Abstract
Multiple health behavior change (MHBC) interventions have great potential for enhancing health and well-being following cancer diagnosis and treatment. However, the characteristics and effects of MHBC interventions remain elusive for cancer survivors. The main purpose of this study was to evaluate the effectiveness of MHBC interventions on healthy eating and physical activity behaviors among cancer survivors. A secondary aim was to examine the effect of using a simultaneous and sequential design approach to MHBC (ie, changing both behaviors at the same time or one after the other). Randomized controlled trials reporting the impact of a MHBC intervention on both healthy eating and physical activity behaviors among cancer survivors were retrieved from MEDLINE, Cochrane Library, and PsycINFO. A total of 27 MHBC interventions were identified; most (92.6%) were designed to promote simultaneous change in both behaviors and assessed end-of-treatment effect among breast cancer survivors. MHBC interventions led by nurses or multidisciplinary teams showed the most compelling evidence for small to moderate improvement in both behaviors, with interventions that lasted ≥17 weeks more likely to improve both behaviors. This study identifies research priorities and provides preliminary evidence for clinical decision making and advancements in MHBC intervention design and delivery for clinical oncology.
Keywords: diet, exercise, systematic review, neoplasm, survivors
‘In particular, body weight and fat mass are related to both diet and physical activity, and obesity and weight gain during cancer treatment are associated with greater risk of morbidity from cancer treatment, greater risk for cancer recurrence, and greater risk for exacerbated treatment-related symptoms (eg, lower functional limitation, lymphedema) and lower quality of life.’
Introduction
Adherence to a healthy diet and regular physical activity is highlighted as an important strategy for improving health and well-being in the aftermath of a cancer diagnosis.1-3 The American Cancer Society (ACS)2 and the World Cancer Research Fund–American Institute of Cancer Research (WCRF-AIRC)3 highlight the benefits of healthy eating (eg, limiting high-fat/caloric foods and increasing fruit and vegetable [F&V]) and physical activity (eg, ≥150 minutes of moderate to vigorous aerobic activity per week that includes at least 2 days of strength training exercises) for improving the health and well-being of cancer survivors. However, the prevalence of unhealthy eating (60% to 85%) and physical inactivity (70% to 80%) are widespread among cancer survivors,4-7 and this combined behavioral pattern represents multiple behavioral risk factors.
Multiple behavioral risk factors may not only have additive but also multiplicative deleterious effects on cancer survivor quality of life4-6,8,9 and survival rate.10-12 In particular, body weight and fat mass are related to both diet and physical activity, and obesity and weight gain during cancer treatment are associated with greater risk of morbidity from cancer treatment, greater risk for cancer recurrence, and greater risk for exacerbated treatment-related symptoms (eg, lower functional limitation, lymphedema) and lower quality of life.1,13 Thus, it is important to acknowledge the potential of multiple health behavior change (MHBC) interventions (eg, targeting both healthy eating and physical activity) for optimal cancer control and survivorship. The term multiple health behavior change intervention is used to describe any treatment manipulation that is designed to improve 2 or more behaviors within a given period of time, either simultaneously (changing both behaviors at the same time) or sequentially (changing 1 behavior at a time, one after the other).14,15 Compared with single health behavior change interventions, it is believed that MHBC interventions offer a unique opportunity to improve health and well-being and reduce health care costs by maximizing intervention contacts between delivery providers and cancer survivors.14,16,17
Uncertainty remains about how best to assist cancer survivors in initiating and maintaining MHBC. The optimal design approach (sequential vs simultaneous behavior change) and dose (frequency of contacts and length of the intervention) of MHBC intervention remain unknown, and this constitutes a complex challenge for many oncology health care teams.1,18 By reviewing empirical evidence from several studies conducted among survivors of various types of cancer, MHBC interventions that offer the most compelling evidence (ie, strongest and most precise effects, replication across survivors of different types of cancer) for changing both healthy eating and physical activity behaviors among cancer survivors could be highlighted. Thus, there is a need to review how MHBC interventions are developed and implemented in order to evaluate them for effectiveness in promoting healthy eating and physical activity behaviors among cancer survivors. Identifying the features of successful interventions can help inform critical research gaps and provide evidence for clinical decision making.
Optimal Design Approach: Sequential or Simultaneous MHBC Interventions?
There are theoretical arguments supporting the use of either sequential or simultaneous MHBC intervention design approaches. Based on social cognitive theory,19,20 initial success in changing a given behavior (ie, mastery experience) leads to increased perceived confidence and capability to change that behavior (ie, self-efficacy), which may subsequently motivate individuals to change another behavior. In addition, breaking up the goal of adhering to 1 healthy behavior and sequentially changing healthy eating and physical activity behaviors may reduce the amount of information received and immediate action required for changing overall lifestyle behavior at a given time of an intervention. As a result, individuals may be less likely to feel overwhelmed and more likely to adhere to the overall lifestyle intervention.21
Alternatively, the compensatory carryover action model22 proposes that experience, knowledge, self-efficacy, and self-regulatory skills (eg, behavioral self-monitoring, goal-setting and planning) acquired while changing a given behavior (eg, healthy eating) may be simultaneously carried over to other behaviors (eg, physical activity). This carryover mechanism, also referred to as transfer or spillover effects,23-27 is likely to occur if targeted behaviors are emotionally related to a higher-level goal (eg, to lose 10% of body fat by the next 26 weeks).22 Thus, targeting several behaviors simultaneously may foster carryover behavioral processes and optimize the synergistic effect of an intervention on both behaviors and relevant health outcomes (eg, decreasing percentage body fat).28,29
Dose (Frequency of Contacts and Length) of the MHBC Intervention
Seminal reviews of MHBC interventions for chronic diseases and disease prevention (mainly cardiovascular disease and diabetes) have elicited key intervention characteristics for positive changes in multiple behaviors.1,16-18,30,31 In a review of MHBC for adults with or at risk for cancer,30 interventions that lasted ≥12 months tended to lead to positive outcomes for at least 2 health behaviors (ie, any combination of 2 behaviors, including healthy eating, physical activity, smoking, and alcohol intake) when compare with those lasting ≤12 months. Although it is recognized that specialized care for improving healthy eating and physical activity behaviors is essential,1 evidence suggests that multidisciplinary- or nurse-led programs may produce optimal changes in health behaviors and outcomes.17,18,31 Finally, theory-based MHBC interventions17 and those that are tailored to the individuals’ needs1,18 are likely to be more effective than atheoretical and nontailored MHBC interventions, respectively. However, evidence from these reviews was largely based on narrative interpretations of P values, which can be misleading because the absence of a statistically significant effect might be the result of a lack of precision (ie, low statistical power) rather than the absence of intervention effect. It is also worth noting that none of these reviews1,16-18,30,31 discuss the impact of the sequential versus simultaneous design approach on MHBC.
Objectives of the Current Study
The objective of this review was to examine the effectiveness of MHBC interventions on healthy eating and physical activity behaviors among cancer survivors. A secondary aim was to examine the effect of using a simultaneous and sequential design approach to MHBC (ie, changing both behaviors at the same time or one after the other) on healthy eating and physical activity behaviors. This work expands on previous systematic reviews16,17,30,31 by summarizing findings of primary studies using study-level and pooled effect sizes for both healthy eating and physical activity behaviors and by focusing on key MHBC interventions characteristics that were delivered to cancer survivors. Given the potential of MHBC interventions for enhancing cancer survivors’ health and well-being, the findings from this study identify research priorities and provide preliminary evidence for clinical decision making and advancements in MHBC intervention design and delivery for clinical oncology.
Methods
The conduct and reporting of this systematic review were realized in reference to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA Statement32).
Study Eligibility Criteria
Randomized controlled trials (RCTs) reporting the effect of a MHBC intervention on both healthy eating and physical activity behaviors among cancer survivors were retrieved. Included studies were written in English, reported in scientific, peer-reviewed journals, and published up to November 2, 2015, inclusively. Cancer survivors (an individual from the time of the diagnosis until end of life33) of any age were considered. RCTs comparing effect of a MHBC intervention condition with a non-MHBC intervention condition were considered. However, studies that combined a MHBC intervention or compared the effect of a MHBC intervention with a pharmacological (eg, metformin) or diet supplement (eg, creatine or vitamin supplement) condition were excluded. The primary outcomes of this review were healthy eating and physical activity behaviors, along with the approach of the MHBC intervention (ie, sequential or simultaneous behavior changes).
