Abstract
Background: Patients undergoing surgery for prostate cancer who have adverse pathological findings experience high rates of recurrence. While there are data supporting adjuvant radiotherapy compared to a wait-and-watch strategy to reduce recurrence rates, there are no randomized controlled trials comparing adjuvant radiotherapy with the other standard of care, salvage radiotherapy (radiotherapy administered at the time of recurrence). Methods: We constructed a health state transition (Markov) model employing two-dimensional Monte Carlo simulation using a lifetime horizon to compare the quality-adjusted survival associated with postoperative strategies using adjuvant or salvage radiotherapy. Prior to analysis, we calibrated and validated our model using the results of previous randomized controlled trials. We considered clinically important oncological health states from immediately postoperative to prostate cancer–specific death, commonly described complications from prostate cancer treatment, and other causes of mortality. Transition probabilities and utilities for disease states were derived from a literature search of MEDLINE and expert consensus. Results: Salvage radiotherapy was associated with an increased quality-adjusted life expectancy (QALE) (58.3 months) as compared with adjuvant radiotherapy (53.7 months), a difference of 4.6 months (standard deviation 8.8). Salvage radiotherapy had higher QALE in 53% of hypothetical cohorts. There was a minimal difference in overall life expectancy (-0.1 months). Examining recurrence rates, our model showed validity when compared with available randomized controlled data. Conclusions: A salvage radiotherapy strategy appears to provide improved QALE for patients with adverse pathological findings following radical prostatectomy, compared with adjuvant radiotherapy. As these findings reflect, population averages, specific patient and tumor factors, and patient preferences remain central for individualized management.
Keywords: quality-adjusted life years, life expectancy, Monte Carlo method, adjuvant radiotherapy, salvage therapy, neoplasm recurrence
For approximately two thirds of patients undergoing radical prostatectomy for prostate cancer, surgery is curative and patients remain disease-free (without biochemical or radiographic evidence of recurrence).1 However, patients with adverse pathologic findings (defined as seminal vesicle invasion, extraprostatic extension, and positive surgical margins [residual tumor at the surgical site]) experience up to a 60% risk of recurrence at 10 years and may require subsequent radiation therapy.2
In three randomized controlled trials, adjuvant radiotherapy improved recurrence-free survival for men with adverse pathologic findings compared with a “watch-and-wait” approach (SWOG S8794,3 EORTC 22911,4 and ARO 96-025). As a result, the American Urological Association (AUA) and American Society for Radiation Oncology (ASTRO) published guidelines recommending adjuvant radiotherapy for these patients, while recommending salvage radiotherapy for patients who do not fit these criteria but who develop biochemical recurrence following surgery.2 Problematically, patients in the “watch-and-wait” strategy received a variety of postoperative treatments including radiotherapy, surgical castration, medical hormonal treatments, and others, administered in a nonsystematic fashion. Furthermore, both the design and execution biased against the “watch-and-wait” approach as only a third of the men in this arm received radiotherapy and it was delayed in nearly half of these. Finally, compared with the endpoint of recurrence following salvage radiotherapy, the endpoint employed (recurrence prior to salvage therapy) favored an adjuvant strategy.6 Thus, due to limitations in the design, analysis, and interpretation of the three randomized trials, many experts do not support the routine use of adjuvant radiotherapy,7,8 despite the AUA/ASTRO guidelines.
An alternative to adjuvant radiotherapy for all patients is the salvage radiotherapy strategy in which radiation is administered at the time of recurrence, thus sparing the 40% of patients with adverse pathologic features who will not recur unnecessary radiotherapy. In observational data, salvage radiotherapy has been shown to provide durable cancer control for patients at high risk for progression to metastasis9 and to decrease the risk of prostate cancer–related mortality compared with observation alone.10 The benefit is maximized when radiotherapy is administered early following biochemical recurrence.10 However, there are no published randomized controlled trials comparing adjuvant and early salvage radiotherapy. A recent trial assessing these two modalities closed prior to reaching planned accrual (RAVES; https://clinicaltrials.gov/ct2/show/NCT00860652).
