Abstract
Osteocytes are differentiated osteoblasts that become surrounded by matrix during the process of bone formation. The acquisition of the osteocyte phenotype is achieved by profound modifications in gene expression that confer adaptation to the changing cellular functions and constitute the molecular signature of osteocytes. The levels of expression of genes characteristic of osteoblasts is altered; and the expression of genes/proteins that impart dendritic cellular morphology, regulate matrix mineralization, and control the function of bone surface cells, is orderly modulated during osteocytogenesis. The discovery of human mutations of osteocytic genes had contributed to a large extent to reveal the role of osteocytes in bone homeostasis. Osteocytes are targets of mechanical force imposed to the skeleton and also play a critical role in integrating mechanosensory pathways with the action of hormones, thereby leading to the orchestrated response of bone to environmental cues. Current, novel therapeutic approaches harness this accumulating knowledge by targeting osteocytic signaling pathways and messengers to improve skeletal health.
OLD THEORIES AND NEW EVIDENCE FOR A CENTRAL ROLE OF OSTEOCYTES IN BONE HOMEOSTASIS
Osteocytes, the most abundant bone cells, are essential for the accomplishment of the functions of the skeleton. Far from being inactive quiescent cells buried in the mineralized tissue (Figure 1), osteocytes coordinate bone acquisition during growth and the maintenance of a healthy skeleton suit for locomotion and protection of essential organs. Osteocytes orchestrate the work of osteoblasts that form bone and osteoclasts that resorb it by producing and secreting factors, thus adapting the skeleton to mechanical needs and hormonal changes. Osteocytes contribute to the endocrine functions of bone by secreting hormones that affect other tissues; and they also regulate mineral homeostasis and hematopoiesis, which are essential for organismal life. The central role of osteocytes was long envisioned by pioneers in the field, who proposed potential mechanisms by which these cells could contribute to the functions of the skeleton, well before such mechanisms were unraveled.
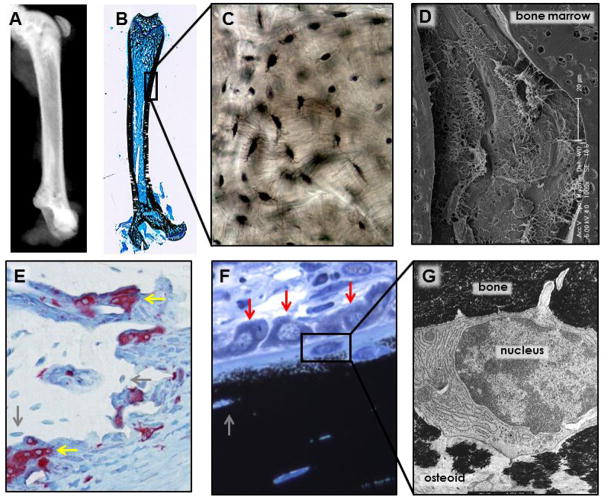
Figure 1. Osteocyte and the hierarchic organization of bone.
(A) X-ray image of a murine femur and (B) rat femur stained with von Kossa and McNeal to show the bone stained in black and bone marrow, stained in blue, showing the bone structure. (C) Image of dry human showing osteocytes and their canaliculi (in black). (D) Scanning electron microscopy of acid etched murine vertebral bone the cellular organization of osteocytes within bone with their projections reaching other osteocytes and cells on the bone marrow. (E) TRAP-stained osteoclasts (in red) on rat bone sections stained with TRAPase and toluidine blue and (F) osteoblasts present on bone surfaces covered by osteoid in rat bone sections stained with von Kossa and McNeal, showing the close proximity of osteocytes (grey arrows) to osteoblasts (red arrows) and osteoclasts (yellow arrows) on the bone surface. (G) Early osteocyte being embedded in bone matrix that is partially (left) and completely (right) mineralized (10,000 x). The boxes in panels B and F delineate the areas enlarged in panels C and F, respectively. Pictures for panels B and F were contributed by Keith Condon, Indiana University School of Medicine, Indianapolis, IN, USA. Picture for panel G was provided by Stephen B. Doty, Hospital for Special Surgery, New York, NY, USA.
Marotti and colleagues showed by microscopic examination of human bone that osteocytes within lacunae extent multiple cytoplasmic projections reaching neighboring osteocytes and cells on bone surfaces.1 They proposed that osteocytes are central players in this cellular network in which cells are connected via gap junctions and are able to sense mechanical and biochemical signals.2 Because osteocytes exhibit longer and more abundant dendrites facing the bone surface than the mineralized bone and the number of osteocytic dendrites in contact with osteoblasts was inversely proportional to the osteoblast size (and thus activity), they proposed that osteocytes send inhibitor signals to osteoblasts to slow bone formation.2 Based on this theory, Martin developed a mathematical model that predicts that during the refilling of resorption cavities there is an initial increase in bone formation followed by a reduction in osteoblast activity as osteocytes mature.3 This model also predicts that the osteoblasts most affected by osteocytic inhibitory signals have higher probability to be buried within the matrix still being synthesized by neighboring osteoblasts, thus becoming the next population of osteocytes. Today, the importance of the osteocyte network and the mechanisms and messengers by which osteocytes control not only bone formation, but also resorption and hematopoiesis, are recognized and some of the molecular mediators have been identified.
Frost in pioneer studies demonstrated decreased osteocyte viability with age,4 proposed that osteocytes regulate water and calcium flow from the canaliculi to the blood compartment,5 and developed the mechanostat theory that proposes that the magnitude of the mechanical stimulation applied to bone dictates whether bone will be increased (by increasing bone formation) or reduced (by increasing bone resorption).6 In this model, osteocytes sense the load imposed to bone and respond by signaling to osteoblasts and osteoclasts to adapt to mechanical changes.7 We now know that one of the osteocyte-derived molecules involved in bone adaptation is Sost/sclerostin.8, 9
Seminal work by Parfitt in the 1970s postulated that osteocytes are involved in the response of the skeleton to parathyroid hormone (PTH).10 Based on the rapid timing of hypercalcemic responses to PTH, the fact that new protein synthesis is not required, and that calcium is released into the circulation at two different rates, Parfitt concluded that osteocytes control the rapid release of calcium, whereas osteoclasts and resorption determines the later phase of calcium released from bone to the circulation induced by PTH. Studies using genetically modified mice have recently demonstrated profound skeletal effects of activation of the PTH receptor in osteocytes11–14 and defective response to PTH of mice lacking this receptor in osteocytes (15 and Bellido et al, unpublished). These studies support and extend Parfitt’s concept that indeed osteocytes are target cells of PTH action. Further, mice lacking the PTH receptor in osteocytes lose less bone with lactation, a condition in which calcium release from the skeleton to the blood induced by PTHrP (the other ligand of the PTH receptor) is required for milk production in the mammary glands.16 In addition, the characteristic increase in osteocyte lacunar size observed in lactating wild type mice is not found mice lacking the PTH receptor in osteocytes, suggesting that removal of perilacunar bone by osteocytes is a source of calcium and contributes to the bone loss. The process of perilacunar remodeling, named earlier by Belanger osteocytic osteolysis,17 is achieved by the expression by osteocytes of genes usually expressed by osteoclasts during bone resorption. These genes encode for proteins that decrease extracellular pH such as carbonic anhydrases and ATPases, degrade the extracellular matrix as MMP13 and cathepsin K, or dissolve the mineral as TRAPase.
Thus, the molecular mechanisms by which osteocytes accomplish the functions postulated by pioneers of the field are being unraveled and it is expected that current research will continue to increase our knowledge of osteocyte biology and pathophysiology.
MOLECULAR AND FUNCTIONAL SIGNATURE OF OSTEOCYTES
An estimated 5–20% of the osteoblasts present on the bone surface become surrounded by matrix proteins that they produce, and differentiate into osteocytes.18,19 The mechanism why which some osteoblasts and not others undergo this process is uncertain. Nevertheless, this transition is characterized by alterations in gene expression and it is accompanied by morphological and functional changes. The highly secretory osteoblasts are characterized by their cuboidal shape with large nucleus located close to the cell basal membrane, enlarged Golgi apparatus on the nuclear apical surface, and extensive endoplasmic reticulum. During osteocytogenesis, the number of organelles markedly reduces and the ratio between nuclear and cytoplasm volume increases as the cell acquires a star-like morphology (Figure 1). The long cytoplasmic processes exhibited by osteocytes run through canaliculi dig in mineralized bone and touch neighboring osteocytes, bone surface cells, and endothelial cells of the blood vessels. This extensive lacunar-canalicular system is maintained by the ability of osteocytes themselves of remodeling their surrounding space, as demonstrated by calcification of the lacuna and reduced numbers of canaliculi when osteocytes are lost by apoptosis. The osteocyte lacunar-canalicular system also distributes molecules secreted by osteocytes among all cells of the bone-bone marrow.