Information Sources and Search Strategies
One reviewer (SA) performed the identification process. Electronic databases (coverage period) search strategy was developed for MEDLINE (PubMed interface; 1950-2015) and adapted for Cochrane Library (via Cochrane Central Register Controlled Trials; 1992-2015) and PsycINFO (1806-2015). For all databases, the following search terms were used to search in titles and abstracts: ((exercise OR “physical activity”) AND (diet OR nutrition*) OR lifestyle) AND (cancer OR neoplasm OR tumor OR lymphoma) AND (intervention). In addition, database-specific Index or Medical Subject Headings (MeSH) terms were used. Full details of the electronic search are presented in Supplementary File 1, Tables 1 to 3 (available at http://ajlm.sagepub.com/supplemental). A hand search of reference lists for all eligible full-text articles retrieved and reference lists of relevant literature reviews13,29,30,34-38 were also screened for sources and assessed for eligibility.
Study Selection and Data Extraction
One reviewer (SA) screened titles and abstracts, and irrelevant citations were excluded, whereas the full-text published articles of remaining potentially relevant citations were retrieved. Two reviewers (SA and AJF) then independently examined the eligibility of each article. Results were compared between the 2 reviewers, and all discrepancies were resolved by discussion, until consensus was reached. Included studies from the same author (or group of authors) were scrutinized to avoid double author counting. For articles reporting results of similar trials, the list of authors, year of publication, number of study participants, study participant characteristics, and outcomes were juxtaposed and verified.
Data Collection Process
After refining a purpose-built extraction sheet, pilot tested by 2 reviewers (SA and AJF) on 3 included studies, the same 2 reviewers independently extracted data from all included articles. Data were compared between the 2 reviewers, and all discrepancies were resolved by discussion until consensus was reached.
Before extracting data, a number of decisions were made. First, it was expected that some studies would evaluate more than 1 treatment condition (eg, 1 control group and 2 intervention conditions). If 2 or more conditions involved a MHBC intervention, the most comprehensive one was selected for data extraction. Second, consistent reporting of behavior change techniques for healthy eating and physical behaviors across study intervention description was performed using the CALO-RE taxonomy.39 This analysis only serves a descriptive purpose and was limited to techniques explicitly related to healthy eating and physical activity behaviors reported in the main article or in the corresponding published study protocol. Finally, data required for effect size calculation (ie, mean, SD, sample size for each condition, F- or t-test values, P value) were extracted. In accordance with the ACS2 and the WCRF-AIRC3 recommendation for healthy lifestyle among cancer survivors, the effect size was calculated for specific (ie, fat intake, F&V intake, physical activity behavior) and general (total energy intake, energy expenditure, and diet quality) healthy eating and physical activity outcomes.
Risk of Bias in Individual Studies
Two reviewers (SA and AJF) with content and methodology expertise in physical activity, diet, behavior change, and oncology independently extracted information on the randomization procedure (sequence generation and allocation concealment), methods used for blinding, and strategies used for handling incomplete data.40 Given the nature of the intervention, blinding of participants to treatment allocation was likely not feasible. Thus, only the blinding of health care providers and outcome assessors to participant group allocation was assessed. Moreover, risk of selective outcome reporting was scrutinized by comparing behavioral outcomes listed in the methods with those reported in the results section. Finally, information on disclosure of any conflicts of interests and financial contribution from industry was retrieved. For each item, 3 qualitative ratings for evaluating the risk of bias were used: low (information reported and methodologically appropriate), high (information reported and methodologically inappropriate), and unclear (information partially or not reported).40
Data Analysis
Study descriptive statistics, such as sample mean age, gender, body mass index (BMI), and type of cancer, were collected and summarized in SAS version 9.3 (SAS Institute, Cary, NC). Given the heterogeneity in the design approach (simultaneous or sequential), content (ie, behavior change techniques and materials), and delivery mode (ie, in-person, phone, computer/Web-assisted) of the MHBC interventions, no meta-analysis combining effects of all MHBC interventions was performed. Rather, a 4-step process was used to summarize the evidence.41 First, a matrix displaying the data reflecting the design approach (sequential or simultaneous), nature (theory-based and tailored MHBC intervention or not), dose (duration of the intervention, number of contacts, intensity of the intervention), and delivery methods (how: in-person, phone, mail, or computer/Web-assisted; by whom: using a multidisciplinary teams/nurse-led intervention or not) of the MHBC interventions was created. Second, visual inspection of the matrix and statistical analyses were conducted to identify subgroups of studies evaluating similar MHBC interventions. Third, the primary citations were reexamined to confirm the similarity of the MHBC intervention with respect to behavior change techniques. Fourth, meta-analyses were performed for subgroups of studies evaluating similar MHBC interventions.
Individual and pooled effect sizes (ie, standardized mean difference [SMD]) and 95% CIs for healthy eating and physical activity outcomes were calculated using Cochrane’s Review Manager 5.3 software.42 Meta-analyses were performed using the inverse-variance method under the random-effects model assumption. Separate meta-analyses were conducted for each behavioral outcome assessed 3 times or more. Effect sizes were qualitatively appraised according to Cohen’s criteria43: SMD ≤ 0.20, trivial; SMD = 0.20, small; SMD = 0.50, medium; SMD = 0.80, large. A positive SMD reflects a larger between-group difference in favor of the MHBC intervention group (ie, increase in F&V intake, diet quality, or physical activity for cancer survivors in the MHBC intervention group). A negative SMD reflected a larger between-group difference in favor of the control group (ie, a decrease in fat or energy intake for cancer survivors in the MHBC intervention group). For some studies,44-54 raw data were transformed or further calculations were made to compute study-level effect size. A complete description of the formula used for all effect size computations is presented in Supplementary File 2.
Variation in the magnitude and direction of effect sizes was assessed using both qualitative and quantitative criteria55: (1) describing the variation in study-level effect sizes; (2) verifying the substantial, minimal, or no overlap in 95% CIs; (3) performing the Cochran Q χ2 test, which tests the hypothesis that all studies share a common effect size (P < .10); and (4) reporting the percentage of total variation in estimated effects that is caused by among-study variation rather than chance (I2). An I2 value of 25% is considered to reflect low heterogeneity, 50% moderate heterogeneity, and 75% high heterogeneity.56
Publication Bias
Assessment of publication bias relied on 2 main assumptions. First, only the largest treatment effects are likely to be statistically significant and published by small-sample-size studies.57,58 Second, there is a stronger likelihood for publication bias in reviews that include small-sample-size studies if those studies are industry sponsored or if investigators report conflicts of interests.59 Therefore, overall likelihood of publication bias was qualitatively appraised by calculating the proportion of small-sample-size studies (n < 100). Next, studies that were at risk of bias for conflict of interest were identified. Finally, a funnel plot was inspected for meta-analyses involving ≥10 study-level effect sizes.60 The presence of publication bias was suspected if the visual inspection demonstrated an asymmetrical rather than a symmetrical funnel plot.