Decision models are useful when examining population-based implementation of established treatments and when assessing complex balances of benefit and harm including quality of life.11 Thus, we used a decision analysis to compare quality-adjusted survival between adjuvant and salvage radiotherapy strategies.
Methods
Decision Model
We constructed a health state transition (Markov) model employing two-dimensional Monte Carlo simulation using TreeAge HealthPro 2015 (TreeAge Software Inc., Williamstown, MA) to compare quality-adjusted life expectancy (QALE) for patients with adverse pathologic findings, defined as positive surgical margins, seminal vesicle invasion, and extraprostatic extension,2 following radical prostatectomy. Model parameters were selected from distributions (outer-loop or second-order iterations), and for each parameter set, hypothetical patients were simulated (inner-loop or first order iterations). Second-order simulation captures parameter-level uncertainly, whereas first-order simulation allows for patient-level variability. The latter facilitates the simulation of the life history of individual hypothetical patients as they traverse through various health states and allows for tracking of complications as they are experienced.
Modelling Details
We modelled a cycle duration of 1 month and used a lifetime time horizon. We discounted QALE at 3% per annum12 and employed a trapezoidal within-cycle correction for bias arising from discrete-time Markov models.13
To account for uncertainty in the probabilities and utilities employed in the model, we sampled the model parameters as distributions using point estimates and measures of uncertainty, such as confidence intervals or standard error. Where published studies did not include these uncertainty measures, we treated the variables as fixed. Further details are provided in the online appendix (Supplementary Table 6).
Outcome
The primary outcome was QALE, which comprises duration of survival and quality of life. Quality of life was assessed using utility weights, derived from a standard gamble technique, which have been previously used in decision analyses of prostate cancer treatments (Table 1).14–16 The effect of complications was assessed using disutility scores. To combine utilities, after sampling the disease-related health state utility from a beta distribution, we subtracted the lowest complication-related disutility.
Table 1.
Utilities and Disutilities Used to Inform Health States
| Health State | Utility | Range | Reference |
|---|---|---|---|
| Postoperative status | 1 | — | — |
| Current radiotherapy | 0.73 | 0–1.0 | Heijnsdijk, NEJM (2012) |
| Previous radiotherapy | 0.78 | 0–1.0 | Heijnsdijk, NEJM (2012) |
| Biochemical recurrence | 0.68 | 0–1.0 | Hayes, JAMA (2010) |
| Metastasis | 0.25 | 0–1.0 | Stewart, Med Care (2005) |
| Death | 0 | — | — |
| Health State | Disutility | Range | Reference |
| Erectile dysfunction | −0.11 | Stewart, Med Care (2005) | |
| Incontinence | −0.17 | Stewart, Med Care (2005) | |
| Bowel dysfunction | −0.29 | Stewart, Med Care (2005) |
The secondary outcome was overall life expectancy (without quality adjustment). We performed a sensitivity analysis without discounting.
Treatment Strategies
For each second-order iteration (parameter set), we considered hypothetical cohorts of 10,000 men with adverse pathologic findings following radical prostatectomy for prostate cancer, beginning 10 to 12 weeks postoperatively. This time was chosen as it represents when patients are likely to initiate radiotherapy in the adjuvant strategy.4,5 Men receive radiotherapy immediately on entering the model (adjuvant) or undergo surveillance with radiotherapy administered for biochemical recurrence (salvage; serum prostate specific antigen < 0.4 ng/mL).
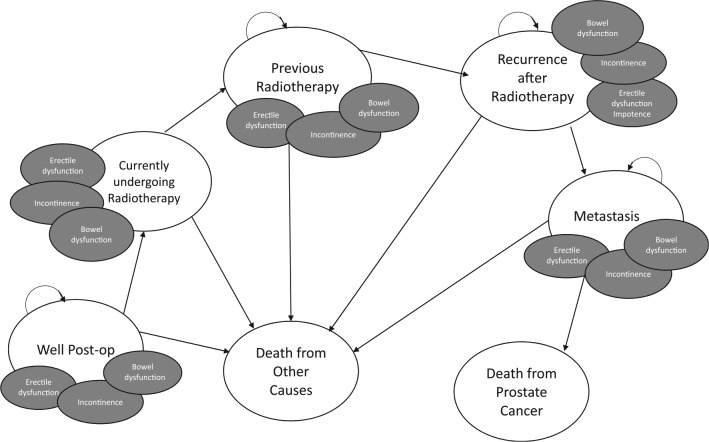
We based this model on a number of disease-related health states (Figure 1) following multidisciplinary consensus involving radiation oncologists (GM and ES), urologists (CJDW, RS, and RKN), and an external methodologist (AJ). Radiotherapy was assumed to last for one cycle. After radiation, patients entered the “previous radiotherapy” state. Subsequently, patients either remained in that state or sequentially moved through disease progression states based on literature-derived transition probabilities.