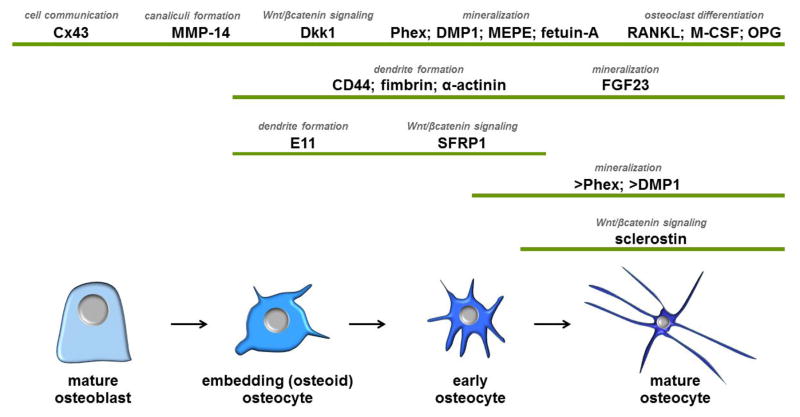
The genes that are regulated during osteocyte development and maturation can be classified based on their function in four main categories: 1) genes related to dendritic morphology and canaliculi formation that facilitate cell embedding into the bone matrix; 2) genes related to phosphate metabolism and matrix mineralization; 3) genes that regulate bone formation; and 4) genes that regulate bone resorption (Figure 2).
Figure 2. Molecular signature of osteocytes.
The pattern of gene expression, changes as osteoblasts become embedded in the bone matrix and differentiate into osteocytes. The function of the different proteins is also shown. > indicate higher expression in these cells, compared to other cells of the osteoblast lineage.
Genes related to formation and maintenance of the osteocytic network
The transition from osteoblasts to osteocytes occurs while the cells become surrounded first by un-mineralized matrix or osteoid, and later, by mineralized bone matrix. Several molecules have been identified as mediators of the development of dendrites by dictating the formation of osteocytic projections or, indirectly, by controlling mineralization and matrix degradation and thus the proper formation of the lacunar-canaliculi system.
E11 was originally described in osteoblastic cells treated by phorbol ester and named OTS-8,20, 21 and subsequently cloned from other tissues and named podoplanin, gp38, or T1α. E11 is expressed in dendritic cells of the kidney, lung, brain, and skin.22, 23 In bone, E11 is expressed in newly embedded osteocytes, but not in more mature cells deep in the bone matrix or in osteoblasts on the bone surface, indicating that is a marker of early osteocyte differentiation.24 E11 expression is required for dendrite elongation induced by loading in vitro and it is increased by mechanical stimulation in vitro and in vivo, suggesting that mechanical signals might control osteocytogenesis.
E11 binds to CD44, a molecule also expressed in neuronal dendrites,25 and expression of either molecule has been associated with osteocytic dendrite branching.26, 27 Osteocytic dendrites also contain α-actinin and fimbrin, actin-bundling proteins required for the cytoskeletal organization of osteocytes isolated from chicken.28, 29 Remarkably, mutations of PLS3, the human homolog of fimbrin that encodes for the protein Plastin 3, causes X-linked osteoporosis with fractures,30 suggesting an association of deficient dendrite formation with bone fragility.
Dentin matrix protein 1 (DMP1) is expressed in mature osteoblasts and its expression increases as osteoblasts differentiate towards osteocytes.31 DMP1 expression is required for proper osteocyte maturation. This is evidenced by abnormally elevated expression of osteoblastic and early osteocytic genes (such as alkaline phosphatase, collagen type 1 and E11) and low expression of sclerostin (a marker of mature osteocytes) in deeply embedded osteocytes in mice lacking the DMP1 gene.32 Further, mineralization is defective in these mice, likely causing the incomplete differentiation of osteocytes as well as the disorganized osteocytic lacunar-canalicular system. Thus the defective osteocyte maturation and the abnormal morphology of osteocytes in DMP1-null mice can be corrected by normalizing phosphate levels using anti-FGF23 antibodies33 or by improving bone mineralization with anti-sclerostin antibodies.34
As DMP1, the expression of matrix metalloproteinases (MMPs) also increases as osteoblasts differentiate into osteocytes. The function of MMPs in osteocytogenesis might be related to their ability to cleave collagen in the matrix surrounding osteocytes, allowing the formation of canaliculi through which osteocytes extend cytoplasmic projections. Consistent with this notion, mice lacking MMP14, a member of the MMP family of proteins, exhibit reduced or absent osteocytic processes.35 Further, the ability of collagen to be degraded by MMP13 is required to maintain osteocyte viability, as demonstrated by increased osteocyte apoptosis in mice expressing a mutated non-degradable collagen1.36
A molecule crucial for the functionality of the osteocyte network is connexin 43 (Cx43), the most abundant member of the connexin family of proteins expressed in osteocytes.37 Cx43 forms gap junction channels between cells participating in cell-to-cell communication within the osteocyte network; and it also forms hemichannels connecting cells with the extracellular milieu. Deletion of the Cx43 gene from osteocytes in mice decreases osteocyte viability and induces changes in the geometry of long bones, which present the features of bones from old mice or humans.38–40 Apoptotic osteocytes and empty lacunae are increased, the bone marrow cavity is enlarged by exaggerated endosteal resorption, and the diameter of the bones is larger due to increased periosteal bone apposition. These effects of Cx43 deletion are intrinsic to osteocytes, as knockdown of the gene in vitro render cells that die spontaneously and exhibit increased RANKL/OPG ratio and ability to support osteoclast formation.40, 41 Further, osteoclasts are found on surfaces adjacent to areas where apoptotic osteocytes accumulate in Cx43 knockout mice, suggesting that signals released by dying osteocytes are required to target osteoclast recruitment. Cx43 expression in osteoblasts/osteocytes is also required for cell survival induced by bisphosphonates or PTH as well as for full bone anabolism in response to PTH.42–45 Cx43 is also involved in the response of osteocytes to mechanical signals. However, whereas PGE2 release induced by in vitro mechanical stimulation requires Cx43 expression in osteocytes, paradoxically Cx43 restrains the response of bone to mechanical stimulation in vivo as deletion of the Cx43 gene from osteocytes enhances the anabolic response of bone to loading.46–48
Osteocytic genes related to phosphate metabolism and matrix mineralization
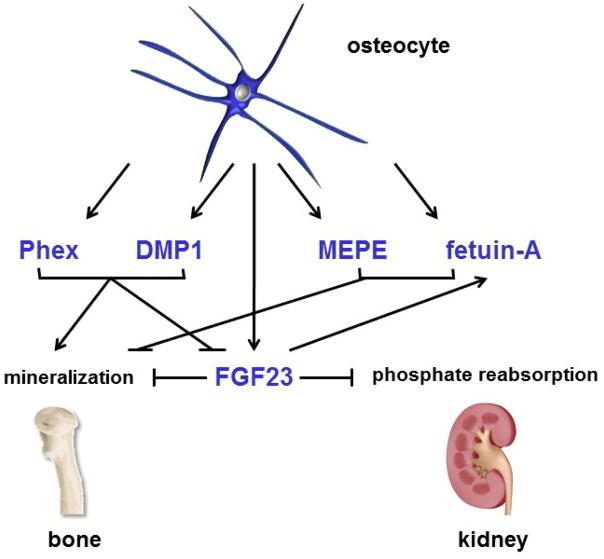
A feature of the process of osteocyte differentiation is the increase in expression of genes related with phosphate metabolism and matrix mineralization.31 These genes encode for fibroblast growth factor 23 (FGF23), the members of short integrin-binding ligand-interacting glycoprotein (SIBLING) family of proteins DMP1 and matrix extracellular phosphoglycoprotein (MEPE), phosphate-regulating gene with homologies to endopeptidases on the X-chromosome (Phex), and fetuin-A (Figure 2 and 3).31, 49
Figure 3. Role of osteocytic genes in the regulation of mineral metabolism.
Osteocytes control bone mineralization and the levels of phosphate in the circulation by producing proteins that act locally on bone cells or as endocrine hormones in kidney.
FGF23 is produced by osteocytes and regulate phosphate metabolism by acting in the kidney. FGF23 binds to FGF receptors and the Klotho co-receptor in the renal proximal tubule leading to inhibition of renal phosphate reabsorption.50 Deletion of FGF23 in mice leads to hyperphosphatemia, decreased bone mineral density, deficient bone formation, and accumulation of unmineralized osteoid.51 FGF23 might also act in an auto/paracrine manner in osteocytes and other bone cells, as receptors for FGF23 (FGFR1 and Klotho) are also expressed in these cells, and could mediate FGF23 functions dependent as well as independent of its co-receptor Klotho.13, 52 Thus, deletion of the renal Na/Pi co-transporter NaPi2a reverses the hyperphosphatemia but not the defective mineralization of mice lacking FGF23, 52 suggesting a direct effect of FGF23 on bone cells. In addition, we have shown that the FGF23 co-receptor Klotho is expressed in bone cells in vivo in long bones and calvaria at the protein and mRNA levels, as well as in isolated osteoblasts and osteocytes.13 Furthermore, the levels of FGF23 are increased in mice with active parathyroid hormone (PTH) receptor signaling in osteocytes (DMP1-caPTHR1 mice), and this leads to increased levels of FGFR1, GALNT3 as well as downstream targets of FGF23 signaling in osteocytes from DMP1-caPTHR1 mice, adding support for a direct effect of FGF23 in bone.