Results
Literature Search
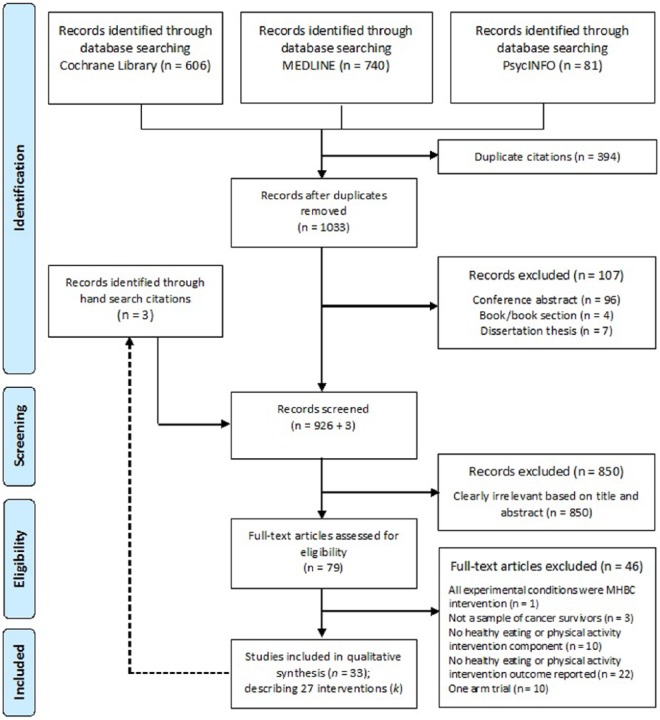
The detailed process used to select studies is summarized in a PRISMA flow diagram depicted in Figure 1. A total of 33 articles describing 27 MHBC interventions (k) promoting healthy eating and physical activity behaviors among cancer survivors were included in this review. Full details regarding the study-level characteristics of the participants, MHBC interventions, control group, and outcome measures are presented in Supplementary File 3; Tables 1 and 2.
Figure 1.
Flow Diagram.a
aThe figure depicts the numbers of studies screened, assessed for eligibility, and included in the systematic review.
Characteristics of the Participants
The number of randomized participants ranged from 14 to 641 (total = 4241; median per study = 83). The mean age of the sample in the studies varied between 7 and 73 years (median = 57 years), with one study that targeted youth cancer survivors.61 Among samples of adult cancer survivors, mean BMI varied between 22.5 and 42.3 kg/m2 (median = 28.9 kg/m2). Participants were mostly female, Caucasian, and breast cancer survivors.44-46,48,62-65 Only 1 study was conducted among a sample of Black and Hispanic breast cancer survivors.66 Other cancer survivors included survivors of colorectal,54,67 endometrial,68,69 haematological,50,61 and prostate47,49,70,71 cancers.
Overall Characteristics of MHBC Interventions
Social cognitive theory was the theory that was used most often to inform the MHBC intervention (k = 12; 44.4%). It was used alone45,62,64,65,68,69 or in combination with the transtheoretical model,53,72-74 the interdependence theory and theory of communal coping,63 or the chronic disease self-management model.51 In all, 11 studies (44%) did not report using any theory or conceptual framework. The length of MHBC intervention varied between 6 and 104 weeks (median = 26 weeks). The number of possible contacts between delivery providers and participants varied between 3 and 88 (median = 19). Interventions were delivered in person (k = 6; 22.2%) or over the phone (k = 2; 7.4%), postal mail (k = 2; 7.4%), or internet (k = 2; 7.4%). Other studies used a mixed mode of delivery (eg, using both phone and in-person delivery [k = 9; 33.3%]; both postal mail and phone delivery [k = 6, 22.2%]). A total of 15 studies (55.6%) used 1 provider: either an exercise physiologist, fitness center staff member, nurse, psychologist, lifestyle coach, or dietician, whereas 7 (25.9%) used a multidisciplinary delivery approach.
Characteristics of the Control Group
Included RCTs reported using a standard care (33.3%), attention/minimal (37.0%), wait-list (delayed treatment; 22.2%), or no-treatment control group (3.7%). Participants in the standard care group were offered standard clinical supervision, received healthy eating and physical activity recommendations from their physician, and/or received counseling sessions regarding overall health concerns. Self-help healthy eating, active lifestyle, and weight management information were distributed to participants of the attention control group by means of brochures and pamphlets freely available from national cancer organizations (eg, National Cancer Institutes, the American Cancer Society, or the Canadian Cancer Society). One study used a control group that included a single-behavior change (ie, physical activity) intervention.63 The nature of the control group was unknown for 1 study (3.7%).75
Behavioral Outcome Measures
Fat, F&V, and total energy intake outcomes were assessed in 20, 17, and 14 RCTs, respectively. In addition, 7 studies assessed overall diet quality.45,52,53,62,73-75 The Food Frequency Questionnaire or dietary recall was used to assess healthy eating outcomes. Other instruments included F&V and fat screener and the Diet Quality Inventory. Physical activity outcomes were assessed in all studies. Leisure-time physical activity, exercise, and sports were the most frequently assessed types of physical activity. Seven studies used a device-based physical activity measure (ie, accelerometer,62,63 pedometer,51,61,69,71 and electronic fitness center attendance records66), and the remaining studies used an interviewer- or a self-report questionnaire.
Risk of Bias in Individual Studies
A summary of overall risk of bias is depicted in Figure 2. Full details regarding risk of bias for each of the included studies are presented in Table 1 and in Supplementary File 4 (Tables 1 to 4). Overall, a majority of studies (≥77%) were at high risk of bias for blinding outcome assessor and incomplete outcome data. Blinding of data collectors and interviewers was not possible in most studies because they all used self-reported questionnaires to assess one or both behavioral outcomes. Therefore, unless otherwise explicitly stated in the article, studies were judged as having a high risk of bias for blinding of outcome assessors. Four studies reported a significantly (P < .05) higher attrition rate in the MHBC intervention than in the control arm,53,62,73,75 whereas 1 study reported a higher attrition rate in the control arm.70 The data analysts were blinded to treatment allocation in 1 study.70 Finally, 4 studies46,47,67,68 discussed the presence of contamination bias, which occurs when control participants also receive, either in part or in full, intervention components that experimental participants receive.76
Figure 2.
Risk of Bias in Individual Studies (k = 27).a
aThe figure depicts the risk of bias (ie, sequence generation and allocation concealment, methods used for blinding health care providers and outcome assessors to participant’s group allocation, strategies used for handling incomplete data, and selective outcome reporting) for each study included in the systematic review.
Table 1.
Risk of Bias in Individual Studies.
| Main Study | Randomization (Selection Bias) | Blinding of Outcome Assessors (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | |
|---|---|---|---|---|
| Sequence Generationa | Allocation Concealmentb | |||
| Andersen et al44 | Unclearc | High | High | Highd |
| Anderson et al45 | Low | Low | High | High |
| Bloom et al46 | High | High | High | High |
| Bourke et al70 | Low | Low | Highe | Low |
| Bourke et al67 | Low | Low | Low | Highd |
| Bourke et al47 | Low | Low | Low | Low |
| Campbell et al72 | Unclearc | Low | Low | High |
| Demark-Wahnefried et al73 | Unclearc | Low | High | High |
| Demark-Wahnefried et al74 | Low | Low | High | Highd |
| Demark-Wahnefried et al63 | Unclearc | High | High | High |
| Demark-Wahnefried et al62 | Unclearc | Low | High | High |
| Djuric et al65 | High | High | High | High |
| Djuric et al64 | Unclearc | Low | High | High |
| Goodwin et al48 | Low | High | High | Highd |
| Greenlee et al66 | Unclearc | High | High | High |
| Hawkes et al54 | Low | Low | Low | High |
| Hébert et al49 | Unclearc | High | High | High |
| Hung et al50 | Low | High | High | High |
| Kim et al75 | Low | High | High | Low |
| James et al51 | Low | Low | High | High |
| Lee et al52 | Low | Low | High | High |
| Morey et al53 | Low | Low | Low | High |
| Moyer-Mileur et al61 | High | High | High | High |
| O’Carrol Bantum et al77 | Low | High | High | High |
| Ornish et al71 | High | Lowf | Low | High |
| von Gruenigen et al68 | Unclearc | High | High | Highd |
| von Gruenigen et al69 | Unclearc | High | High | Low |
Reporting both method (eg, using a computer algorithm, tossing a coin, or a table of random numbers) and type (eg, simple, stratified block, or permuted block) of randomization.
Used remote randomization or sealed envelopes.
Randomization method not explicitly stated.
Inappropriate handling of missing data (eg, last observation carried forward, worst case scenario, complete cases analysis).
Data analyst was blinded to group assignment; method for blinding is unknown.
Allocation concealment was inferred as “low” risk of bias since a randomized consent design was used.