Figure 1.
Diagram of health states. Patients in the adjuvant radiotherapy arm initially enter the model at the “Currently undergoing radiotherapy” state, whereas those in the salvage arm enter at the “Well Post-op” state. We assumed that patients then sequentially progressed through the health states based on literature-derived probabilities. A decision analytic tree is provided in the appendix as Supplementary Figure 1.
Independent of current state membership, we explicitly modeled complications of erectile dysfunction, urinary incontinence, and bowel dysfunction as they significantly affect patient quality of life and have been used in other decision analyses in prostate cancer.15,17 Postsurgical complications of erectile dysfunction and urinary incontinence recovered according to literature derived probabilities within 1 and 2 years of surgery, respectively.18,19 We assumed that adjuvant radiotherapy did not increase the probability of erectile dysfunction or incontinence nor their probabilities of recovery compared with salvage, based on previous work.20,21 For patients receiving radiotherapy, we modeled radiotherapy-associated bowel dysfunction, which we assumed to recover after 1 year.
Derivation of Model Probabilities
Transition probabilities were determined from a MEDLINE literature search as of 1 November 2015, supplemented by hand search of references from retrieved studies, review articles, previous decision analyses, and expert consultation (Table 2). The multidisciplinary panel appraised the acquired literature. Each transition probability and utility was determined by consensus following panel discussion. Priority was given to results derived from randomized trials, followed by multi-institutional cohort studies. We assumed the risk of metastasis after recurrence and prostate cancer mortality following metastasis were the same in both arms. Annual age-specific probabilities of other cause death were obtained from life tables.
Table 2.
Transition Probabilities Used to Inform Monte Carlo Microsimulation Model
| Probability | Range | Source | |
|---|---|---|---|
| Per cycle probabilities | |||
| Probability of recurrence after radical prostatectomy | |||
| Adjuvant radiotherapy | N/A | ||
| Salvage radiotherapy | 0.010 | 0–0.1 | Compositea |
| Probability of biochemical recurrence following radiotherapy | |||
| Adjuvant radiotherapy | 0.005 | 0–0.05 | Compositea |
| Salvage radiotherapy | 0.015 | 0–0.05 | Compositea |
| Probability of metastasis following recurrence after radiotherapy | |||
| Adjuvant radiotherapy | 0.0018 | 0–0.01 | Compositea |
| Salvage radiotherapy | 0.0018 | 0–0.01 | Compositea |
| Probability of prostate cancer death following metastasis | |||
| Adjuvant radiotherapy | 0.00585 | 0–0.01 | Compositea |
| Salvage radiotherapy | 0.00585 | 0–0.01 | Compositea |
| Probability of death from other causes | Age-dependent | 0–0.3 | Statistics Canada |
| Probability of developing new erectile dysfunction, after erectile function regained (age-related erectile dysfunction) | Age-dependent | Johannes, J Urol (2000) | |
| Probability of regaining erectile function after surgeryb | 0.066 | 0–0.1 | Rabanni, J Urol (2000) |
| Probability of developing new incontinence, after continence regained (age-related incontinence) | 0 | Assumption | |
| Probability of regaining continence after surgeryc | 0.037 | 0–0.1 | Suardi, Eur Urol (2014) |
| Probability of bowel dysfunction | 0.0013 | 0–0.1 | Alibhai, JCO (2003) |
| Instantaneous probabilities | |||
| Probability of erectile dysfunction immediately post–radical prostatectomy | 0.77 | Hayes, JAMA (2010) | |
| Probability of incontinence immediately post–radical prostatectomy | 0.5 | Sacco, BJU Int (2006) | |
Derivation of the composite estimates is provided in Supplementary Table 1.
