: vasodilatation (nitric oxide) and neovascularization. Both have previously been found to occur in animal model skin and musculoskeletal tissues.17,29-32 There is evidence of enhanced recruitment of progenitor cells to the site of both ischemic and nonischemic tissue after shock wave therapy, most likely by these chemoattractants.17,33 There have been a few small, single-center human studies that suggest improved exercise tolerance anginal symptoms in the human model34-36 The present study is the first multicenter pilot study in the United States that suggests that ESMR is a safe, clinically feasible treatment modality. The present data also suggest that ESMR may be associated with improved exercise treadmill time, which is comparable to more invasive therapeutic modalities.11
Although other studies have described similar findings, the present study makes several important contributions. First, the multicenter design of this pilot study makes it distinct from previously published studies. The multicenter design of the present trial reduces bias that may be inevitable in single-center studies. It also allows for the diversity of patient population, making it more applicable to the generalized population. This study is supported by several basic science studies in the animal model that have provided a mechanistic explanation to the clinical improvement in symptoms seen with the use of ESMR. Furthermore, we report greater progression in ischemia in untreated myocardial regions as compared with regions treated with ESMR. A comparison of regions treated with ESMR and regions not treated with ESMR allows for an internal control not described previously. In the present study, we report that myocardial segments treated with ESMR did not have progression of ischemia, compared with nontreated segments, potentially suggesting that ESMR could have stopped the progression of flow impairments in these segments. The actual mechanism behind this beneficial effect remains to be determined, although it could be hypothesized that it may have to do with changes in endothelial function37 and stimulation of proangiogenic factors described previously. Decreased progression of ischemia with ESMR is an important finding not reported previously. The ability to compare treated and untreated regions in this way provides an internal control, further corroborating the beneficial effect of ESMR.
In the present study, we have also recorded patients’ reason for termination and found that angina was more often reported as a reason for termination before ESMR vs after ESMR.
Finally, it is important to mention the limitations of this study: This was a pilot feasibility study to assess safety and possible efficacy of ESMR and no control group was included. It is important to note the pilot nature of this study, making it difficult to draw conclusions. The present data suggest limited adverse events with ESMR therapy and increased exercise tolerance in patients receiving ESMR. However, because of the pilot nature of this study and the small number of participants, the present data must be interpreted with caution. Larger standardized studies are required to better assess the potential benefits of this technique. This study is also limited in its size. We report a small number of patients who have been treated with ESMR. Ideally with a small study of this nature, the patients should be highly standardized with strict entry criteria. We attempted to standardize the patients to the best of our ability, but inherent differences can be more meaningful when conducting a small study. Thus, larger confirmatory studies, including sham-controlled randomized trials, are needed to ascertain the beneficial effect of ESMR in patients with CAD and advanced angina and limited revascularization options.
CONCLUSION
In this multicenter study, we find that ESMR is a safe and possibly efficacious noninvasive treatment for patients with refractory angina pectoris. However, more data from larger, randomized, placebo-controlled trials are required to confirm these promising findings before they can be used widely.
Supplementary Material
Acknowledgments
Grant Support: This study was supported by an unrestricted grant from MediSpec Ltd.
Abbreviations and Acronyms:
- BNP
brain natriuretic peptide
- CAD
coronary artery disease
- CK
creatine kinase
- ECG
electrocardiogram/electrocardiographic
- ESMR
extracorporeal shockwave myocardial revascularization
- ETT
exercise treadmill test
- SDS
summed difference score
- SPECT
single-photon emission computed tomography
- SRS
summed rest score
- SSS
summed stress score
- SWA
shock wave applicator
Footnotes
SUPPLEMENTAL ONLINE MATERIAL
Supplemental material can be found online at http://www.mayoclinicproceedings.org.
Contributor Information
Andrew Cassar, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN.
Megha Prasad, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN.
Martin Rodriguez-Porcel, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN.
Guy S. Reeder, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN.
Darshak Karia, Division of Cardiovascular Diseases, Albert Einstein Medical Center, Philadelphia, PA.
Anthony N. DeMaria, Division of Cardiovascular Diseases, University of California, San Diego, La Jolla.