Results of Individual Studies
Design Approach to MHBC Intervention
The simultaneous MHBC intervention approach was used in 25 of the 27 MHBC intervention trials (92.6%). Among MHBC interventions that opted for a simultaneous approach, 23 used the full simultaneous approach (ie, healthy eating and physical activity behaviors were targeted at the same time for all the duration of MHBC intervention), whereas 4 interventions47,66,67,70 used a tapered simultaneous approach (ie, physical activity was targeted first and healthy eating behavior was targeted sometime after, but both behaviors were eventually targeted together). Therefore, the findings of simultaneous and sequential MHBC interventions were summarized together.
Visual Inspection of the Matrix of Imputed Intervention Characteristics
The visual inspection of the matrix of intervention characteristics (Supplementary File 4, Table 1) and χ2 tests suggest that MHBC interventions delivered by an exercise specialist or dietician were less likely to be tailored and theory based [χ2(df = 2) = 7.18; P = .03] and more likely to be delivered in person [χ2(df = 2) = 5.87; P = .02] than multidisciplinary- or nurse-led MHBC interventions. The number of contacts between the delivery provider(s) and cancer survivors as well as the intensity of the MHBC intervention were higher for such MHBC interventions relative to MHBC interventions led by a nurse or delivered by a multidisciplinary team of health care providers. However, these differences were not statistically significant (P = .09 and .12 for the number of contacts and intensity, respectively). Based on the visual inspection of the matrix of intervention characteristics, 3 different subgroups of similar MHBC interventions were identified: (1) exercise specialist–led intensive MHBC interventions; (2) dietician-led intensive MHBC interventions; and (3) nurse-led or multidisciplinary teams–led MHBC interventions, which also included computed/web-assisted tailored MHBC interventions. The similarity in the use of behavior change techniques for each subgroup of MHBC interventions is presented in Table 2.
Table 2.
Between-Group Postintervention MHBC Effects on Healthy Eating and Physical Activity Behaviors.a
| Categories of MHBC Interventions and Description | Behavior | k | Q | I 2 | SMD | 95% CI |
|---|---|---|---|---|---|---|
| 1. Exercise specialist–led MHBC interventions (k = 4; n = 210) All MHBC interventions opted for a tapered simultaneous design approach, where the physical activity component of the intervention was more intensive than the healthy eating component The physical activity component included providing instructions on how to perform physical activity, prompting practice (ie, prompting the participant to exercise 2-3 times/wk at moderate to vigorous intensity), as well as prompting self-monitoring of physical activity. In contrast, the healthy eating component included instructions on how to eat a low-fat/low-calorie diet. All MHBC interventions last ≤13 weeks and were delivered in person |
Fat intake | 4 | 7.88* | 62% | −0.51* | [−1.00, −0.02] |
| Physical activity | 4 | 4.61 | 35% | 1.11*** | [0.72, 1.51] | |
| 2. Dietitian-led MHBC interventions (k = 4; n = 195) All MHBC interventions opted for a simultaneous approach and involved either telephone or in-person nutrition education or counseling with a dietitian. They include the provision of instructions on how to eat a well-balanced diet (increasing F&V and decreasing fat intake) and the review of behavioral goals for both healthy eating and physical activity behaviors. The details of the nutrition education and counseling strategies were not clearly specified in 1 study71 All MHBC interventions lasted ≤13 weeks. The primary objective of all these MHBC interventions was to improve health (ie, to slow the progression of prostate cancer,71 or to control or reduce body weight61,64,65) |
Fat Intake | 3 | 8.71* | 77% | −0.95* | [−1.86, −0.05] |
| Physical Activity | 2 | 0.00 | 0% | 0.52** | [0.14, 0.89] | |
| 3. Nurse-led or multidisciplinary team–led MHBC interventionb,c
Nontailored: theory- (k = 4) and nontheory (k = 3)-based (n = 1306) All opted for a simultaneous design approach to the MHBC intervention Most MHBC interventions included the following: self-monitoring, goal setting, providing feedback on behavioral performance, and providing information on the consequence of behaviors as behavior change techniques for both healthy eating and physical activity behaviors. Tailored: theory-based MHBC interventions (k = 10; n = 2003) Most MHBC interventions opted for a simultaneous design approach to the intervention; 2 MHBC interventions opted for a sequential design approach73,74 Most MHBC interventions included self-monitoring, goal setting, providing feedback on behavioral performance, and providing information on the consequence of behaviors as behavior change techniques for both healthy eating and physical activity behaviors Tailoring refers to the creation of individualized intervention materials or strategies based on cancer survivor’s behavior change needs (eg, levels of self-efficacy and stage of change) |
Fat intake/length of the intervention | 10 | 22.44* | 60% | −0.30*** | [−0.45, −0.16] |
| ≤13 weeks | 3 | 1.23 | 0% | −0.06 | [−0.29, 0.17] | |
| 17-26 weeks | 5 | 15.10* | 74% | −0.42* | [−0.72, −0.12] | |
| ≥43 weeks | 2 | 0.50 | 0% | −0.37*** | [−0.48, −0.26] | |
| F&V intake/length of the intervention | 11 | 14.37 | 30% | 0.29*** | [0.19, 0.40] | |
| ≤13 weeks | 3 | 3.52 | 43% | 0.17 | [−0.16, 0.51] | |
| 17-26 weeks | 5 | 2.15 | 0% | 0.26*** | [0.16, 0.37] | |
| ≥43 weeks | 3 | 5.51* | 64% | 0.36** | [0.11, 0.60] | |
| Physical activity/length of the intervention | 16 | 16.85 | 11% | 0.24*** | [0.15, 0.32] | |
| ≤13 weeks | 5 | 6.30 | 37% | 0.42** | [0.10, 0.73] | |
| 17-26 weeks | 7 | 5.65 | 0% | 0.20** | [0.07, 0.32] | |
| ≥43 weeks | 4 | 1.80 | 0% | 0.23*** | [0.13, 0.34] |
Abbreviations: F&V, fruit and vegetable; MHBC, multiple health behavior change; SMD, standardized mean difference.
*P < .10; **P < .01; ***P < .001.
The intervention provider was only identified as an “instructor” in 1 study.49 These 4 MHBC interventions were included in the nurse-led or multidisciplinary team–led MHBC interventions subgroup because their behavior change techniques and intervention characteristics were similar to other MHBC interventions.
Postintervention Effects on Behaviors
Between-group (control vs MHBC intervention) postintervention pooled effect sizes are presented in Table 2. Exercise specialist–led intense MHBC interventions47,66,67,70 resulted in large improvements in physical activity (pooled SMD = 1.11; P < .0001) and in small to moderate reductions in total energy intake (pooled SMD = −0.41; P = .001; data not shown in Table 2). Study-level SMD for fat intake ranging from −0.43 to −1.00 was reported in 3 studies,47,67,70 whereas 1 trivial increase in fat intake (SMD = 0.16) was reported.66 Dietician-led intense MHBC interventions61,64,65,71 resulted in a wide range of effects for fat intake. Excluding 1 MHBC intervention that targeted children and reported a nonsignificant change in F&V intake, fat intake, and energy expenditure,61 the statistically detected variation in reduction in fat intake were within the range of small to moderate (SMD = −0.34), moderate to large (SMD = −0.73), and large (SMD = −2.29) effects. With respect to physical activity, 1 study conducted among young cancer survivors reported a significant increase in number of steps,61 and 1 reported a nonsignificant change in physical activity64; in both cases, data necessary for effect size calculation were not reported. The 2 other studies reported a moderate increase in physical activity.65,71
Nurse-led or multidisciplinary teams–led MHBC interventions reported a small to moderate increase in F&V intake (pooled SMD = 0.29; P < .0001), fat intake (pooled SMD = −0.30; P < .0001), and physical activity (pooled SMD = 0.25; P < .0001). The statistically detected variation in the fat intake effect size distribution was within the range of small (SMD = −0.17), medium (SMD = −0.52), and large (SMD = −1.35) effects, with the exception of 2 reported trivial effects (SMD = −0.07 and 0.09).45,74 Moreover, there was considerable overlap in 95% CIs, with the exception of the 95% CI for the reported large effect (SMD = −1.35).63 Given that almost all study-level effect sizes for fat intake were within the range of small and large effects, the variability (ie, heterogeneity) was deemed of questionable clinical importance. It is also worth noting that the findings for F&V and fat intake behaviors were reflected by an increase in diet quality (pooled SMD = 0.37; P < .0001); one potential outlier study effect size (SMD = −0.92)75 was removed from the pooled effect size calculation). However, no change in total energy intake was observed (pooled SMD = −0.11; P = .27; data not shown in Table 2). Post hoc subgroup analyses were also performed according to the length of the intervention for the nurse-led or multidisciplinary teams–led MHBC interventions. Results presented in Table 2 suggested that the length of the MHBC intervention affected the effectiveness of healthy eating and physical activity behaviors. Specifically, the effects of MHBC interventions on F&V and fat intake were trivial (SMD = 0.17 and −0.06, respectively) for the intervention that lasted ≤13 weeks (3 months), whereas it was small to moderate for interventions that lasted ≥17 weeks (4 months). In contrast, the effect of MHBC interventions on physical activity was small to moderate for interventions that lasted ≤13 weeks (SMD = 0.42), whereas it was small (albeit significant) for interventions that lasted ≥17 weeks.