May recover for first 12 months following radical prostatectomy.
May recover for first 24 months following radical prostatectomy.
Model Calibration
We calibrated the values of the probabilities of recurrence following radical prostatectomy, recurrence following radiotherapy in the adjuvant and salvage arms, metastasis following recurrence, prostate cancer mortality following metastasis, and other cause mortality using biochemical recurrence as the outcome. To do so, we multiplied each literature-derived probability by a calibration factor and compared the resulting value for biochemical recurrence with the results of EORTC 22911.4 As the trial did not have a salvage radiotherapy arm, we used the probability of recurrence following radical prostatectomy for our salvage arm and the probability of recurrence following radiotherapy for our adjuvant arm as calibration outcomes. We selected the combination of calibration values, which resulted in the best approximation of the trial.22,23 Further details are available in the appendix.
Analysis
We used two-dimensional simulation in order to capture both parameter-level uncertainty and patient-level variability. We drew 10,000 parameter samples from the distributions specified from the literatures search and, for each, simulated cohorts of 10,000 hypothetical patients. On each inner-loop iteration, each hypothetical patient traversed both strategies and the incremental benefit was calculated as the difference in quality-adjusted survival between the two strategies. The incremental benefits were then averaged across the 10,000 inner-loop iterations. These average incremental benefits were then averaged in turn across the 10,000 outer-loop parameter samples to yield the overall model output.
To better understand the relationship between treatment strategy and QALE, we plotted a histogram of the incremental benefit of salvage radiotherapy across the total number of parameter samples. Further details are in the appendix.
Results
Model Validation
Following calibration, the model closely approximated the results of EORTC 22991 (Figure 2). Five-year biochemical recurrence rates were 26% for patients in the adjuvant group and 44% for patients in the salvage group, similar to literature values (Supplementary Table 5).3–5 Thus, we considered the model adequately calibrated and validated.
Figure 2.
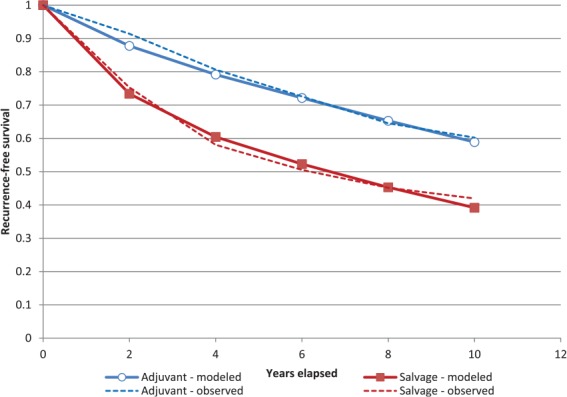
Kaplan-Meier analysis of biochemical recurrence comparing model-derived results (modeled) following calibration with the EORTC 22911 trial (observed).4
Quality-Adjusted Life Expectancy
A salvage radiotherapy strategy was associated with increased QALE (mean 58.3 months), compared with adjuvant radiotherapy (53.7 months) for men with adverse pathological findings. The mean incremental benefit was 4.6 months (standard deviation 8.8 months).
Among 10,000 outer-loop iterations of the model, salvage radiotherapy had a higher QALE in 5,270 (52.7%) iterations while adjuvant was preferred in 4,730 (47.3%). The distribution of incremental benefit was heavily skewed (Figure 3), with a much larger incremental benefit among iterations favoring salvage radiotherapy (9.4 months) than among iterations favoring adjuvant (0.8 months; Figure 3).
Figure 3.
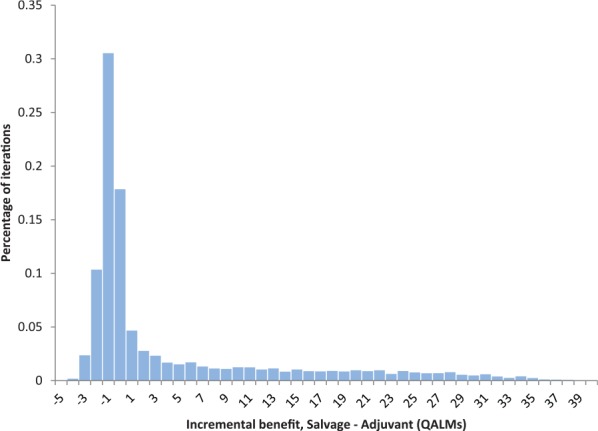
Distribution of incremental benefit of salvage radiotherapy, compared with adjuvant radiotherapy, across 10,000 outer-loop iterations of the decision analytic model.