Other osteocyte produced molecules regulate FGF23 expression and/or function, thus indirectly affecting phosphate homeostasis. For example, human inactivating mutations of DMP1 or Phex result in high FGF23 levels and hypophosphatemia.53 Because reducing FGF23 and correcting the hypophosphatemia rescue only partially the osteomalacia, it is likely that the defective bone mineralization observed in these diseases results from a combination of FGF23-mediated effects in the kidney and direct action of DMP1 and Phex in bone. Further, studies in animal models and humans showed that altered levels of FGF23, Phex, DMP1, and MEPE are associated with impaired glucose metabolism likely by decreasing undercarboxylated osteocalcin.54 Therefore, this group of osteocyte-derived genes directly or indirectly modulates the homeostasis of bone, kidney and other tissues/organs.
As discussed above, DMP1 is required for proper bone mineralization, whereas MEPE is a mineralization inhibitor and its deletion from the mouse genome results in increased bone mineral density55 (Figure 2 and 3). DMP1 and MEPE expression is upregulated by loading and these proteins might be responsible for the local effects of mechanical stimulation in the matrix surrounding osteocytes.56
Enzymatic degradation of MEPE generates an acidic serine aspartate-rich MEPE-associated (ASARM) peptide that blocks mineralization in vitro and in vivo.57 Phex is a metalloendopeptidase that binds to MEPE and its ASARM peptide. Phex deletion in mice results in osteomalacia and abnormal osteocytic lacuna-canalicular system.58
The liver protein fetuin-A is an inhibitor of bone mineralization and ectopic calcification, which is also expressed in bone and it is enriched in osteocytes compared to osteoblasts.49 Fetuin-A might be involved in the formation of cellular processes by slowing calcification of the matrix that surrounds developing osteocytes.
The regulation of phosphate metabolism appears to result from highly interrelated functions of all these osteocytic proteins, as changes in the levels of expression of one of these molecules alters the expression of others, constituting a cascade that ultimately regulate bone mineralization (Figure 3). Some examples of this interplay are the high levels of FGF23 exhibited in humans in the absence of DMP1 and Phex function,53 the decrease in active FGF23 levels due to Phex binding to DMP1, the reversal of the hypophosphatemia of Phex-deficient mice by FGF23 deletion,51 and the increase in fetuin-A expression in osteocytes by an autocrine function of FGF23.49 Altered expression of some of the osteocytic genes described above causes disorders of phosphate homeostasis in humans.53 Excess production of FGF23 or gain-of-function mutations of this hormone results in hypophosphatemia, renal phosphate wasting, low vitamin D3 levels and vitamin D-resistant rickets, a condition known as autosomal dominant hypophosphatemic rickets (ADHR). Increased FGF23 is found in chronic kidney disease (CKD) and is associated with higher risk of cardiovascular disease and death. The high levels of FGF23 in late stages of CKD are partially due to increased PTH, as evidenced by the reduction in serum FGF23 following parathyroidectomy in animal models and patients with CKD. FGF23 was found increased in the circulation of a patient with Jansen’s metaphyseal chondrodysplasia, characterized by expression of a constitutively active PTH receptor 1 (PTHR1).59 Similarly, mice expressing the same PTHR1 mutant in osteocytes exhibit increased expression of FGF23.13 These findings demonstrate that some of the actions of PTH in controlling mineral metabolism are mediated through regulation of osteocyte-derived FGF23.
Other forms of hypophosphatemia resulting from changes in osteocytic genes are X-linked hypophosphatemic rickets (XLH) due to inactivating mutations of the Phex gene and autosomal recessive hypophosphatemic rickets (ARHR) due to inactivating mutations of DMP1.53
Osteocytic genes that regulate bone formation
One of the most important events of the last 2 decades that advanced our understanding of skeletal biology was the discovery of the role of Wnt/βcatenin signaling in bone.60 The so-called canonical Wnt signaling pathway controls fate of mesenchymal stem cells (MSC) and their differentiation into the various cell lineages. Canonical Wnt signaling restrains chondrogenic and adipogenic differentiation while enhances osteoblastic differentiation from MSC. In addition, Wnt/βcatenin signalling promotes osteoblast maturation and survival of osteoblasts and osteocytes, and inhibits osteoclastogenesis, although indirectly by increasing the expression of OPG in osteoblasts and osteocytes.
Osteocytes are critical players in the regulation of this signaling pathway both as targets of the Wnt ligands and as producers of molecules that modulate their action. Wnt/βcatenin signaling is activated by binding of Wnt proteins to a receptor complex composed of frizzled receptors and co-receptors of the low density lipoprotein receptor-related protein (LRP) family, LRP5 and 6. This event stabilizes βcatenin, induces its translocation to the nucleus, and activates gene transcription. Activation of this pathway is critical for bone acquisition and maintenance through increased bone formation and decreased resorption.
Dickkopf-related protein 1 (Dkk1) is a Wnt antagonist expressed in osteoblasts and at higher levels in osteocytes.61 Dkk1 binds to LRP5/6 preventing the binding of Wnt ligands. Mice overexpressing Dkk1 in osteoblastic cells exhibit decreased bone formation and low bone mass, consistent with the stimulatory role of the Wnt/βcatenin pathway on bone formation (Figure 4).62 Another Wnt inhibitor, secreted frizzle-related protein 1 (sFRP1) is expressed in early osteocytes and its levels decrease as osteocytes mature.63 Consistent with the role of sFRP1 as an inhibitor of anabolic Wnt/βcatenin signaling, its deletion from the mouse genome results in increased bone mass.60
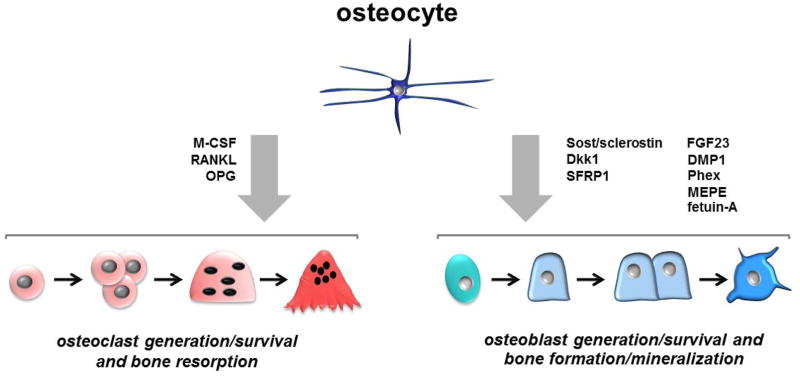
Figure 4. Osteocytes as regulators of bone formation and resorption.
Osteocytes produce and secrete molecules that stimulate or inhibit osteoclast or osteoblast differentiation, viability, and function.
Another antagonist of Wnt signaling is sclerostin, a protein encoded by the Sost gene, which is primarily expressed by mature osteocytes but not by early osteocytes or osteoblasts.64 Sclerostin binds to the Wnt co-receptors LRP5/6 antagonizing downstream signalling.65 Sclerostin also binds to LRP4, another member of the LRP family of proteins, which acts as a chaperone and is required for the inhibitory action of sclerostin on Wnt/βcatenin signaling.66 Absence of sclerostin expression or secretion in humans causes sclerosteosis, van Buchem disease, or craniodiaphyseal dysplasia, inherited high bone mass conditions characterized by exaggerated bone formation.60 Further, inactivating mutations of the Wnt co-receptor LRP5 result in low bone mass (osteoporosis pseudoglioma); and, conversely, activating LRP5 mutations lead to high bone mass partially due to decreased binding affinity of the mutated LRP5 for sclerostin. Consistent with the requirement of LRP4 for the inhibitory function of sclerostin, individuals with LRP4 inactivating mutations exhibit high bone mass. Moreover, deletion of the Sost or LRP4 genes in mice or neutralizing antibodies for sclerostin or LRP4 reproduces the high bone mass phenotype found in humans lacking Sost or LRP4 activity,67–71 whereas overexpression of Sost/sclerostin decreases bone mass.9, 12, 72, 73 Remarkably, osteocyte-targeted deletion of LRP5 or overexpression of high bone mass LRP5 mutants only in osteocytes reproduces the low or high bone mass, respectively, exhibited by mice or humans with the genetic modifications in all cells, suggesting that activation of the pathway in osteocytes is sufficient to elicit bone formation downstream of LRP5.74, 75 This conclusion is consistent with recent findings demonstrating that osteocytes mediate the anabolic actions of canonical Wnt/βcatenin signaling in bone.76
Osteocalcin, the most abundant non-collagenous protein present in bone, is an inhibitor of bone formation, as evidenced by the high bone mass in the absence of defective bone mineralization or bone resorption in mice lacking the osteocalcin gene.77 Osteocytes are more abundant than osteoblasts, and studies in mice showed that osteocalcin is expressed at a higher level by osteocytes;78 therefore, it is tempting to speculate that osteocytes as well as osteoblasts contribute to the pool of osteocalcin in the circulation. However, the chief cellular source of this hormone is not known. Studies in mice showed that, in addition to its role in bone, osteocalcin in its undercarboxylated form can regulate insulin production/secretion by pancreatic β cells, insulin sensitivity and glucose uptake in muscle, and fat metabolism.79 In addition, osteocalcin might regulate testosterone production80 and brain development and brain function.81 However, the extra-skeletal role of osteocalcin as a molecule with endocrine properties remains controversial and additional studies are required to determine the relevance of the purported extra-skeletal functions of osteocalcin in humans both under physiological and pathological conditions.