Amir Lerman, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association [published correction appears in Circulation. 2012;125(22):e1002]. Circulation. 2012;125(1):e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams B, Menon M, Satran D, et al. Patients with coronary artery disease not amenable to traditional revascularization: prevalence and 3-year mortality. Catheter Cardiovasc Interv 2010;75(6):886–891. [DOI] [PubMed] [Google Scholar]
- 3.Kiernan TJ, Boilson BA, Sandhu GS, et al. Nonrevascularizable coronary artery disease following coronary artery bypass graft surgery: a population-based study in Olmsted County, Minnesota. Coron Artery Dis 2009;20(2):106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGillion M, L’Allier PL, Arthur H, et al. Recommendations for advancing the care of Canadians living with refractory angina pectoris: a Canadian Cardiovascular Society position statement. Can J Cardiol 2009;25(7):399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson SR, Scirica BM, Braunwald E, et al. Efficacy of ranolazine in patients with chronic angina observations from the randomized, double-blind, placebo-controlled MERLIN-TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Segment Elevation Acute Coronary Syndromes) 36 Trial. J Am Coll Cardiol 2009;53(17):1510–1516. [DOI] [PubMed] [Google Scholar]
- 6.Tarolif JC, Ponikowski P, Kahan T, ASSOCIATE Study Investigators. Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4 month, randomized, placebo-controlled trial. Eur Heart J 2009;30(5):540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barsness G, Feldman, Holmes DR, Holubkov R, Kelsey SF, Kennard ED. The International EECP Patient Registry (IEPR): design, methods, baseline characteristics, and acute results. Cin Cardiol 2001;24(6):435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hautvast RW, DeJongste MJ, Staal MJ, van Gilst WH, Lie KI. Spinal cord stimulation in chronic intractable angina pectoris: a randomized, controlled efficacy study. Am Heart J 1998; 136(6):l 114–1120. [DOI] [PubMed] [Google Scholar]
- 9.Saririan M, Eisenberg MJ. Myocardial laser revascularization for the treatment of end-stage coronary artery disease. J Am Coll Cardiol 2003;41(2):173–183. [DOI] [PubMed] [Google Scholar]
- 10.van Ramshorst J, Bax JJ, Beeres SL, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301(l9):l997–2004. [DOI] [PubMed] [Google Scholar]
- 11.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res 2011;109(4):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friis T, Haack-Sørensen M, Mathiasen AB, et al. Mesenchymal stromal cell derived endothelial progenitor treatment in patients with refractory angina. Scand Cardiovasc J 2011;45(3):161–168. [DOI] [PubMed] [Google Scholar]
- 13.Kastrup J, Jørgensen E, Rüick A, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris: a randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol 2005;45(7):982–988. [DOI] [PubMed] [Google Scholar]
- 14.Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107(10):1359–1365. [DOI] [PubMed] [Google Scholar]
- 15.Nishida T, Shimokawa H, Oi K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004; 110(19):3055–3061. [DOI] [PubMed] [Google Scholar]
- 16.Mariotto S, de Prati AC, Cavalieri E, Amelio E, Marlinghaus E, Suzuki H. Extracorporeal shock wave therapy in inflammatory diseases: molecular mechanism that triggers anti-inflammatory action. Curr Med Chem 2009;16(19):2366–2372. [DOI] [PubMed] [Google Scholar]
- 17.Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114(25):2823–2830. [DOI] [PubMed] [Google Scholar]
- 18.Uwatoku T, Ito K, Abe K, et al. Extracorporeal cardiac shock wave therapy improves left ventricular remodeling after acute myocardial infarction in pigs. Coron Artery Dis 2007;18(5):397–404. [DOI] [PubMed] [Google Scholar]
- 19.Zuoziene G, Laucevičius A, Leibowitz D. Extracorporeal shockwave myocardial revascularization improves clinical symptoms and left ventricular function in patients with refractory angina. Coron Artery Dis 2012;23(1):62–67. [DOI] [PubMed] [Google Scholar]
- 20.Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res 1971;3(6):323–332. [PubMed] [Google Scholar]
- 21.Gibbons RJ, Balady GJ, Bricker JT, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) [published correction appears in J Am Coll Cardiol. 2006;48(8): 1731]. J Am Coll Cardiol 2002;40(8):1531–1540. [DOI] [PubMed] [Google Scholar]
- 22.Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS, Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13(6):e80–e90. [DOI] [PubMed] [Google Scholar]
- 23.Cerqueira MD, Weissman NJ, Dilsizian V, et al. ; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4): 539–542. [DOI] [PubMed] [Google Scholar]
- 24.Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock-waves. Lancet. 1980; 2(8207):1265–1268. [DOI] [PubMed] [Google Scholar]
- 25.Preminger GM, Tiselius HG, Assimos DG, et al. ; EAU/AUA Nephrolithiasis Guideline Panel. 2007 guideline for the management of Ureteral calculi. J Urol 2007;178(6):2418–2434. [DOI] [PubMed] [Google Scholar]
- 26.Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol 2001;281(3):L529–L533. [DOI] [PubMed] [Google Scholar]
- 27.Wang FS, Wang CJ, Huang HJ, Chung H, Chen RF, Yang KD. Physical shock wave mediates membrane hyperpolarization and Ras activation for osteogenesis in human bone marrow stromal cells. Biochem Biophys Res Commun 2001;287(3): 648–655. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida J, Ohmori K, Takeuchi H, et al. Treatment of ischemic limbs based on local recruitment of vascular endothelial growth factor-producing inflammatory cells with ultrasonic microbubble destruction. J Am Coll Cardiol 2005;46(5):899–905. [DOI] [PubMed] [Google Scholar]
- 29.Yan X, Zeng B, Chai Y, Luo C, Li X. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann Plast Surg 2008;61(6):646–653. [DOI] [PubMed] [Google Scholar]
- 30.Wang CJ, Huang HY, Pai CH. Shock wave-enhanced neovascularization at the tendon-bone junction: an experiment in dogs. J Foot Ankle Surg 2002;41(1):16–22. [DOI] [PubMed] [Google Scholar]
- 31.Young SR, Dyson M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol 1990;16(3):261–269. [DOI] [PubMed] [Google Scholar]
- 32.Stephenson TJ, Johnson AG, Ross B. Short-term effects of extracorporeal shock wave lithotripsy on the human gallbladder. J Pathol 1989;158(3):239–246. [DOI] [PubMed] [Google Scholar]
- 33.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. [DOI] [PubMed] [Google Scholar]
- 34.Fukumoto Y, Ito A, Uwatoku T, et al. Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coron Artery Dis 2006; 17(1):63–70. [DOI] [PubMed] [Google Scholar]
- 35.Khattab AA, Brodersen B, Schuermann-Kuchenbrandt D, et al. Extracorporeal cardiac shock wave therapy: first experience in the everyday practice for treatment of chronic refractory angina pectoris. Int J Cardiol 2007;121(1):84–85. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi Y, Ito K, Ito Y, et al. Double-blind and placebo-controlled study of the effectiveness and safety of extracorporeal cardiac shock wave therapy for severe angina pectoris. Circ J 2010;74(3):589–591. [DOI] [PubMed] [Google Scholar]
- 37.Hasdai D, Gibbons RJ, Holmes DR Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96(10): 3390–3395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.