Overall Life Expectancy
The mean difference in overall survival between the two radiotherapy strategies was 0.07 months (2 days; standard deviation 0.4 months) favoring an adjuvant radiotherapy approach, with mean survival of 64 months in both groups. In an analysis without discounting, the mean difference was 0.2 months (6 days; standard deviation 0.6 months), again favoring an adjuvant approach.
Discussion
We evaluated the optimal radiation strategy for patients with adverse pathological findings following radical prostatectomy for prostate cancer using a health state transition (Markov) model employing two-dimensional Monte Carlo simulation. A salvage radiotherapy therapy strategy was associated with QALE of 58.3 months, whereas adjuvant radiotherapy had QALE of 53.7 months, an incremental benefit of 4.6 quality-adjusted life months. We found a marked skew in incremental benefit with a much larger benefit in those iterations favoring salvage radiotherapy. Our model showed validity when compared with published randomized data assessing adjuvant radiotherapy.
Wright and Weinstein suggested that interpretation of an incremental benefit in QALE can be made by comparing with other, similar interventions aimed at similar patient populations.24 The incremental benefit of adjuvant chemotherapy for women with node-positive breast cancer is 3.2 quality-adjusted life months.24 Thus, the benefit seen with salvage radiotherapy compared with adjuvant is likely meaningful. However, these authors also point out that such population average effects do not preclude the possibility of conflicting outcomes for individual patients.
Elliott and others have previously examined this research question using a similar methodology6 and demonstrated that salvage radiotherapy is associated with improved QALE when accounting for the disutility of radiotherapy. Our analysis employs more mature data from both the EORTC4 and ARO5 randomized trials and probabilistic sensitivity analyses, which account for uncertainty and variability in all parameters simultaneously, rather than deterministic sensitivity analyses. This allowed us to capture the distribution of the incremental benefit around its mean. Furthermore, its highlights the two dimensions of the policy question: first, what treatment most benefits the entire population of patients following radical prostatectomy (an economic, utilitarian perspective), and second, what treatment is best for each individual patient? We sought to assess the former. In doing so, we found that a salvage radiotherapy strategy is unambiguously superior. However, this does not preclude adjuvant radiotherapy benefiting a subset of men, supported by the finding that adjuvant radiotherapy was preferred in 47% of iterations. However, the incremental benefit of adjuvant radiotherapy in these iterations was quite small. Conversely, in iterations favoring salvage radiotherapy, the incremental benefit was large.
In order to better inform individual patient decision making, the use of genomic classifiers may assist in the identification of patients who are at high risk of biochemical recurrence or metastasis following radical prostatectomy.25–27 Furthermore, genomic markers may distinguish between patients who benefit from adjuvant radiotherapy and those who do not.28,29 Thus, adjuvant radiotherapy is likely to benefit patients who are identified as being at high risk of biochemical recurrence and metastasis.30
As there were very small differences in unadjusted life expectancy, the observed differences in QALE were driven by differences in quality of life in keeping with previous analyses.6 This likely stems from the avoidance of radiotherapy complications in a large proportion of patients in the salvage radiotherapy group who never recur. In a subset of 217 patients in SWOG S8794, adjuvant therapy was associated with worse bowel and urinary function and with increased symptom distress but not with significant differences in other general measures of health-related quality of life.31 However, Moinpour and others employed the SWOG quality of life questionnaire, whereas the US Panel on Cost-Effectiveness in Health and Medicine and the UK National Institute of Health and Clinical Excellence recommend the use of QALEs based on utilities32 such as we used to inform health care decision making.