Osteocytic genes that regulate bone resorption and the role of osteocyte apoptosis
Osteocytes produce cytokines that regulate osteoclast formation and survival (Figure 4). Osteoclastogenesis involves activation of the receptor activator of nuclear factor κ B (RANK) in osteoclast precursors induced by RANK ligand (RANKL) produced by stromal/osteoblastic cells as well as by osteocytes.31 Osteocytes also secrete the RANKL decoy receptor osteoprotegerin (OPG), which binds RANKL competing with RANK and thus inhibiting osteoclast differentiation. Studies using genetically modified mice showed that deletion of the RANKL gene from osteocytes renders an osteopetrotic phenotype, due to reduced number of osteoclasts, decreased resorption, and progressive increase in bone mass,82, 83 suggesting that osteocytes are an important source of RANKL in bone.
Macrophage colony stimulating factor (M-CSF), produced by bone cells including osteocytes,61 promotes osteoclast precursor proliferation and survival and is required for osteoclast formation.84 Similar to mice lacking M-CSF in all tissues,85 mice lacking M-CSF only from osteocytes exhibit reduced osteoclast number and osteopetrosis demonstrating the osteocytes are an important source of the cytokine in bone.86 Osteocytes also express receptors for M-CSF,61 and mice lacking osteocytic M-CSF have osteocytes with abnormal morphology, high prevalence of apoptosis, increased ROS production, and reduced gap junction communication.86 These findings suggest that osteocytes are not only an important source of M-CSF, but also a target cell for this cytokine, which controls osteocyte viability.
The anti-osteoclastogenic cytokine OPG is expressed in both osteoblasts and osteocytes, and OPG mRNA expression is more abundant in osteocytes than in osteoblasts isolated by serial digestions of bones from 6-week-old mice and at least as abundant in cells from 4-month-old mice.87 Moreover, OPG is a target gene of canonical Wnt signaling and mice lacking βcatenin from osteoblasts/osteocytes or only from osteocytes exhibit similar reduced OPG expression, increased osteoclasts and low bone mass.87–89 These findings suggest that the regulation of osteocytic OPG by Wnt/βcatenin signaling plays an important role in the control of bone resorption.
Osteocyte viability is an important component of the cascade of events that directs osteoclasts to particular bone surfaces, so-called targeted bone remodeling.90–92 Accumulation of apoptotic osteocytes in certain areas of bone promotes pre-osteoclast recruitment, local osteoclast differentiation, and increased resorption. Although the mechanisms underlying this phenomenon are still unclear, it is recognized that osteocyte apoptosis precedes temporally and spatially osteoclastic resorption. Induction of osteocyte apoptosis by injecting diphtheria toxin to transgenic mice expressing the diphtheria toxin receptor in osteocytes is sufficient to increase osteoclasts and trigger resorption in the vicinity of dead osteocytes.93 Moreover, osteocyte death is accompanied by increased osteoclasts in nearby bone in rodent models of unloading, excessive mechanical forces, or ovariectomy, opening the possibility that increased osteocyte apoptosis is a generalized mechanism to induce localized bone resorption.
One of the potential mechanisms by which increased osteocyte apoptosis could trigger local bone resorption is by increasing RANKL expression in osteocytes located close to dying cells. This notion is supported by the fact that inhibition of apoptosis by administering anti-apoptotic bisphosphonates or caspase inhibitors blocks the increase in osteocytic RANKL found in the above described animal models.90–92, 94, 95 Further, deletion of RANKL from osteocytes and mature osteoblasts prevents the decrease in bone mass and the increase in osteoclast number in tail-suspended mice.82 Moreover, inhibition of osteocyte/osteoblast apoptosis with a bisphosphonate that does not affect osteoclasts or with a pan caspase inhibitor prevented the increase in osteocytic RANKL induced by unloading.94 However, the bisphosphonate did not prevent the loss of bone94 whereas the caspase inhibitor did.95 Nevertheless, these findings demonstrate a cause-effect relationship between osteocyte apoptosis and osteocytic RANKL and suggest that at least under certain unloading conditions osteocytic RANKL is central for osteoclast formation and bone resorption.
In addition to osteocytic genes or pathways that regulate either bone formation or bone resorption, genetic manipulations targeting osteocytes can simultaneously control both arms of remodelling. For example, activation of βcatenin in osteocytes increases bone mass in the context of elevated bone formation and bone resorption, leading to high bone remodeling with bone gain.76 This effect contrasts with the findings that activation of βcatenin in osteoblasts/osteocytes increases bone mass due to reduced bone resorption, without affecting osteoblast function.89
INTEGRATION OF BONE MECHANICAL AND HORMONAL STIMULI BY OSTEOCYTES
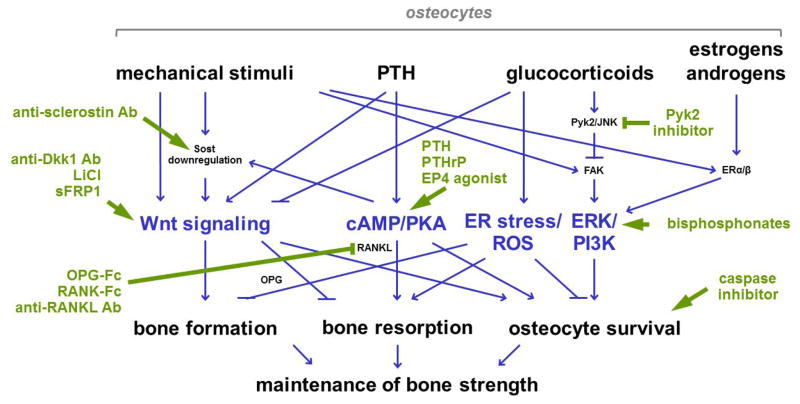
Due to their strategic location, osteocytes have been long considered the ideal cells to mediate bone adaptation to changes in the mechanical environment; and extensive evidence accumulated during the past several decades revealed several mechanisms potentially involved in this phenomenon. Changes in mechanical force profoundly affect the viability of osteocytes (as mentioned when discussing the role of osteocytes on bone resorption). Further, mechanical stimulation alters the expression of osteocytic genes and induces the release of small molecules, which, in turn, regulate the generation and function of osteoblasts and osteoclasts, thus impacting bone modeling and remodeling. Osteocytes are also target cells of the action of systemic hormones; which activate similar as well as different pathways than mechanical stimuli (Figure 5). There is also crosstalk between hormonal and mechanical signaling in osteocytes demonstrated by modulation of mechano-responses by systemic hormones or by changes in the expression of hormone receptors.
Figure 5. Osteocytic signaling pathways as therapeutic targets.
Therapeutic approaches (green lines and text) take advantage of pathways activated by mechanical and hormonal stimuli in osteocytes (blue lines and text). Ab, antibody.
Regulation of osteocyte lifespan by mechanical signals
Lack of mechanical stimulation, as with immobilization or paralysis, decreases bone mass and increases the risk of fractures. This is associated with high prevalence of osteocyte apoptosis in mice and rats in vivo.96, 97 Conversely, increasing levels of loading within the physiological range reduce the density of apoptotic osteocytes in rat ulnae.98
The elevation of osteocyte apoptosis with unloading might be due to the absence of survival signals provided by normal physical activity. Indeed, in vitro mechanical stimulation opposes the action of several pro-apoptotic stimuli; and the combination of several mechanisms are likely to be involved in promoting survival.99 Integrin engagement and activation of the focal adhesion kinase (FAK) is induced by mechanical stimulation, followed by activation of the Src/MEK/ERK signaling cascade in vitro.100 Furthermore, nuclear accumulation of the ERK survival kinase as well as ERK-mediated gene transcription are required for anti-apoptosis. In vivo studies showed that integrins are involved in the attachment of osteocytes to the lacunar/canalicular wall.101, 102 Moreover, deletion of β1 integrin from osteocytes results in abnormal response to unloading,103 adding support to a role of integrins in mechanotransduction.