Long-Term/Maintenance Effects on Behaviors
Long-term/maintenance end points were defined as a period of no contact with participants from the end of the intervention to the time where behavioral outcomes were assessed. In all, 9 studies reported MHBC treatment effect at follow-up (median = 26 months following the end of the intervention).44,46,51,54,68,69,73,74,77 Based on the findings, a trivial/small long-term improvement or maintenance of a change in F&V intake (pooled SMD = 0.13; P = .05; k = 7) and fat intake (pooled SMD = −0.17; P = .009; k = 5) for nurse-led or multidisciplinary teams–led MHBC interventions was observed. No long-term/maintenance effect was detected for physical activity (pooled SMD = 0.13; P = .06; k = 8).
Publication Bias
Overall potential for publication bias was qualitatively appraised for total energy intake, F&V intake, fat intake, and physical activity. A majority of the reviewed studies (k = 16; 59.3%) were conducted among small samples of cancer survivors (n < 100). Of these, 2 studies focused on fat intake and physical activity were at high risk of bias for conflict of interests.64,71 In addition, potential for publication bias for postintervention pooled effect for F&V intake, fat intake, and physical activity was assessed using the funnel plot. Asymmetry in the funnel plot for fat intake and physical activity was detected (Supplementary File 5, Figures 1 to 3), and this suggests the presence of publication bias.
Smaller studies may have suffered from greater study limitations; consequently, they may be more likely to report larger treatment effects.59 As depicted in Supplementary File 6, Tables 1 to 4, a more heterogeneous effect size distribution (particularly for F&V and fat intake) and extreme effect size values (for all behavioral outcomes) were observed in the subgroup of studies having a high or unclear risk of bias. For instance, one small study (n = 40)65 also reported the largest effect size for F&V (1.66 [0.81, 2.52]); however, participants who dropped out had lower baseline levels of F&V intake (0.93 serving/d) than participants who completed the trial (2.9 servings/d). We also acknowledge that smaller studies may have generated larger treatment effects because a more restrictive and responsive sample population was studied.59 For instance, 3 MHBC trials (n ≤ 75)64,68,69 were conducted among samples of obese women (mean BMI ≥ 35.5 kg/m2). Being overweight or obese was found to be a predictor of improved fat intake and physical activity following a computer-tailored MHBC intervention among apparently healthy adults.78 The largest effect size for fat (−2.29 [−3.52, −1.07]) and total energy intake (−1.58 [−2.65, −0.51]) was reported by 1 of the 3 studies conducted among obese cancer survivors64; however, this study was also at high risk of bias for all assessed methodological criteria.
In summary, the presence of publication bias was suspected, especially for physical activity and fat intake outcomes. However, the possibility that this apparent manifestation of bias was a reflection of an artifact of bias resulting from study limitations or specific characteristics of the population (ie, obese women) of the reviewed trials cannot be ruled out.
Discussion
The primary objective of this review was to examine the effectiveness of MHBC interventions on healthy eating and physical activity behaviors among cancer survivors. A secondary aim was to examine the effect of using a simultaneous and sequential design approach to MHBC (ie, changing both behaviors at the same time or one after the other) on healthy eating and physical activity behaviors. A systematic search of the literature identified 27 RCTs of MHBC interventions. Healthy eating and physical activity behaviors were the primary outcomes of this review, as both are essential lifestyle behaviors for improving cancer survivor’s health and well-being.2,3 MHBC interventions have been delivered predominantly simultaneously and target F&V and fat intake, and leisure-time physical activity (including sports and exercise) as primary healthy behavior markers.
Irrespective of MHBC intervention design approaches and intervention end point, a wide range (from trivial to large and negative to positive) of treatment effect sizes were detected for both healthy eating and physical activity behaviors. Qualitative and quantitative syntheses suggested that there were 3 main categories of MHBC interventions: exercise specialist–led intensive MHBC interventions, dietician-led intensive MHBC interventions, and nurse-led or multidisciplinary teams–led (as well as computer-/web-assisted) MHBC interventions. MHBC interventions in the latter category were more likely to be tailored to the needs of cancer survivors and theory based than the exercise specialist– or dietician-led MHBC intensive interventions.
Specifically, exercise specialist– and dietician-led intensive MHBC interventions resulted in a large postintervention increase in physical activity and a large postintervention decrease in fat intake, respectively. Smaller treatment effects were reported for the behavior that does not fall in the primary area of expertise of the delivery provider. This observation for the exercise specialist–led intensive MHBC interventions is not surprising because the frequency of contacts and duration of exposition to a given set of behavior change techniques were higher for the physical activity than the healthy eating component of the intervention. Both the exercise specialist–led and dietician-led MHBC interventions were more intense (greater frequency of contact between participants/wk) and were delivered in person. This can be considered as additional evidence supporting increases in contact frequency and intensity for greater improvements in healthy eating or physical activity behaviors.17,30 However, this finding may only be generalized to a subset of cancer survivors because intensive MHBC intervention trials may only enroll participants who are more motivated to make behavior changes and comply with intense study procedures.17
Nurse-led or multidisciplinary teams–led MHBC interventions (as well as computer/web-assisted interventions) generate postintervention improvement in both healthy eating and physical activity behaviors, reflected in a small to moderate increase in F&V intake, fat intake, and physical activity.43 The reported effect size for physical activity is similar, albeit lower, than those reported in previous reviews of studies conducted among cancer survivors (SMD = 0.33 and 0.38).38,79 Most of the reviewed studies incorporated behavior change techniques such as self-monitoring (behavior), goal setting (behavior), and feedback on behavioral performance, which are theoretically informed and supported by evidence from the physical activity and healthy eating behavior change domain.17,80,81
Post hoc subgroup analysis suggests that shorter nurse-led or multidisciplinary teams–led MHBC (as well as computer/web-assisted) intervention duration (≤13 weeks; 3 months) was associated with stronger posttreatment effects for physical activity compared with a longer intervention duration (>17 weeks; 4 months). However, because only nonsignificant trivial/small between-group changes in F&V and fat intake were reported for shorter MHBC interventions (see Table 2), longer MHBC interventions (>17 weeks; 4 months) might be more likely to generate improvement in both healthy eating and physical activity behaviors. This is consistent with the observation made by Green et al,30 where longer intervention duration was associated with improved healthy eating and physical activity behaviors among individuals at risk of cancer. Because this interpretation is based on the findings of a post hoc subgroup analysis, it should best be viewed as a new research hypothesis that needs to be verified in future studies.
Few MHBC trials reported healthy eating and physical activity behaviors long term or maintenance effect (follow-up), which limits the generalization of current systematic review findings to end-of-treatment effects (postintervention). This observation is similar to that reported in other oncology healthy eating and physical activity behavior change intervention systematic reviews.38,82-85 Although wait-list delayed-treatment crossover design does not allow for between-group comparison at follow-up, trials using such a design may nonetheless provide within-group evidence for the maintenance effect. For instance, improvement in healthy eating and physical activity behaviors measured at postintervention were maintained at the 12-month follow-up for cancer survivors participating in simultaneous, tailored-print, and telephone-delivered MHBC interventions.86 In summary, there is limited and inconclusive evidence supporting long-term and maintenance effects of MHBC interventions on both healthy eating and physical activity behaviors among cancer survivors.