We did not perform traditional deterministic sensitivity analyses in which one or two probability or utilities values were varied in order to assess the sensitivity of the model to variation in a single parameter because variation in the model results is due to the simultaneous uncertainty of all parameters. Therefore, we used probabilistic analyses in which all parameters were varied simultaneously (by using probability distributions). The net effect of the global uncertainty is reflected in the distribution of incremental benefits around the mean.33 Furthermore, we examined the frequency with which each strategy is optimal.33 As the distribution of net benefit shifts when assessing unadjusted life expectancy, the model appears to be sensitive to quality of life assessment as reflected in utility scores, as others have shown.34
Typically, gains in QALE necessitate active medical intervention. In contrast, this analysis demonstrates that prostate cancer patients with adverse pathological findings benefit when postoperative radiotherapy is withheld until there is evidence of recurrence. In this analysis, we considered quality-adjusted and overall life expectancy outcomes and did not model costs. This was done in order to increase the generalizability of the research findings as there is wide variation in radiotherapy costs across regions and jurisdictions, a minority of which is due to patient factors.35 While costs were not explicitly examined, in keeping with the framework of Choosing Wisely,36 routine adoption of a salvage radiotherapy strategy offers an opportunity to improve patient outcomes while reducing health care utilization.
There are several limitations of this study. First, we assumed that salvage radiotherapy is administered early in the time course of biochemical recurrence. While this may not reflect routine clinical practice, it is appropriate for comparative assessment of adjuvant and salvage radiotherapy. Second, true clinical complexity is underestimated in this model. We used estimates derived from the primary analyses of each of the randomized controlled trials. Many authors have pointed out the significant heterogeneity of outcomes within this group,7 including the lack of survival benefit to adjuvant radiotherapy for patients with negative surgical margins37 and possible harm for patients older than 70 years.4 Furthermore, recent observational data suggest that patients treated with early salvage radiotherapy (prostate specific antigen 0.2–0.5 ng/mL) have a similar risk of recurrence or metastasis as those treated with adjuvant radiotherapy.38 In addition, there are limitations regarding the modeling of complications. First, we considered these dichotomously rather than as a spectrum of severity as they exist in clinical practice. Second, we considered the risk of incontinence and erectile dysfunction to be equivalent between the groups based on literature review including data from SWOG S8794.3,20 However, there is recent observational evidence that functional outcomes may be compromised by early postoperative radiotherapy.18 Additional complications beyond erectile dysfunction, incontinence, and bowel dysfunction may also be important following radiotherapy.39 Inclusion of these would be expected to increase the difference between the two strategies as a greater proportion of patients in the adjuvant arm are expected to experience these complications. Thus, in each instance, we have used conservative assumptions that favor adjuvant radiotherapy and therefore underestimate the true benefit of routine adoption of a salvage radiotherapy strategy. Finally, we assumed that each patient in the salvage radiotherapy strategy would receive early radiotherapy. While in clinical practice, a proportion of patients may be lost to follow-up or have delayed recognition of recurrence for another reason, such an assumption is reasonable when assessing the population-level effects of a salvage approach.
The quality of the conclusions that can be drawn from this decision model depends on the data underpinning our transition probabilities. In many cases, we were unable to obtain transition probabilities for the adjuvant and salvage radiotherapy arms from the same study population given the heterogeneous treatment offered to patients in the “watch-and-wait” arms.
Methodologically, there is debate regarding the most appropriate manner to combine utilities with options including addition, multiplication, selection of the lowest utility, and selection of the highest utility. We selected disease-related health states independently from beta distributions. We considered the treatment-related complications to be short-term outcomes and thus subtracted the associated disutilities from the disease-related health state utilities, as has been recommended.40 Distribution-based sampling as we performed produces a range of possible values that encompasses the results of multiplicative, additive, and minimum approaches.
Recently, the addition of androgen deprivation therapy to salvage radiotherapy following prostatectomy was shown to improve recurrence and progression-free survival.41 Should this practice become standard of care, survival is likely to improve for all patients with adverse pathologic factors following radical prostatectomy, but it is not clear how this may affect the incremental benefit of salvage compared with adjuvant radiotherapy.