The canonical Wnt/βcatenin pathway is also activated by loading,104 in part due to decreased expression of the antagonists of the pathway Dkk1 and sclerostin.8, 104 Because Wnts prevent apoptosis whereas the Wnt antagonists sFRP1 and sclerostin induce apoptosis in cultured osteocytic and osteoblastic cells,63, 105, 106 activation of this pathway could contribute to the maintenance of osteocyte/osteoblast survival with physiologic levels of loading. Further, βcatenin accumulation induced by mechanical stimulation is required for preventing glucocorticoid-induced osteocyte apoptosis in vitro,107, 108 suggesting that canonical Wnt signaling also promotes survival under pathological conditions. Moreover, in vivo activation of Wnt/βcatenin signaling, as in mice lacking the Wnt antagonist sFRP160 or expressing LRP5 activating mutations,109 leads to decreased prevalence of apoptotic osteocytes.
Mechanical stimulation of osteocytes also induces release of prostaglandin E2 (PGE2), produced by cyclooxygenase 2 (COX-2), an enzyme induced by mechanical stimulation.110 Binding of PGE2 to the EP2 receptor and subsequent activation of cAMP/PKA and PI3K/Akt/βcatenin inhibits apoptosis of osteocytic cells induced by glucocorticoids.108
Some of these pro-survival pathways activated by loading in osteocytes have been also shown to be required for the anabolic effect induced by mechanical stimulation. Thus, mice lacking the Wnt co-receptor LRP5 or overexpressing Sost do not exhibit an anabolic response to loading,9, 111 and inhibition of prostaglandin synthesis blocks bone formation induced by mechanical stimulation.112
Crosstalk between mechanical and hormonal signals in osteocytes
One example of a pro-survival pathway activated by mechanical stimuli that is also involved in hormonal responses is Src/ERK signaling, which is activated by mechanical stimulation downstream of integrins/FAK and by estrogens/androgens through non-genotropic signaling mediated by extra-nuclear functions of the estrogen (ER) or androgen receptors.100, 113 (Figure 5). ERα or ERβ also play a permissive role in mechanotransduction, as their presence in the osteocytic cell membrane within caveolae is required for the ERK-mediated survival effect of mechanical stimulation in a ligand independent fashion.114 In addition, estrogen as well as mechanical stimulation induce ERα phosphorylation in ERK- and PKA- dependent manner.115 Consistent with these in vitro findings, mice lacking ERα or ERβ exhibit a reduced anabolic response to loading.116, 117
Like mechanical stimulation, systemic elevation of PTH reduces the expression of Sost/sclerostin, which might contribute to the increase in bone formation by the hormone.91, 118 Consistent with this notion, the increase in bone mass and in bone formation exhibited by transgenic mice expressing an activated PTHR1 in osteocytes is abrogated by Sost overexpression.12 Moreover, PTH signaling might interplay with mechanotransduction, as the anabolic response to mechanical stimulation is enhanced when PTH is administered together with loading119–122 and is decreased in parathyroidectomized rats.119 In addition, the increase in cancellous bone volume and improvement of mechanical properties induced by exercise is abolished when activation of the PTHR1 is inhibited using PTH(7-34) in mice.123 The effect of PTH in combination with mechanical stimulation is blocked by verapamil,124 suggesting the involvement of L-type voltage channels in the synergistic effect between the hormone and loading.
PTHR1 can also be activated by binding of the other ligand of this receptor, PTH related peptide (PTHrP). PTHrP expression is increased by mechanical stimulation in osteocytic and osteoblastic cells,125, 126 and the anti-apoptotic effect of mechanical stimulation is mimicked by treatment with PTHrP and abolished by silencing PTHR1 in vitro.126, 127 PTHrP, as PTH, decreases Sost expression in vitro in primary cultures of osteoblastic cells containing osteocytes.13 Taken together, this evidence strongly suggests that local increases in PTHrP induced by mechanical loading could contribute to maintaining osteocyte survival and to the anabolic action of mechanical loading.
In contrast to the overlapping and potentially synergistic signaling activated by mechanical signals and sex steroids or PTH, glucocorticoids antagonize survival and bone-maintaining signaling activated by mechanical stimulation. Glucocorticoids induce osteocyte and osteoblast apoptosis in vitro and in vivo in mice and humans, thus opposing the survival effect of mechanical stimuli on these cells.99, 128–132 Glucocorticoids inhibit whereas mechanical stimulation increases Wnt/βcatenin signaling. Further, whereas mechanical stimulation activates the FAK/ERK survival pathway, glucocorticoids-induced apoptosis is preceded by cell detachment (known as anoikis) through the activation of the FAK-related kinase Pyk2, and the kinase JNK.100, 133 The findings that glucocorticoids oppose the FAK/ERK pathway through activation of Pyk2 raise the possibility that Pyk2 could be inhibited to increase the mechanoresponsive FAK signaling. Indeed, a Pyk2 inhibitor has been shown to increase bone mass in rodents through stimulation of bone formation and inhibition of bone resorption.134, 135 In addition, part of the pro-apoptotic actions of glucocorticoids on osteocytes and osteoblasts is mediate through endoplasmic reticulum (ER) stress, and inhibition of eIF2α dephosphorylation, which leads to ER stress, prevents apoptosis and the loss of bone mass induced by glucocorticoid excess in mice.136
Elevation of endogenous glucocorticoids may contribute to the effect of reduced mechanical stimulation, as evidenced by delayed increase in osteoblast apoptosis, and in the reduction of serum osteocalcin and vertebral strength in mice expressing the glucocorticoid-inactivating enzyme 11-β hydroxysteroid dehydrogenase in osteoblastic cells (OG2-11-β HSD2 mice) and subject to tail suspension.96 On the other hand, osteocytes still exhibit apoptosis in tail-suspended OG2-11-β HSD2 mice indicating that the effect of unloading on osteocytes is not a consequence of increased endogenous glucocorticoids, but is entirely caused by reduced mechanical loading.
OSTEOCYTIC SIGNALING PATHWAYS AS THERAPEUTIC TARGETS
Our increased understanding of mechanosensory pathways provide opportunities of developing therapeutic approaches to improve bone health (Figure 5).
Agents that prevent osteocyte apoptosis preserve bone strength.137 Such is the case of bisphosphonates, which their anti-fracture efficacy cannot be completely explained by the effect of the drugs on bone mass. Bisphosphonates prevent osteocyte apoptosis induced by glucocorticoid excess and lack of mechanical forces, and this effect does not require the anti-catabolic activity of the drugs, as it is exerted by bisphosphonates that inhibit apoptosis of osteoblastic cells but do not affect osteoclast function.94, 129, 138 Estrogens and androgens have also been shown to preserve osteocyte viability, and removal of sex steroids increase osteocyte apoptosis.113, 139 In addition, intermittent PTH administration decreases osteocyte apoptosis and increases osteocyte density.140, 141 Activation of Wnt/βcatenin signaling either by blocking inhibitors of the pathway directly or through mechanical stimulation, or as a consequence of PTH administration, promotes osteocyte survival.108, 118, 142, 143 Further, inhibition of osteocyte apoptosis with caspase inhibitors decreases resorption following fatigue loading or ovariectomy-induced thinning of cortical bone,92 and prevent the increase in bone resorption and the loss of bone induced by immobilization in the appendicular skeleton.95 These pieces of evidence support the possibility that by preventing osteocyte (and osteoblast) apoptosis, bone mass can be maintained and the effect of deleterious stimuli on bone can be at least in part neutralized.
Several ways of activating Wnt/βcatenin signaling by blocking antagonists of the pathway through pharmacologic interventions have been designed. Studies using humanized neutralizing anti-Dkk1 increase bone mass in growing female mice and in ovariectomized adult rhesus monkeys.144, 145 In addition, a small molecule inhibitor of sFRP1 increases bone volume in calvaria organ cultures.146 Further, inhibition of GSK3β thus stabilizing β-catenin and activating canonical Wnt signaling, protects bone integrity, as shown in preclinical studies as well as in epidemiologic studies in which the GSK3β inhibitor lithium chloride was used to treat psychological disorders.60 However, since the Wnt pathway is active in numerous tissues and both Dkk1 and sFRP1 are widely expressed, the use of these inhibitors might need to be restricted to local bone applications, to avoid unwanted effects in other organs. Nevertheless, anti-Dkk1 antibodies are currently being tested for the treatment of skeletal complications of multiple myeloma.147
More recently, a neutralizing antibody directed to Sost/sclerostin was developed. Sclerostin expression is restricted to osteocytes among bone cells and genetic increase in Sost expression leads to an almost exclusive bone phenotype. This makes sclerostin an excellent target to improve bone health without affecting other tissues. Preclinical studies showed that inhibition of sclerostin using the antibody prevents the decrease in bone mass induced by ovariectomy, excess glucocorticoid administration, ulcerative colitis, immobilization, and aging.148 Phase 2 studies have been carried out and show increased bone mineral density, increased bone formation and inhibition of resorption induced by treatment with the anti-sclerostin antibody.149
Sclerostin protein was also detected in chondrocytes150–152 raising the possibility that treatment with the anti-sclerostin antibody has an impact in cartilage. In fact, deletion of Sost in mice worsens the osteoarthritic phenotype induced by medial meniscus destabilization.153 However, no difference in the development of age-dependent osteoarthritis was found between wild type and Sost KO mice.152 Moreover, intraarticular administration of an anti-sclerostin antibody did not alter the osteoarthritic phenotype induced by medial meniscus destabilization in rats;152 and no adverse osteoarthritic events have been reported in the human trials with anti-sclerostin antibodies.154 Overall, these studies suggest that even when sclerostin is expressed in cartilage, neutralization of its activity might not significantly affect the functionality of this tissue.