Study-Level Limitations
Most MHBC trials (84.6%) were at risk for incomplete outcome data. Moreover, 4 studies53,62,73,75 reported a higher drop-out rate in the MHBC intervention than in the control condition. A similar finding was reported in a systematic review of exercise oncology trials; drop-out rates were, on average, greater among cancer survivors allocated to the exercise (intervention) compared with the control condition.76 With respect to MHBC intervention, Schulz et al21 reported high drop-out rates for apparently healthy adults allocated to either simultaneous or sequential MHBC interventions (71%). The main reasons for intervention noncompletion were the amount of time required to complete the MHBC intervention and information overload, which reflects the increased burden resulting from trying to change several behaviors at once.87 This suggests that incomplete outcome data may not only hamper the internal validity (in an unpredictable direction) of the findings reported in the reviewed studies,88 but it may also reflect cancer survivors (lower) acceptability of MHBC interventions.
Given the nature of behavior change interventions, blinding of the participants to treatment allocation is almost impossible; and this may have led to an increased likelihood of contamination bias.76 As such, 4 studies discussed the possibilities of how participants randomized to the control group might also increase their physical activity.46,47,67,68 The risk of contamination bias across all reviewed studies was not addressed, and therefore, it cannot be determined if this post hoc observation is restricted to these 4 MHBC trials. Nonetheless, contamination bias may have resulted in an underestimation of MHBC intervention effect sizes.
Although we reviewed studies that evaluated the effectiveness of MHBC interventions to improve both healthy eating and physical activity behaviors, none of the trials used multivariate analysis (eg, MANOVA) that can handle multiple behavioral outcomes within a single analysis. Compared with univariate analysis, multivariate analysis would provide protection against inflated type 1 error rate (false-positive results) resulting from multiple test of potentially correlated behavioral outcomes. In addition, multivariate analyses combining healthy eating and physical activity behavior outcomes may occasionally capture differences that would not otherwise be detected when performing several statistical tests considering each behavior separately.89
Review-Level Limitations
There are some limitations to this current review that should be acknowledged. First, only articles written in English and published in peer-reviewed scientific journals were included in the current review, which may have been reflected in the risk of publication bias assessment. Second, publication bias might account for some of the treatment effect sizes observed in retrieved studies. Smaller MHBC trials tended to have larger treatment effects, especially for physical activity and fat intake outcomes. However, smaller studies tend to be performed with less methodological rigor and among specific cancer survivor sub-populations (obese women), which may also partially explain the apparent association between treatment effect and sample size. Therefore, this suggests additional caution in interpreting the findings of low-sample-size MHBC trials. Third, although published protocols were retrieved,90-96 no attempt was made to contact trial authors to obtain unreported methodological information and data-related results. As a result, we were unable to calculate behavioral effect sizes for 5 MHBC trials.44,48,61,64,72
Implications for Research
Additional research evaluating the effect of sequential MHBC interventions among cancer survivors is needed. These studies should also focus on the long-term or maintenance effect of MHBC interventions. This would provide more robust evidence-based support to oncology health care teams for the development and implementation of MHBC interventions.
Future studies should be designed to evaluate the efficacy of MHBC interventions using an appropriate control group. MHBC interventions should be tested against a single health behavior change intervention (ie, either a healthy eating or physical activity only condition). This type of evidence is timely because findings from systematic reviews within and outside the oncology context suggest that physical activity–only interventions have a larger impact than MHBC interventions (mainly healthy eating and physical activity) on physical activity behavior.28,38,97 The current review identified 1 study that provided a direct comparison between a physical activity–only arm (with calcium intake) and a full simultaneous MHBC arm (physical activity, and F&V and fat intake, with calcium intake).63 Similarly, future studies should be conducted to directly compare MHBC intervention approaches (simultaneous and sequential) with each other.
Additionally, a more thorough description of tested MHBC interventions, specifically in terms of behavior change techniques and intervention dose (frequency of contacts and duration of exposition to a given set of behavior change techniques) for each targeted healthy behavior and for both the MHBC and control groups, is warranted. These steps are essential to accurately evaluate the specific contribution of a set of behavior change techniques, intervention dosage, and approach (sequential or simultaneous) of MHBC interventions. The use of the CALO-RE taxonomy39 could be useful to improve standard reporting of behavior change technique in MHBC intervention oncology trials.
Furthermore, special attention should be devoted to minimizing the impact of selection bias on internal and external validity of study findings. MHBC intervention trials were conducted mostly among small samples of breast cancer survivors who are Caucasian, English speaking, and highly educated. As proposed by Phillips et al,98 data on nonparticipants should be collected and mixed methods should be used in future studies to identify factors influencing cancer survivors’ enrollment and retention in MHBC trials. A careful selection of control group could also contribute to reduced rate of study noncompletion. The drop-out rate (as well as contamination) was found to be minimal in oncology exercise trials when participants of the control group were offered an intervention during and/or after study completion.76 Although the most optimal types of control groups have not been identified,76 the use of an alternative treatment (eg, single behavior change intervention) or a wait-list delayed-treatment control group should be considered to minimize drop-out rate.
As a final suggestion for improving the evidence on MHBC interventions in oncology care, information on personal (eg, health care provider preferences) and contextual (eg, resources available, intervention cost) factors should be collected and reported in future MHBC intervention trials. For instance, the cost of 2 computer-tailored MHBC interventions was estimated at US$800 to US$1000 per participant,53,92 whereas the cost of a telephone-delivered MHBC intervention was estimated at US$300 per participant.99 If efficacy of the MHBC intervention is proven, efficiency argument could facilitate dialogue between investigators, health care providers, and stakeholders and accelerate its dissemination and implementation in the real-world setting.98
Conclusion
A wide range of treatment effects were detected for both healthy eating and physical activity behaviors. Most MHBC interventions targeted healthy eating and physical activity behaviors simultaneously, which precludes the identification of the most effective design approach to MHBC. Nonetheless, nurse-led or multidisciplinary teams–led (as well as computer/web-assisted) MHBC interventions showed the most compelling evidence for improving both healthy eating and physical activity behaviors among cancer survivors. Furthermore, post hoc subgroup analyses suggest that nurse-led or multidisciplinary teams–led MHBC interventions that last ≥17 weeks (≥4 months) are more likely to improve both healthy eating and physical activity behaviors at postintervention than those that last ≤13 weeks (≤3 months). However, this finding should best be viewed as a new hypothesis that needs to be verified in future studies. Few MHBC trials reported healthy eating and physical activity behaviors long term or maintenance effect, which limits the generalization of current systematic review findings to end-of-treatment effects. The risk of selection bias in the reviewed primary study was high, and the presence of publication bias is suspected. Therefore, the interpretation of the review findings should be made in light of these limitations.
The identification of intervention characteristics associated with effective MHBC intervention is essential for maximizing success of MHBC interventions at changing multiple behaviors, and promoting health and well-being in cancer survivors. Thus, this study makes a significant contribution to the oncology literature by identifying research priorities and providing preliminary evidence for clinical decision making and advancements in MHBC intervention design and delivery for clinical oncology.
Supplementary Material
Acknowledgments
This research was conducted while SA was supported by a postdoctoral research training award from the Fonds de recherche du Québec—Santé (FRQS). CMS is supported by the Canada Research Chairs program. The findings of this research were presented at the Canadian Society for Psychomotor Learning and Sport Psychology annual scientific conference (October 16, 2015, Edmonton, AB, Canada). The views expressed in this systematic review are those of the authors and not an official position of the institutions to which the authors are affiliated. SA, AJF, and CMS declare that they have no competing interests. No funding agency was involved in the planning, conduct, and report of this systematic review.