In conclusion, while randomized data have shown that adjuvant radiotherapy reduces biochemical recurrence compared with a “watch-and-wait” approach, expert opinion and clinical practice has shown a decreasing use of this treatment strategy.42 This decision analysis supports the preferred use of a salvage radiotherapy strategy for patients with adverse pathologic findings following radical prostatectomy in order to maximize QALE. These findings, in addition to forthcoming data to come from ongoing randomized trials, will help guide urologists and radiation oncologists in the provision of postoperative radiotherapy.
Supplementary Material
Footnotes
Financial support for this study was provided in part by a peer-reviewed grant from the Canadian Urological Association Scholarship Foundation (Canadian Urological Association—Pfizer Urology Resident Grant) to CJDW and the Ajmera Family Chair in Urologic Oncology held by RKN. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
All authors declare that they have no conflicts of interest or disclosures to declare.
CJDW and RKN had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The online appendix for this article is available on the Medical Decision Making Policy & Practice Web site at http://journals.sagepub.com/doi/suppl/10.1177/2381468317709476
References
- 1. Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”). Urology. 2005;66(5 Suppl):83–94. [DOI] [PubMed] [Google Scholar]
- 2. Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J Urol. 2013;190(2):441–9. [DOI] [PubMed] [Google Scholar]
- 3. Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380(9858):2018–27. [DOI] [PubMed] [Google Scholar]
- 5. Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66(2):243–50. [DOI] [PubMed] [Google Scholar]
- 6. Elliott SP, Wilt TJ, Kuntz KM. Projecting the clinical benefits of adjuvant radiotherapy versus observation and selective salvage radiotherapy after radical prostatectomy: a decision analysis. Prostate Cancer Prostatic Dis. 2011;14(3):270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walsh PC, Lawrentschuk N. Immediate adjuvant radiation therapy following radical prostatectomy should not be advised for men with extraprostatic extension who have negative surgical margins. Eur Urol. 2016;69(2):191–2. [DOI] [PubMed] [Google Scholar]
- 8. Stephenson AJ, Bolla M, Briganti A, et al. Postoperative radiation therapy for pathologically advanced prostate cancer after radical prostatectomy. Eur Urol. 2012;61(3):443–51. [DOI] [PubMed] [Google Scholar]
- 9. Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291(11):1325–32. [DOI] [PubMed] [Google Scholar]
- 10. Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299(23):2760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Owens DK, Whitlock EP, Henderson J, et al. Use of decision models in the development of evidence-based clinical preventive services recommendations: methods of the U.S. Preventive Services Task Force. Ann Intern Med. 2016;165(7):501–8. [DOI] [PubMed] [Google Scholar]
- 12. Doyle JR. Survey of time preference, delay discounting models. Judgment Decis Making. 2013;8(2):116–35. [Google Scholar]
- 13. Naimark DM, Bott M, Krahn M. The half-cycle correction explained: two alternative pedagogical approaches. Med Decis Making. 2008;28(5):706–12. [DOI] [PubMed] [Google Scholar]
- 14. Stewart ST, Lenert L, Bhatnagar V, Kaplan RM. Utilities for prostate cancer health states in men aged 60 and older. Med Care. 2005;43(4):347–55. [DOI] [PubMed] [Google Scholar]
- 15. Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304(21):2373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med. 2012;367(7):595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alibhai SM, Naglie G, Nam R, Trachtenberg J, Krahn MD. Do older men benefit from curative therapy of localized prostate cancer? J Clin Oncol. 2003;21(17):3318–27. [DOI] [PubMed] [Google Scholar]
- 18. Suardi N, Gallina A, Lista G, et al. Impact of adjuvant radiation therapy on urinary continence recovery after radical prostatectomy. Eur Urol. 2014;65(3):546–51. [DOI] [PubMed] [Google Scholar]
- 19. Rabbani F, Stapleton AM, Kattan MW, Wheeler TM, Scardino PT. Factors predicting recovery of erections after radical prostatectomy. J Urol. 2000;164(6):1929–34. [PubMed] [Google Scholar]