Recent studies have shown that administration of an inactivating FGF23 antibody reversed the hypophosphatemia and increased 1,25(OH)2 vitamin D levels in patients with XLH.155 The antibody also increased circulating markers of bone formation, suggesting an improvement in bone mineralization. Consistent with this possibility, administration of the anti-FGF23 antibody improved mineralization in adult hyp mice, a murine model of XLH.156 Furthermore, a pan-specific FGF receptor inhibitor reversed the abnormal serum levels of phosphate, calcium, PTH and 1,25(OH)2 vitamin D, and improved the skeletal manifestations of hypophosphatemia in hyp mice, as well as in mice lacking DMP1, a model of ARHR.157
Attempts were made over the years to block osteoclast differentiation and therefore, to reduce bone loss, by targeting the RANKL/RANK/OPG signaling pathway.158–160 Because the current evidence suggest that osteocytes are one of the sources of RANKL and OPG in bone, therapeutic approaches modulating this pathway are examples of the potential benefits of targeting osteocytes to improve skeletal health.
PGE2 has a bone anabolic effect when administered in an intermittent fashion.161 However, due to the widespread systemic distribution and the side effects associated with PGE2 administration, it is not used currently in the clinic. PGE2 acts in bone through the activation of the EP2 and EP4 receptors. Deletion of EP4 results in osteopenia in mice, with increased osteoclasts and decreased bone formation.162 EP4 agonists have been developed and were shown to stimulate bone formation and inhibit bone resorption in ovariectomized rats.163 To avoid the systemic effect of activation of the receptor, a bisphosphonate-conjugated agonist was developed for bone targeting.164 The EP4 agonist-bisphosphonate conjugate increased bone formation and improved bone strength in ovariectomized rats. The levels of EP2, the other PGE2 receptor expressed in bone, is increased by mechanical stimulation and is involved in the effects of PGE2 on osteocytic cells upon mechanical stimulation in vitro.37 Further, deletion of EP2 receptor in mice results in impaired osteoclastogenesis and decreased bone strength.165 EP2 agonists have been developed and induce periosteal bone formation upon local administration in rats. Whether activation of the receptor in osteocytes contribute to the effect of the EP agonists remains unknown.
Conclusions and future directions
Over the last two decades, our understanding of the function of osteocytes both as regulators of the activity of bone forming and resorbing cells and as modulators of the function of other tissues has markedly increased. Human mutations and experimental models have uncovered novel signaling pathways activated in osteocytes that can be targeted with therapeutic goals to improve bone health. Further, it is also now apparent that some stimuli that affect osteocytes and other bone cells affect skeletal muscle and vice versa. In particular, mechanical stimulation is required for maintaining both bone and muscle homeostasis. Thus, the crosstalk between the two tissues might be exploited in the future to provide opportunities for the combined of musculoskeletal integrity as a whole.
Acknowledgments
This research was supported by grants from the National Institutes of Health R01-AR053643 and R01-AR067210 to LIP and R01-AR059357, R01-DK076007, and S10-RR023710 to TB; and from the Veteran’s Administration Merit Review 1 I01 BX002104-01 to TB.
References
- 1.Marotti G, Palumbo C. The mechanism of transduction of mechanical strains into biological signals at the bone cellular level. Eur J Histochem. 2007;51(Suppl 1):15–19. [PubMed] [Google Scholar]
- 2.Marotti G. The structure of bone tissues and the cellular control of their deposition. Ital J Anat Embryol. 1996;101:25–79. [PubMed] [Google Scholar]
- 3.Martin RB. Does osteocyte formation cause the nonlinear refilling of osteons? Bone. 2000;26:71–78. doi: 10.1016/s8756-3282(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 4.Frost HM. In vivo osteocyte death. J Bone Joint Surg Am. 1960;42-A:138–143. [PubMed] [Google Scholar]
- 5.Arnold JS, Frost HM, Buss RO. The osteocyte as a bone pump. Clin Orthop Relat Res. 1971;78:47–55. doi: 10.1097/00003086-197107000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 7.Frost HM. Bone’s mechanostat: A 2003 update. Anat Rec. 2003;275A:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 8.Robling AG, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 9.Tu X, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parfitt AM. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone diseases. II PTH and bone cells: bone turnover and plasma calcium regulation. Metabolism. 1976;25:909–955. doi: 10.1016/0026-0495(76)90124-4. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien CA, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee Y, et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling. J Bone Miner Res. 2011;26:1035–1046. doi: 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee Y, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49:636–643. doi: 10.1016/j.bone.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee Y, et al. Resorption controls bone anabolism driven by PTH receptor signaling in osteocytes. J Biol Chem. 2013;288:29809–29820. doi: 10.1074/jbc.M113.485938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saini V, et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013;288:20122–20134. doi: 10.1074/jbc.M112.441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qing H, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27:1018–1029. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belanger LF. Osteocytic osteolysis. Calcif Tissue Res. 1969;4:1–12. doi: 10.1007/BF02279101. [DOI] [PubMed] [Google Scholar]
- 18.Parfitt AM. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part I of IV parts: mechanisms of calcium transfer between blood and bone and their cellular basis: morphological and kinetic approaches to bone turnover. Metabolism. 1976;25:809–844. doi: 10.1016/0026-0495(76)90151-7. [DOI] [PubMed] [Google Scholar]
- 19.Martin RB, Burr DB, Sharkey NA. Skeletal tissue mechanics. Springer-Verlag; New York: 1998. [Google Scholar]
- 20.Nose K, Saito H, Kuroki T. Isolation of a gene sequence induced later by tumor-promoting 12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells (MC3T3-E1) and expressed constitutively in ras-transformed cells. Cell Growth Differ. 1990;1:511–518. [PubMed] [Google Scholar]
- 21.Wetterwald A, et al. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 1996;18:125–132. doi: 10.1016/8756-3282(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 22.Boucherot A, Schreiber R, Pavenstadt H, Kunzelmann K. Cloning and expression of the mouse glomerular podoplanin homologue gp38P. Nephrol Dial Transplant. 2002;17:978–984. doi: 10.1093/ndt/17.6.978. [DOI] [PubMed] [Google Scholar]
- 23.Rishi AK, et al. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol. 1995;167:294–306. doi: 10.1006/dbio.1995.1024. [DOI] [PubMed] [Google Scholar]
- 24.Zhang K, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–4552. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skupien A, et al. CD44 regulates dendrite morphogenesis through Src tyrosine kinase-dependent positioning of the Golgi. J Cell Sci. 2014;127:5038–5051. doi: 10.1242/jcs.154542. [DOI] [PubMed] [Google Scholar]
- 26.Hughes DE, Salter DM, Simpson R. CD44 expression in human bone: a novel marker of osteocytic differentiation. J Bone Min Res. 1994;9:39–44. doi: 10.1002/jbmr.5650090106. [DOI] [PubMed] [Google Scholar]
- 27.Ohizumi I, et al. Association of CD44 with OTS-8 in tumor vascular endothelial cells. Biochim Biophys Acta. 2000;1497:197–203. doi: 10.1016/s0167-4889(00)00063-x. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka-Kamioka K, Kamioka H, Ris H, Lim SS. Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin–rich projections. J Bone Min Res. 1998;13:1555–1568. doi: 10.1359/jbmr.1998.13.10.1555. [DOI] [PubMed] [Google Scholar]
- 29.Kamioka H, Sugawara Y, Honjo T, Yamashiro T, Takano-Yamamoto T. Terminal differentiation of osteoblasts to osteocytes is accompanied by dramatic changes in the distribution of actin-binding proteins. J Bone Miner Res. 2004;19:471–478. doi: 10.1359/JBMR.040128. [DOI] [PubMed] [Google Scholar]
- 30.van Dijk FS, et al. PLS3 mutations in X-linked osteoporosis with fractures. N Engl J Med. 2013;369:1529–1536. doi: 10.1056/NEJMoa1308223. [DOI] [PubMed] [Google Scholar]
- 31.Bellido T, Plotkin LI, Bruzzaniti A. In: Bone cells in Basic and Applied Bone Biology. Burr D, Allen M, editors. Elsevier; 2014. pp. 27–45. [Google Scholar]
- 32.Feng JQ, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang R, et al. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res. 2010 doi: 10.1002/jbmr.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren Y, Lin S, Jing Y, Dechow PC, Feng JQ. A novel way to statistically analyze morphologic changes in Dmp1-null osteocytes. Connect Tissue Res. 2014;55:129–133. doi: 10.3109/03008207.2014.923879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmbeck K, et al. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 2005;118:147–156. doi: 10.1242/jcs.01581. [DOI] [PubMed] [Google Scholar]
- 36.Zhao W, Byrne MH, Wang Y, Krane SM. Osteocyte and osteoblast apoptosis and excessive bone deposition accompany failure of collagenase cleavage of collagen. J Clin Invest. 2000;106:941–949. doi: 10.1172/JCI10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone. 2013;52:157–166. doi: 10.1016/j.bone.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu S, Rao DS, Palnitkar S, Parfitt AM. Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone. 2002;31:313–318. doi: 10.1016/s8756-3282(02)00819-0. [DOI] [PubMed] [Google Scholar]