Footnotes
Authors’ Note: This is a systematic review for which ethical approval is not required.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
References
- 1. Demark-Wahnefried W, Rogers LQ, Alfano CM, et al. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J Clin. 2015;65:167-189. [DOI] [PubMed] [Google Scholar]
- 2. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243-274. [DOI] [PubMed] [Google Scholar]
- 3. World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 4. Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198-2204. [DOI] [PubMed] [Google Scholar]
- 5. Eakin EG, Youlden DR, Baade PD, et al. Health behaviors of cancer survivors: data from an Australian population-based survey. Cancer Causes Control. 2007;18:881-894. [DOI] [PubMed] [Google Scholar]
- 6. LeMasters TJ, Madhavan SS, Sambamoorthi U, Kurian S. Health behaviors among breast, prostate, and colorectal cancer survivors: a US population-based case-control study, with comparisons by cancer type and gender. J Cancer Surviv. 2014;8:336-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884-8893. [DOI] [PubMed] [Google Scholar]
- 8. Blanchard CM, Stein KD, Baker F, et al. Association between current lifestyle behaviors and health-related quality of life in breast, colorectal, and prostate cancer survivors. Psychol Health. 2004;19:1-13. [Google Scholar]
- 9. Inoue-Choi M, Lazovich D, Prizment AE, Robien K. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations for cancer prevention is associated with better health-related quality of life among elderly female cancer survivors. J Clin Oncol. 2013;31:1758-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inoue-Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:792-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. George S, Irwin M, Smith A, et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control. 2011;22:589-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demark-Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematol Oncol Clin North Am. 2008;22:319-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prochaska JJ, Prochaska JM. Multiple risk behavior change: what most individuals need. In: Shumaker SA, Ockene JK, Riekert KA. eds. The Handbook of Health Behavior Change. New York, NY: Springer; 2009:287-305. [Google Scholar]
- 15. Prochaska JJ, Spring B, Nigg CR. Multiple health behavior change research: an introduction and overview. Prev Med. 2008;46:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prochaska JJ, Prochaska JO. A review of multiple health behavior change interventions for primary prevention. Am J Lifestyle Med. 2011;5:208-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldstein MG, Whitlock EP, DePue J. Multiple behavioral risk factor interventions in primary care. Am J Prev Med. 2004;27(2, suppl):61-79. [DOI] [PubMed] [Google Scholar]
- 18. Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology Position Statement on Obesity and Cancer. J Clin Oncol. 2014;32:3568-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191-215. [DOI] [PubMed] [Google Scholar]
- 20. Bandura A. Self-efficacy: The Exercise of Control. New York, NY: W H Freeman; 1997. [Google Scholar]
- 21. Schulz DN, Schneider F, de Vries H, van Osch LA, van Nierop PW, Kremers SP. Program completion of a web-based tailored lifestyle intervention for adults: differences between a sequential and a simultaneous approach. J Med Internet Res. 2012;14:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lippke S. Modelling and supporting complex behavior change related to obesity and diabetes prevention and management with the compensatory carry-over action model. J Diabetes Obes. 2014;1:1-5.25599089 [Google Scholar]
- 23. Fleig L, Kerschreiter R, Schwarzer R, Pomp S, Lippke S. “Sticking to a healthy diet is easier for me when I exercise regularly”: cognitive transfer between physical exercise and healthy nutrition. Psychol Health. 2014;29:1361-1372. [DOI] [PubMed] [Google Scholar]
- 24. Lippke S, Nigg CR, Maddock JE. Health-promoting and health-risk behaviors: theory-driven analyses of multiple health behavior change in three international samples. Int J Behav Med. 2012;19:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mata J, Silva MN, Vieira PN, et al. Motivational “spill-over” during weight control: increased self-determination and exercise intrinsic motivation predict eating self-regulation. Health Psychol. 2009;28:709-716. [DOI] [PubMed] [Google Scholar]
- 26. King TK, Marcus BH, Pinto BM, Emmons KM, Abrams DB. Cognitive-behavioral mediators of changing multiple behaviors: smoking and a sedentary lifestyle. Prev Med. 1996;25:684-691. [DOI] [PubMed] [Google Scholar]
- 27. Barnett SM, Ceci SJ. When and where do we apply what we learn? A taxonomy for far transfer. Psychol Bull. 2002;128:612-637. [DOI] [PubMed] [Google Scholar]
- 28. Sweet SN, Fortier MS. Improving physical activity and dietary behaviours with single or multiple health behaviour interventions? A synthesis of meta-analyses and reviews. Int J Environ Res Public Health. 2010;7:1720-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer. 2011;105(suppl 1):S52-S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Green AC, Hayman LL, Cooley ME. Multiple health behavior change in adults with or at risk for cancer: a systematic review. Am J Health Behav. 2015;39:380-394. [DOI] [PubMed] [Google Scholar]
- 31. McAlister FA, Lawson FME, Teo KK, Armstrong PW. Randomised trials of secondary prevention programmes in coronary heart disease: systematic review. BMJ. 2001;323:957-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Cancer Institute. Dictionary of cancer terms. http://www.cancer.gov/dictionary?cdrid=445089. Accessed August 15, 2014.
- 34. Demark-Wahnefried W, Pinto BM, Gritz ER. Promoting health and physical function among cancer survivors: potential for prevention and questions that remain. J Clin Oncol. 2006;24:5125-5131. [DOI] [PubMed] [Google Scholar]
- 35. Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50:167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohamad H, McNeill G, Haseen F, N’Dow J, Craig LCA, Heys SD. The effect of dietary and exercise interventions on body weight in prostate cancer patients: a systematic review. Nutr Cancer. 2015;67:43-60. [DOI] [PubMed] [Google Scholar]
- 37. Goode AD, Lawler SP, Brakenridge CL, Reeves MM, Eakin EG. Telephone, print, and web-based interventions for physical activity, diet, and weight control among cancer survivors: a systematic review. J Cancer Surviv. 2015;9:660-682. [DOI] [PubMed] [Google Scholar]
- 38. Stacey F, James E, Chapman K, Courneya K, Lubans D. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Surviv. 2015;9:305-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011;26:1479-1498. [DOI] [PubMed] [Google Scholar]
- 40. Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins J, Green S. eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]; 2011. http://handbook.cochrane.org/. Accessed January 23, 2015.
- 41. Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs. 2005;52:546-553. [DOI] [PubMed] [Google Scholar]
- 42. 5.3 V. Review Manager (RevMan) [computer program]. Version 5.3 ed. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 43. Cohen J. A power primer. Psychol Bull. 1992;112:155-159. [DOI] [PubMed] [Google Scholar]
- 44. Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol. 2004;22:3570-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anderson DJ, Seib C, McCarthy AL, et al. Facilitating lifestyle changes to manage menopausal symptoms in women with breast cancer: a randomized controlled pilot trial of The Pink Women’s Wellness Program. Menopause. 2015;22:937-945. [DOI] [PubMed] [Google Scholar]
- 46. Bloom JR, Stewart SL, D’Onofrio CN, Luce J, Banks PJ. Addressing the needs of young breast cancer survivors at the 5 year milestone: can a short-term, low intensity intervention produce change? J Cancer Surviv. 2008;2:190-204. [DOI] [PubMed] [Google Scholar]
- 47. Bourke L, Gilbert S, Hooper R, et al. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: a randomised controlled trial. Eur Urol. 2014;65:865-872. [DOI] [PubMed] [Google Scholar]
- 48. Goodwin PJ, Segal RJ, Vallis M, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32:2231-2239. [DOI] [PubMed] [Google Scholar]
- 49. Hébert JR, Hurley TG, Harmon BE, Heiney S, Hebert CJ, Steck SE. A diet, physical activity, and stress reduction intervention in men with rising prostate-specific antigen after treatment for prostate cancer. Cancer Epidemiol. 2012;36:e128-e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hung YC, Bauer JD, Horsely P, Coll J, Bashford J, Isenring EA. Telephone-delivered nutrition and exercise counselling after auto-SCT: a pilot, randomised controlled trial. Bone Marrow Transplant. 2014;49:786-792. [DOI] [PubMed] [Google Scholar]
- 51. James EL, Stacey FG, Chapman K, et al. Impact of a nutrition and physical activity intervention (ENRICH: Exercise and Nutrition Routine Improving Cancer Health) on health behaviors of cancer survivors and carers: a pragmatic randomized controlled trial. BMC Cancer. 2015;15:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee MK, Yun YH, Park HA, Lee ES, Jung KH, Noh DY. A web-based self-management exercise and diet intervention for breast cancer survivors: pilot randomized controlled trial. Int J Nurs Stud. 2014;51:1557-1567. [DOI] [PubMed] [Google Scholar]
- 53. Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;13:1883-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hawkes AL, Chambers SK, Pakenham KI, et al. Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. J Clin Oncol. 2013;31:2313-2321. [DOI] [PubMed] [Google Scholar]
- 55. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294-1302. [DOI] [PubMed] [Google Scholar]
- 56. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005;2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277-1282. [DOI] [PubMed] [Google Scholar]
- 60. Stern JAC, Egger M, Moher D. Adressing reporting biases. In: Higgins J, Green S. eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]; 2011. http://handbook.cochrane.org/. Accessed July 18, 2016.