- 20. Hegarty SE, Hyslop T, Dicker AP, Showalter TN. Radiation therapy after radical prostatectomy for prostate cancer: evaluation of complications and influence of radiation timing on outcomes in a large, population-based cohort. PLoS One. 2015;10(2):e0118430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Showalter TN, Hegarty SE, Rabinowitz C, et al. Assessing adverse events of postprostatectomy radiation therapy for prostate cancer: evaluation of outcomes in the Regione Emilia-Romagna, Italy. Int J Radiat Oncol Biol Phys. 2015;91(4):752–9. [DOI] [PubMed] [Google Scholar]
- 22. Weinstein MC. Recent developments in decision-analytic modelling for economic evaluation. Pharmacoeconomics. 2006;24(11):1043–53. [DOI] [PubMed] [Google Scholar]
- 23. van der Steen A, van Rosmalen J, Kroep S, et al. Calibrating parameters for microsimulation disease models: a review and comparison of different goodness-of-fit criteria. Med Decis Making. 2016;36(5):652–65. [DOI] [PubMed] [Google Scholar]
- 24. Wright JC, Weinstein MC. Gains in life expectancy from medical interventions—standardizing data on outcomes. N Engl J Med. 1998;339(6):380–6. [DOI] [PubMed] [Google Scholar]
- 25. Cuzick J, Swanson GP, Fisher G, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nam RK, Amemiya Y, Benatar T, et al. Identification and validation of a five microRNA signature predictive of prostate cancer recurrence and metastasis: a cohort study. J Cancer. 2015;6(11):1160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ross AE, Johnson MH, Yousefi K, et al. Tissue-based genomics augments post-prostatectomy risk stratification in a natural history cohort of intermediate- and high-risk men. Eur Urol. 2016;69(1):157–65. [DOI] [PubMed] [Google Scholar]
- 28. Den RB, Yousefi K, Trabulsi EJ, et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. J Clin Oncol. 2015;33(8):944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao SG, Chang SL, Spratt DE, et al. Development and validation of a 24-gene predictor of response to postoperative radiotherapy in prostate cancer: a matched, retrospective analysis. Lancet Oncol. 2016;17(11):1612–20. [DOI] [PubMed] [Google Scholar]
- 30. Lobo JM, Stukenborg GJ, Trifiletti DM, Patel N, Showalter TN. Reconsidering adjuvant versus salvage radiation therapy for prostate cancer in the genomics era. J Comp Eff Res. 2016;5(4):375–82. [DOI] [PubMed] [Google Scholar]
- 31. Moinpour CM, Hayden KA, Unger JM, et al. Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol. 2008;26(1):112–20. [DOI] [PubMed] [Google Scholar]
- 32. Weinstein MC, Torrance G, McGuire A. QALYs: the basics. Value Health. 2009;12(Suppl 1):S5–S9. [DOI] [PubMed] [Google Scholar]
- 33. Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5(2):157–77. [DOI] [PubMed] [Google Scholar]
- 34. Cowen ME, Miles BJ, Cahill DF, Giesler RB, Beck JR, Kattan MW. The danger of applying group-level utilities in decision analyses of the treatment of localized prostate cancer in individual patients. Med Decis Making. 1998;18(4):376–80. [DOI] [PubMed] [Google Scholar]
- 35. Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11(5):403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801–2. [DOI] [PubMed] [Google Scholar]
- 37. Van der Kwast TH, Bolla M, Van Poppel H, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25(27):4178–86. [DOI] [PubMed] [Google Scholar]
- 38. Briganti A, Wiegel T, Joniau S, et al. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol. 2012;62(3):472–87. [DOI] [PubMed] [Google Scholar]
- 39. Wallis CJ, Mahar A, Cheung P, et al. New rates of interventions to manage complications of modern prostate cancer treatment in older men. Eur Urol. 2016;69(5):933–41. [DOI] [PubMed] [Google Scholar]
- 40. Naglie G, Krahn MD, Naimark D, Redelmeier DA, Detsky AS. Primer on medical decision analysis: part 3—estimating probabilities and utilities. Med Decis Making. 1997;17(2):136–41. [DOI] [PubMed] [Google Scholar]
- 41. Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17(6):747–56. [DOI] [PubMed] [Google Scholar]
- 42. Sineshaw HM, Gray PJ, Efstathiou JA, Jemal A. Declining use of radiotherapy for adverse features after radical prostatectomy: results from the National Cancer Database. Eur Urol. 2015;68(5):768–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.