- 39.Almeida M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bivi N, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Min Res. 2012;27:374–389. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis HM, et al. Reduction in microRNA21 promotes apoptosis and increases RANKL in osteocytes: a mechanism for enhanced resorption in the absence of Cx43 in osteoblastic cells and with aging. J Bone Miner Res. 2015;30:S101. [Google Scholar]
- 42.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 43.Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with βarrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem. 2011;112:2920–2930. doi: 10.1002/jcb.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung D, et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119:4187–4198. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 45.Pacheco-Costa R, et al. Defective cancellous bone structure and abnormal response to PTH in cortical bone of mice lacking Cx43 cytoplasmic C-terminus domain. Bone. 2015;81:632–643. doi: 10.1016/j.bone.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimston SK, Watkins MP, Brodt MD, Silva MJ, Civitelli R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS ONE. 2012;7:e44222. doi: 10.1371/journal.pone.0044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE. 2011;6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bivi N, et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res. 2013;31:1075–1081. doi: 10.1002/jor.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattinzoli D, et al. FGF23-regulated production of Fetuin-A (AHSG) in osteocytes. Bone. 2015;83:35–47. doi: 10.1016/j.bone.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Shimada T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sitara D, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sitara D, et al. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008;4:e1000154. doi: 10.1371/journal.pgen.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng JQ, Clinkenbeard EL, Yuan B, White KE, Drezner MK. Osteocyte regulation of phosphate homeostasis and bone mineralization underlies the pathophysiology of the heritable disorders of rickets and osteomalacia. Bone. 2013;54:213–221. doi: 10.1016/j.bone.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowe PS. The chicken or the egg: PHEX, FGF23 and SIBLINGs unscrambled. Cell Biochem Funct. 2012;30:355–375. doi: 10.1002/cbf.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gowen LC, et al. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278:1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 56.Harris SE, et al. DMP1 and MEPE expression are elevated in osteocytes after mechanical loading in vivo: Theoretical role in controlling mineral quality in the perilacunar matrix. J Musculoskelet Neuronal Interact. 2007;7:313–315. [PMC free article] [PubMed] [Google Scholar]
- 57.Rowe PS. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryot Gene Expr. 2012;22:61–86. doi: 10.1615/critreveukargeneexpr.v22.i1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan B, et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118:722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown WW, et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen’s metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2009;94:17–20. doi: 10.1210/jc.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 61.Paic F, et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45:682–692. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 63.Bodine PV, et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem. 2005;96:1212–1230. doi: 10.1002/jcb.20599. [DOI] [PubMed] [Google Scholar]
- 64.Poole KE, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 65.Li X, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 66.Leupin O, et al. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J Biol Chem. 2011;286:19489–19500. doi: 10.1074/jbc.M110.190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim SJ, et al. Identification of signal peptide domain SOST mutations in autosomal dominant craniodiaphyseal dysplasia. Hum Genet. 2011;129:497–502. doi: 10.1007/s00439-011-0947-3. [DOI] [PubMed] [Google Scholar]
- 68.Balemans W, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 69.Li X, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 70.Chang MK, et al. Disruption of Lrp4 function by genetic deletion or pharmacological blockade increases bone mass and serum sclerostin levels. Proc Natl Acad Sci U S A. 2014;111:E5187–E5195. doi: 10.1073/pnas.1413828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McClung MR. Emerging Therapies for Osteoporosis. Endocrinol Metab (Seoul) 2015 doi: 10.3803/EnM.2015.30.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niziolek PJ, et al. High Bone Mass-Causing Mutant LRP5 Receptors Are Resistant to Endogenous Inhibitors In Vivo. J Bone Miner Res. 2015;30:1822–1830. doi: 10.1002/jbmr.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kramer I, Loots GG, Studer A, Keller H, Kneissel M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res. 2010;25:178–189. doi: 10.1359/jbmr.090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui Y, et al. LRP5 functions in bone to regulate bone mass. Nature Medicine. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Little RD, et al. A Mutation in the LDL Receptor-Related Protein 5 Gene Results in the Autosomal Dominant High-Bone-Mass Trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tu X, et al. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci U S A. 2015;112:E478–E486. doi: 10.1073/pnas.1409857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ducy P, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 78.Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12:2014–2023. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 79.Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009 doi: 10.1016/j.mce.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oury F, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oury F, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–241. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiong J, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakashima T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 84.Suda T, Nakamura I, Jimi E, Takahashi N. Regulation of osteoclast function. J Bone Miner Res. 1997;12:869–879. doi: 10.1359/jbmr.1997.12.6.869. [DOI] [PubMed] [Google Scholar]
- 85.Harris SE, et al. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone. 2012;50:42–53. doi: 10.1016/j.bone.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abboud-Werner SL. CSF in osteocytes. J Bone Miner Res. 2013 [Google Scholar]
- 87.Kramer I, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30:3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holmen SL, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 89.Glass DA, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 90.Plotkin LI. Apoptotic osteocytes and the control of targeted bone resorption. Curr Osteoporos Rep. 2014;12:121–126. doi: 10.1007/s11914-014-0194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bellido T. Osteocyte-Driven Bone Remodeling. Calcif Tissue Int. 2013;94:25–34. doi: 10.1007/s00223-013-9774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: Master Orchestrators of Bone. Calcif Tissue Int. 2013;94:5–24. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tatsumi S, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Plotkin LI, et al. Inhibition of Osteocyte Apoptosis Prevents the Increase in Osteocytic RANKL but it does not Stop Bone Resorption or the Loss of Bone Induced by Unloading. J Biol Chem. 2015 doi: 10.1074/jbc.M115.642090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cabahug-Zuckerman P, et al. Osteocyte Apoptosis Caused by Hindlimb Unloading is Required to Trigger Osteocyte RANKL Production and Subsequent Resorption of Cortical and Trabecular Bone in Mice Femurs. J Bone Miner Res. 2016 doi: 10.1002/jbmr.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aguirre JI, et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Min Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 97.Morse LR, et al. Severe Spinal Cord Injury Causes Immediate Multicellular Dysfunction at the Chondro-Osseous Junction. Transl Stroke Res. 2011;2:643–650. doi: 10.1007/s12975-011-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noble BS, et al. Mechanical loading: biphasic osteocyte survival and the targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284:C934–C943. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- 99.Bellido T. Antagonistic interplay between mechanical forces and glucocorticoids in bone: a tale of kinases. J Cell Biochem. 2010;111:1–6. doi: 10.1002/jcb.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Plotkin LI, et al. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–C643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 101.McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. Anat Rec (Hoboken) 2009;292:355–363. doi: 10.1002/ar.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci U S A. 2007;104:15941–15946. doi: 10.1073/pnas.0707246104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Phillips JA, et al. Role for beta1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol. 2008 doi: 10.1016/j.matbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 104.Robinson JA, et al. WNT/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 105.Sutherland MK, et al. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone. 2004;35:828–835. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 106.Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280:41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 107.Gortazar AR, Martin-Millan M, Bravo B, Plotkin LI, Bellido T. Crosstalk between caveolin-1/ERKs and β-catenin survival pathways in osteocyte mechanotransduction. J Biol Chem. 2013;288:8168–8175. doi: 10.1074/jbc.M112.437921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kitase Y, Barragan L, Jiang JX, Johnson ML, Bonewald LF. Mechanical induction of PGE(2) in osteocytes blocks glucocorticoid induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25:2657–2668. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Babij P, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–974. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 110.Santos A, Bakker AD, Klein-Nulend J. The role of osteocytes in bone mechanotransduction. Osteoporos Int. 2009 doi: 10.1007/s00198-009-0858-5. [DOI] [PubMed] [Google Scholar]