- 61. Moyer-Mileur LJ, Ransdell L, Bruggers CS. Fitness of children with standard-risk acute lymphoblastic leukemia during maintenance therapy: response to a home-based exercise and nutrition program. J Pediatr Hematol Oncol. 2009;31:259-266. [DOI] [PubMed] [Google Scholar]
- 62. Demark-Wahnefried W, Jones LW, Snyder DC, et al. Daughters and Mothers Against Breast Cancer (DAMES): main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer. 2014;15:2522-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Demark-Wahnefried W, Case LD, Blackwell K, et al. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin Breast Cancer. 2008;8:70-79. [DOI] [PubMed] [Google Scholar]
- 64. Djuric Z, Ellsworth JS, Weldon AL, et al. A diet and exercise intervention during chemotherapy for breast cancer. Open Obes J. 2011;3:87-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Djuric Z, DiLaura NM, Jenkins I, et al. Combining weight-loss counseling with the weight watchers plan for obese breast cancer survivors. Obes Res. 2002;10:657-665. [DOI] [PubMed] [Google Scholar]
- 66. Greenlee HA, Crew KD, Mata JM, et al. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity. 2013;21:65-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bourke L, Thompson G, Gibson DJ, et al. Pragmatic lifestyle intervention in patients recovering from colon cancer: a randomized controlled pilot study. Arch Phys Med Rehabil. 2011;92:749-755. [DOI] [PubMed] [Google Scholar]
- 68. von Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecol Oncol. 2008;109:19-26. [DOI] [PubMed] [Google Scholar]
- 69. von Gruenigen V, Frasure H, Kavanagh MB, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2012;125:699-704. [DOI] [PubMed] [Google Scholar]
- 70. Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2011;20:647-657. [DOI] [PubMed] [Google Scholar]
- 71. Ornish D, Weidner G, Fair WR, et al. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174:1065-1069. [DOI] [PubMed] [Google Scholar]
- 72. Campbell MK, Carr C, Devellis B, et al. A randomized trial of tailoring and motivational interviewing to promote fruit and vegetable consumption for cancer prevention and control. Ann Behav Med. 2009;38:71-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Demark-Wahnefried W, Clipp EC, Morey MC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from project LEAD. J Clin Oncol. 2006;24:3465-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709-2718. [DOI] [PubMed] [Google Scholar]
- 75. Kim SH, Shin MS, Lee HS, et al. Randomized pilot test of a simultaneous stage-matched exercise and diet intervention for breast cancer survivors. Oncol Nurs Forum. 2011;38:E97-E106. [DOI] [PubMed] [Google Scholar]
- 76. Steins Bisschop CN, Courneya KS, Velthuis MJ, et al. Control group design, contamination and drop-out in exercise oncology trials: a systematic review. PLoS One. 2015;10:e0120996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. O’Carroll Bantum E, Albright CL, White KK, et al. Surviving and thriving with cancer using a web-based health behavior change intervention: randomized controlled trial. J Med Internet Res. 2014;16:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vandelanotte C, Reeves MM, Brug J, De Bourdeaudhuij I. A randomized trial of sequential and simultaneous multiple behavior change interventions for physical activity and fat intake. Prev Med. 2008;46:232-237. [DOI] [PubMed] [Google Scholar]
- 79. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87-100. [DOI] [PubMed] [Google Scholar]
- 80. Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. 2009;28:690-701. [DOI] [PubMed] [Google Scholar]
- 81. Dombrowski SU, Sniehotta FF, Avenell A, Johnston M, MacLennan G, Araújo-Soares V. Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: a systematic review. Health Psychol Rev. 2012;6:7-32. [Google Scholar]
- 82. Spark LC, Reeves MM, Fjeldsoe BS, Eakin EG. Physical activity and/or dietary interventions in breast cancer survivors: a systematic review of the maintenance of outcomes. J Cancer Surviv. 2013;7:74-82. [DOI] [PubMed] [Google Scholar]
- 83. White SM, McAuley E, Estabrooks PA, Courneya KS. Translating physical activity interventions for breast cancer survivors into practice: an evaluation of randomized controlled trials. Ann Behav Med. 2009;37:10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Short CE, James EL, Stacey F, Plotnikoff RC. A qualitative synthesis of trials promoting physical activity behaviour change among post-treatment breast cancer survivors. J Cancer Surviv. 2013;7:570-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jankowski CM, Ory MG, Friedman DB, Dwyer A, Birken SA, Risendal B. Searching for maintenance in exercise interventions for cancer survivors. J Cancer Surviv. 2014;8:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Demark-Wahnefried W, Morey MC, Sloane R, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30:2354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nigg CR, Allegrante JP, Ory M. Theory-comparison and multiple-behavior research: common themes advancing health behavior research. Health Educ Res. 2002;17:670-679. [DOI] [PubMed] [Google Scholar]
- 88. Heitjan DF. Incomplete data: what you don’t know might hurt you. Cancer Epidemiol Biomarkers Prev. 2011;20:1567-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th ed. Boston, MA: Pearson Education; 2007. [Google Scholar]
- 90. Demark-Wahnefried W, Clipp EC, McBride C, et al. Design of FRESH START: a randomized trial of exercise and diet among cancer survivors. Med Sci Sports Exerc. 2003;35:415-424. [DOI] [PubMed] [Google Scholar]
- 91. Demark-Wahnefried W, Morey MC, Clipp EC, et al. Leading the Way in Exercise and Diet (Project LEAD): intervening to improve function among older breast and prostate cancer survivors. Control Clin Trials. 2003;24:206-223. [DOI] [PubMed] [Google Scholar]
- 92. Demark-Wahnefried W. Print-to-practice: designing tailored print materials to improve cancer survivors’ dietary and exercise practices in the FRESH START trial. Nutr Today. 2007;42:131-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Snyder DC, Morey MC, Sloane R, et al. Reach out to ENhancE Wellness in Older Cancer Survivors (RENEW): design, methods and recruitment challenges of a home-based exercise and diet intervention to improve physical function among long-term survivors of breast, prostate, and colorectal cancer. Psychooncology. 2009;18:429-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hawkes AL, Pakenham KI, Courneya KS, et al. A randomised controlled trial of a tele-based lifestyle intervention for colorectal cancer survivors (“CanChange”): study protocol. BMC Cancer. 2009;9:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ornish DM, Lee KL, Fair WR, Pettengill EB, Carroll PR. Dietary trial in prostate cancer: early experience and implications for clinical trial design. Urology. 2001;57(4, suppl 1):200-201. [DOI] [PubMed] [Google Scholar]
- 96. James EL, Stacey F, Chapman K, et al. Exercise and nutrition routine improving cancer health (ENRICH): the protocol for a randomized efficacy trial of a nutrition and physical activity program for adult cancer survivors and carers. BMC Public Health. 2011;11:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Conn VS, Hafdahl AR, Brown SA, Brown LM. Meta-analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Educ Couns. 2008;70:157-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Phillips SM, Alfano CM, Perna FM, Glasgow RE. Accelerating translation of physical activity and cancer survivorship research into practice: recommendations for a more integrated and collaborative approach. Cancer Epidemiol Biomarkers Prev. 2014;23:687-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gordon LG, Patrao T, Kularatna S, Hawkes AL. A telephone-delivered multiple health behaviour change intervention for colorectal cancer survivors: making the case for cost-effective healthcare. Eur J Cancer Care. 2015;24:854-861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.