- 111.Sawakami K, et al. The WNT co-receptor LRP5 is essential for skeletal mechanotransduction, but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 112.Chow JW, Chambers TJ. Indomethacin has distinct early and late actions on bone formation induced by mechanical stimulation. Am J Physiol. 1994;267:E287–E292. doi: 10.1152/ajpendo.1994.267.2.E287. [DOI] [PubMed] [Google Scholar]
- 113.Kousteni S, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 114.Aguirre JI, et al. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem. 2007;282:25501–25508. doi: 10.1074/jbc.M702231200. [DOI] [PubMed] [Google Scholar]
- 115.Jessop HL, et al. Mechanical strain and estrogen activate estrogen receptor alpha in bone cells. J Bone Miner Res. 2001;16:1045–1055. doi: 10.1359/jbmr.2001.16.6.1045. [DOI] [PubMed] [Google Scholar]
- 116.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424:389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 117.Lee KC, Jessop H, Suswillo R, Zaman G, Lanyon LE. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and -beta. J Endocrinol. 2004;182:193–201. doi: 10.1677/joe.0.1820193. [DOI] [PubMed] [Google Scholar]
- 118.Bellido T, Saini V, Divieti Pajevic P. Effects of PTH on osteocyte function. Bone. 2013;54:250–257. doi: 10.1016/j.bone.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chow JW, Fox S, Jagger CJ, Chambers TJ. Role for parathyroid hormone in mechanical responsiveness of rat bone. Am J Physiol. 1998;274:E146–E154. doi: 10.1152/ajpendo.1998.274.1.E146. [DOI] [PubMed] [Google Scholar]
- 120.Ma Y, et al. Parathyroid hormone and mechanical usage have a synergistic effect in rat tibial diaphyseal cortical bone. J Bone Min Res. 1999;14:439–448. doi: 10.1359/jbmr.1999.14.3.439. [DOI] [PubMed] [Google Scholar]
- 121.Hagino H, Okano T, Akhter MP, Enokida M, Teshima R. Effect of parathyroid hormone on cortical bone response to in vivo external loading of the rat tibia. J Bone Miner Metab. 2001;19:244–250. doi: 10.1007/s007740170027. [DOI] [PubMed] [Google Scholar]
- 122.Sugiyama T, et al. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. Bone. 2008;43:238–248. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 123.Gardinier JD, Mohamed F, Kohn DH. PTH Signaling During Exercise Contributes to Bone Adaptation. J Bone Miner Res. 2015;30:1053–1063. doi: 10.1002/jbmr.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J, Duncan RL, Burr DB, Gattone VH, Turner CH. Parathyroid hormone enhances mechanically induced bone formation, possibly involving L-type voltage-sensitive calcium channels. Endocrinology. 2003;144:1226–1233. doi: 10.1210/en.2002-220821. [DOI] [PubMed] [Google Scholar]
- 125.Wysolmerski JJ. Parathyroid hormone-related protein: an update. J Clin Endocrinol Metab. 2012;97:2947–2956. doi: 10.1210/jc.2012-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen X, Macica CM, Ng KW, Broadus AE. Stretch-Induced PTH-Related Protein Gene Expression in Osteoblasts. J Bone Miner Res. 2005;20:1454–1461. doi: 10.1359/jbmr.2005.20.8.1454. [DOI] [PubMed] [Google Scholar]
- 127.Maycas M, et al. Role of the parathyroid hormone type 1 receptor (PTH1R) as a mechanosensor in osteocyte survival. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2439. [DOI] [PubMed] [Google Scholar]
- 128.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 129.Plotkin LI, et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 132.Gu G, Hentunen TA, Nars M, Harkonen PL, Vaananen HK. Estrogen protects primary osteocytes against glucocorticoid-induced apoptosis. Apoptosis. 2005;10:583–595. doi: 10.1007/s10495-005-1893-0. [DOI] [PubMed] [Google Scholar]
- 133.Plotkin LI, Manolagas SC, Bellido T. Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival: evidence for inside-out signaling leading to anoikis. J Biol Chem. 2007;282:24120–24130. doi: 10.1074/jbc.M611435200. [DOI] [PubMed] [Google Scholar]
- 134.Gil-Henn H, et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. J Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Buckbinder L, et al. Proline-rich tyrosine kinase 2 regulates osteoprogenitor cells and bone formation, and offers an anabolic treatment approach for osteoporosis. Proc Natl Acad Sci U S A. 2007;104:10619–10624. doi: 10.1073/pnas.0701421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sato AY, Tu X, McAndrews KA, Plotkin LI, Bellido T. Prevention of glucocorticoid induced-apoptosis of osteoblasts and osteocytes by protecting against endoplasmic reticulum (ER) stress in vitro and in vivo in female mice. Bone. 2014;73:60–68. doi: 10.1016/j.bone.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jilka RL, et al. Apoptosis in bone cells. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of Bone Biology. Academic Press; San Diego, San Francisco, New York, London, Sydney, Tokyo: 2008. pp. 237–261. [Google Scholar]
- 138.Plotkin LI, Bivi N, Bellido T. A bisphosphonate that does not affect osteoclasts prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids in mice. Bone. 2011;49:122–127. doi: 10.1016/j.bone.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kousteni S, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 140.Bellido T, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 141.Jilka RL, et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lin C, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 143.Bodine PV. Wnt signaling control of bone cell apoptosis. Cell Res. 2008;18:248–253. doi: 10.1038/cr.2008.13. [DOI] [PubMed] [Google Scholar]
- 144.Glantschnig H, et al. A rate-limiting role for Dickkopf-1 in bone formation and the remediation of bone loss in mouse and primate models of postmenopausal osteoporosis by an experimental therapeutic antibody. J Pharmacol Exp Ther. 2011;338:568–578. doi: 10.1124/jpet.111.181404. [DOI] [PubMed] [Google Scholar]
- 145.Glantschnig H, et al. Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J Biol Chem. 2010;285:40135–40147. doi: 10.1074/jbc.M110.166892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bodine PV, et al. A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation. Bone. 2009;44:1063–1068. doi: 10.1016/j.bone.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 147.Jelinek T, Hajek R. Monoclonal antibodies - A new era in the treatment of multiple myeloma. Blood Rev. 2015 doi: 10.1016/j.blre.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 148.Costa AG, Bilezikian JP. Sclerostin: therapeutic horizons based upon its actions. Curr Osteoporos Rep. 2012;10:64–72. doi: 10.1007/s11914-011-0089-5. [DOI] [PubMed] [Google Scholar]
- 149.McClung MR, et al. Romosozumab in Postmenopausal Women with Low Bone Mineral Density. N Engl J Med. 2014 doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 150.Van Bezooijen RL, et al. Sclerostin in mineralized matrices and van Buchem disease. J Dent Res. 2009;88:569–574. doi: 10.1177/0022034509338340. [DOI] [PubMed] [Google Scholar]
- 151.Van Bezooijen RL, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Roudier M, et al. Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Rheum. 2013;65:721–731. doi: 10.1002/art.37802. [DOI] [PubMed] [Google Scholar]
- 153.Bouaziz W, et al. Loss of sclerostin promotes osteoarthritis in mice via beta-catenin-dependent and -independent Wnt pathways. Arthritis Res Ther. 2015;17:24. doi: 10.1186/s13075-015-0540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Drake MT, Farr JN. Inhibitors of sclerostin: emerging concepts. Curr Opin Rheumatol. 2014;26:447–452. doi: 10.1097/BOR.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Imel EA, et al. Prolonged Correction of Serum Phosphorus in Adults With X-Linked Hypophosphatemia Using Monthly Doses of KRN23. J Clin Endocrinol Metab. 2015;100:2565–2573. doi: 10.1210/jc.2015-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aono Y, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–1888. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 157.Wohrle S, et al. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J Bone Miner Res. 2013;28:899–911. doi: 10.1002/jbmr.1810. [DOI] [PubMed] [Google Scholar]
- 158.Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther. 2007;9(Suppl 1):S7. doi: 10.1186/ar2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tanaka S, Nakamura K, Takahasi N, Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev. 2005;208:30–49. doi: 10.1111/j.0105-2896.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 160.Reid IR. Denosumab after 8 years. Osteoporos Int. 2015 doi: 10.1007/s00198-015-3347-z. [DOI] [PubMed] [Google Scholar]
- 161.Sibai T, Morgan EF, Einhorn TA. Anabolic agents and bone quality. Clin Orthop Relat Res. 2011;469:2215–2224. doi: 10.1007/s11999-010-1722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li M, et al. Osteopenia and impaired fracture healing in aged EP4 receptor knockout mice. Bone. 2005;37:46–54. doi: 10.1016/j.bone.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 163.Ke HZ, et al. A nonprostanoid EP4 receptor selective prostaglandin E2 agonist restores bone mass and strength in aged, ovariectomized rats. J Bone Miner Res. 2006;21:565–575. doi: 10.1359/jbmr.051110. [DOI] [PubMed] [Google Scholar]
- 164.Liu CC, et al. Novel EP4 receptor agonist-bisphosphonate conjugate drug (C1) promotes bone formation and improves vertebral mechanical properties in the ovariectomized rat model of postmenopausal bone loss. J Bone Miner Res. 2015;30:670–680. doi: 10.1002/jbmr.2382. [DOI] [PubMed] [Google Scholar]
- 165.Li M, Thompson DD, Paralkar VM. Prostaglandin E(2) receptors in bone formation. Int Orthop. 2007;31:767–772. doi: 10.1007/s00264-007-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]